Abstract
Purpose
For many years, our laboratory has been investigating different biological substrates for the effects of electromagnetic stimulation proposed in orthopaedic treatments. The results show an acceleration of differentiation at the expense of proliferation. This study using microarray analysis is focused on the cellular mechanisms involved.
Methods
A microarray analysis (Affymetrix) allowing the screening of the expression of 38,500 genes was used on epidermal cells sampled from three different human donors and distributed within each donor in seven groups of 12 explants, stimulated at different times, to compare control. Modifications of the expression of BMP-2, 4 and 7 were studied at days four, seven and 12.
Results
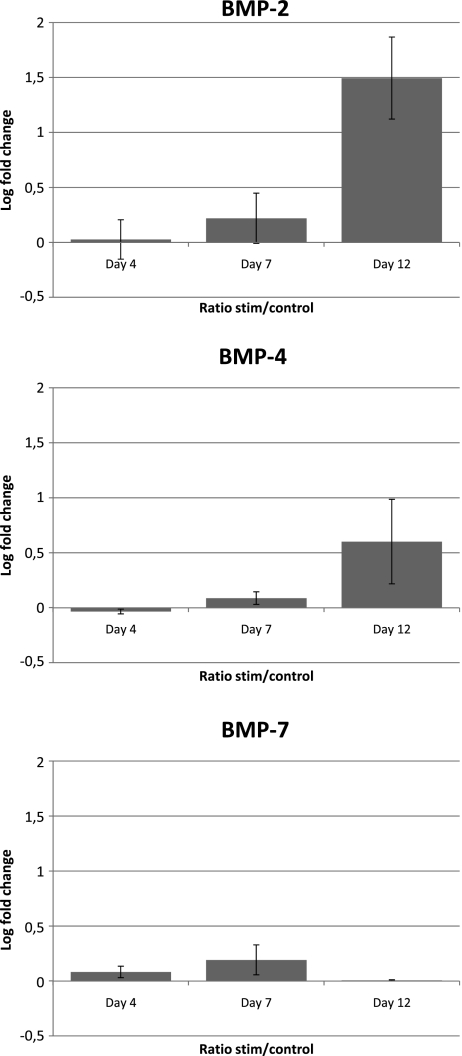
The expression of BMP-2 was significantly increased at day 12 on the stimulated samples. J4 and J7 did not show any significant difference nor did the expression of BMP-4 and 7 at the different times.
Conclusion
The results obtained in previous experiments on cellular substrates, bone embryonic tissue and clinical series were all consistent with the increase of BMP-2. Other publications have confirmed an increase of BMP-2 under electric or electromagnetic stimulation. The increase of BMP-2 appears as an effect of the electromagnetic field stimulations applied in orthopaedics. This observation contributes towards possible indications and a better understanding of the cellular mechanism.
Introduction
During the 1980s, the stimulation of bone healing by electromagnetic fields [1] or electric fields [8] gained a significant interest in the orthopaedic community. Too many indications and sometimes empirical applications led to confusing clinical results. In our clinical studies a few observations encouraged us to explore more fundamental aspects of this stimulation on in vitro and in vivo embryonic bone tissue and on animal models of fresh fracture [19, 20].
The results using low frequency and low amplitude electromagnetic fields with a carrier frequency of 4 KHz (pulse train) modulated by a fundamental frequency of 15 Hz shows:
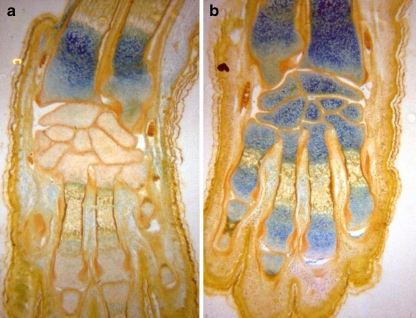
In limb buds of mice embryos exposed in vitro, an increased concentration of acid glycosaminoglycans in the cartilaginous matrix of bones [14, 19, 28] (Fig 1);
In chicken embryos exposed in vivo, a relative acceleration in the ossification at the primary ossification point [17, 19, 29];
In quail embryos, a relation between the ossification rate and the amplitude of local electric fields [18, 19].
