Abstract
The purpose of this study was to evaluate the relationship between the pelvic osteolytic volume on computed tomography (CT) and clinical outcome in patients with cementless acetabular components. We reviewed 87 patients (104 hips) who met the following inclusion criteria: (1) there was evidence of pelvic osteolysis on CT at a minium of five years postoperatively, (2) all cups and stems were radiographically stable at the time of CT, (3) the follow-up period after CT was a minimum of two years clinically. The mean pelvic osteolytic volume was 2.3 ± 6.9 cm3. The mean Harris hip score (HHS) at CT was 92.3 ± 7.9 points. Inversely moderate correlation (r = −0.569, P < 0.05) was found between the HHS at CT and pelvic osteolytic volume. In ten cases of hips with acetabular revisions, the mean pelvic osteolytic volume was 16.3 ± 26.9 cm3. The mean HHS at CT and HHS at reoperation was 87.6 ± 9.2 points and 73.4 ± 8.8 points, respectively, with significant difference (P < 0.05). The area under curve (ROC) analysis showed that the optimal cutoff value of the osteolytic volume was 4.8 cm3 with 100% each for sensitivity and specificity. We conclude that the amount of pelvic osteolytic volume on CT may be used to guide treatment decision-making in patients with well-fixed cementless acetabular components who show evidence of pelvic osteolysis.
Introduction
Periprosthetic osteolysis is primarily induced by the presence of wear particles and is potentiated by mechanical factors [1, 2]. Wear particles are thought to initiate the biological reactions that lead to increased bone resorption and decreased bone formation [3], and many types of cells have been involved in inducing the osteolytic response [4]. The entire pathway is not well understood. Osteolysis can eventually produce implant instability. Implant instability can accelerate the inflammatory response and destabilise the implant [5, 6], and many efforts have been made to reduce wear particles. The progression of pelvic osteolysis generally makes bone defects more serious, and the revision process more difficult, and is the most important complication in terms of implant longevity [7, 8].
Compared with cemented acetabular cup fixation cases, cementless acetabular cup fixation shows different patterns of pelvic osteolysis [7]. It is clinically silent until huge bone defects cause the loosening of the acetabular components and pain develops clinically [1].
It is therefore necessary to detect pelvic osteolyis for patients to reduce their suffering, to decrease the expense for treatment, and to improve treatment outcomes [8, 9]. Plain radiographs are less sensitive than computed tomography (CT) scans [10, 11], because of factors such as lesion location, patient size, position of the pelvis, and magnification [12, 13]. Currently, CT scan with metal artifact reducing software is considered to be the standard to detect pelvic osteolysis and evaluate the shape, volume, and location of pelvic osteolysis [10, 11]. There has been a surveillance algorithm [14] for monitoring pelvic osteolysis following total hip arthroplasty (THA); however, there is no general treatment guideline dealing with the timing of reoperation in the well-fixed patients with established pelvic osteolysis on CT scan.
In this study, we examined the correlation between pelvic osteolysis on CT and clinical function. We also followed-up the patients clinically for a minimum of two years and observed their clinical outcomes. The aims of the study were to evaluate the amount of pelvic osteolytic volume on CT which can be a clinical risk factor for reoperation in patients with well-fixed cementless acetabular cups.
