Abstract
The lower cervical segments are commonly the level responsible for cervical spondylotic myelopathy; however, we rarely encounter stenosis at the upper cervical segment in a clinical setting. We assumed that there might be some differences between the pathogenetic mechanisms underlying the development of cervical canal stenosis at different segments. We performed positional MRI in the weight-bearing position for 295 consecutive symptomatic patients. All subjects were classified into four groups (A: normal; B: C3-4 stenosis; C: C5-6 stenosis; D: two-level cervical segments stenosis, stenosis at C3-4 and C5-6). Age, sagittal cervical canal diameter, cervical intervertebral disc degeneration, cervical cord compression, and cervical mobilities were evaluated for each group. Group B showed a narrow cervical spinal canal structure at the C3 to C4 pedicle levels, while groups C and D showed narrow structures at the C4 to C6 pedicle levels in the cervical spine. Additionally, the sagittal cervical canal diameters at all pedicle levels, except C7, in group D were significantly smaller than those observed in group C. We demonstrated the differences in the pathogenetic processes for the development of cervical spinal canal stenosis between C3-4, C5-6, and two-level cervical segments stenosis. Our results suggest that the developmental morphological structure of the cervical spinal canal plays an important role in the development of cervical canal stenosis at different segments. Moreover, individuals with sagittal cervical canal diameters of less than 13 mm may be exposed to an increased risk for future development of cervical spinal canal stenosis at the upper cervical segments following stenosis at the lower cervical segments.
Introduction
The most common causes of cervical spondylotic myelopathy (CSM) in patients older than 50 years of age are spondylosis or degenerative changes. Moreover, a congenitally narrow cervical spinal canal has been established as an important risk factor in the development of CSM in patients with cervical spondylosis [1–4].
Cervical spondylosis begins with intervertebral disc degeneration. Due to altered mechanical function of the disc, degenerative changes also begin to occur posteriorly in the facet joints [5–9]. The spondylotic changes in anterior structures, such as bulging, ossified, or herniated discs, as well as anterior osteophytic spurs are generally responsible for cord compression in CSM. Disorders in posterior structures, such as hypertrophy, or rarely, ossification of the ligamentum flavum or facet joints may also contribute to cord compression, but do so less commonly than disorders in the anterior structures. Spondylotic changes in the cervical spine are most prevalent at the C5-6 segment, followed by C6-7 and C4-5 [10]. The lower cervical segments are commonly the level responsible for CSM; however, we rarely encounter stenosis at the upper cervical segment (C3-4) in a clinical setting. We assumed that there might be some differences between the pathogenetic mechanisms underlying the development of cervical canal stenosis at different segments.
CSM is a disorder that is typically treated surgically. Early treatment before the onset of permanent cord injury is recommended [11]. Therefore, it is important for physicians to recognise the differences between the pathogenetic mechanisms of cervical canal stenosis at different segments for the surgical planning or management of these disorders. Numerous studies have reported the pathogenesis of cervical canal stenosis. However, to the best of our knowledge, few reports have thus far described the differences between the pathogenetic mechanisms of cervical canal stenosis at different segments. The objective of this study was to elucidate these mechanisms by using positional magnetic resonance imaging (MRI).
Materials and methods
From February 2006 to May 2007, 295 symptomatic patients (135 men and 160 women) with an average age of 43.8 years (range, 17–93 years) were examined. The subjects comprised consecutive patients experiencing neck pain with or without neurogenic symptoms induced by cervical spondylosis. None of the subjects had previously undergone spinal surgery. The Institutional Review Board approved this study.
Positional MRI
All patients underwent cervical positional MRI; the scanning was performed on a 0.6 Tesla MRI scanner (Upright Multi-Position™; Fonar Corporation, New York, NY) in the weight-bearing position.
The data obtained from the MR images were recorded on a computer for subsequent measurements, and all calculations were automatically performed using an MR analyser (True MRI Corporation, Bellflower, CA).
The sagittal diameter of the cervical cerebrospinal fluid (CSF) column at five pedicle levels (C3, C4, C5, C6, and C7) were calculated. Based on the results of previous studies [12–14], we established the sagittal diameter of the cervical CSF column at a given pedicle level to be the sagittal diameter of the cervical spinal canal at that level.
