Abstract
The amygdala is a key limbic structure strongly implicated in both epilepsy and anxiety disorders. Epilepsy-like mechanisms involve increased glutamatergic activity, whereas disturbances in serotonin (5-HT) systems are associated with anxiety-like behavior. Previous studies suggest low 5-HT increases amygdala excitability, but the molecular mechanisms are not well-characterized. Here we explore the ability of low serotonin to increase glutamate receptor transcription. Using quantitative RT-PCR, we found that rats treated with p-chlorophenylalanine, an inhibitor of tyrosine-5-hydroxylase, resulted in a 21-fold increase in glutamate receptor 1 (GluR1) mRNA expression in the amygdala. These results suggest that low 5-HT induces hyperexcitability of LA neurons by increasing GluR1 transcription, and the upregulation of amygdala GluR1 may be important in the pathophysiology of anxiety disorders.
Keywords: amygdala, glutamate, 5-HT, qRT-PCR
Introduction
The amygdala is a key limbic structure that is strongly implicated in the pathophysiology of both epilepsy [1,2] and mood and anxiety disorders [3]. The amygdala functions as a convergence point for sensory input that determines the emotional salience of the input. The emotional content of sensory information is relayed to cortical and brainstem areas involved in fear to coordinate appropriate physiological and behavioral responses to environmental danger [4,5]. However, hyperexcitability of amygdala circuitry has been shown in temporal lobe epilepsy (TLE) [2]. Patients with TLE may have comorbid psychiatric symptoms [6] and anti-epileptic drugs can be therapeutic in psychiatric disorders [7]. Additionally, in the kindling model of epilepsy, seizures are associated with abnormal emotional behaviors such as increased aggression and anxiety-like behavior [1,8,9]. Together, this suggests that epilepsy-like mechanisms may be involved in abnormal emotional conditions. It is therefore suggested that anxiety and mood disorders may be the result of sub-seizure hyperexcitability in the amygdala [3,10].
Epilepsy-like mechanisms involve increased glutamatergic activity, whereas dysfunction in the serotonin (5-hydroxytryptamine, 5-HT) system is associated with abnormal emotion [11,12]. In the amygdala, 5-HT decreases excitability [13,14]. Conversely 5-HT deficits increase amygdala excitability [10,15] and contribute to abnormal emotional behavior [16,17]. The molecular mechanisms underlying low 5-HT-induced hyperexcitability have not been elucidated completely. However it has been shown that changes in amygdala glutamate receptors (GluRs) are critical for conditioned fear [18,19] and conditioned fear is increased in 5-HT-depleted rats [16]. Therefore, we hypothesized that 5-HT depletion by systemic administration of p-chlorophenylalanine (PCPA) is associated with increased glutamate receptor expression. Our results show that GluR1 transcription is upregulated in PCPA-treated animals.
Methods
Animals
All experimental animal procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and conform to a protocol approved by Baylor University Animal Care and Use Committee. Male Sprague-Dawley rats approximately 150 g (n=6; Harlan, Houston, TX) were group housed in a light controlled 12 hr light/dark cycle and temperature controlled (23 °C) room. Food and water were available ad libitum.
Experimental Design
Individually housed rats were treated on days 1, 2, and 10 with either p-chlorophenylalanine (PCPA, 300 mg/kg, n=3) or phosphate buffered saline (PBS, n=3). On day 14, rats were euthanized by rapid decapitation with a small animal guillotine. Brains were dissected in cold (2 – 4 °C) PBS and 2 mm slices containing the basolateral complex the amygdala (taken between 4.5 mm and 2.5 mm posterior to Bregma) were dissected on dry ice. Tissue samples were taken bilaterally from the amygdala, dorsal hippocampus, and overlying cortex using a core sampler (1.0 mm diameter). Samples were weighed and flash frozen in dry ice/EtOH and stored at −80 °C until used.