These results and the observations of clinical studies suggest an acceleration of the maturation of the cartilaginous matrix preceding the ossification. This explains the good results obtained by the stimulation of hypertrophic non union of the tibia with a pre-existing fibrocartilage and the weak results obtained on fresh fractures [13, 15, 16].
Fig. 1.
Mice embryos, distal epiphysis of the forearm, carpal and metacarpal bones in control (a) and exposed to electromagnetic field (b). More pronounced blue coloration represents a higher concentration of acid glycosaminoglycans (Hale’s method: colloidal iron) [19, 28]
In parallel to these earlier studies on bone tissue, a cytofluorometry analysis after cell exposure shows an increase in RNA production and modification of the DNA configuration [9, 10, 12].
To analyse further the effect on cell differentiation during the healing process, we used a more simple protocol using in vitro culture of human epidermal cells exposed to a low frequency electric field. The results showed a decrease in the growth area surrounding the explant, a better stratification of the keratinocyte and a decreased percentage of cells marked with H3-thymidin. These observations confirmed the same effect observed previously on bone tissue: an acceleration of the maturation at the expense of proliferation [21]. A recent study on the same biological substrate using microarray, the analysis of the mRNA expression of 38,500 genes confirms the activation of cellular pathway involved in differentiation [11]. In this paper, we investigated the effect on BMP-2, 4 and 7 using the same protocol.
Materials and methods
The biological model, the stimulation device and the experimental protocol were exhaustively described in previous publications [11, 21]. Human epidermal cells from three different subjects were cultured in vitro on dermal support close to physiologic conditions. Eighty-four explants from each subject were divided into control and exposed groups and distributed in 14 Petri dishes. Samplings for microarray analysis were done at days 1, 4, 7 and 12. After sampling, the total RNA was extracted from a pool of 12 explants in each sampling condition.
Stimulation is realised with a generator producing a biphasic, asymmetric, charge-balanced signal with a carrier frequency of 40 Hz and a peak current amplitude of 20 mA. The stimulus is repeated every four seconds followed by a four second break for 40 minutes per day for 11 days.
Microarray experiments and part of the data analysis were performed by PartnerChip (Evry, France) following the procedure recommended by Affymetrix (Santa Clara, CA). The gene expressions are analysed using Affymetrix microarray U133 Plus 2.0 chips. Quality control was assessed based on 3′/5′ ratios of glyceraldehyde 3-phosphate dehydrogenase and b-actin control probe sets.
Normalisation and statistical analysis of microarray data were performed for variance analysis (ANOVA) and k-means analysis. ANOVA analyses were conducted on the results of control samples (J1control, J4control, J7control, J12control) and on the results of stimulated samples (J1control, J4stim, J7stim, J12stim). Probe sets were defined as differentially expressed for one of the J(1, 4, 7, or 12)stim versus J1control or J(1, 4, 7, or 12)stim versus J(1, 4, 7, or 12)control time points, if the fold change (FC) was ≥ 2 or ≤ −2 and the P-value was ≤ 0.05 after unpaired t-test.
Real-time rtPCR was used to validate microarray data on TXNRD1, ATF3, MME, DKK1, and MACF1 genes. They showed a good correlation with microarray results [11]. All these genes play roles in the proliferation or differentiation mechanism.
Results
In agreement with our previous observations, the global analysis shows an increased activity of some cellular pathways involved in cell differentiation [11]:
The analysis of gene expression at the three stimulated times compared with their three respective controls shows three genes (TXNRD1, ATF3, MME) up-regulated during the entire stimulation time. All are involved in cell proliferation and differentiation.
Dickkopf Homolog 1 (DKK1) plays a role in the negative regulation of Wnt receptor signalling pathway. The effect of the Wnt down-regulation was a reduction of cell proliferation and an induction of terminal cell differentiation.
Microtubule-actin cross-linking factor 1 (MACF1) plays the role of a positive downstream regulator in the Wnt/b-catenin signalling pathway. Down-regulation of MACF1 has the same effect as the action of DKK1 on the Wnt molecule: an inactivation of the Wnt pathway and a decrease in b-catenin concentration.
Regarding BMP-2, there was no difference at days four and seven when comparing the control and stimulated explants, but a significant difference appeared at day 12. At that time, we observed a significant upregulation of the RNAm responsible for the BMP-2 production (fold change J4stim/J4control = 2.82 and P = 0.038) (Fig 2).
