Abstract
Juvenile neuronal ceroid lipofuscinosis (JNCL; CLN3 disease; Batten disease) is an autosomal recessive neurodegenerative disease of childhood that typically presents at school age with vision loss followed by progressive cognitive decline, motor dysfunction, seizures, and behavior problems. No therapy has been shown to slow the progression of disease in JNCL patients, and all current treatments are symptomatic. Flupirtine has been shown in vitro to reduce apoptosis in CLN3 lymphocytes. Based on that preclinical study, several children with JNCL were given flupirtine by their parents. The purpose of this study was to determine if there was evidence of attenuated disease progression in any JNCL symptom domain. We administered a survey to parents of JNCL children to qualitatively assess flupirtine efficacy. We used the Unified Batten Disease Rating Scale (UBDRS) to determine specific aspects of disease progression and investigated three age-related factors: loss of independent ambulation, loss of intelligible speech, and loss of ability to perform independent activities of daily living. The median scores for the UBDRS physical, behavior, and capability subscales were determined in flupirtine-exposed subjects and compared to age-, sex-, and genotype-matched subjects who had never taken flupirtine. Twenty-one percent of survey responders reported administering flupirtine to their JNCL child, and 56% of these families perceived beneficial changes that they attributed to flupirtine. However, our quantitative, prospectively obtained data did not show any change in JNCL disease progression that could be attributed to flupirtine. This study highlights the need for prospective experimental therapeutic research.
Introduction
The neuronal ceroid lipofuscinoses (NCLs) are the most prevalent neurodegenerative diseases of childhood (Santavuori et al. 2000). The NCLs represent a heterogeneous group of inherited disorders characterized clinically by blindness secondary to retinal degeneration, seizures, progressive decline of motor and cognitive abilities, and premature death (Kohlschutter and Schulz 2009). Although age at onset, symptom chronology, and the associated single-gene defect differ among specific types, all NCLs are characterized by accumulation of autofluorescent lipoprotein (ceroid lipofuscin) in neurons and other tissues (Boustany 1996). The main subtypes are infantile (INCL, CLN1 disease), late infantile (LINCL, CLN2 disease), and juvenile (JNCL, Batten disease, CLN3 disease). Identification of specific genetic mutations has enhanced the traditional classification system by age at onset and pathology, leading to recognition of additional variant forms (Jalanko and Braulke 2009).
The juvenile subtype, JNCL, is the most common NCL (Santavuori et al. 2000). Classic JNCL is caused by mutations at the CLN3 locus on chromosome 16, resulting in loss of function of battenin, a lysosomal membrane protein of unknown function. Children with JNCL typically present at school age with vision loss followed by progressive cognitive decline, motor dysfunction, seizures, and behavior problems (Marshall et al. 2005). Death usually occurs early in the third decade of life. The most common mutation associated with JNCL is an approximately 1 kb deletion including exons 7 and 8 of CLN3 (Phillips et al. 2005). Approximately 85% of affected children are homozygous for this common deletion; the majority of remaining individuals are compound heterozygotes with one copy of the common mutation and a second, novel mutation (Munroe et al. 1997; Phillips et al. 2005).
No therapy has been shown to slow, stop, or reverse disease progression in JNCL patients, and all current treatments are symptomatic. It has been suggested that flupirtine, a centrally acting nonopioid analgesic, has the potential to slow disease progression in JNCL and LINCL (Boustany 1996; Dhar et al. 2002). Although the biochemical defect and pathobiology in CLN2 disease and CLN3 disease are different, accelerated apoptosis has been shown to be the mechanism of neuronal and photoreceptor cell death in animal models of both diseases (Boustany 1996). Flupirtine protects CLN3-deficient human lymphoblasts and prevents CLN2- and CLN3-deficient human-derived neurons from apoptosis (Dhar et al. 2002). It has been proposed that flupirtine inhibits apoptosis by increasing cellular BCL2, a protein that operates at the mitochondria to prevent the release of cytochrome c, a key event in the apoptotic pathway. It may also improve calcium retention within the intermembrane mitochondrial space during oxidative stress and prevent NMDA receptor-induced death. Despite these promising cell-based studies, flupirtine has not been tested in animal models or human clinical trials.