Cervical intervertebral disc
A comprehensive grading system for cervical disc degeneration was obtained by previously reported systems of classifying cervical intervertebral disc degeneration based on degenerative changes in the cervical functional spinal unit (FSU) [15]. Accordingly, T2-weighted sagittal images of 1,475 cervical intervertebral discs from 295 subjects were classified into four grades (Table 1) by the primary author and were judged eligible for inclusion in the study.
Table 1.
The grading system for cervical intervertebral disc degeneration
| Grade | Nucleus signal intensity | Disc height | Structure of FSU |
|---|---|---|---|
| 1 | Hyperintense | Normal | Without disc herniation |
| 2 | Intermediate/hypointense | Normal | With/without disc herniation |
| 3 | Hypointense | Decreased | With disc herniation/osteophyte |
| 4 | Hypointense | Collapsed | With disc herniation/osteophyte |
Cervical cord compression
We estimated cervical cord compression in each segment by examining the T2-weighted sagittal images. We defined cervical cord compression as the obliteration of the subarachnoid space in each segment resulting from compression due to disc herniation, osteophyte formation, or hypertrophy of the ligamentum flavum. Cervical cord compression in each segment was rated on a 3-point scale (range, 0–2), in which 0 indicated no cervical cord compression; 1, anterior or posterior cervical cord compression not affecting cord alignment; and 2, anterior or posterior cervical cord compression affecting cord alignment [15].
More than 1 point for each individual segment was regarded as a significant stenosis. Based on this scoring system, all subjects were classified into four groups. Group A: normal, with no stenosis at any individual segment; group B: C3-4 stenosis, with stenosis only at the C3-4 segment; group C: C5-6 stenosis, with stenosis only at the C5-6 segment; and group D: two-level cervical segments stenosis, with stenosis at least at the C3-4 and C5-6 segments.
Cervical mobility
We defined the total sagittal motion of the cervical spine as the total absolute value of the individual sagittal angular motions (C2-3 + C3-4 + C4-5 + C5-6 + C6-7) in degrees, and the contribution of each segment to the total angular mobility of the cervical spine between flexion and extension as percentage segmental mobility [(sagittal angular motion of each segment in degrees)/(total sagittal angular motion in degrees) × 100].
Statistical analysis
Mann-Whitney U tests were used for statistical analyses. A P value of less than 0.05 was considered statistically significant.
Results
Age
The subjects were classified into four groups based on the degree of cervical cord compression: Group A comprised 90 subjects with an average age of 38.6 years; group B was 14 subjects with an average age of 44.1 years; group C was 42 subjects with an average age of 42.0 years; and group D was 40 subjects with an average age of 47.9 years. The average age in groups B, C, and D tended to be higher than that in group A, and a significant difference was observed only between groups A and D (p < 0.001). Group B tended to have a higher age than group C; however, the difference was not significant. Additionally, group D had a significantly higher age than group C (p < 0.01).
Sagittal cervical canal diameter
Table 2 shows the sagittal cervical canal diameter at all pedicle levels for each group. The sagittal cervical canal diameter at the C3 and C4 pedicle levels in group B and at all pedicle levels in groups C and D were significantly smaller than those observed in group A. When the sagittal cervical canal diameters of groups B and C are compared, the value at the C3 pedicle level tended to be lower in group B, and the value at the C4 pedicle level was almost identical in both groups; the values at the other pedicle levels, i.e., C5, C6, and C7, tended to be lower in group C. However, no significant differences were observed at any pedicle level. When the sagittal cervical canal diameters of groups C and D were compared, the values at all pedicle levels, except C7, were significantly smaller in group D.
Table 2.