Fig. 2.
Ratio after logarithm normalisation of the concentration of the BMPs mRNA between stimulated and control at days four, seven and 12. For BMP-2 at day 12, the fold change was 2.82 (log 1.49). The concentration was increased 2.82 times in the stimulated samples compared to the control. For BMP-2 at days four and seven and BMP-4 and BMP-7, at any time, the mRNA did not show significant differences between stimulated and control
Comparing control and stimulated explants for BMP-4 and 7, there was no significant difference at any time (days four, seven or 12).
Discussion
The role of BMPs and, in particular BMP-2 in osteogenesis and fracture healing has already been recognised [2, 4, 25–27].
After 12 days’ exposure to time varying electric field, our microarray analysis showed a significant increase of BMP-2 mRNA in human epidermal cells. The known effects of BMP-2 on these cells were previously observed on the same biological model [21]. Stelnicki et al. [32] showed an identical effect produced by BMP-2 alone with a marked epidermal thickening and keratinisation also representing an effect on the cell maturation.
Our previous studies on embryonic bone, exposure to time varying electromagnetic fields (EMF) showed an acceleration of the maturation of the cartilage, which immediately precedes, in the normal physiological sequence, the enchondral ossification [14, 19, 28] and an acceleration of membranous ossification, which are known effects of BMP-2 [17, 19, 29].
This observation was confirmed by Jansen et al. [23] who showed, using a pulsed electromagnetic field, an enhanced mineralisation on bone marrow derived stromal cells in parallel with the increase of the mRNA levels of BMP-2 measured by real time rtPCR. They also concluded that there was an induction of differentiation at the expense of proliferation.
Upregulation of BMP-2 mRNA was reported with biphasic electrical current on mesenchymal straumal cells [24], pulsed electromagnetic field on a rat osteoblast [3] and capacitive coupling on murine bone cells [33]. Synergic effects between BMP-2 and pulsed electromagnetic fields were reported on rat osteoblastic cells [31] and mesenchymal stem cells [30].
Our previous animal models and clinical studies on fresh fractures exposed to EMF showed early rigidity of the callus with less periosteal callus formation [13, 15, 19] suggesting an acceleration of the early membranous ossification compatible with the effects of an increase in BMP-2 observed in the literature [5, 22].
A clinical analysis of the results of 308 cases of non unions showed that hypertrophic non unions have a significantly better prognosis of healing under ELF (87.8%) in comparison to the others. Again, an acceleration of the maturation of the pre-existing fibrocartilage may explain this result and suggests an increased production of BMP-2 [6, 7, 16].
Conclusion
The microarray analysis of human epidermal cells exposed to time varying low frequency electric fields shows an upregulation of the BMP-2 mRNA at day 12.
In our experience, the effects observed for more than 30 years on cells, embryonic bone tissue, animal models of fresh fracture and clinical studies of fresh fracture and non union can all be explained by the increase of BMP-2.
This observation sheds light on the effects of ELF electromagnetic stimulation on osteogenesis and bone healing, focussing our attention on the effect on BMP-2 pathway. A better definition of the indications may be expected together with a better understanding of the involved cellular mechanisms.
Acknowledgements
The authors are grateful to the Belgian BioElectroMagnetic Group (BBEMG) for its financial grant support. We also thank P. Soularue and S. Baulande from PartnerChip, CEA Genopole, Evry, France, for their collaboration in the microarray analysis.