During an ongoing longitudinal study of JNCL, we encountered several families who administered flupirtine to their JNCL children and believed it provided clinical benefit. Therefore, the purpose of this investigation was to evaluate parent-reported effects of flupirtine and to quantify any differences in disease progression in children exposed to flupirtine in comparison to those without any exposure to this drug.
Methods
Flupirtine survey
We created a questionnaire to gather information regarding (1) prior use of flupirtine, including duration and dose, (2) indication, (3) reasons for discontinuation, and (4) parent-perceived changes (positive or negative) attributed to flupirtine use. We mailed surveys to the 125 families who had previously participated in our research and had agreed to be contacted for future studies. We also conducted the survey during subjects’ in-person visits at the 2010 Annual Meeting of Batten Disease Support and Research Association (BDSRA) and at the University of Rochester Batten Disease Diagnostic and Clinical Research Center in Rochester, New York. We received a total of 53 completed surveys from 50 families (some families had multiple affected children). This study was approved by the University of Rochester Research Subjects Review Board, and all parents provided written, informed permission for their child’s participation.
Subject demographics
Subject diagnosis was reported by the parents and was usually based on clinical course plus electron microscopy or genetic testing for CLN3 mutations. For those subjects with genetic data available (n = 42), JNCL was confirmed by mutations in both CLN3 genes (Rothberg et al. 2004). Two subjects reported by parents as having JNCL were diagnosed by skin biopsy only and were later determined not to be JNCL either by normal CLN3 analysis (n = 1) or clinical picture (n = 1). They were excluded from further analyses.
Clinical assessment
We have developed a clinical rating instrument for JNCL, the Unified Batten Disease Rating Scale (UBDRS) (Marshall et al. 2005). The UBDRS is a reliable and valid tool that quantitatively assesses five aspects of JNCL: physical ability, seizure activity, behavioral problems, functional capability, and global clinical impression of disease. We used the components of the UBDRS to determine specific aspects of disease progression. The UBDRS physical subscale contains multiple elements to quantify different aspects of physical impairment in JNCL. We selected two specific items from this scale that were most likely to reflect important aspects of function: gait and speech. We determined the age at which subjects became unable to walk without assistance and the age at which speech became unintelligible. Using the functional capability subscale, we assessed the ability to independently perform activities of daily living (ADLs). Since the ability to perform ADLs is affected by cognitive ability, physical ability, and vision, performance of ADLs is a broad measure of disease severity in JNCL. We determined the age at which subjects were no longer able to perform ADLs independently.
Data analysis
All statistical analyses were performed using Statistica v. 6.1. Due to the small sample size, the mean age (in years) for crossing these thresholds was not normally distributed. Thus, a Mann Whitney U-test was performed to assess mean age in years at which loss of independent gait, loss of intelligible speech, and loss of ability to perform independent ADLs occurred. The age at which subjects progressed to loss of independent ADLs was also assessed by constructing a Kaplan Meier survival curve in which survival was defined as the ability to independently perform ADLs. Subjects were categorized as either on- or off-flupirtine based on parent-reported use of flupirtine at time of assessment. Survival curves were not calculated for gait or speech due to the small number of subjects crossing the thresholds for independent ambulation or intelligible speech.
In addition to assessing specific measures within the UBDRS subscales, total subscale scores for the physical, behavior, and functional capability domains were analyzed. The UBDRS subscale scores ranged from 0–84 points for physical, 0–54 points for behavior, and 0–28 points for functional capability. Higher scores reflected greater severity in the physical and behavior subscales. Lower scores reflected greater severity in the functional capability subscale. The physical and functional capability subscale scores correlate with disease progression.
Subjects who were ever exposed to flupirtine were randomly matched by sex, age at assessment (within 1 year), and genotype (homozygous for common deletion or compound heterozygote) to control subjects with no flupirtine exposure. To minimize ascertainment bias, subject matching was performed by sorting each group in numerical order of the individual subject identification numbers. The first control fulfilling the criteria described above was assigned as a match to a flupirtine subject. The UBDRS median physical, behavior, and functional capability subscale scores were determined for the last assessment at which flupirtine-exposed subjects were known to be actively taking the medication. These scores were compared to those of the matched controls at the same age by performing a Wilcoxon matched pairs test.
A two-sample t-test was performed to compare the mean age (at date of survey completion) of flupirtine-exposed to non-exposed subjects. One JNCL subject was deceased at the time of survey completion and was excluded from the age analysis.