Primers and plasmids
Primers were obtained from Maxim Biotech (San Francisco, CA). The GluR1 forward primer was 5' - TCG TAC CAC CAT TTG TTT TTC A - 3' and the reverse primer for GluR1 was 5' - AAG AGG GAC GAG ACC AGA CAA C - 3'. Primers for 18S rRNA were 5’ - CCG CAG CTA GGA ATA ATG GAA TAG GAC - 3’ (forward) and 5’ - GTT AGC ATG CCG AGA GTC TCG TTC - 3’ (reverse).
GluR1 plasmids were a gift from R. L. Huganir (John Hopkins Medical Institute, Baltimore, MD). The plasmids were Amp+ and contained a CMV promoter. DH5α cells were transformed with plasmids, which were then extracted using QIAprep spin Miniprep Kit (QIAgen, Valencia, CA). Concentrations of the cloned plasmids were measured spectrophotometrically. The plasmids were diagnostically verified by double enzyme digest and PCR with specific primers. PCR was performed on a Techne TC512 Thermo Cycler using Sigma RedTaq Jumpstart Ready Mix PCR reaction mix (Sigma, St. Louis, MO). Annealing temperature of the PCR reaction was optimized using a gradient of temperatures. PCR was performed using 30 cycles of 94 °C for 30 s, gradient annealing temperature (ranging from 55.9 to 64.2 °C) for 30s and 72 °C for 5 min. Products were separated on a 1% agarose gel and visualized with ethidium bromide to determine optimal PCR conditions.
Quantitative RT-PCR
Whole cell RNA was extracted using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH) following the manufacturer’s instructions. Concentration of total RNA was determined by spectrophotometry (λ=260 nm). Extraction was followed by cDNA synthesis and real-time PCR using Dynamo SYBR Green 2 step qRT-PCR kit (NEB, Ipswich, MA) in a total reaction volume of 25 µl. Samples were run in duplicates. A ‘no template’ condition served as a negative control. GluR1-containing plasmids were used to standardize separate runs and 18S rRNA was an internal control. The reaction was performed on a Corbett Rotor-Gene 6000 with the initial denaturation at 95 °C 15 min, subsequent denaturation at 94 °C for 10 s, annealing at 59.4 °C for 30 s, extension 72 °C for 30 s, and a final extension at 72 °C for 10 min. Melting curves were performed at the end of each cycle from 72–95 °C with 90 s intervals, and showed only a single peak near 80 °C. PCPA-induced changes in GluR1 transcripts were quantified using the comparative ΔΔC(T) method. The number of cycles to threshold [C(T)] ranged between 19 and 34 for GluR1 and between 12 and 36 for 18S rRNA. Relative quantity of GluR1 mRNA from each sample was calculated as the difference in C(T) for GluR1 minus C(T) for 18S rRNA [ΔC(T)], and calibrated to C(T) of PBS-treated control sample [ΔΔC(T)]. Fold-change in transcription is expressed as 2[−ΔΔC(T)] [20].
Statistical Analysis
Student’s unpaired t-test (two-tailed) was used to compare the relative number of PCR cycles to threshold (ΔC(T)) in samples from PCPA and PBS-treated animals in each of the 3 brain regions examined. Statistical significance is defined as p < 0.05. Data are reported as the mean ± standard error. Estimates of variance in 2[−ΔΔC(T)] were calculated using the mean ΔΔC(T) ± SEM of ΔΔC(T).
