Abstract
Lower-extremity wounds are a major complication of diabetes. Hemoglobin A1c (HbA1c) reflects glycemia over 2–3 months and is the standard measure used to monitor glycemia in diabetic patients, but results from studies have not shown a consistent association of HbA1c with wound healing. We hypothesized that elevated HbA1c would be most associated with poor wound healing. To test this hypothesis we conducted a retrospective cohort study of 183 diabetic individuals treated at the Johns Hopkins Wound Center. Our primary outcome was wound-area healing rate (cm2/day). Calibrated tracings of digital images were used to measure wound area. We estimated coefficients for healing rate using a multiple linear regression model controlling for clustering of wounds within individuals and other common clinic variables. The study population was 45% female and 41% black with mean age of 61 years. Mean HbA1c was 8.0% and there were 2.3 wounds per individual (310 wounds total). Of all measures assessed, only HbA1c was significantly associated with wound-area healing rate. Specifically, for each 1.0% point increase in HbA1c, the daily wound-area healing rate decreased by 0.028 cm2/day (95% CI: 0.003, 0.0054, p=0.027). Our results suggest that glycemia, as assessed by HbA1c, may be an important biomarker in predicting wound healing rate in diabetic patients.
INTRODUCTION
Approximately 170 million individuals are affected by diabetes worldwide including 23.6 million individuals in the United States (7.8% of the population of the United States) (Cowie et al., 2009). Of these individuals, 28.5% have diabetic peripheral neuropathy (Gregg et al., 2004). Poor glycemic control is associated with the presence of neuropathy and increased risk for wounds and amputations (Gregg et al., 2004). Wounds of the foot are common in individuals with diabetes, with an annual incidence of 1–2% (Boulton et al., 2005). Approximately 15% of diabetic individuals in the United States will develop a foot wound at some point in their lives (Margolis et al., 2003). The cost of diabetic wounds includes interventions to prevent wounds, management to heal wounds, and management and care for amputation surgery and disability. In 2001, the cost of diabetic wounds in the United States was 10.9 billion dollars (Gordois et al., 2003) and this number is expected to rise as the prevalence of diabetes is expected to increase from the current prevalence of 26 million (prevalence of 8.3%) by 2050 (Centers for Disease Control and Prevention, 2011).
Given the large burden of diabetic wounds, it is important to identify modifiable factors that could aid healing and help optimize wound care. Previous studies in diabetic populations have identified prognostic factors associated with wound healing, but most of these have focused on non-modifiable variables such as baseline wound area (Ince et al., 2007; Jeffcoate et al., 2006; Margolis et al., 2000), duration of wound (Margolis et al., 2002), age (Apelqvist and Agardh, 1992), and sex (Marston, 2006). These studies have typically examined neuropathic foot ulcers (healed versus not healed) (Ince et al., 2007; Jeffcoate et al., 2006), need for amputation (Apelqvist and Agardh, 1992), or total time to complete healing (Margolis et al., 2000). Few studies have quantified healing rates. It remains unclear in the literature whether hemoglobin A1c (HbA1c), a standard measure of glycemia over 2–3 months, is related to wound healing rate (Apelqvist and Agardh, 1992; Margolis et al., 2000; Markuson et al., 2009).
Our objective was to identify the common clinical variable(s) most strongly related to healing rate in a clinic-based population of persons with diabetes. We hypothesized that among common laboratory tests and clinical measures, higher HbA1c values would be the most associated with decreased daily wound healing rate.
RESULTS
The demographic and clinical characteristics of the 183 individuals at the time of the first clinic visit are shown both overall and stratified by HbA1c categories in Table 1a. In the total sample, the mean age was 61 years, 45% were female, and 41% were African American. Sixty percent of the sample had peripheral neuropathy and 29% had peripheral artery disease. The mean HbA1c was 8.0% and there were a mean of 2.3 wounds per individual (310 wounds total).
Table 1a.