Results
Flupirtine survey
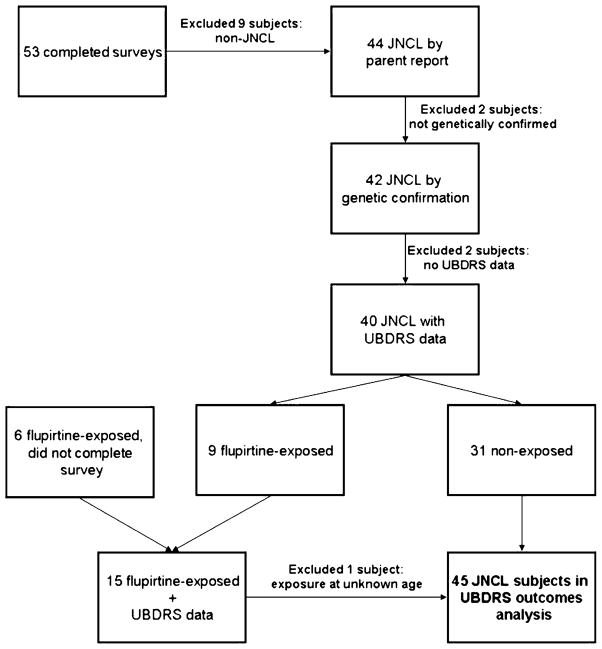
Of the 53 completed surveys (25 females, 28 males), 42 subjects had genetically confirmed JNCL (Fig. 1). These 42 subjects were used for analysis. Thirty subjects were homozygous for the common deletion, and 12 subjects were compound heterozygous for the common deletion and another, uncommon mutation (Table 1). The mean age of genetically confirmed subjects at time of survey completion was 16.8 years (SD 4.9).
Fig 1.
Flowchart depicting subject inclusion and exclusion criteria for UBDRS outcomes analyses
Table 1.
Demographic data on genetically confirmed JNCL subjects whose parents completed the survey on flupirtine use. Age of subject at survey completion excluded one subject who was deceased at time of survey
| Have taken flupirtine | Never taken flupirtine | |
|---|---|---|
| JNCL subjects (n) | 9 | 33 |
| Sex (F/M) | 3/6 | 15/18 |
| Mean (SD) age at survey completion (years) | 15.0 (5.2) | 17.3 (4.7) |
| Genotype: homozygous for common deletion (n) | 8 | 22 |
| Genotype: compound heterozygous (n) | 1 | 11 |
Of the 42 JNCL subjects with genetic confirmation, 9 (3 females, 6 males) had taken in the past or were currently taking flupirtine (21%) and 33 (79%) had never been exposed to flupirtine. The mean age of subjects at the time of survey completion was not statistically different between flupirtine-exposed and non-exposed groups (Table 1). Of those who had taken flupirtine, eight were homozygous for the common deletion and one was a compound heterozygote. Of those who had never taken flupirtine, 22 were homozygous for the common deletion, and 11 were compound heterozygous for the common deletion and another, noncommon mutation. The mean duration of flupirtine use was 3.8 years (SD 3.0) and median duration was 5 years (range 6 months to 8 years). All subjects exposed to flupirtine had initiated treatment between 5 and 15 years of age. Of those children whose parents reported flupirtine exposure, three have since discontinued the medication. Those who discontinued flupirtine used it for a mean of 2.2 years (SD 2.5) and a median of 1 year (range 6 months to 5 years), while those subjects still on flupirtine had taken it for a mean of 4.7 years (SD 3.0) and a median of 5.5 years (range 1–8 years).
Parent-reported reasons for discontinuation include concern for possible adverse effects (n = 1), perceived lack of benefit (n = 2), financial cost (n = 1), or a combination. Families of five subjects reported beneficial changes that they attributed to flupirtine use. Beneficial changes included parent perceptions of slowed disease progression (n = 3), improved mood (n = 2), improved seizure activity (n = 1), and decreased pain (n = 1). Families of three subjects noted no change. In addition to two families who discontinued flupirtine for this reason, one other family has continued to use the medication despite no clear parent-observed change in their child’s function. One additional family was uncertain whether there was benefit or not but has continued to use flupirtine. This family reported diarrhea as a possible negative effect of flupirtine.