References
- 1.Bassett CA, Mitchell SN, Gaston SR. Treatment of ununited tibial diaphyseal fractures with pulsing electromagnetic fields. J Bone Joint Surg Am. 1981;63(4):511–523. [PubMed] [Google Scholar]
- 2.Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31(6):721–727. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodamyali T, Bhatt B, Hughes FJ, Winrow VR, Kanczler JM, Simon B, Abbott J, Blake DR, Stevens CR. Pulsed electromagnetic fields simultaneously induce osteogenesis and upregulate transcription of bone morphogenetic proteins 2 and 4 in rat osteoblasts in vitro. Biochem Biophys Res Commun. 1998;250(2):458–461. doi: 10.1006/bbrc.1998.9243. [DOI] [PubMed] [Google Scholar]
- 4.Borovecki F, Pecina-Slaus N, Vukicevic S. Biological mechanisms of bone and cartilage remodelling-genomic perspective. Int Orthop. 2007;31(6):799–805. doi: 10.1007/s00264-007-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostrom MP, Lane JM, Berberian WS, Missri AA, Tomin E, Weiland A, Doty SB, Glaser D, Rosen VM. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J Orthop Res. 1995;13(3):357–367. doi: 10.1002/jor.1100130309. [DOI] [PubMed] [Google Scholar]
- 6.Bostrom MP. Expression of bone morphogenetic proteins in fracture healing. Clin Orthop Relat Res. 1998;355(Suppl):S116–S123. doi: 10.1097/00003086-199810001-00013. [DOI] [PubMed] [Google Scholar]
- 7.Bostrom MP, Camacho NP. Potential role of bone morphogenetic proteins in fracture healing. Clin Orthop Relat Res. 1998;355(Suppl):S274–S282. doi: 10.1097/00003086-199810001-00028. [DOI] [PubMed] [Google Scholar]
- 8.Brighton CT, Hozack WJ, Brager MD, Windsor RE, Pollack SR, Vreslovic EJ, Kotwick JE. Fracture healing in the rabbit fibula when subjected to various capacitively coupled electrical fields. J Orthop Res. 1985;3(3):331–340. doi: 10.1002/jor.1100030310. [DOI] [PubMed] [Google Scholar]
- 9.Chiabrera A, Hinsenkamp M, Pilla A, Ryaby J, Ponta D, Belmont A, Beltrame F, Grattarola M, Nicolini C. Cytofluorometry of electromagnetically controlled cell dedifferentiation. J Histochem Cytochem. 1979;27:375–381. doi: 10.1177/27.1.86566. [DOI] [PubMed] [Google Scholar]
- 10.Chiabrera A, Viviani R, Parodi G, Vernazza G, Hinsenkamp M, Pilla A, Ryaby J, Beltrame F, Grattarola M, Nicolini C. Automated absorption image cytometry of electromagnetically exposed frog erythrocytes. Cytometry. 1980;1:42–48. doi: 10.1002/cyto.990010110. [DOI] [PubMed] [Google Scholar]
- 11.Collard JF, Mertens B, Hinsenkamp M. In vitro study of the effects of ELF electric fields on gene expression in human epidermal cells. Bioelectromagnetics. 2011;32(1):28–36. doi: 10.1002/bem.20608. [DOI] [PubMed] [Google Scholar]
- 12.Hinsenkamp M, Chiabrera A, Ryaby J, Pilla A, Bassett CAL. Cell behaviour and DNA modification in pulsing electromagnetic fields. Acta Orthop Belg. 1978;44:636–650. [PubMed] [Google Scholar]
- 13.Hinsenkamp M, Bourgois R, Bassett CAL, Chiabrera A, Burny F, Ryaby J. Electromagnetic stimulation of fracture repair. Influence on healing of fresh fractures. Acta Orthop Belg. 1978;44:671–698. [PubMed] [Google Scholar]
- 14.Hinsenkamp M, Rooze MA. Morphological effect of electromagnetic stimulation on the skeleton of fetal or newborn mice. Acta Orthop Scand Suppl. 1982;196:39–50. doi: 10.3109/17453678209158548. [DOI] [PubMed] [Google Scholar]
- 15.Hinsenkamp M, Burny F, Donkerwolcke M, Coussaert E. Electromagnetic stimulation of fresh fractures treated with Hoffmann external fixation. Orthopedics. 1984;7:411–416. doi: 10.3928/0147-7447-19840301-08. [DOI] [PubMed] [Google Scholar]
- 16.Hinsenkamp M, Ryaby J, Burny F. Treatment of non union by pulsing electromagnetic field: European multicenter study of 308 cases. Reconstr Surg Traumatol. 1985;19:147–151. [PubMed] [Google Scholar]
- 17.Hinsenkamp M, Tuerlinckx B, Rooze M (1985) Effect of ELF fields on bone growth and fracture repair. In Grandolfo M, Michaelson S, Rindi A (eds) Biological effects and dosimetry of static and ELF electromagnetic fields. Plenum Press, pp 441–476