Materials and methods
Ninety-seven patients (118 hips) who had undergone primary THA between January 1996 and February 2001 at our hospital were given CT scans at a minimum of five-years postoperatively between April 2006 and June 2006. All CT scans were acquired using the same machine. All the acetabular components had hemispherical, porous-coated titanium shells with multiple holes (Harris-Galante cup, Zimmer, Warsaw, USA) and all the femoral heads were of cobalt-chrome alloy with a diameter of 28 mm. In 63 hips, polyethylene (PE) liners used were ultra high molecular weight polyethylene sterilised by gamma radiation in air; and highly cross-linked PE sterilised with gas plasma [15] was used in the remaining 55 hips. Among these patients, we retrospectively reviewed 87 patients (104 hips) who met the following inclusion criteria: (1) there was evidence of pelvic osteolysis on CT scans, (2) all cups and stems were radiographically stable at the time of CT scans, and (3) the follow-up period after CT scans was a minimum of two years clinically. We considered acetabular revisions during follow-up as a failure, whereas we excluded patients who had liner exchange and/or bone grafting as part of a femoral revision. The male/female ratio was 57/30, and mean patient age at the time of surgery was 48.5 years (range, 21–65 years). The most common reason for THA was avascular necrosis (AVN) of the femoral head in 78 hips (75%). The mean time interval between surgery and CT scans was 81.4 months (range, 61–146 months). The mean follow-up period after CT scans was 30 months (range, 6–37 months). This study was approved by our local institutional review board and all patients provided informed consent.
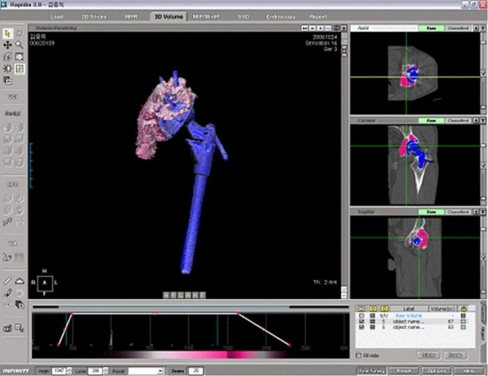
All CT images were acquired using a 16-channel multidetector row CT (Sensation 16; Siemens Medicals, Erlangen, Germany) using a detector collimation of 16 × 0.75 mm, a tube energy and current of 120 kV and 250 mAs, respectively, and a 0.7-mm beam pitch in Osteo scanning mode. Areas from 5 cm above the dome of the acetabular cup to the distal limit of the ischium were evaluated. Display section thicknesses were 1 or 2 mm and image thicknesses for secondary raw data used for multiplanar reformatting were 0.5 or 1 mm. The images were obtained in the axial plane, and coronal and sagittal planes were reconstructed from the data obtained. A medium bone algorithm which is bone kernel with a mid range edge enhancement algorithm was used to correct for image degradation caused by hip prostheses. A surgeon and a radiologist, who were unaware of the radiographic results, performed measurements independently using 3D software (Rapidia 2.8; Infinite, Seoul, Korea). Interpretation was done independently and interobserver reliability in measuring osteolysis, as expressed by Cronbach’s alpha value, were 0.93. Pelvic osteolysis was defined as any sharply demarcated area adjacent to the socket or screws without osseous trabecular defects, and was classified as osteolysis when it met the following criteria [16, 17]: (1) a well-defined sclerotic border, (2) a clear communication between the defect and the cup, (3) the absence of trabecular bone, and (4) bone defects present on CT scans that were not evident on the six-week postoperative radiographs. Bone defects seen on the CT scan and the six-week postoperative radiographs were defined as pre-existing lesions. Criterion (1) is excluded if the lesions are located at the most inferior or superior area. The software automatically computed the total volumes of pelvic bone defects from segmented areas of osteolysis, measured on slices, and CT image slice thickness (Fig. 1).
Fig. 1.
Measurement of osteolytic volume on computed tomography scan
Linear PE wear was determined as described by Dorr and Wan [18] using digitalised AP radiographs. For the Dorr and Wan method, radiographs were magnified to 200%, printed, and readings taken with digital caliper with an accuracy of 0.01 mm (digimatic calipers, Mitutoyo Corporation, Kawasaki, Kanagawa, Japan). This method uses only the edge of a ball along the face of a cup, because it is almost always well visualised, and is a convenient method for determining PE wear after THA with cementless acetabular cups [19, 20]. This method has a reported accuracy of 0.6 mm for linear wear [19]. A study showed no statistically significant difference between the mean errors of clinical measurement of PE wear with Dorr and Wan, Livermore, computerised Polyware, and Hip 32 Suite analysis methods [19].