The sagittal cervical canal diameter at each pedicle level
| Group | C3† | C4† | C5† | C6†† | C7 |
|---|---|---|---|---|---|
| A | 14.39 ± 1.87 | 14.27 ± 1.85 | 14.29 ± 1.77 | 14.35 ± 1.81 | 14.42 ± 1.70 |
| B | 13.39 ± 3.05* | 13.39 ± 2.88* | 13.82 ± 2.65 | 13.94 ± 2.90 | 13.96 ± 2.29 |
| C | 13.75 ± 2.36* | 13.37 ± 2.08** | 13.36 ± 1.98** | 13.43 ± 1.81** | 13.83 ± 1.72* |
| D | 13.13 ± 2.27*** | 12.79 ± 2.09*** | 12.77 ± 1.95*** | 12.57 ± 2.00*** | 13.60 ± 1.74** |
Compared with group A: *p < 0.05, **p < 0.01, ***p < 0.001
Compared between groups C and D: †p < 0.05, ††p < 0.01, †††p < 0.001
In addition, structure of the cervical spinal canal in group B demonstrated narrow structure at the C3 and C4 pedicle levels, while those in groups C and D demonstrated narrow structure at the C4, C5, and C6 pedicle levels.
Cervical intervertebral disc degeneration
Table 3 shows the grade of cervical intervertebral disc degeneration at all segments for each group. The C3-4 segment in group B, the C5-6 segment in group C, and all segments, except C2-3, in group D showed significantly higher degeneration grades than the corresponding segments in group A. Significant differences were observed between the C3-4 and C5-6 segments of groups B and C. Moreover, all segments, except C2-3, in group D showed significantly higher degeneration grades than those in group C.
Table 3.
The intervertebral disc degeneration grade at each segment
| Group | C2-3 | C3-4 | C4-5 | C5-6 | C6-7 |
|---|---|---|---|---|---|
| A | 1.39 ± 0.49 | 1.58 ± 0.54 | 1.68 ± 0.63 | 1.82 ± 0.70 | 1.49 ± 0.60 |
| B | 1.43 ± 0.65 | 2.29 ± 0.73*** | 2.00 ± 0.78 | 2.00 ± 0.68 | 1.64 ± 0.63 |
| †† | †† | ||||
| C | 1.29 ± 0.46 | 1.67 ± 0.53 | 1.83 ± 0.49 | 2.60 ± 0.63*** | 1.67 ± 0.69 |
| ††† | †† | † | ††† | ||
| D | 1.45 ± 0.50 | 2.33 ± 0.62*** | 2.30 ± 0.76*** | 2.95 ± 0.78*** | 2.38 ± 0.93*** |
Compared with group A: *p < 0.05, **p < 0.01, ***p < 0.001
Compared between groups B and C or groups C and D: †p < 0.05, ††p < 0.01, †††p < 0.001
Cervical cord compression
The cervical cord compression score was 1.36 ± 0.5 at the C3-4 segment in group B, and 1.31 ± 0.47 at the C5-6 segment in group C. The scores in group D were 0.03 ± 0.16 at the C2-3 segment, 1.35 ± 0.66 at the C3-4 segment, 0.83 ± 0.9 at the C4-5 segment, 1.70 ± 0.79 at the C5-6 segment, and 0.5 ± 0.82 at the C6-7 segment. No significant difference was observed in the score at the C3-4 segment between groups B and D. However, there was a significant difference in the score at the C5-6 segment between groups C and D (p < 0.05).
Cervical mobility
Table 4 shows the cervical mobility at all segments for each group. The total angular mobility in groups B, C, and D tended to be smaller than that in group A, and a significant difference was observed only between groups A and D.
Table 4.
