Abstract
Background and Purpose
Various radiotherapy planning methods for locally advanced squamous cell carcinoma of the head and neck (SCCHN) have been proposed to decrease normal tissue toxicity. We compare IMRT, adaptive IMRT, proton therapy (IMPT), and adaptive IMPT for SCCHN.
Materials and Methods
Initial and re-simulation CT images from 10 consecutive patients with SCCHN were used to quantify dosimetric differences between photon and proton therapy. Contouring was performed on both CTs, and plans (n=40 plans) and dose volume histograms were generated.
Results
The mean GTV volume decreased 53.4% with re-simulation. All plans provided comparable PTV coverage. Compared with IMRT, adaptive IMRT significantly reduced the maximum dose to the mandible (p=0.020) and mean doses to the contralateral parotid gland (p=0.049) and larynx (p=0.049). Compared with IMRT and adaptive IMRT, IMPT significantly lower the maximum doses to the spinal cord (p<0.002 for both) and brainstem (p<0.002 for both) and mean doses to the larynx (p<0.002 for both) and ipsilateral (p=0.004 IMRT, p=0.050 adaptive) and contralateral (p<0.002 IMRT, p=0.010 adaptive) parotid glands. Adaptive IMPT significantly reduced doses to all critical structures compared with IMRT and adaptive IMRT and several critical structures compared with non-adaptive IMPT.
Conclusions
Although adaptive IMRT reduced dose to several normal structures compared with standard IMRT, non-adaptive proton therapy had a more favorable dosimetric profile than IMRT or adaptive IMRT and may obviate the need for adaptive planning. Protons allowed significant sparing of the spinal cord, parotid glands, larynx, and brainstem and should be considered for SCCHN to decrease normal tissue toxicity while still providing optimal tumor coverage.
Keywords: head and neck cancer, proton therapy, adaptive radiotherapy, IMRT, treatment planning
INTRODUCTION
Radiation therapy with concurrent cisplatin-based chemotherapy is standard treatment for patients with locally advanced SCCHN [1]. Chemoradiation can potentially allow for organ preservation and improve patient quality of life compared with surgery [2]. Concurrent chemoradiation in this region, however, is associated with significant morbidity. The close proximity to vital organs makes it difficult to deliver definitive radiation doses to disease sites without compromising normal tissue function. Xerostomia from salivary gland dysfunction commonly results and is strongly associated with dysphagia, difficulty with social eating, increased oral bacteria colonization, dental caries, and decreased quality of life [3–5]. Radiation to the glottic larynx can result in poor voice function, weight loss, swallowing dysfunction, and decreased quality of life [6]. Mucositis [7], osteoradionecrosis from dose to the mandible [8], and nausea from dose to the brainstem [7] can also occur. Conformal techniques such as intensity-modulated radiotherapy (IMRT) can decrease dose to defined critical normal tissues and achieve dose escalation to SCCHN target volumes [9].
Adaptive radiotherapy for SCCHN is increasingly being employed to account for anatomic changes that can lead to overtreatment of normal tissues or undertreatment of tumors. Since there is a steep dose gradient with conformal techniques between target and normal tissues, interfractional anatomical changes become significant. Interfractional patient weight loss or deformation of tumor or normal tissues are not accounted for with standard radiotherapy but can markedly alter head and neck anatomy and modify treatment parameters [10]. SCCHN often have rapid favorable responses to therapy with significant tumor shrinkage [11–15]. Such response can affect other nearby nontarget structures, particularly the parotid glands. As primary tumors regress, parotid glands in the involved treatment fields often shrink and are displaced medially, potentially increasing radiation exposure [15–18].
Adaptive radiotherapy aims to modify treatment according to changes that occur during therapy. For SCCHN, this involves re-simulating patients during their radiotherapy and modifying target volumes and treatment plans to attempt to minimize effects of anatomic changes in tumors and surrounding structures. Preliminary studies assessing adaptive photon radiotherapy have reported improved sparing of organs at risk (OARs) [12–14].
Particle therapy may also provide a more favorable toxicity profile than IMRT. Proton therapy allows energy to be deposited at a specific depth known as the Bragg peak, with rapid energy falloff beyond this point [19]. Therefore, normal tissues on the distal side of the target volume can be spared. A single-institution report has documented excellent local control rates and reduced doses to OARs for the treatment of SCCHN with proton therapy [20].
To date, there is limited data directly comparing different radiotherapy modalities and treatment strategies for SCCHN, and to the authors knowledge, no data exists comparing adaptive photon radiotherapy to particle radiotherapy or assessing adaptive particle radiotherapy for SCCHN. This is the first study comparing dose volume histograms (DVHs) of target volumes and normal tissue structures in photon-based versus proton-based plans using both fixed target volumes and adaptive planning for patients with locally advanced SCCHN.
MATERIALS AND METHODS
Ten consecutive patients with Stage IV locally advanced SCCHN treated at the National Institutes of Health Clinical Research Center (six patients) or Walter Reed Army Medical Center (four patients) from 1/2008 to 9/2009 who required repeat simulation during their course of radiotherapy due to changes in anatomy or difficulties that arose during treatment relating to patient set-up or thermoplastic immobilization devices were included in the present study. Most patients had oropharynx primary malignancies (seven patients), and all patients had N2 nodal disease, with three having bilateral nodal involvement and all but one having multiple positive lymph nodes [AJCC, 6th Ed.] (Table 1). All patients underwent a single repeat simulation planning session that occurred on average 2.9 weeks into treatment with concurrent chemoradiation and were treated to revised target volumes based on the second CT data set following repeat simulation. All patients were treated with IMRT in 2Gy daily fractions to 70Gy over 35 fractions with concurrent cisplatin.