Clinical functions were evaluated using Harris hip score (HHS) [21]. HHS was checked during preoperative assessment for the primary hip surgery, CT scan taken, and last follow-up.
Correlation analysis was undertaken using Spearman rank correlation coefficient to assess the relationship between HHS and pelvic osteolytic volume. Receiver operating characteristic (ROC) analysis was used to determine optimal cut-off values of various pelvic osteolytic volumes in order to predict reoperation. It was made using measures of sensitivity and specificity based on these various values. We also compared area under curve (AUC) for four types of ROC curves, which were plotted by HHS, total wear amount, annual wear rate, and osteolytic volume. Statistical analysis was performed using SAS software (version 9.12, SAS, Cary, NC), and P values of < 0.05 were considered statistically significant.
Results
The mean pelvic osteolytic volume of the 87 patients (104 hips) on CT scans was 2.3 ± 6.9 cm3 (range, 0.2–61.7 cm3). Of these, 75 hips had pelvic osteolytic volumes of less 1 cm3, 19 had volumes between 1 and 6 cm3, and ten had volumes greater than 6 cm3. The mean annual linear wear rate was 0.08 ± 0.06 mm/year (range, 0–0.27 mm/year). The mean linear wear in 63 hips with conventional PE was 0.11 ± 0.07 mm/year (range, 0.01–0.27 mm/year), whereas it was 0.05 ± 0.04 mm/year (range, 0–0.20 mm/year) in 55 hips with highly cross-linked PE (p = 0.001). The mean HHS at CT scan was 92.3 ± 7.9 points (range, 74–100 points). Inversely moderate correlation (r = -0.569, P < 0.05) was found between the HHS at CT scan and pelvic osteolytic volume.
Of the 87 patients (104 hips) who showed pelvic osteolysis on CT scans, 14 patients (15 hips) (14.4%) underwent acetabular and/or femoral revisions during the follow-up, including stem change with liner exchange for stem loosening in five hips, cup change for cup loosening in two hips, cup and stem change for both loosening in three hips, and liner exchange with bone grafting for aggravated hip pain in five hips.
In ten cases of hips with acetabular revisions (Table 1), the mean time interval between THA and reoperation was 142 ± 13.2 months (range, 102–234 months), and the mean time interval between CT scan and reoperation was 13.3 ± 4.7 months (range, 6–29 months). The mean pelvic osteolytic volume was 16.3 ± 26.9 cm3 (range, 6.1–61.7 cm3). The mean pelvic osteolytic volume in cases of cup change in five hips was 24.7 ± 13.4 cm3 (range, 8.0–61.7 cm3) and was 7.2 ± 3.2 cm3 (range, 6.1–11.7 cm3) in cases of liner exchange with bone grafting in five hips. The mean annual linear wear rate was 0.17 ± 0.09 mm/year (range, 0.02–0.27 mm/year). The mean HHS at CT scan and HHS at reoperation was 87.6 ± 9.2 points (range, 74–98 points) and 73.4 ± 8.8 points (range, 59–85 points), respectively, with significant difference (P < 0.05).
Table 1.