Results
PCPA treatment increased GluR1 expression in the amygdale
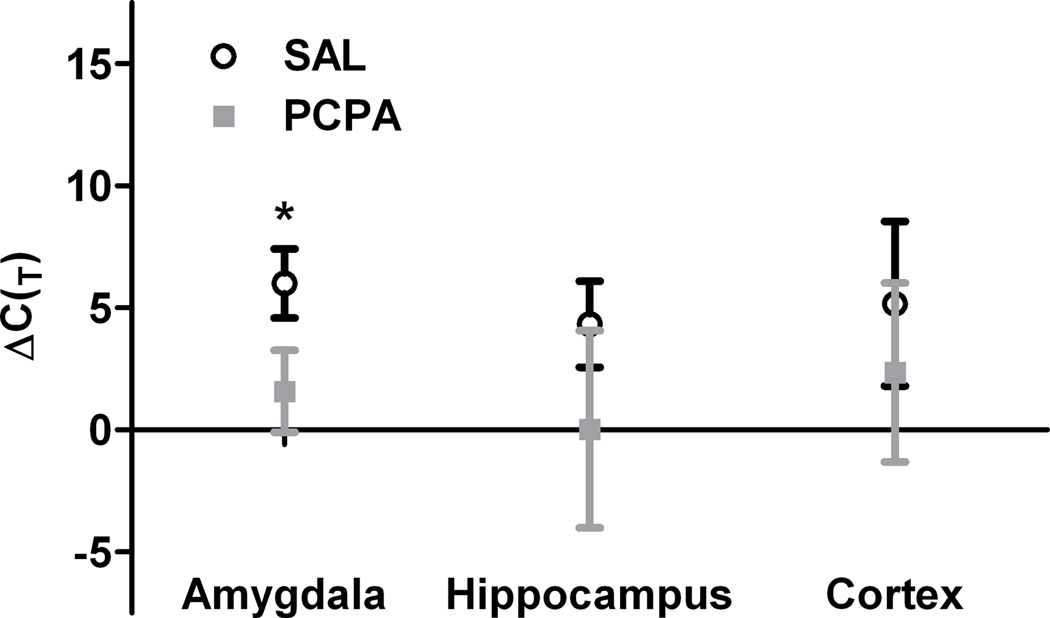
Systemic administration of p-chlorophenylalanine (PCPA) increases fear-potentiation of the startle reflex [16]. Since fear-conditioning requires upregulation of the GluR1 subunit of AMPA receptors [19] we investigated the regulation of GluR1 transcription in PCPA-treated animals by quantitative RT-PCR. Figure 1 shows the number of PCR cycles to detection threshold for GluR1 transcripts relative to 18S rRNA (ΔC(T); see methods) in the amygdala, hippocampus and cortex. ΔC(T) was decreased from 6.0 ± 1.4 to 1.6 ± 1.7 cycles (p < 0.05, t(4)=2.45) in samples from PBS-treated control and PCPA-treated rats, respectively. Fewer PCR cycles to threshold reflects a quantitative increase in the amount of mRNA for GluR1. In hippocampal samples there was a small, but insignificant decrease in cycle numbers (control: ΔC(T) = 4.3 ±1.7 cycles; PCPA: ΔC(T) = 0.0 ± 7.0 cycles; p > 0.05). Similarly, ΔC(T) was unchanged in samples from cortex (control: ΔC(T) = 5.2 ±3.4 cycles; PCPA: ΔC(T) = 2.4 ± 3.7 cycles; p > 0.05).
Figure 1.
Quantitative RT-PCR reveals an increase in relative GluR1 transcription in the amygdala from PCPA-treated rats. mRNA levels measured by qRT-PCR of amygdala, hippocampus and cortex samples was normalized to 18S rRNA and the ΔC(t) values were calculated for each sample. PCPA treatment (gray squares, n = 3) reduced ΔC(t) in samples from the amygdala (left), but not hippocampus (middle) or cortex (right). *p < 0.05 (unpaired Student’s t-test) relative to samples from PBS-treated control rats (open circles, n = 3). Data represent mean ± S.E.M.