UBDRS data
Of the 42 genetically confirmed JNCL subjects, UBDRS quantitative data were available for 40 subjects. In addition to our survey subjects, there were six additional JNCL subjects who reported use of flupirtine in our database but who did not respond to our survey. Therefore, we have a total of 46 JNCL subjects with quantitative data who either completed our survey or had previously reported using flupirtine according to our records.
Of the 46 subjects on whom we have quantitative data from the UBDRS, almost one-third (n = 15) had ever taken flupirtine. One subject exposed to flupirtine did not start the medication until after our most recent clinical assessment and was therefore considered a nonflupirtine subject for this analysis. One subject was only on flupirtine for a total of 6 months during an unknown time and thus was excluded from our analyses since we do not know if the subject was on flupirtine at any point before or during the UBDRS assessments. Most subjects had multiple assessments over a period of up to 8 years, for an average of 3.6 assessments per subject.
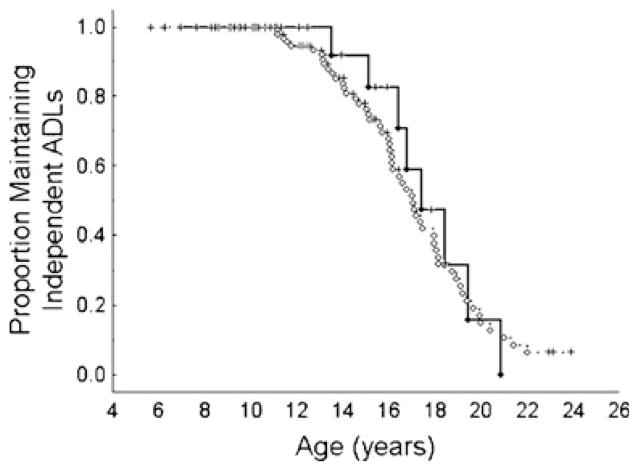
The mean age at which subjects lost the ability to ambulate independently, speak intelligibly, and independently perform ADLs was not different between subjects who had taken flupirtine and those who had not (Table 2). However, there was a trend toward earlier age at loss of intelligible speech in the flupirtine-treated group. When plotted as a Kaplan-Meier survival curve, subjects followed the same trajectory in loss of independent ADLs regardless of flupirtine use (Fig. 2).
Table 2.
Mean (SD) age at which flupirtine-exposed and nonexposed subjects crossed each threshold of disease progression
| Used flupirtine: mean age (SD) | Never used flupirtine: mean age (SD) | |
|---|---|---|
| Age at loss of independent gait (P = 0.93) | 15.3 years (3.9 years) (n = 3) | 16.1 years (2.2 years) (n = 9) |
| Age at loss of intelligible speech (P = 0.10) | 14.9 years (2.5 years) (n = 4) | 17.5 years (3.4 years) (n = 10) |
| Age at loss of independent ADLs (P = 0.85) | 14.1 years (2.3 years) (n = 7) | 14.4 years (2.8 years) (n = 10) |
n represents the number of subjects who crossed each threshold
Flupirtine-exposed subjects did not lose their ability to walk independently, speak intelligibly, and perform independent ADLs at a significantly different mean age than nonexposed subjects
Fig 2.
Change in number of subjects still able to perform ADLs independently with age. Age at which subjects were no longer able to perform ADLs independently is similar in flupirtine-exposed and nonexposed subjects. Solid line indicates subjects on flupirtine. Dotted line indicates subjects not on flupirtine. Circles represent loss of ADLs and plus signs independent ADLs
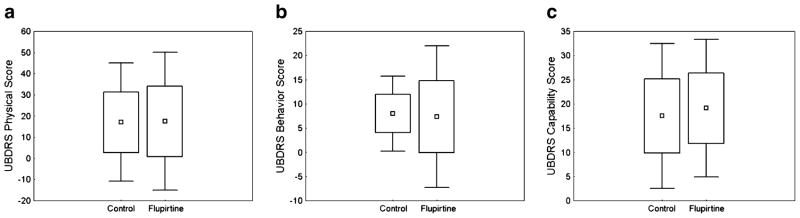
The median scores for the UBDRS physical, behavior, and capability subscales were determined for the last assessment at which subjects were known to be on flupirtine and compared to subjects matched for age (in years), sex, and homozygote vs. compound heterozygote condition who had never taken flupirtine. Four subjects from the flupirtine group were excluded from this analysis for the following reasons: not assessed while on flupirtine (n=2), on flupirtine for 6 months at an unknown time (n=1), and lack of suitable matched control (n=1). There were no differences between the flupirtine group and the control group on measures of physical impairment, behavioral impairment, or functional capability (Fig. 3).