Characteristics of participants at time of first wound, overall and by HbA1c category.
| All Participants (N=183) | HbA1c < 7.0% (n=71) | HbA1c 7.0 – 8.0% (n=42) | |
|---|---|---|---|
| Age (years), mean (SD) | 61.0 (12.0) | 61.8 (12.0) | 65.9 (10.8) |
| Females, n (%) | 83 (45.4) | 34 (47.9) | 15 (35.7) |
| Race | |||
| Caucasian, n (%) | 100 (54.6) | 43 (60.6) | 29 (69.1) |
| African American, n (%) | 75 (41.0) | 25 (35.2) | 13 (31.0) |
| Other, n (%) | 8 (4.4) | 3 (4.2) | 0 (0.0) |
| Body Mass Index (kg/m2), mean (SD) | 35.4 (9.8) | 34.4 (10.1) | 35.2 (9.5) |
| HbA1c (%), mean (SD) | 7.97 (2.28) | 6.03 (0.60) | 7.39 (0.26) |
| Total Cholesterol (mg/dL), mean (SD) | 154 (44.7) | 148 (44.7) | 153 (40.6) |
| LDL Cholesterol (mg/dL), mean (SD) | 83.9 (41.5) | 75.4 (32.2) | 83.2 (32.0) |
| HDL Cholesterol (mg/dL), mean (SD) | 43.4 (15.9) | 44.1 (15.0) | 41.6 (13.2) |
| Triglycerides (mmol/L), mean (SD) | 142 (75.4) | 141 (78.1) | 147 (77.2) |
| Systolic Blood Pressure (mmHg), mean (SD) | 139 (23.3) | 136 (24.5) | 143 (24.9) |
| Diastolic Blood Pressure (mmHg), mean (SD) | 76.5 (13.5) | 76.0 (13.6) | 77.2 (14.1) |
| Pulse (bpm), mean (SD) | 79.4 (14.3) | 80.4 (13.5) | 75.8 (13.0) |
| Temperature (°Fahrenheit), mean (SD) | 98.0 (0.58) | 97.9 (0.56) | 98.0 (0.67) |
| White Blood Cell Count (cells/microliter), mean (SD) | 7882.4 (2490.4) | 7949.2 (2574.7) | 7862.4 (2379.8) |
| Smoking | |||
| Never, n (%) | 62 (33.9) | 17 (23.9) | 22 (52.4) |
| Former, n (%) | 70 (38.3) | 37 (52.1) | 12 (28.6) |
| Current, n (%) | 51 (27.9) | 17 (23.9) | 8 (19.1) |
| Neuropathy, n (%) | 109 (59.7) | 38 (53.5) | 23 (54.8) |
| Peripheral Artery Disease, n (%) | 53 (29.0) | 19 (26.8) | 12 (28.6) |
| Wound Number, mean (SD) | 2.28 (1.54) | 2.30 (1.62) | 2.05 (1.32) |
We divided the wounds into two broad categories of foot wounds with documented neuropathy (n=197) that are classic diabetic wounds versus chronic wounds elsewhere (n=113), which are predominately surgical wounds that have failed to heal. The demographic and clinical characteristics of the individuals at the time of the first clinic visit are shown stratified by neuropathy status in Supplemental Table 1a. Characteristics of the first wound are shown stratified by neuropathy status in Supplemental Table 1b. Participants with and without neuropathy differed by body mass index, LDL cholesterol, HbA1c, and by wound measurements and change in wound size per day.
The most common location for wounds in this diabetic population was the lower extremity, with 43.9% of wounds occurring on the leg or ankle and 41.2% of wounds occurring on the foot. The remainder of the wounds occurred on the buttocks (5.5%), the abdomen or chest (4.5%), the arm (2.3%), the groin (1.0%), the hip (1.0%), and the back (0.6%).