Table 1.
Patient Characteristics
| Patient Number | Primary Disease | T Stage* | N Stage* | GTV Initial Volume (cm3) | GTV Rescan Volume (cm3) | PTV70 Initial Volume (cm3) | PTV70 Rescan Volume (cm3) |
|---|---|---|---|---|---|---|---|
| 1 | Supraglottic Larynx | T2 | N2c | 41.2 | 30.6 | 164.4 | 124.6 |
| 2 | Oropharynx: Tonsil | T3 | N2b | 57.4 | 5.1 | 214.0 | 77.8 |
| 3 | Oroharynx: Base of Tongue | T4 | N2c | 89.7 | 54.8 | 490.2 | 370.0 |
| 4 | Supraglottic Larynx | T2 | N2b | 40.4 | 6.6 | 193.8 | 120.3 |
| 5 | Oropharynx: Tonsil | T1 | N2a | 36.7 | 17.7 | 282.3 | 144.7 |
| 6 | Oropharynx: Tonsil | T2 | N2b | 173.0 | 62.2 | 514.0 | 268.1 |
| 7 | Oropharynx: Tonsil | T2 | N2b | 71.1 | 33.4 | 386.2 | 175.8 |
| 8 | Oropharynx: Tonsil | T4 | N2b | 23.0 | 11.7 | 194.9 | 131.0 |
| 9 | Nasopharynx | T2 | N2 (bilateral) | 160.5 | 100.1 | 878.7 | 543.5 |
| 10 | Oropharynx: Tonsil | T1 | N2b | 67.6 | 32.4 | 403.5 | 288.0 |
AJCC, 6th Ed.
The CT images from these patients were used to quantify dosimetric differences between photon and proton therapy. CT data were acquired with a slice thickness of 3mm. CT images were imported into a photon and proton commercial treatment planning system (Eclipse, Varian Medical Systems, Palo Alto, CA) for defining target and nontarget structures. OARs and planning target volumes (PTVs) were contoured on the initial and re-simulation CT images. Target and nontarget structure sets for a given patient CT image set were held constant for all plans. Assessed OARs included the bilateral parotid glands, glottic larynx, spinal cord, brainstem, and mandible. CT streak artifacts from metal were contoured and assigned a CT value equivalent to tissue prior to calculating all photon and proton plans. Artifacts from teeth were assigned a tissue equivalent value for all proton plans and as necessary for photon plans.
Gross Tumor Volume (GTV) was defined as the maximum extent of all known gross disease determined from clinical examination, endoscopy, or CT, MRI, or PET imaging. In all directions circumferentially, the margin between GTV and Clinical Target Volume 70 (CTV70) was 1cm to include all volumes of known tumor and suspected microscopic spread. This margin was reduced to ≥2mm for tumors in close proximity to bone or air not at risk for subclinical disease. High risk nodal regions, including small volume lymph nodes and all potential routes of spread for primary and nodal disease, were contoured and designated CTV64. Nodal regions at lower risk of disease spread were designated CTV50.
To account for set-up variation and organ and patient motion, a uniform margin of 5mm was added around each corresponding CTV to define PTV70, PTV64, and PTV50, respectively. To account for proton beam properties and range uncertainties, proton beam range compensators were designed to provide proximal and distal margins relative to each PTV, and blocking was designed to create a lateral margin relative to each PTV. These margins were individualized for each patient on the basis of the formulas by Moyers et al. [21]. PTV70 was planned to 70Gy for photon plans or 70 cobalt Gray equivalents (CGE) for proton plans, with proton doses corrected with the accepted relative biologic effectiveness value of 1.1 [19]. PTV64 was planned to 64Gy or 64CGE, whereas PTV50 was planned to 50Gy or 50CGE. Plans were devised to initially target PTV50, followed by a conedown to PTV64, followed by a second conedown to PTV70, with no integrated boost administration planned.
Four treatment plans were generated for each patient (n=40 plans): 1) photon IMRT, with treatment planned to the target volumes and normal structures from the initial CT image set, 2) adaptive photon IMRT, with treatment planned to the target volumes and normal structures from the initial CT image set to 36Gy and to the second CT simulation image set to 34Gy, 3) spot scanning proton IMPT, with treatment planned to the target volumes and normal structures from the initial CT image set, and 4) adaptive proton IMPT, with treatment planned to the target volumes and normal structures from the initial CT image set to 36Gy and to the second CT simulation image set to 34Gy. For all adaptive plans, following the first 36 Gy planned to the initial PTV50 based on the initial CT data set, the remaining 14Gy planned to a revised PTV50 and both conedown doses to PTV64 and PTV70 were planned to new PTVs devised using the second CT data set. The initial and subsequent CT data sets were fused together to allow for plan sums and composite DVHs to be generated.