Summary of failure cases
| Case number | Gender/Age | HHS | Indication | Revision | Osteolysis (cm3) |
|---|---|---|---|---|---|
| 1 | F/56 | 98/74 | Aggravated pain | Liner exchange (15 cc B.G) | 6.1 |
| 2 | F/61 | 84/74 | Aggravated pain | Liner exchange (90 cc B.G) | 6.4 |
| 3 | M/54 | 91/85 | Aggravated pain | Liner exchange (120 cc B.G) | 7.0 |
| 4 | F/48 | 82/78 | Both loosening | Cup change (60 cc B.G) | 8.0 |
| 5 | F/48 | 88/59 | Aggravated pain | Liner exchange (90 cc B.G) | 8.6 |
| 6 | F/61 | 94/77 | Aggravated pain | Liner exchange (90 cc B.G) | 11.7 |
| 7 | M/65 | 88/74 | Both loosening | Cup change (60 cc B.G) | 13.4 |
| 8 | M/63 | 74/56 | Both loosening | Cup change (180 cc B.G) | 19.4 |
| 9 | M/49 | 91/82 | Cup loosening | Cup change (120 cc B.G) | 21.0 |
| 10 | M/55 | 86/75 | Cup loosening | Cup change (150 cc B.G) | 61.7 |
M male, F female, HHS Harris hip score
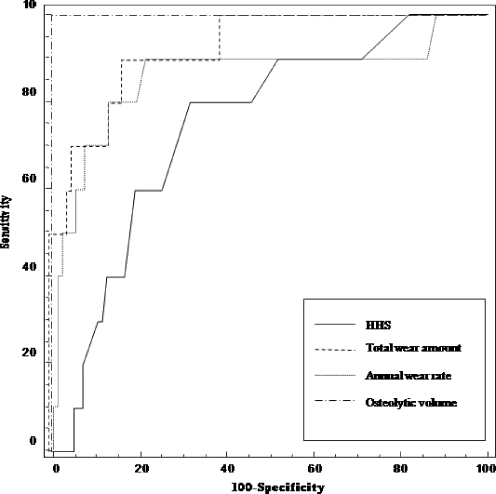
ROC analysis showed that the optimal cutoff value of the osteolytic volume was 4.8 cm3 with 100% each for sensitivity and specificity. ROC curve of ostolytic volume had the greatest AUC (1.00; 95% CI, 0.97–1.00) and that of total wear amount was the second (92%) (Fig. 2). The difference between these two AUCs was not statistically significant (p = 0.079).
Fig. 2.
Four types of receiver operation characteristic curves used to determine optimal cutoff value for the prediction of acetabular reoperation. The area under the curve of osteolytic volume was 1.00
Discussion
Both anteroposterior and oblique radiographs of the hips provide the most convenient means of screening for detection of osteolysis because of the lower cost and easy accessibility [22]. However, pelvic osteolysis has been underdiagnosed in radiographs [10]. Currently, CT scan with metal artifact-reducing software is considered to be the most sensitive method for diagnosing the presence and location of osteolysis and the most accurate method for measuring osteolytic volume [10, 11]. Egawa et al. [23] reported a strong correlation (r = 0.93, r2 = 0.87) between the actual volume of pelvic osteolysis measured on CT scan and osteolytic volumes calculated on radiographs. However, values calculated on radiographs deviated from osteolytic volumes measured on CT scan; therefore, it was concluded that CT is the preferred diagnostic modality in terms of determining accurate pelvic osteolytic volumes.
The pelvic osteolytic volumes (mean, 2.3 cm3) in our recent study [22] were substantially lower than those found in several other studies (Table 2) [10, 16, 23–25], and this is most likely due to multiple factors. These include the fact that our study population was not composed of a high risk group for osteolysis [14], the time interval between THAs and CT scans was shorter, and the wear amounts were lower compared to other established studies. Additionally, the type of polyethylene liner could affect the result of lower pelvic osteolytic volumes in our study. Currently, the clinical protocol for patients who have had THA done at our institution includes obtaining CT scans according to the surveillance algorithm proposed by Stulberg et al. [14].
Table 2.