Fig 3.
Flupirtine-exposed subjects (n=11) had similar physical (P=0.88) (a), behavior (P=0.80) (b), and functional capability (P=0.89) (c) UBDRS subscale scores as the age-, sex-, and genotype-matched nonexposed controls. Boxes indicate mean score ± SD, and error bars indicate ±1.96SD
Discussion
Juvenile neuronal ceroid lipofuscinosis is a neurodegenerative disease in which affected children lose physical and functional capabilities over time. The aim of the present study was to evaluate whether flupirtine, which had been shown in vitro to reduce apoptosis in CLN3 lymphocytes, is associated with attenuation of disease progression in any symptom domain. If flupirtine slowed disease progression, we would have expected subjects who had taken flupirtine to be older when they lost skills of independent ambulation, intelligible speech, and performance of ADLs. However, there was no difference in age at which these endpoints were reached. In fact, there was a trend toward earlier loss of intelligible speech in the flupirtine-treated group, which further argues against a disease-modifying benefit. There was no difference in median physical, behavior, and functional capability UBDRS subscale scores between subjects who had taken flupirtine and the age-, sex-, and genotype-matched controls. Thus, we were not able to demonstrate that flupirtine slowed disease progression at a quantitatively detectable rate in this sample of JNCL subjects.
Fifty-six percent of families of JNCL subjects who had taken flupirtine perceived beneficial changes that they attributed to flupirtine. Three families specifically perceived a slower than expected progression of disease. Our data are in contradiction to these perceptions. There are several reasons why this discrepancy may exist. First, it is possible that our outcome measurements are insensitive to subtle changes in the rate of disease progression in flupirtine users. Second, flupirtine may exert a placebo effect such that families perceive a benefit where none exists. Since these families were not blinded to the use of flupirtine, their perception of benefit may be affected by knowing that their child used flupirtine. Finally, because flupirtine has analgesic properties, it is possible that parent perception of improvement may have been related to reduced pain or improved mood as was noted in three children. Our analyses were limited by a small sample size and the inability to control for other factors that might be related to disease progression. Of the 45 JNCL subjects considered in the analysis of UBDRS endpoints, only a small number had documented loss of independent ambulation (n=12), loss of intelligible speech (n=14), or loss of independent ADLs (n=17). Additionally, we were not able to control for timing of flupirtine use in relation to the timing of UBDRS exams. Finally, since this is a nonrandomized sample, it is possible that that there was a preferential use of flupirtine by families of children whose disease had progressed further in comparison to children not on flupirtine.
The proposed role of flupirtine in JNCL disease is based on in vitro cell-culture data (Dhar et al. 2002). In addition, there are animal data suggesting that AMPA receptor inhibition can improve function in the Cln3-knockout mouse, presumably by reducing excitoxicity-mediated apoptosis (Kovacs et al. 2011). However, no data exist on the efficacy of flupirtine in JNCL patients. Flupirtine is not yet FDA-approved for use in any disease and is thus not available in the United States, making it expensive and difficult to obtain. In fact, one family cited expense as a reason for discontinuing the medication. Flupirtine was well-tolerated in most children. Other than one reported case of diarrhea, no adverse effects of the drug were noted in this sample. However, while flupirtine is generally considered a safe medication, safety and appropriate dosing in JNCL children have not been established.
Although BCL2-dependent apoptotic pathways have been implicated in JNCL and LINCL neurodegeneration, other pathways are likely involved in the disease process. In a mouse model of LINCL (Kim et al 2009), the affected mice were genetically modified to alter levels of TP53 or BCL2, proteins critical to the apoptotic pathway. LINCL mice with absent TP53 (pro-apoptotic) or increased BCL2 (anti-apoptotic) did not demonstrate a slowed disease course or change in survival rates. If apoptotic pathways were central to the NCL disease process, absence of TP53 or elevated expression of BCL2 would be expected to decrease apoptosis and slow disease progression. This suggests that alternative pathways may be involved in the disease process and that pharmacologically targeting apoptosis is unlikely to prevent neurodegeneration in LINCL. Together with our data, these observations oppose the hypothesis that flupirtine can slow the rate of disease progress in either LINCL or JNCL. However, we do not know if flupirtine actually exerts an anti-apoptotic activity on neurons in vivo; consequently we cannot entirely rule out a role for inhibition of apoptosis in the therapy of JNCL.