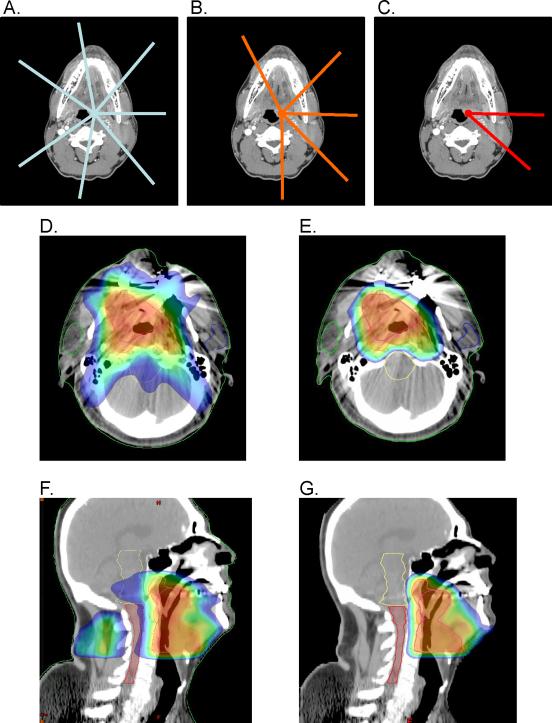
IMRT and adaptive IMRT plans were designed with seven equally spaced beams every 50° beginning at 30° (30°, 80°, 130°, 180°, 230°, 280°, 330°) centered on each corresponding PTV (Figure 1A). These beam angles corresponded to the class solution employed by our clinic for treating locally advanced SCCHN. For proton and adaptive proton plans, patients were planned with five beams centered on PTV50 and PTV64, with beam angles individualized for each patient. For the final three fractions of proton therapy, patients were planned with an individualized two-field technique, with beams centered on PTV70 (Figure 1B–1C). For photon plans, 6 MV photons were used, whereas the maximum clinical beam energy was 235 MeV for proton plans.
Figure 1.
Beam arrangements and treatment planning images. Representative beam arrangements used for A) photon plans to treat PTV70 with a seven-field technique, B) proton plans to treat PTV50 and PTV60 with a five-field technique, and C) proton plans to treat PTV70 with a two-field technique. Beam angles for photon plans were equally spaced and centered on each corresponding PTV. Beam angles for proton plans were individualized for each patient to minimize dose to critical structure and maintain optimal PTV coverage and dose homogeneity throughout the target volumes. Representative treatment planning images for a patient with cT4N2cM0 stage IVA squamous cell carcinoma of the oropharynx (base of tongue) in axial planes for D) IMRT and E) proton therapy, and in sagittal planes for F) IMRT and G) proton therapy. Images depict treatment to PTV70, the final treatment conedown targeting gross disease with margin. The same slices from the same initial CT data set were employed for Figures 1D and 1E and Figures 1F and 1G. Color coding: red = 100% to blue = 50% of 6Gy in 2Gy fractions.
For optimization purposes, dose objectives were created for PTVs and OARs. All plans were optimized via Helios Inverse Treatment Planning (Varian Medical Systems) to minimize dose to critical structure by increasing constraints on OARs and OARs with margin (spinal cord, brainstem), while maintaining optimal PTV coverage and dose homogeneity throughout target volumes. Planning was performed to achieve maximum doses to the spinal cord less than 45Gy, brainstem less than 54Gy, and mandible no more than 1cc to exceed 75Gy, as well as mean doses to the parotid glands less than 26Gy (or at least 50% of one gland less than 30Gy) and glottic larynx less than 45Gy. All plans were optimized to ensure 100% of the prescription dose covered ≥97% of each PTV. DVHs of PTVs and OARs were generated for IMRT, adaptive IMRT, IMPT, and adaptive IMPT plans to compare doses to tumor volumes and normal structures.
Various methods were used to minimize bias in the present study. Ten consecutive patients who required re-planning were included in this study to minimize sampling bias. All contours were performed by a single radiation oncologist (CS) and approved by at least one additional radiation oncologist. A standard treatment planning optimization strategy was used for all photon and proton plans. Conformity indexes (see definition in Table 2) and DVHs were assessed to ensure comparable PTV coverage between plans to minimize any bias when comparing normal tissue dosimetry between plans. However, despite these measures, as with any retrospective study [22], it is possible that bias existed in the current study that may have favored a certain type of planning strategy. Furthermore, despite recontouring on and fusing the second CT data sets to the first CT data sets, as deformable registration was not employed and small changes were noted in normal tissue positioning on the second CT data sets following tumor response, a margin of error may exist in the dose accumulate between the initial and subsequent CTs.
Table 2.
Comparison† of the Mean Dose to Target Volumes and Conformity Index* for IMRT, Adaptive IMRT, IMPT, and Adaptive IMPT
| Volume of PTV Receiving 100% of the Prescribed Dose | Conformity Index | |||||
|---|---|---|---|---|---|---|
| PTV50 | PTV64 | PTV70 | PTV50 | PTV64 | PTV70 | |
| IMRT | 99.9% (±0.2%) | 99.1% (±0.3%) | 98.6% (±0.7%) | 1.97 (±0.48) | 1.59 (±0.28) | 1.49 (±0.26) |
| Adaptive IMRT | 99.8% (±0.4%) | 99.0% (±0.5%) | 99.0% (±0.8%) | 2.06 (±0.56) | 1.53 (±0.39) | 1.75 (±0.69) |
| IMPT | 99.9% (±0.1%) | 99.5% (±0.5%) | 99.0% (±0.7%) | 1.52 (±0.31) | 1.30 (±0.18) | 1.23 (±0.11) |
| Adaptive IMPT | 99.9% (±0.1%) | 99.5% (±0.5%) | 99.4% (±0.7%) | 1.64 (±0.38) | 1.40 (±0.19) | 1.54 (±0.46) |
Values listed for PTV volumes and conformity indexes are the mean values for the study population. The standard deviations for each comparison are listed in brackets.