Case study comparison
| Study | Polyethylene liner | CT scan /Osteolysis No. of hips) | Time interval (years) | Mean osteolysis (cm3) | Wear rate |
|---|---|---|---|---|---|
| Egawa et al. [23] | UHMWPE | 78/40 | 7.7 | 19 | 0.24 mm/year |
| Howie et al. [24] | UHMWPE | 35/32 | 15 | 12.6 | 56 mm3/year |
| Kitamura et al. [16] | UHMWPE | 126/116 | 10.9 | 6.4 | 0.17 mm/year |
| Puri et al. [10] | UHMWPE | 50/26 | 7.6 | 4.9 | 0.14 mm/year |
| Looney et al. [25] | UHMWPE | 20/20 | 10.8 | 3 | 94 mm3/year |
| Our study [20] | 55 X-linked/ 63 UHMWPE | 118/104 | 6.8 | 2.3 | 0.08 mm/year |
Many published data have shown that pelvic osteolysis had no correlation with clinical function [26–28]. In particular, pelvic osteolysis in cementless acetabular fixation cases may be clinically asymptomatic until loosening occurs [1]. A few published studies have shown a correlation between pelvic osteolysis and clinical function, but the results are rather ambiguous [25, 29]. Looney et al. [25] and Schwarz et al. [29] reported that HHS tends to correlate inversely with CT scan measures of pelvic osteolysis, but this correlation did not reach significance (r = −0.418, P = 0.066). In our study, pelvic osteolytic volume tended to correlate inversely with the HHS (r = −0.569, P < 0.05); however, this did not reach significance. Schwarz et al. [29] reported progression of osteolysis for over one year in 20 patients with established pelvic osteolysis, and Howie et al. [24] found that hips with larger osteolytic lesions on CT scan were most likely to show progression. We also found a significant difference between HHS at CT scan and HHS at reoperation in the failure group (>6 cm3 pelvic osteolytic volumes). Total hip arthroplasty is a successful operation because of pain relief. Pain disappears because the natural symptomatic joint is replaced with a neuropathic spacer functioning within a foreign body bursa; and clinical functions, mainly pain, can only arise from living tissues, e.g. bone or muscle. Pelvic osteolysis can produce microscopic implant instability if it will progress. Microscopic implant instability can accelerate the inflammatory response, and then destabilise the implant macroscopically [5, 6]. It causes pain due to inflammation and mechanical irritation around the periprosthethic tissues. Our results suggested that pelvic osteolysis may be clinically asymptomatic until a threshold amount of bone loss occurs, which then produces implant instability macroscopically which reduces clinical function. In our study, however, there was no specification of the items of HHS, and no serial CT scans to confirm this highly plausible theory. Additional investigation is obviously needed.
Our study focused on which amount of pelvic osteolytic volume on CT scan can clinically be the risk factor for reoperation in patients who had well-fixed cementless acetabular cup and had no clinically important pain or other symptoms. The patterns and progression of pelvic osteolysis are evidently different between cemented and cementless acetabular cup fixation cases [7, 26]. For cemented acetabular components, a generalised-linear pattern of pelvic osteolysis develops at the cement–bone interface and progresses towards the dome of the acetabular component. As a consequence, pelvic osteolysis compromises implant stability, and a revision procedure is frequently inevitable. On the other hand, for cementless acetabular components, a localised-expansive pattern of pelvic osteolysis develops at the cup–bone intersurface and extensive bone loss can occur without affecting implant stability. These patients who have “silent osteolysis” can clinically be asymptomatic despite significant pelvic bone destruction [8, 10]. Maloney et al. [26] classified cementless acetabular component, which had evidence of pelvic osteolysis, into three categories based on the radiographic stability of porous-coated shells, and examined the possibility of retaining the shell and substituting a polyethylene liner for surgical treatment of pelvic osteolysis. This principle may now be applied to patients who have neither symptoms nor evidence of osteolysis on radiographs, but have evidence of osteolysis on CT scans [10]. The early recognition of pelvic osteolysis may increase the likelihood of successful medical or surgical intervention [8, 9]. Nevertheless, the proper timing of surgical intervention under a well-fixed porous-coated socket is debatable, and little information is available to guide treatment in the face of this growing problem [26]. In our study, clinical outcomes were found to be associated positively with the amount of pelvic osteolytic volumes (AUC, 100%). Interestingly, all failures (ten cases) occurred in the cases of pelvic osteolytic volumes of >6 cm3 (Figs. 3 and 4). The optimal cutoff value of pelvic osteolytic volume for the prediction of acetabular reoperation was 4.8 cm3 with 100% sensitivity and 100% specificity. Howie et al. [24] reported that larger lesions (>10 cm3) were more likely to progress when compared with smaller lesions around cementless acetabular components. These results imply that large lesions are associated with a high risk of progression and the size of pelvic osteolytic volumes can be considered as one of the guidelines for surgical intervention. However, associations other than these variables may also exist, and a two-year follow-up period may be too short to establish such associations. With serial CT scans and longer follow-up periods in more patients, the ability to detect associations would be strengthened.