This analysis of flupirtine use in genetically confirmed JNCL subjects highlights the need for data from animal models or prospective trials in human subjects. Although cell-culture studies suggest a potential role for flupirtine in modifying the CLN3 disease pathway, there are no human data to support the use of flupirtine in the clinical management of JNCL. JNCL is a fatal diagnosis with no therapies proven to slow, stop, or reverse disease progression in patients. It is tempting to turn to medications with potential benefits based on cell-culture studies. However, our data do not support efficacy of flupirtine in JNCL. We recognize the need to explore novel treatment strategies in rare metabolic disorders. This exploration should be based on careful experimental therapeutic research.
Acknowledgments
The authors would like to thank Denia Ramirez-Montealegre, MD, PhD, MPH, Erika Wexler, MD, Tiffani McDonough, MD, and Leon Dure, MD for contributions to data collection and Will Wester for thoughtful discussions. We also thank the patients and families. This work was supported by NIH Grants R01NS060022, K12NS066098, K23NS058756, and TL1RR024136, by the Batten Disease Support and Research Association, the Strong Children’s Research Center, and the Geoffrey Waasdorp Pediatric Neurology Fund.
Footnotes
Competing interests: None declared
Communicated by: Robert Steiner
References
- Boustany R-M. Batten disease or neuronal ceroid lipofuscinosis. In: Moser HW, editor. Handbook of clinical neurology. Vol. 22. Elsevier; Amsterdam: 1996. pp. 671–700. [Google Scholar]
- Dhar S, Bitting RL, Rylova SN, et al. Flupirtine blocks apoptosis in Batten patient lymphoblasts and in human postmitotic cln3- and cln2-deficient neurons. Ann Neurol. 2002;51:448–466. doi: 10.1002/ana.10143. [DOI] [PubMed] [Google Scholar]
- Jalanko A, Braulke T. Neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2009;1793:697–709. doi: 10.1016/j.bbamcr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Kim KH, Sleat DE, Bernard O, Lobel P. Genetic modulation of apoptotic pathways fails to alter disease course in tripeptidyl-peptidase 1 deficient mice. Neurosci Lett. 2009;453:27–30. doi: 10.1016/j.neulet.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlschutter A, Schulz A. Towards understanding the neuronal ceroid lipofuscinoses. Brain Dev. 2009;31:499–502. doi: 10.1016/j.braindev.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Kovacs AD, Saje A, Wong A, et al. Temporary inhibition of ampa receptors induces a prolonged improvement of motor performance in a mouse model of juvenile Batten disease. Neuropharmacology. 2011;60:405–409. doi: 10.1016/j.neuropharm.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall FJ, de Blieck EA, Mink JW, et al. A clinical rating scale for Batten disease: reliable and relevant for clinical trials. Neurology. 2005;65:275–279. doi: 10.1212/01.wnl.0000169019.41332.8a. [DOI] [PubMed] [Google Scholar]
- Munroe PB, Mitchison HM, O’Rawe AM, et al. Spectrum of mutations in the Batten disease gene, cln3. Am J Hum Genet. 1997;61:310–316. doi: 10.1086/514846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SN, Benedict JW, Weimer JM, Pearce DA. Cln3, the protein associated with Batten disease: structure, function and localization. J Neurosci Res. 2005;79:573–583. doi: 10.1002/jnr.20367. [DOI] [PubMed] [Google Scholar]
- Rothberg PG, Ramirez-Montealegre D, Frazier SD, Pearce DA. Homogeneous polymerase chain reaction nucleobase quenching assay to detect the 1-kbp deletion in cln3 that causes Batten disease. J Mol Diagn. 2004;6:260–263. doi: 10.1016/S1525-1578(10)60519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santavuori P, Lauronen L, Kirveskari E, Åberg L, Sainio K, Autti T. Neuronal ceroid lipofuscinoses in childhood. Neurol Sci. 2000;21:S35–S41. doi: 10.1007/s100720070038. [DOI] [PubMed] [Google Scholar]