Conformity index was calculated as the ratio of the reference isodose volume to the volume of each planning target volume being assessed, with the 95% isodose line of each PTV selected as the reference isodose volume in accordance with ICRU 50 guidelines.
Statistical analysis was performed using JMP 7.0 (SAS, Cary, NC). Because the population was not normally distributed, a non-parametric statistical hypothesis was utilized. The Wilcoxon Signed-rank test was used to evaluate the differences between pairwise comparisons. A two-tailed p-value was utilized, and statistical significance was defined as p≤0.050.
RESULTS
Patient Statistics
The mean GTV volume from the initial CT image set obtained before concurrent chemoradiation was 76.1cm3 [range 23.0–173.0cm3] (Table 1). The mean GTV volume from the second CT image set decreased by 53.4% to 35.5cm3 [5.1–100.1cm3]. The corresponding mean PTV70 volume decreased 39.7%, from 372.2cm3 [164.4–878.7cm3] to 224.4cm3 [77.8–543.5cm3]. No appreciable difference in tumor volume reduction was noted by tumor primary location.
Dose Coverage
IMRT, adaptive IMRT, IMPT, and adaptive IMPT plans all provided acceptable target volume coverage, with no significant difference in coverage to PTV70, PTV64, or PTV50 among the different plans (Table 2). In all cases, 100% of the prescription dose covered ≥97% of each PTV, and no point dose within or outside PTVs was >114% of the prescribed dose (Figure 1D–1G). Overall, proton plans and adaptive proton plans had superior conformity than either IMRT plans or adaptive IMRT plans, and they delivered less dose outside of target volumes, particularly among low to intermediate dose volumes (Table 2).
IMRT versus Adaptive IMRT
Compared with IMRT plans, adaptive IMRT significantly decreased the maximum point dose to the mandible (p=0.020) and mean doses to the left parotid gland (p<0.002) and glottic larynx (p=0.049) (Table 3, Supplemental Table 1). The maximum dose to the brainstem was somewhat lower with adaptive plans, although this difference was not statistically significant (p=0.084). There was no difference in the maximum dose to the spinal cord (p=0.770) or mean dose to the right parotid gland (p=0.846). When assessing the parotid glands relative to the site of primary disease for each patient, adaptive IMRT decreased the mean dose to the contralateral (p=0.049) but not the ipsilateral parotid gland (p=0.160) [Supplemental Figure 1].
Table 3.
Comparison of the Average Maximum or Mean Doses to Normal Tissues Between IMRT, Adaptive IMRT, IMPT, and Adaptive IMPT
| Spinal Cord | Left Parotid | Right Parotid | Ipsilateral Parotid | Contralateral Parotid | Glottic Larynx | Brain stem | Mandible | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max† (Gy) | Mean (Gy) | V20* | Mean (Gy) | V20* | Mean (Gy) | V20* | Mean (Gy) | V20* | Mean (Gy) | Max† (Gy) | Max† (Gy) | V60* | V70* | |
| IMRT | 42.1 | 37.8 | 60.4% | 32.0 | 55.5% | 43.1 | 68.4% | 26.8 | 47.5% | 45.4 | 44.8 | 75.1 | 34.2% | 15.0% |
| Adaptive IMRT | 41.7 | 32.8 | 54.0% | 31.6 | 54.8% | 39.0 | 63.6% | 25.3 | 45.2% | 41.8 | 42.2 | 73.0 | 25.3% | 6.7% |
| IMPT | 30.5 | 27.2 | 47.9% | 25.3 | 45.8% | 32.9 | 54.5% | 19.5 | 39.2% | 35.3 | 31.3 | 73.8 | 22.8% | 8.9% |
| Adaptive IMPT | 28.4 | 25.0 | 43.3% | 23.1 | 43.5% | 29.8 | 51.3% | 18.3 | 35.7% | 31.0 | 29.0 | 71.1 | 19.5% | 5.2% |
Max = average maximum point dose
V20/60/70 = volume receiving 20/60/70 Gy
IMRT versus IMPT
Proton therapy significantly reduced the mean and maximum doses to most OARs examined. Compared with IMRT plans, IMPT reduced the maximum doses to the spinal cord (p<0.002) and brainstem (p<0.002), as well as the mean doses to the left (p<0.002) and right (p=0.004) parotid glands and larynx (p<0.002). Both the ipsilateral (p=0.004) and contralateral (p<0.002) parotid glands showed significant sparing with proton therapy. Only the maximum dose to the mandible (p=0.275) was not significantly reduced with proton therapy, although the mandible V60 (p<0.002) and V70 (p=0.010) were lower with IMPT (Figure 1D–1G).
Adaptive IMRT versus IMPT
When compared to adaptive IMRT, IMPT provided a significant dose reduction to all OARs other than the mandible (p=0.826). Proton therapy reduced the maximum doses to the spinal cord (p<0.002) and brainstem (p<0.002), as well as the mean doses to the left (p=0.010) and right (p=0.050) parotid glands, ipsilateral (p=0.050) and contralateral (p=0.010) parotid glands, and larynx (p<0.002).