The sagittal mobility of the cervical spine
| Group | Total mobility (°) | Percent segmental mobility (%) | ||||
|---|---|---|---|---|---|---|
| C2-3 | C3-4 | C4-5 | C5-6 | C6-7 | ||
| A | 47.19 ± 12.81 | 15.20 ± 9.94 | 19.14 ± 11.23 | 22.68 ± 11.86 | 24.54 ± 13.63 | 18.44 ± 10.54 |
| B | 46.17 ± 13.62 | 12.22 ± 8.05 | 20.99 ± 11.49 | 23.39 ± 10.20 | 26.00 ± 14.60 | 17.41 ± 9.17 |
| C | 45.61 ± 13.54 | 16.05 ± 8.64 | 18.08 ± 10.06 | 24.54 ± 10.95 | 20.87 ± 11.94 | 20.46 ± 11.37 |
| D | 41.30 ± 10.52* | 16.22 ± 11.46 | 21.33 ± 11.51 | 25.14 ± 11.22 | 19.04 ± 12.58* | 18.27 ± 11.20 |
Compared with group A: *p < 0.05, **p < 0.01, ***p< 0.001
With respect to percent segmental mobility in groups A and B, the C5-6 segment had the highest value, followed by C4-5, while in group C, the C4-5 segment had the highest value, followed by C5-6. Moreover, in group D, the C4-5 segment had the highest value, followed by C3-4. Compared with the values in group A, only the C5-6 segment in group D showed a significant difference.
Discussion
In our study, all stenosis groups demonstrated significantly narrower cervical spinal canal at all pedicle levels when compared with the normal group, except at C5, C6, and C7 in the subjects with C3-4 stenosis. The cervical spinal canals were significantly narrower at both the upper and lower pedicle levels adjacent to all of the stenosed segments. These results affirm previous studies [1–4] that have shown that a congenitally narrow cervical spinal canal is an important risk factor in the development of cervical spinal canal stenosis.
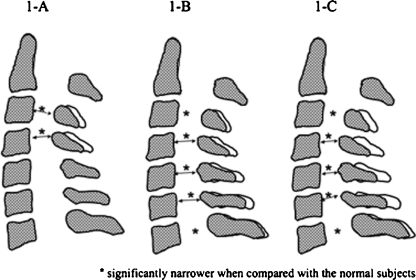
We found that the developmental morphological structure of the cervical spinal canal was different between the subjects with C3-4 stenosis and those with C5-6 stenosis. The cervical spinal canal diameter at the C4 pedicle level in both groups was almost identical; however, the values at the other pedicle levels were different. The subjects with C3-4 stenosis demonstrated significantly narrower sagittal cervical spinal canal diameters only at the C3 and C4 pedicle levels in comparison to normal subjects, and a narrow cervical spinal canal structure at the C3 to C4 pedicle levels in the cervical spine in comparison to other segments in the same subjects (Fig. 1A). In contrast, the subjects with C5-6 stenosis demonstrated significantly narrower sagittal cervical spinal canal diameters at all pedicle levels in comparison to normal subjects, and a narrow cervical spinal canal structure at the C4 to C6 pedicle levels in the cervical spine in comparison to other segments in the same subjects (Fig. 1B). The subjects with C3-4 stenosis had a significant degenerative intervertebral disc only at the C3-4 segment, while those with C5-6 stenosis had a significant degenerative intervertebral disc only at the C5-6 segment when compared with the normal subjects. These two stenosis subjects demonstrated different pathological processes for the development of cervical spinal canal stenosis.
Fig. 1.
Developmental morphological structures of the cervical spinal canal. A C3-4 stenosis. B C5-6 stenosis. C Multiple cervical segments stenosis
With regard to kinematic changes of the cervical spine, there were few changes in the distribution of the cervical segmental mobility between the subjects with C3-4 stenosis and the normal subjects, mostly at the C5-6 segment, followed by C4-5. However, changes in the distribution of the cervical segmental mobility in the subjects with C5-6 stenosis were larger, mostly at the C4-5 segment, followed by C5-6. Miyazaki et al. [16] reported that the C5-6 and C4-5 segments contributed the most to the total angular mobility of the cervical spine in the subjects with normal cervical intervertebral discs. However, the role of the C5-6 and C4-5 segments in the total angular mobility significantly decreased with severe intervertebral disc degenerative changes. They hypothesised that cervical intervertebral disc degenerative changes begin at the C5-6 and C4-5 segments because these segments withstand the largest mechanical loading. In our normal subjects, the C5-6 segment had the largest sagittal segmental mobility in the cervical spine, followed by C4-5, which was consistent with the results of Miyazaki et al. Our results demonstrated that the subjects with C3-4 stenosis had a less degenerative change at the C5-6 segment, which contributes the most to the total mobility of the cervical spine; therefore, this change may have a lesser effect on the distribution of the cervical segmental mobility. However, the subjects with C5-6 stenosis had a significant degenerative change at the C5-6 segment; therefore, the distribution of the cervical segmental mobility may be affected to a greater extent, and may shift to the upper adjacent segment, i.e., the C4-5 segment, and then to C5-6.