We noted in compiling wound-healing histories that individuals with lower HbA1c had faster healing. For example, the clinical course of two individuals who presented to the Johns Hopkins Wound Clinic with foot wounds is shown in Figure 1. The individual with HbA1c of 5.6% had a wound-healing rate of 0.35 cm2 per day (calculated using first and last visits to clinic) and the wound was completely resolved 64 days after initial presentation (Figure 1a. – 1d.). The individual with HbA1c of 11.1% had a more variable clinical course where the wound area increased and decreased over time and 727 days after initial presentation, the wound was not resolved (Figure 1e. – 1i.). During the time when the wound was decreasing in size (March to November 2009), the wound-healing rate was 0.001 cm2 per day. This suggested that a relationship might exist between faster wound healing rate and low HbA1c levels.
Figure 1.
Baseline HbA1c correlates to poor wound healing in patients with diabetes. Wound-healing in an individual with HbA1c of 5.6% (a – d) and in an individual with HbA1c of 11.1% (e – i). Wound-healing per day is 0.35 cm2 for the individual with HbA1c of 5.6% (a). The wound is shown at first clinic visit (b, c) and healed at last clinic visit 64 days later (d). Wound-healing per day is 0.001 cm2 for the individual with HbA1c of 11.1% (e). The wound is shown at first clinic visit (f, g), and at the last clinic visit 727 days later (h, i).
The dimensions and healing rates of the baseline wound for each individual are shown in Table 1b. The mean baseline area was 7.22 cm2 and the mean change in area per day was a closure rate of 0.12 cm2 per day. Despite being the smallest size (4.14±5.29 cm2) at baseline, wounds at the highest level of HbA1c healed at the slowest rate (0.08±0.24 cm2 per day) (Table 1b). Conversely, the ulcers with larger baseline size in the lowest and intermediate HbA1c categories both had greater healing rates than the ulcers with smaller baseline size in the highest HbA1c category. Although, there trended to be an inverse association between baseline wound size and healing rate, statistical significance was not reached after adjusting for HbA1c and other variables in our model (p > 0.2).
Table 1b.
Characteristics of wounds, overall and by HbA1c category.
| All Participants (N=183) | HbA1c < 7.0 (n=71) | HbA1c 7.0 – 8.0 (n=42) | |
|---|---|---|---|
| Baseline area (cm2), mean (SD) | 7.22 (12.99) | 6.07 (11.36) | 7.60 (14.28) |
| Baseline length (cm), mean (SD) | 2.65 (2.68) | 2.00 (2.16) | 2.09 (1.77) |
| Baseline width (cm), mean (SD) | 2.21 (1.98) | 2.35 (2.81) | 2.72 (3.01) |
| Change in area per day (cm2), mean (SD) | 0.12 (0.61) | 0.16 (0.76) | 0.17 (0.53) |
| Change in length per day (cm), mean (SD) | 0.01 (0.18) | 0.03 (0.11) | 0.02 (0.04) |
| Change in width per day (cm), mean (SD) | 0.03 (0.12) | 0.04 (0.13) | 0.04 (0.08) |
Our results from the multiple linear regression models for change in wound area per day are found in Table 2. HbA1c was inversely related to healing rate. For each 1.0% point increase in HbA1c, the wound-area healing rate per day decreased by 0.028 cm2 (95% CI: 0.003, 0.054, p = 0.027). Diastolic blood pressure was also inversely association with wound area-healing rate per day, but the association was of borderline statistical significance (p = 0.060). Age, sex, race/ethnicity, smoking, body mass index, wound number, cholesterol, triglycerides, systolic blood pressure, pulse, temperature, white blood cell count and peripheral artery disease, neuropathy showed no association. Therefore, the only characteristic significantly associated with wound-area-healing rate per day was HbA1c.
Table 2.