IMRT versus Adaptive IMPT
Adaptive IMPT significantly reduced the mean and maximum doses to all OARs examined. Compared with IMRT plans, adaptive IMPT reduced the maximum doses to the spinal cord (p<0.002), brainstem (p<0.002), and mandible (0.020), as well as the mean doses to the left (p=0.004) and right (p=0.004) parotid glands, ipsilateral (p=0.004) and contralateral (p=0.004) parotid glands, and larynx (p<0.002).
Adaptive IMRT versus Adaptive IMPT
When compared to adaptive IMRT, adaptive IMPT significantly reduced dose to all OARs examined. Adaptive IMPT reduced the maximum doses to the spinal cord (p<0.002), brainstem (p<0.002), and mandible (p=0.049), as well as the mean doses to the left (p=0.020) and right (p=0.010) parotid glands, ipsilateral (p=0.010) and contralateral (p=0.020) parotid glands, and larynx (p<0.002).
IMPT versus Adaptive IMPT
When compared to IMPT, adaptive IMPT provided a further reduction in dose to several OARs. Adaptive IMPT reduced the maximum doses to the spinal cord (p=0.004) and mandible (p=0.006) and mean doses to the larynx (p=0.010) and ipsilateral parotid gland (p=0.020). Non-significantly lower mean doses to left (p=0.084) and right (p=0.065) parotid glands and maximum dose to the brainstem (p=0.106) were also demonstrated with adaptive IMPT. There was no difference in mean dose to the contralateral parotid gland (p=0.160).
DISCUSSION
This study demonstrated that adaptive photon radiotherapy for locally advanced SCCHN can significantly decrease the maximum dose to the mandible and mean doses to the contralateral parotid gland and glottic larynx when compared with IMRT, while still maintaining optimal tumor coverage. Proton radiotherapy, however, allowed further benefits over standard IMRT and adaptive IMRT plans by decreasing the maximum doses to the spinal cord and brainstem and mean doses to the bilateral parotid glands and larynx, while still delivering optimal tumor coverage. Adaptive proton radiotherapy further reduced doses received by the spinal cord, ipsilateral parotid gland, glottic larynx, and mandible compared with non-adaptive proton plans, although this reduction was less clinically significant.
High dose homogeneity and target volume coverage were achieved despite a diverse patient population with varying primary tumor locations and extents of nodal involvement. Importantly, no significant difference in PTV coverage was demonstrated between all plans, thus minimizing bias when comparing normal tissue dosimetry. However, the sensitivity of proton plans to anatomic and target volume changes was generally greater than for photon plans. Plans with fewer beams and with beams in the direction of shrinkage of target volumes were most sensitive. For target volumes in this study that exhibited significant shrinkage following a partial course of chemoradiation prior to repeat CT simulation for adaptive radiotherapy, the actual doses delivered to critical structures just distal to shrinking target volumes varied from the doses expected based on the initial CT data sets more with protons than photons.
Adaptive Radiotherapy
Supporting a potential benefit of adaptive radiotherapy in our study, when contours from the initial CT image sets were compared with those from the re-planning CT image sets, a significant reduction in mean GTV was demonstrated that was similar to tumor reductions reported in previous studies with an average reduction in GTV noted between 69–73% [11–15]. Several patients in this study underwent re-planning early in the course of their radiotherapy and, therefore, may have had more GTV reduction if re-planning was performed later in therapy.
Preliminary research on adaptive radiotherapy has demonstrated potential improvements in radiation treatment delivery which was also noted in our study. By evaluating serial CT images throughout radiotherapy, Barker et al. reported significant changes in size and positions of target volumes and OARs, suggesting dosimetric underdosing of PTVs or overdosing of parotid glands may occur if such changes are not accounted for with re-planning [13]. Another study demonstrated lack of re-planning decreased dose coverage of originally planned PTVs in 92% of patients and increased the maximum doses delivered to the spinal cord in 100% and brainstem in 85% [14]. Another study showed the dose delivered to parotid glands can be 5–7Gy higher than what was planned at the time of initial simulation in 45% of patients [18]. Although small absolute differences in dose delivered to such organs as the mandible and spinal cord may be less clinically significant, such an increase in dose to parotid glands is not trivial. Parotid glands exhibit a steep dose-response relationship, with functioning impaired after a mean dose as low as 10Gy and grade 4 xerostomia occurring in 70% of patients receiving mean doses greater than 26Gy [4–5].
Although attempt was made to maintain the mean bilateral parotid gland dose under 26Gy, PTVs and ipsilateral parotid glands overlapped in most patients. As the priority of sparing the parotid glands was lower than that for achieving the prescribed PTV coverage, few patients had mean ipsilateral parotid gland doses below 26Gy, and the reduction in ipsilateral parotid gland mean dose from 43.1Gy with IMRT to 39.0Gy with adaptive IMRT in this study was not significant (p=0.160). The contralateral parotid gland mean dose was lower with adaptive IMRT (25.3Gy vs. 26.8Gy, p=0.049), which is in line with the magnitude of parotid gland dose reduction of up to 10% reported in prior studies assessing adaptive photon radiotherapy [12]. The mean doses to the ipsilateral and contralateral parotid glands with IMPT of 32.9Gy and 19.5Gy, respectively, and adaptive IMPT of 29.8Gy and 18.3Gy, suggest an even larger potential clinical benefit in preservation of parotid gland functioning with protons.