Mihara et al. [17] reported that pathological changes were noted at the C3-4 segment in 40.9 % of the elderly CSM patients they studied, and that this incidence was five times higher than that observed in their younger counterparts. Furthermore, they postulated that an age-related reduction in the mobility of the lower cervical segments may promote mechanical stresses on the upper cervical segments, leading to canal stenosis at the C3-4 segment. This is contradictory to our findings that the segmental mobility of C4-5, C5-6, and C6-7 segments were not reduced in the subjects with C3-4 stenosis. They only discussed the pathogenesis of C3-4 stenosis that developed following stenosis at the lower cervical segments. We also assumed that the mechanism underlying the pathogenesis of cervical spinal canal stenosis at two-level segments is different from those underlying the pathogenesis of cervical spinal canal stenosis at the C3-4 or C5-6 segments.
The morphological cervical spinal canal structure in two-level cervical segment stenosis was similar to that in C5-6 stenosis, i.e., it had a narrow cervical spinal canal structure at the C4 to C6 pedicle levels in the cervical spine. Additionally, cervical spinal canals at all pedicle levels, except C7 in two-level cervical segments stenosis were significantly narrower than those in C5-6 stenosis (Fig. 1C). The average age of the subjects with multiple cervical segments stenosis was significantly higher, and the degenerative changes in the intervertebral discs at all segments, except C2-3, showed more significant deterioration than those noted in C5-6 stenosis. Moreover, the amount of stenosis at the C5-6 segment was significantly greater in two-level cervical segments stenosis than that in C5-6 stenosis. Based on these results, we hypothesise that two-level cervical segments stenosis may develop following initial stenosis at the C5-6 segment. Those with narrower cervical spinal canals at multiple segments (less than 13 mm) may be exposed to an increased risk for degeneration in cervical FSU at multiple segments.
We previously demonstrated [15] that cervical cord compression greatly affected the sagittal segmental motion of the cervical spine. The sagittal segmental mobility at all levels was significantly reduced in the segments with severe cord compression when compared to those with no cord compression. The spinal cord may shift horizontally to prevent lesions that develop due to cord compression. However, in severe cord compression, which affects spinal cord alignment and causes cord impingement, the spinal cord cannot shift away and escape compression, and therefore may be affected by restriction of segmental motion. In this study, with severe cord compression at the C5-6 segment in the subjects with two-level cervical segments stenosis, both the sagittal segmental mobility at the C5-6 segment and total mobility of the cervical spine decreased significantly. In such cases, the distribution of segmental mobility may shift to the upper segment, mostly at the C4-5 segment, followed by C3-4. The increasing mechanical stresses on the upper cervical segments may also contribute to the development of cervical spinal canal stenosis at the upper segments.
We demonstrated the differences in the pathogenetic processes for the development of cervical spinal canal stenosis and cervical kinematics between C3-4, C5-6, and two-level cervical segments stenosis. Our results suggest that the developmental morphological structure of the cervical spinal canal plays an important role in the development of cervical canal stenosis at different segments. Moreover, individuals with sagittal cervical spinal canal diameters of less than 13 mm may be exposed to an increased risk for future development of cervical spinal canal stenosis at the upper cervical segments following stenosis at the lower cervical segments.
Nevertheless, some issues remain unresolved even in this study. We have not discussed the clinical manifestations, such as myelopathy symptoms, in each of the study groups. Therefore, using this investigation as a pilot study, further research involving a larger patient population may help resolve several issues left unanswered. Additionally, the details of the pathogenetic mechanisms of cervical spinal stenosis may be clarified further.