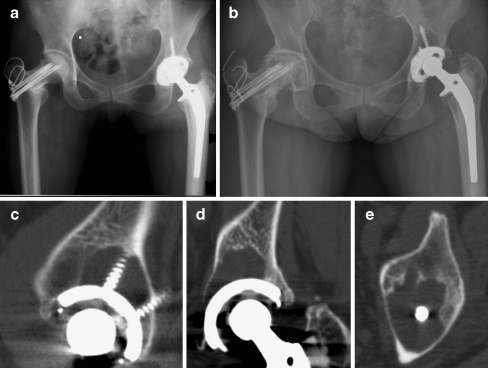
Fig. 3.
A 48-year-old woman who underwent total hip arthroplasty in the left hip joint. a Initial postoperative (left) radiograph of the hip. b Postoperative 12.5-year radiograph of the hip. Harris hip score was 88 points on the left side. c–e Selected images (sagittal, coronal, and axial) from the computed tomography scan showing areas of osteolysis around the acetabular component. Total pelvic osteolytic volume was 8.6 cm3
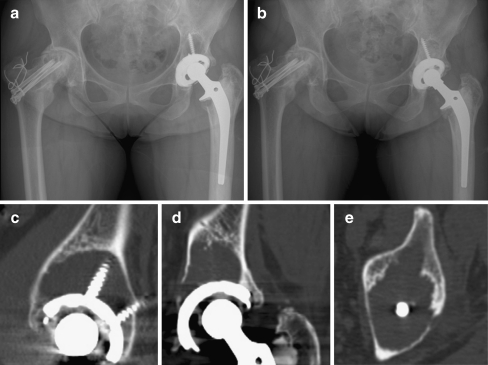
Fig. 4.
A 48-year-old woman who underwent total hip arthroplasty in the left hip joint. a Postoperative 14.7-year radiograph of the hip in the same patient. Harris hip score was 59 points in the left side. b Liner exchange with bone graft was performed c–e Selected images (sagittal, coronal, and axial) from the computed tomography scan showing increased areas of osteolysis around the acetabular component compared to Fig. 1. c–e Total pelvic osteolytic volume was 22 cm3
Compared with pelvic osteolytic volumes (mean, 7.2 cm3; range 6.1–11.7 cm3) in five cases of liner exchange with bone graft, pelvic osteolytic volumes (mean, 24.7 cm3; range, 8.0–61.7 cm3) in five cases of cup change were very large. These results suggest that the greater the pelvic osteolytic volumes, the more the extensive surgery and that an early recognition of osteolysis may decrease the extent of surgery.
Our study had several limitations. First, we could not confirm the presence or measure the volumes of pelvic osteolytic lesions during reoperation or in retrieved specimens. Nevertheless, a previous study has established that CT scan provides an accurate means of assessing osteolysis [17]. Second, we did not examine correlations between clinical function and location of pelvic osteolysis [16] or cup surface involved area of pelvic osteolysis [29], which might influence clinical function. Third, the magnification factor used to measure pelvic osteolysis in our study was different from those used in previous studies [10, 11]. Lastly, we only used the HHS in the analysis of clinical functions, and did not specify the items of HHS.
In summary, this study demonstrates that pelvic osteolysis may be clinically asymptomatic until a threshold amount of bone loss occurs. Furthermore, the amount of pelvic osteolytic volume on CT scan may be used to guide treatment decision-making in patients with well-fixed cementless acetabular components that show evidence of pelvic osteolysis.