Several concerns exist with adaptive treatment planning. Since adaptive radiotherapy is labor intensive, deformable image registration, automated target delineation, and higher computational power likely will become more important. Additionally, the timing of when to re-plan during chemoradiation is not well-defined. While some practitioners utilize dose thresholds to perform re-simulation, others use anatomical thresholds such as GTV reduction. In this study, patients underwent a single repeat simulation. As has been demonstrated in prior studies [12], a greater benefit with adaptive radiotherapy might have been seen if re-planning occurred more frequently during radiotherapy.
Controversy also exists regarding local control when treating smaller target volumes based on re-planning CT data [23–24]. With tumor responses after both induction chemotherapy and a partial course of concurrent chemoradiation, concern exists that substantial numbers of tumor cells may remain in tissue volumes previously occupied by gross disease that are below the threshold of radiographic detection [23–25]. To evaluate these issues, there are currently three pilot trials lead by U.T.M.D. Anderson Cancer Center, Washington University School of Medicine, and University Hospital Ghent (Belgium) that aim to assess adaptive radiotherapy for the treatment of SCCHN [26]. A recent consensus conference assessing induction chemotherapy recommended treating patients based on prechemotherapy target volumes, regardless of tumor response, to avoid risking marginal recurrences. While no such consensus exists for adaptive radiotherapy, the multidisciplinary team concluded that “many of the same issues and recommendations will apply to adaptive RT…” [27].
Proton Radiotherapy
Particle radiotherapy has also been shown in preliminary reports and modeling studies to improve tumor dose distribution and decrease normal tissue toxicity in the treatment of many cancers [28–30] SCCHN compared with photon therapy [31–36]. Cozzi et al. performed a treatment planning comparison of mixed photon-electron, 3D conformal photon, IMRT, and proton therapy (passively scattered and spot scanned) for patients with advanced SCCHN. Proton plans provided improved dose homogeneity and delivered the least dose to the spinal cord and parotid glands [31]. Investigators from Loma Linda University Medical Center treated 29 patients with stage II-IV oropharyngeal cancers using an accelerated fractionation schedule with a combination of photons and protons to 75.9CGE in 45 fractions. Their reported 84% locoregional control and 65% disease-free survival rates at five years compare very favorably to historical controls without increasing treatment toxicity [20]. Another dosimetric study of hypopharyngeal patients similarly demonstrated lower doses to non-target tissues with protons than photon IMRT [32].
The dosimetric advantages of proton radiotherapy demonstrated in this study might improve the therapeutic ratio for patients with locally advanced SCCHN. Based on historical dose-response relationships, with significantly lower radiation doses to several OARs demonstrated in this study, patients treated with protons may have improved quality of life and reduced rates of xerostomia, dental problems, voice changes, weight loss, swallowing dysfunction, mucositis, nausea, and other radiation-induced toxicities. Longitudinal studies examining normal tissue toxicities from photon and proton radiotherapy are needed to confirm the clinical significance of our findings.
In this study, although adaptive photon radiotherapy reduced dose to several OARs compared with standard IMRT, non-adaptive proton plans were dosimetrically superior to all photon plans despite planning to treat larger target volumes than were planned with adaptive photon radiotherapy. To date, no previous data exist assessing adaptive proton therapy for SCCHN, and adaptive proton therapy has only previously been evaluated in a single dosimetric analysis of patients with non-small cell lung cancer [37]. Although adaptive proton plans in this study, compared with IMPT, significantly lowered the radiation doses to the spinal cord, ipsilateral parotid gland, glottic larynx, and mandible, this dose reduction was less clinically significant and the magnitude of this reduction compared with IMPT plans was much less than the reduction in dose achieved by non-adaptive proton plans over IMRT and adaptive IMRT photon plans. With concerns regarding adaptive treatment strategies and the limited resources of the few proton therapy centers worldwide at this time, it is unlikely that adaptive proton therapy will become a clinically utilized modality for treating patients with SCCHN solely in an attempt to minimize the volumes of targets treated, particularly in light of the significant benefit demonstrated in this study with standard proton therapy over all photons plans. However, as proton plans are more sensitive than photon plans to interfractional changes in tumor volumes and patient anatomy, care must be taken to account for these changes or otherwise ensure accuracy of the beam range when using protons to treat SCCHN. The role of adaptive proton radiotherapy may best be answered in the context of a clinical trial with serially planning repeat CT simulations.
The possible advantages to proton therapy are being assessed in three phase II trials for SCCHN. Researchers at University of Florida and Massachusetts General Hospital are investigating proton therapy for the treatment of nasopharygeal carcinomas, and investigators at University of Florida are assessing proton therapy to treat oropharynx cancers. Each of these trials is enrolling patients with considerably less advanced disease than was included in the present study [26].
Standard vs. Adaptive Radiotherapy
This study assessed a population of SCCHN patients with a high disease burden, all of whom had stage IV non-metastatic disease. The advanced nature of their diseases may have allowed for a greater advantage for adaptive plans over non-adaptive plans following an initial tumor response to chemoradiation. However, an even greater benefit to adaptive radiotherapy may have been demonstrated if the study population had been limited only to patients with clinically significant responses to initial partial courses of chemoradiation. Additionally, neither photon technique could maintain optimal PTV coverage while sparing OARs to the same extent as either proton technique. It is possible that the dosimetric advantage to OARs with protons demonstrated in this study would be even greater in patients with less advanced disease. As such, the study results may not be applicable to patients with early-stage SCCHN and may underestimate the potential benefit of proton therapy over photon therapy. However, it is also possible that the spot scanning proton techniques utilized in this study allowed for a greater benefit with proton therapy than would be seen in centers treating SCCHN patients with scattered beam delivery.