Acknowledgments
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Edwards WC, LaRocca H. The developmental segmental sagittal diameter of the cervical spinal canal in patients with cervical spondylosis. Spine. 1983;8:20–27. doi: 10.1097/00007632-198301000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Gore DR. Roentgenographic findings in the cervical spine in asymptomatic persons: a ten-year follow-up. Spine. 2001;26:2463–2466. doi: 10.1097/00007632-200111150-00013. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi H, Okada K, Hamada M, Tada K, Ueno R. Etiologic factors of myelopathy: a radiographic evaluation of the aging changes in the cervical spine. Clin Orthop. 1987;214:200–209. [PubMed] [Google Scholar]
- 4.Torg JS, Naranja RJ, Jr, Pavlov H, Galinat BJ, Warren R, Stine RA. The relationship of developmental narrowing of the cervical spinal canal to reversible and irreversible injury of the cervical spinal cord in football players. J Bone Joint Surg [Am] 1996;78:1308–1314. doi: 10.2106/00004623-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Bailey RW. The cervical spine. Philadelphia, PA: Lippincott-Raven; 1974. [Google Scholar]
- 6.Boijsen E. The cervical spinal canal in intraspinal expansive process. Acta Radiol. 1954;42:101–115. doi: 10.3109/00016925409175101. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Chen D, Wang X, Lu X, Guo Y, He Z, Tian H. Anterior corpectomy and fusion for severe ossification of posterior longitudinal ligament in the cervical spine. Int Orthop. 2009;33:477–482. doi: 10.1007/s00264-008-0542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DePalma A, Rothman R. The intervertebral disc. Philadelphia, PA: W. B. Saunders Co.; 1970. pp. 35–46. [Google Scholar]
- 9.Friedenberg ZB, Miller WT. Degenerative disc disease of the cervical spine: A comparative study of asymptomatic and symptomatic patients. J Bone Joint Surg [Am] 1963;45:1171–1178. [PubMed] [Google Scholar]
- 10.Heller JG (2002) Surgical treatment of degenerative cervical disc disease. Orthopaedic Knowledge Update, Spine 2. AAOS, 32, pp 299-309
- 11.Schmitt-Sody M, Kirchhoff C, Buhmann S, Metz P, Birkenmaier C, Troullier H, et al. Timing of cervical spine stabilization and outcome in patients with rheumatoid arthritis. Int Orthop. 2008;32:511–516. doi: 10.1007/s00264-007-0349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herzog RJ, Weins JJ, Dillingham MF, Sontag MJ. Normal cervical spine morphometry and cervical spine stenosis in asymptomatic professional football players. Spine. 1991;16:178–186. doi: 10.1097/00007632-199106001-00001. [DOI] [PubMed] [Google Scholar]
- 13.Okada Y, Ikata T, Yamada H, Sakamoto R, Katoh S. Magnetic resonance imaging study on the results of surgery for cervical compression myelopathy. Spine. 1993;18:2024–2029. doi: 10.1097/00007632-199310001-00016. [DOI] [PubMed] [Google Scholar]
- 14.Payne E, Spillane J. The cervical spine and anatomico-pathological study of 70 specimens with particular reference to the problem of cervical spondylosis. Brain. 1957;80:571–596. doi: 10.1093/brain/80.4.571. [DOI] [PubMed] [Google Scholar]
- 15.Morishita Y, Hida S, Miyazaki M, Hong SW, Zou J, Wei F, et al. The effect of the degenerative changes in the functional spinal unit on the kinematics of the cervical spine. Spine. 2008;33:E178–E182. doi: 10.1097/BRS.0b013e318166f059. [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki M, Hong SW, Yoon SH, Zou J, Tow B, Alanay A, et al. Kinematic analysis of the relationship between the grade of disc degeneration and motion unit of the cervical spine. Spine. 2008;33:187–193. doi: 10.1097/BRS.0b013e3181604501. [DOI] [PubMed] [Google Scholar]
- 17.Mihara H, Ohnari K, Hachiya M, Kondo S, Yamada K. Cervical myelopathy caused by C3-4 spondylosis in elderly patients. Spine. 2000;25:796–800. doi: 10.1097/00007632-200004010-00006. [DOI] [PubMed] [Google Scholar]