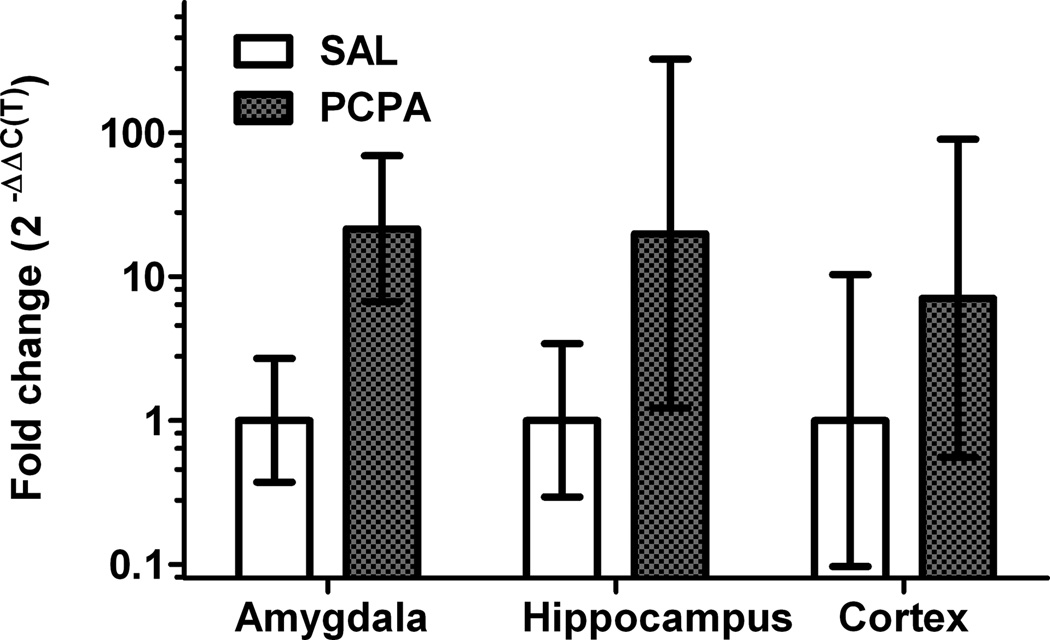
The fold change in GluR1 transcript expression was quantified by the ΔΔC(t) method [20] and is shown in Figure 2. Using the ΔΔC(T) method, the data were transformed to reflect quantitative changes in number of GluR1 transcripts. In samples from the amygdala, PCPA treatment is associated with a 21-fold increase (SEM: 7- to 69-fold increase) in GluR1 transcripts. GluR1 transcript expression did not differ significantly with PBS/PCPA treatment in either the hippocampus (mean: 20-fold increase in PCPA treated samples; SEM: 1- to 323-fold increase) or the cortex (mean: 7-fold increase in PCPA treated samples; SEM: 40% decrease to 89-fold increase).
Figure 2.
The relative expression of GluR1 transcripts is increased in the amygdala of PCPA treated animals (n = 3, stippled bars). mRNA levels were analyzed by qRT-PCR and normalized using the ΔΔC(T) method [20]. PCPA-induced increase in GluR1 transcripts is expressed as fold change relative to samples from PBS-treated control animals (n = 3, open bars) in samples from the amygdala (left) hippocampus (middle) and cortex (right).
Discussion
In the present study, we show that systemic PCPA treatment increases GluR1 transcription in the amygdala. PCPA is a competitive inhibitor of the rate-limiting enzyme in 5-HT synthesis, tryptophan-5-hydroxylase. Chronic administration of PCPA, as used in the current study, consistently depletes 5-HT levels in several models. We (Hughes, Tran & Keele, submitted) and others have shown previously that PCPA causes over 90% reduction in 5-HT and its primary metabolite 5-hydroxy-indoleacetic acid (5-HIAA) [21,22]. Additionally, 5-HT turnover (the ratio of 5-HIAA to 5-HT) is significantly reduced in PCPA-treated rats. Further, we have shown previously the PCPA treatment increases both aggressive behavior [17] and learned fear behavior [16] in rats. Altogether, these data suggest that disrupting 5-HT levels results in upregulation of excitatory glutamatergic transmission. The low-5-HT-induced increase in excitability of amygdala neurons may be an important mechanism involved in abnormal emotional behavior [10].