CONCLUSION
For patients with locally advanced SCCHN, adaptive photon radiotherapy offers some benefit over standard IMRT in reducing dose to several regional OARs. Proton therapy has a more favorable dosimetric profile than either standard IMRT or adaptive IMRT and significantly lower radiation doses to the spinal cord, bilateral parotid glands, glottic larynx, and brainstem. As such, the dosimetric advantage demonstrated with non-adaptive proton therapy in this study may obviate the need for adaptive treatment planning for patients with SCCHN. With decreased doses delivered to OARs, patients treated with proton therapy may benefit from fewer radiation-induced side effects. Proton therapy should be considered for patients with locally advanced SCCHN to decrease normal tissue toxicity while still providing optimal tumor coverage.
Supplementary Material
Acknowledgements
This research was supported in part by U.S. Army Medical Research and Materiel Command under Contract Agreement No.DAMD17-W81XWH-04-2-0022, in conjunction with the Roberts Proton Therapy Center at University of Pennsylvania Health System. Research support was also through the Intramural Research Program of the NIH. D.L. was supported by the Clinical Research Training Program, a public-private partnership supported jointly by NIH and Pfizer, Inc. Opinions, interpretations, conclusions, and recommendations are those of the authors and not necessarily endorsed by the U.S. Army.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: none.
REFERENCES
- 1.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91(24):2081–6. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 2.Fung K, Lyden TH, Lee J, et al. Voice and swallowing outcomes of an organ-preservation trial for advanced laryngeal cancer. Int J Radiat Oncol Biol Phys. 2005;63(5):1395–9. doi: 10.1016/j.ijrobp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Bussels B, Maes A, Flamen P, et al. Dose-response relationships within the parotid gland after radiotherapy for head and neck cancer. Radiother Oncol. 2004;73(3):297–306. doi: 10.1016/j.radonc.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Taylor JM, Ten Haken RK, Eisbruch A. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67(3):660–9. doi: 10.1016/j.ijrobp.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco AI, Chao KS, El Naqa I, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62(4):1055–69. doi: 10.1016/j.ijrobp.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 6.Dornfeld K, Simmons JR, Karnell L, et al. Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet- and speech-related quality of life. Int J Radiat Oncol Biol Phys. 2007;68(3):750–7. doi: 10.1016/j.ijrobp.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal DI, Chambers MS, Fuller CD, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;72(3):747–55. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melzner WJ, Lotter M, Sauer R, Strnad V. Quality of interstitial PDR-brachytherapy-implants of head-and-neck-cancers: predictive factors for local control and late toxicity? Radiother Oncol. 2007;82(2):167–73. doi: 10.1016/j.radonc.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Corvò R. Evidence-based radiation oncology in head and neck squamous cell carcinoma. Radiother Oncol. 2007;85(1):156–70. doi: 10.1016/j.radonc.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Chencharick JD, Mossman KL. Nutritional consequences of the radiotherapy of head and neck cancer. Cancer. 1983;51(5):811–5. doi: 10.1002/1097-0142(19830301)51:5<811::aid-cncr2820510511>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Fang FM, Tsai WL, Go SF, et al. Implications of quantitative tumor and nodal regression rates for nasopharyngeal carcinomas after 45 Gy of radiotherapy. Int J Radiat Oncol Biol Phys. 2001;50(4):961–9. doi: 10.1016/s0360-3016(01)01531-0. [DOI] [PubMed] [Google Scholar]
- 12.Wu Q, Chi Y, Chen PY, Krauss DJ, Yan D, Martinez A. Adaptive replanning strategies accounting for shrinkage in head and neck IMRT. Int J Radiat Oncol Biol Phys. 2009;75(3):924–32. doi: 10.1016/j.ijrobp.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 13.Barker JL, Jr, Garden AS, Ang KK, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;59(4):960–70. doi: 10.1016/j.ijrobp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Hansen EK, Bucci MK, Quivey JM, Weinberg V, Xia P. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;64(2):355–62. doi: 10.1016/j.ijrobp.2005.07.957. [DOI] [PubMed] [Google Scholar]
- 15.Castadot P, Geets X, Lee JA, Christian N, Grégoire V. Assessment by a deformable registration method of the volumetric and positional changes of target volumes and organs at risk in pharyngo-laryngeal tumors treated with concomitant chemo-radiation. Radiother Oncol. 2010;95(2):209–17. doi: 10.1016/j.radonc.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Broggi S, Fiorino C, Dell'Oca I, et al. A two-variable linear model of parotid shrinkage during IMRT for head and neck cancer. Radiother Oncol. 2010;94(2):206–12. doi: 10.1016/j.radonc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Lee C, Langen KM, Lu W, et al. Evaluation of geometric changes of parotid glands during head and neck cancer radiotherapy using daily MVCT and automatic deformable registration. Radiother Oncol. 2008;89(1):81–8. doi: 10.1016/j.radonc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 18.O'Daniel JC, Garden AS, Schwartz DL, et al. Parotid gland dose in intensity-modulated radiotherapy for head and neck cancer: is what you plan what you get? Int J Radiat Oncol Biol Phys. 2007;69(4):1290–6. doi: 10.1016/j.ijrobp.2007.07.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerweck LE, Kozin SV. Relative biological effectiveness of proton beams in clinical therapy. Radiother Oncol. 1999;50(2):135–42. doi: 10.1016/s0167-8140(98)00092-9. [DOI] [PubMed] [Google Scholar]