References
- 1.Schmalzried TP, Guttmann D, Grecula M, Amstutz HC. The relationship between the design, position, and articular wear of acetabular components inserted without cement and the development of pelvic osteolysis. J Bone Joint Surg Am. 1994;76(5):677–688. doi: 10.2106/00004623-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;460:240–252. doi: 10.1097/BLO.0b013e31804b4147. [DOI] [PubMed] [Google Scholar]
- 3.Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3–16. [PubMed] [Google Scholar]
- 4.Goodman SB, Huie P, Song Y, Lee K, Doshi A, Rushdieh B, Woolson S, Maloney W, Schurman D, Sibley R. Loosening and osteolysis of cemented joint arthroplasties. A biologic spectrum. Clin Orthop Relat Res. 1997;337:149–163. doi: 10.1097/00003086-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Jones LC, Frondoza C, Hungerford DS. Effect of PMMA particles and movement on an implant interface in a canine model. J Bone Joint Surg Br. 2001;83(3):448–458. doi: 10.1302/0301-620X.83B3.10734. [DOI] [PubMed] [Google Scholar]
- 6.Skoglund B, Aspenberg P. Pmma particles and pressure—a study of the osteolytic properties of two agents proposed to cause prosthetic loosening. J Orthop Res. 2003;21(2):196–201. doi: 10.1016/S0736-0266(02)00150-X. [DOI] [PubMed] [Google Scholar]
- 7.Zicat B, Engh CA, Gokcen E. Patterns of osteolysis around total hip components inserted with and without cement. J Bone Joint Surg Am. 1995;77(3):432–439. doi: 10.2106/00004623-199503000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Lavernia CJ. Cost-effectiveness of early surgical intervention in silent osteolysis. J Arthroplasty. 1998;13(3):277–279. doi: 10.1016/S0883-5403(98)90172-3. [DOI] [PubMed] [Google Scholar]
- 9.Engelbrecht DJ, Weber FA, Sweet MB, Jakim I. Long-term results of revision total hip arthroplasty. J Bone Joint Surg Br. 1990;72(1):41–45. doi: 10.1302/0301-620X.72B1.2298793. [DOI] [PubMed] [Google Scholar]
- 10.Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84-A(4):609–614. doi: 10.2106/00004623-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura N, Pappedemos PC, Duffy PR, 3rd, Stepniewski AS, Hopper RH, Jr, Engh CA, Jr, Engh CA. The value of anteroposterior pelvic radiographs for evaluating pelvic osteolysis. Clin Orthop Relat Res. 2006;453:239–245. doi: 10.1097/01.blo.0000246554.41058.8d. [DOI] [PubMed] [Google Scholar]
- 12.Jerosch J, Steinbeck J, Fuchs S, Kirchhoff C. Radiologic evaluation of acetabular defects on acetabular loosening of hip alloarthroplasty. Unfallchirurg. 1996;99(10):727–733. doi: 10.1007/s001130050048. [DOI] [PubMed] [Google Scholar]
- 13.Wenz JF, Hauser DL, Scott WW, Robertson DD, Tsapakos MJ, Kearney DK, Bluemke DA, Naiman DO, Brooker AF, Chao EY. Observer variation in the detection of acetabular bone deficiencies. Skeletal Radiol. 1997;26(5):272–278. doi: 10.1007/s002560050234. [DOI] [PubMed] [Google Scholar]
- 14.Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: An algorithm for surveillance. J Bone Joint Surg Am. 2002;84-A(Suppl 2):116–122. doi: 10.2106/00004623-200200002-00016. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs CA, Christensen CP, Greenwald AS, McKellop H. Clinical performance of highly cross-linked polyethylenes in total hip arthroplasty. J Bone Joint Surg Am. 2007;89(12):2779–2786. doi: 10.2106/JBJS.G.00043. [DOI] [PubMed] [Google Scholar]