- 18.Hinsenkamp M, Rooze M, Noorbergen M, Tuerlinckx B, Coussaert E (1985) Topography of EM exposure and its relationship to biological effects on tissues. In Chiabrera A, Nicolini C, Schwan H (eds) Interactions between electromagnetic fields and cells. Plenum Press, pp 557–567
- 19.Hinsenkamp M (1994) Stimulation électromagnétique de l'ostéogénèse et de la consolidation des fractures. Académie Royale de Belgique, Classe des Sciences, Bruxelles, pp 336
- 20.Hinsenkamp M. 15 years experience in electromagnetic stimulation of bone growth and repair. J Jpn Bioelect Res Soc. 1994;8:1–10. [Google Scholar]
- 21.Hinsenkamp M, Jercinovic A, Graef C, Wilaert F, Heenen M. Effects of low frequency pulsed electrical current on keratinocytes in vitro. Bioelectromagnetics. 1997;18(3):250–254. doi: 10.1002/(SICI)1521-186X(1997)18:3<250::AID-BEM8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Ishidou Y, Kitajima I, Obama H, Maruyama I, Murata F, Imamura T, Yamada N, Dijke P, Miyazono K, Sakou T. Enhanced expression of type I receptors for bone morphogenetic proteins during bone formation. J Bone Miner Res. 1995;10(11):1651–1659. doi: 10.1002/jbmr.5650101107. [DOI] [PubMed] [Google Scholar]
- 23.Jansen JH, Jagt OP, Punt BJ, Verhaar JA, Leeuwen JP, Weinans H, Jahr H. Stimulation of osteogenic differentiation in human osteoprogenitor cells by pulsed electromagnetic fields: an in vitro study. BMC Musculoskelet Disord. 2010;11:188. doi: 10.1186/1471-2474-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim IS, Song JK, Song YM, Cho TH, Lee TH, Lim SS, Kim SJ, Hwang SJ. Novel effect of biphasic electric current on in vitro osteogenesis and cytokine production in human mesenchymal stromal cells. Tissue Eng A. 2009;15(9):2411–2422. doi: 10.1089/ten.tea.2008.0554. [DOI] [PubMed] [Google Scholar]
- 25.Kwong FN, Hoyland JA, Evans CH, Freemont AJ. Regional and cellular localisation of BMPs and their inhibitors' expression in human fractures. Int Orthop. 2009;33(1):281–288. doi: 10.1007/s00264-008-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int Orthop. 2007;31(6):729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pecina M, Vukicevic S. Biological aspects of bone, cartilage and tendon regeneration. Int Orthop. 2007;31(6):719–720. doi: 10.1007/s00264-007-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rooze M, Hinsenkamp M. "In vitro" histochemical modifications induced by electromagnetic stimulation. Acta Orthop Scand Suppl. 1982;196:51–62. doi: 10.3109/17453678209158549. [DOI] [PubMed] [Google Scholar]
- 29.Rooze M, Hinsenkamp M. In vivo modifications induced by electromagnetic stimulation of chicken embryos. Reconstr Surg Traumatol. 1985;19:87–92. [PubMed] [Google Scholar]
- 30.Schwartz Z, Simon BJ, Duran MA, Barabino G, Chaudhri R, Boyan BD. Pulsed electromagnetic fields enhance BMP-2 dependent osteoblastic differentiation of human mesenchymal stem cells. J Orthop Res. 2008;26(9):1250–1255. doi: 10.1002/jor.20591. [DOI] [PubMed] [Google Scholar]
- 31.Selvamurugan N, Kwok S, Vasilov A, Jefcoat SC, Partridge NC. Effects of BMP-2 and pulsed electromagnetic field (PEMF) on rat primary osteoblastic cell proliferation and gene expression. J Orthop Res. 2007;25(9):1213–1220. doi: 10.1002/jor.20409. [DOI] [PubMed] [Google Scholar]
- 32.Stelnicki EJ, Longaker MT, Holmes D, Vanderwall K, Harrison MR, Largman C, Hoffman WY. Bone morphogenetic protein-2 induces scar formation and skin maturation in the second trimester fetus. Plast Reconstr Surg. 1998;101(1):12–19. doi: 10.1097/00006534-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Clark CC, Brighton CT. Up-regulation of bone morphogenetic proteins in cultured murine bone cells with use of specific electric fields. J Bone Joint Surg Am. 2006;88(5):1053–1065. doi: 10.2106/JBJS.E.00443. [DOI] [PubMed] [Google Scholar]