GluR1 expression has an important role in synaptic plasticity. Upregulation of GluR1 underlies enhanced magnitude of excitatory neurotransmission in hippocampal LTP [23]. LTP in sensory pathways afferent to the amygdala is the neural correlate of learned fear [18], and thus GluR1 expression is also increased in the amygdala of rodents exhibiting learned fear [19]. In these studies, the upregulation of GluR1 seems to occur without similar changes in GluR2 subunits since the degree of rectification of glutamatergic synaptic currents is enhanced (reflecting a decrease in the relative contribution of GluR2-containing AMPA-R’s), which is also accompanied by increased Ca2+-permeability of AMPA receptors. Although the selectivity of PCPA on GluR1 expression was not determined in the current study, our previous results showing that PCPA enhances fear learning suggests that GluR1 expression may be selectively upregulated in PCPA-treated rats.
While the pharmacological effect of PCPA is selective, we cannot confirm the direct role of 5-HT and/or 5-HT receptors in the observed increase of GluR1 transcripts. 5-HT systems are known to interact (cross-talk) with other aminergic systems. For example, it has been shown that 5-HT receptors can modulate mesolimbic dopamine synthesis [24]. Since the amygdala receives DA-ergic input from the midbrain [25], an alternate explanation of our data may involve changes in DA levels as the critical determinant of GluR1 transcription. That is, in the absence of 5-HT in PCPA-treated animals, DA synthesis (and release) could be enhanced and it is the increased DA concentration in the amygdala that initiates the signal transduction cascade causing upregulation of GluR1 transcription. However, the distribution of postsynaptic DA and 5-HT receptors in the amygdala argue against this possibility. DA receptors are located primarily on inhibitory GABAergic interneurons but 5-HT receptors are located on both interneurons and glutamatergic projection neurons. Activation of 5-HT receptors on interneurons are depolarizing and increase GABA release [13,14]; whereas 5-HT receptors on glutamatergic neurons are hyperpolarizing [14]. In light of the differential distribution of receptors, the most parsimonious interpretation of our data is that increased transcription of GluR1 in PCPA-treated rats is a direct result of 5-HT depletion.
5-HT has primarily an inhibitory role on amygdala output, by both increasing local-circuit GABAergic inhibition and by decreasing glutamatergic excitatory output. 5-HT depletion, therefore, can increase excitability by removing these acute inhibitory components. In vitro recordings from amygdala neurons of PCPA-treated rats are consistent with low 5-HT-induced hyperexcitability [15]. Here our data reveal an additional mechanism, specifically increased GluR1 transcription, following chronic 5-HT depletion. This further suggests there may be several avenues whereby dysfunction of 5-HTergic mechanisms can increase excitability in the amygdala, both directly at the level of the membrane, and indirectly through changing gene expression. We and others [3,9,10] have suggested that hyperexcitability in the amygdala circuitry mediating fear could lead to symptoms of mood and anxiety disorders. Increased transcription of GluR1 may be an important biological correlate involved in these disorders where regulation of 5-HT systems is perturbed.
Conclusion
Glutamate receptor 1 (GluR1) transcription is enhanced 21-fold in the amygdala of rats treated chronically with p-chlorophenylalanine (PCPA) to deplete brain serotonin (5-HT). These data suggest that increased GluR1 may underlie amygdala hyperexcitability involved in psychiatric disorders, such as mood and anxiety disorders, where 5-HT function is disrupted.
Acknowledgements
L. T. conducted all experiments, analyzed data and wrote the first draft of the manuscript. N.B.K. conceptualized the hypothesis, supervised the experiments, and finalized the manuscript. All authors have read and approved the manuscript.
Support: This work was supported by National Institute of Mental Health grant MH-80400 (to NBK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: The authors declare that they have no competing financial interests.