- 16.Kitamura N, Leung SB, Engh CA., Sr Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop Relat Res. 2005;441:291–297. doi: 10.1097/01.blo.0000192359.12573.15. [DOI] [PubMed] [Google Scholar]
- 17.Leung S, Naudie D, Kitamura N, Walde T, Engh CA. Computed tomography in the assessment of periacetabular osteolysis. J Bone Joint Surg Am. 2005;87(3):592–597. doi: 10.2106/JBJS.D.02116. [DOI] [PubMed] [Google Scholar]
- 18.Dorr LD, Wan Z. Ten years of experience with porous acetabular components for revision surgery. Clin Orthop Relat Res. 1995;319:191–200. [PubMed] [Google Scholar]
- 19.Ebramzadeh E, Sangiorgio SN, Lattuada F, Kang JS, Chiesa R, McKellop HA, Dorr LD. Accuracy of measurement of polyethylene wear with use of radiographs of total hip replacements. J Bone Joint Surg Am. 2003;85-A(12):2378–2384. doi: 10.2106/00004623-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Wan Z, Boutary M, Dorr LD. Precision and limitation of measuring two-dimensional wear on clinical radiographs. Clin Orthop Relat Res. 2006;449:267–274. doi: 10.1097/01.blo.0000218758.35181.93. [DOI] [PubMed] [Google Scholar]
- 21.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: Treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737–755. [PubMed] [Google Scholar]
- 22.Shon WY, Gupta S, Biswal S, Han SH, Hong SJ, Moon JG. Pelvic osteolysis relationship to radiographs and polyethylene wear. J Arthroplasty. 2009;24(5):743–750. doi: 10.1016/j.arth.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Egawa H, Powers CC, Beykirch SE, Hopper RH, Jr, Engh CA, Jr, Engh CA. Can the volume of pelvic osteolysis be calculated without using computed tomography? Clin Orthop Relat Res. 2009;467(1):181–187. doi: 10.1007/s11999-008-0522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howie DW, Neale SD, Stamenkov R, McGee MA, Taylor DJ, Findlay DM. Progression of acetabular periprosthetic osteolytic lesions measured with computed tomography. J Bone Joint Surg Am. 2007;89(8):1818–1825. doi: 10.2106/JBJS.E.01305. [DOI] [PubMed] [Google Scholar]
- 25.Looney RJ, Boyd A, Totterman S, Seo GS, Tamez-Pena J, Campbell D, Novotny L, Olcott C, Martell J, Hayes FA, O'Keefe RJ, Schwarz EM. Volumetric computerized tomography as a measurement of periprosthetic acetabular osteolysis and its correlation with wear. Arthritis Res. 2002;4(1):59–63. doi: 10.1186/ar384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maloney WJ, Paprosky W, Engh CA, Rubash H. Surgical treatment of pelvic osteolysis. Clin Orthop Relat Res. 2001;393:78–84. doi: 10.1097/00003086-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Hozack WJ, Mesa JJ, Carey C, Rothman RH. Relationship between polyethylene wear, pelvic osteolysis, and clinical symptomatology in patients with cementless acetabular components. A framework for decision making. J Arthroplasty. 1996;11(7):769–772. doi: 10.1016/S0883-5403(96)80175-6. [DOI] [PubMed] [Google Scholar]
- 28.Schmalzried TP, Fowble VA, Amstutz HC. The fate of pelvic osteolysis after reoperation. No recurrence with lesional treatment. Clin Orthop Relat Res. 1998;350:128–137. doi: 10.1097/00003086-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz EM, Campbell D, Totterman S, Boyd A, O'Keefe RJ, Looney RJ. Use of volumetric computerized tomography as a primary outcome measure to evaluate drug efficacy in the prevention of peri-prosthetic osteolysis: A 1-year clinical pilot of etanercept vs. placebo. J Orthop Res. 2003;21(6):1049–1055. doi: 10.1016/S0736-0266(03)00093-7. [DOI] [PubMed] [Google Scholar]