- 20.Slater JD, Yonemoto LT, Mantik DW, et al. Proton radiation for treatment of cancer of the oropharynx: early experience at Loma Linda University Medical Center using a concomitant boost technique. Int J Radiat Oncol Biol Phys. 2005;62(2):494–500. doi: 10.1016/j.ijrobp.2004.09.064. [DOI] [PubMed] [Google Scholar]
- 21.Moyers MF, Miller DW, Bush DA, Slater JD. Methodologies and tools for proton beam design for lung tumors. Int J Radiat Oncol Biol Phys. 2001;49(5):1429–38. doi: 10.1016/s0360-3016(00)01555-8. [DOI] [PubMed] [Google Scholar]
- 22.Brown ML, Gersh BJ, Holmes DR, Bailey KR, Sundt TM., 3rd From randomized trials to registry studies: translating data into clinical information. Nat Clin Pract Cardiovasc Med. 2008;5(10):613–20. doi: 10.1038/ncpcardio1307. [DOI] [PubMed] [Google Scholar]
- 23.Eisbruch A, Gregoire V. Balancing risk and reward in target delineation for highly conformal radiotherapy in head and neck cancer. Semin Radiat Oncol. 2009;19(1):43–52. doi: 10.1016/j.semradonc.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Xivry JO, Castadot P, Janssens G, et al. Evaluation of the radiobiological impact of anatomic modifications during radiation therapy for head and neck cancer: Can we simply summate the dose? Radiother Oncol. 2010;96(1):131–138. doi: 10.1016/j.radonc.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Klug C, Keszthelyi D, Ploder O, et al. Neoadjuvant radiochemotherapy of oral cavity and oropharyngeal cancer: evaluation of tumor response by CT differs from histopathologic response assessment in a significant fraction of patients. Head Neck. 2004;26(3):224–31. doi: 10.1002/hed.10373. [DOI] [PubMed] [Google Scholar]
- 26.ClinicalTrials.gov Search for Clinical Trials. 2010 May 11; < http://clinicaltrials.gov/> (11 May 2010)
- 27.Salama JK, Haddad RI, Kies MS, et al. Clinical practice guidance for radiotherapy planning after induction chemotherapy in locoregionally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;75(3):725–33. doi: 10.1016/j.ijrobp.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz M, Pierelli A, Fiorino C, et al. Helical tomotherapy and intensity modulated proton therapy in the treatment of early stage prostate cancer: A treatment planning comparison. Radiother Oncol. 2011;98(1):74–80. doi: 10.1016/j.radonc.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 29.Hoppe BS, Huh S, Flampouri S, et al. Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: A dosimetric comparison with photon-based stereotactic body radiotherapy. Radiother Oncol. 2010;97(3):425–430. doi: 10.1016/j.radonc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Toscas JI, Linero D, Rubio I, et al. Boosting the tumor bed from deep-seated tumors in early-stage breast cancer: A planning study between electron, photon, and proton beams. Radiother Oncol. 2010;96(2):192–198. doi: 10.1016/j.radonc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Cozzi L, Fogliata A, Lomax A, Bolsi A. A treatment planning comparison of 3D conformal therapy, intensity modulated photon therapy and proton therapy for treatment of advanced head and neck tumours. Radiother Oncol. 2001;61(3):287–97. doi: 10.1016/s0167-8140(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 32.Johansson J, Blomquist E, Montelius A, Isacsson U, Glimelius B. Potential outcomes of modalities and techniques in radiotherapy for patients with hypopharyngeal carcinoma. Radiother Oncol. 2004;72(2):129–38. doi: 10.1016/j.radonc.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Brown AP, Urie MM, Chisin R, Suit HD. Proton therapy for carcinoma of the nasopharynx: a study in comparative treatment planning. Int J Radiat Oncol Biol Phys. 1989;16(6):1607–14. doi: 10.1016/0360-3016(89)90970-x. [DOI] [PubMed] [Google Scholar]
- 34.Taheri-Kadkhoda Z, Björk-Eriksson T, Nill S, et al. Intensity-modulated radiotherapy of nasopharyngeal carcinoma: a comparative treatment planning study of photons and protons. Radiother Oncol. 2008;3:4. doi: 10.1186/1748-717X-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noël G, Boisserie G, Dessard-Diana B, et al. Comparison with dose-volume histograms of two conformal irradiation techniques used for the treatment of T2N0M0 nasopharyngeal cancer, one with association of photons and protons and another with photons alone. Cancer Radiother. 2002;6(6):337–48. doi: 10.1016/s1278-3218(02)00222-6. [DOI] [PubMed] [Google Scholar]
- 36.Steneker M, Lomax A, Schneider U. Intensity modulated photon and proton therapy for the treatment of head and neck tumors. Radiother Oncol. 2006;80(2):263–7. doi: 10.1016/j.radonc.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 37.Hui Z, Zhang X, Starkschall G, et al. Effects of interfractional motion and anatomic changes on proton therapy dose distribution in lung cancer. Int J Radiat Oncol Biol Phys. 2008;72(5):1385–95. doi: 10.1016/j.ijrobp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.