References
- 1.Adamec R, Blundell J, Burton P. Anxiolytic effects of kindling role of anatomical location of the kindling electrode in response to kindling of the right basolateral amygdala. Brain Res. 2004;1024:44–58. doi: 10.1016/j.brainres.2004.06.074. [DOI] [PubMed] [Google Scholar]
- 2.Schubert M, Siegmund H, Pape HC, Albrecht D. Kindling-induced changes in plasticity of the rat amygdala and hippocampus. Learn Mem. 2005;12:520–526. doi: 10.1101/lm.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- 4.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 5.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 6.Devinsky O, Bear D. Varieties of aggressive behavior in temporal lobe epilepsy. Am J Psychiatry. 1984;141:651–656. doi: 10.1176/ajp.141.5.651. [DOI] [PubMed] [Google Scholar]
- 7.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med. 2004;10:685–692. doi: 10.1038/nm1074. [DOI] [PubMed] [Google Scholar]
- 8.Kalynchuk LE. Long-term amygdala kindling in rats as a model for the study of interictal emotionality in temporal lobe epilepsy. Neurosci Biobehav Rev. 2000;24:691–704. doi: 10.1016/s0149-7634(00)00031-2. [DOI] [PubMed] [Google Scholar]
- 9.Post RM. Kindling and sensitization as models for affective episode recurrence, cyclicity, and tolerance phenomena. Neurosci Biobehav Rev. 2007;31:858–873. doi: 10.1016/j.neubiorev.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Keele NB. The role of serotonin in impulsive and aggressive behaviors associated with epilepsy-like neuronal hyperexcitability in the amygdala. Epilepsy Behav. 2005;7:325–335. doi: 10.1016/j.yebeh.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 11.de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526:125–139. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 12.Lucki I. Serotonin receptor specificity in anxiety disorders. J Clin Psychiatry. 1996;57 Suppl 6:5–10. [PubMed] [Google Scholar]
- 13.Jiang XL, Xing GQ, Yang CH, Verma A, Zhang L, Li H. Stress Impairs 5-HT2A Receptor-Mediated Serotonergic Facilitation of GABA Release in Juvenile Rat Basolateral Amygdala. Neuropsychopharmacology. 2009;34:410–423. doi: 10.1038/npp.2008.71. [DOI] [PubMed] [Google Scholar]
- 14.Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- 15.Keele NB, Randall DR. Altered modulation of excitatory neurotransmission in the amygdala by serotonin in an animal model of impulsive aggression. Ann N Y Acad Sci. 2003;985:528–532. [Google Scholar]
- 16.Hughes CR, Keele NB. Phenytoin normalizes exaggerated fear behavior in p-chlorophenylalanine (PCPA)-treated rats. Epilepsy Behav. 2006;9:557–563. doi: 10.1016/j.yebeh.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keele NB. Phenytoin inhibits isolation-induced aggression specifically in rats with low serotonin. Neuroreport. 2001;12:1107–1112. doi: 10.1097/00001756-200105080-00012. [DOI] [PubMed] [Google Scholar]
- 18.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 19.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Dewar KM, Grondin L, Carli M, Lima L, Reader TA. [3H]paroxetine binding and serotonin content of rat cortical areas, hippocampus, neostriatum, ventral mesencephalic tegmentum, and midbrain raphe nuclei region following p-chlorophenylalanine and p-chloroamphetamine treatment. J Neurochem. 1992;58:250–257. doi: 10.1111/j.1471-4159.1992.tb09303.x. [DOI] [PubMed] [Google Scholar]
- 22.Vergnes M, Depaulis A, Boehrer A. Parachlorophenylalanine-induced serotonin depletion increases offensive but not defensive aggression in male rats. Physiol Behav. 1986;36:653–658. doi: 10.1016/0031-9384(86)90349-5. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Nichols DE. 5-HT2 receptor-mediated potentiation of dopamine synthesis and central serotonergic deficits. Eur J Pharmacol. 1993;238:291–296. doi: 10.1016/0014-2999(93)90859-g. [DOI] [PubMed] [Google Scholar]
- 25.Fallon JH, Koziell DA, Moore RY. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol. 1978;180:509–532. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]