Adjusted β-coefficients and 95% confidence intervals for the relationship between change in wound area per day and other variables.
| Variable* | Change in Wound Area Per Day (β) (cm2/day) | P-Value |
|---|---|---|
| Age (per 12 years) | 0.017 (−0.065, 0.100) | 0.680 |
| Female (versus male) | 0.038 (−0.132, 0.209) | 0.659 |
| African American race (versus white race) | −0.063 (−0.174, 0.048) | 0.263 |
| Body mass index (per 9.76 kg/m2), | 0.053 (−0.032, 0.139) | 0.220 |
| Wound number (per 1.54 wounds) | −0.001 (−0.059, 0.058) | 0.982 |
| Total cholesterol (per 44.7 mg/dl) | −0.060 (−0.137, 0.017) | 0.126 |
| LDL cholesterol (per 41.5 mg/dl) | 0.035 (−0.025, 0.094) | 0.250 |
| HDL cholesterol (per 15.9 mg/dl) | −0.020 (−0.069, 0.029) | 0.414 |
| Triglycerides (per 75.4 mmol/l) | −0.016 (−0.085, 0.052) | 0.641 |
| Systolic blood pressure (per 23.3 mmHg) | 0.020 (−0.063, 0.102) | 0.641 |
| Diastolic blood pressure (per 13.5 mmHg) | −0.074 (−0.151, 0.003) | 0.060 |
| Pulse (per 14.3 bpm) | 0.025 (−0.037, 0.087) | 0.432 |
| Temperature (per 0.58 ° Fahrenheit) | 0.015 (−0.048, 0.078) | 0.639 |
| White blood cell count (per 2490.42 cells/microliter) | 0.118 (−0.085, 0.266) | 0.119 |
| Former smoker (versus never smoker) | −0.015 (−0.224, 0.067) | 0.862 |
| Current smoker (versus never smoker) | 0.054 (−0.103, 0.212) | 0.496 |
| Neuropathy (versus no neuropathy) | −0.079 (−0.224, 0.067) | 0.287 |
| Peripheral artery disease (versus no peripheral artery disease) | −0.053 (−0.144, 0.038) | |
| HbA1c (per 1.0% point) | −0.028 (−0.054, −0.003) | 0.027 |
All coefficients are expressed per SD except Hba1c, which is expressed per 1.0-% point.
To assess the possibility of differential healing rates between neuropathic wounds of the foot that are predominantly diabetic wounds versus wounds in other locations, we performed a sensitivity analysis stratified by wound location and documented neuropathy. For neuropathic foot wounds, each 1.0% point increase in HbA1c was associated with a decrease in wound-area-healing rate of 0.022 cm2 per day (95% CI: 0.001, 0.043, p = 0.043). For all other wound locations, each 1.0% point increase in HbA1c was associated with a decrease of 0.018 cm2 per day (95% CI: −0.020, 0.056, p = 0.337). Documented neuropathy alone was not significantly associated with healing rates (Table 2).
A sensitivity analysis was also performed stratified by peripheral artery disease status. In participants without peripheral artery disease, HbA1c was not significantly related to wound healing rate (p = 0.576). In participants with peripheral artery disease, each 1.0% point increase in HbA1c was associated with a decrease of 0.030 cm2 per day (95% CI: 0.001, 0.060, p = 0.046).
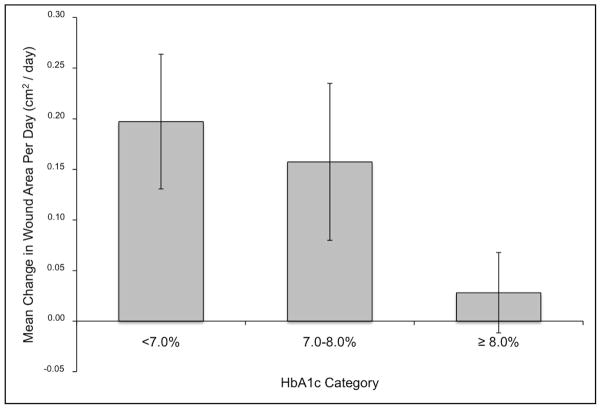
The mean daily change in wound area adjusted for age, sex, and race/ethnicity by HbA1c clinical category is shown in Figure 2. For individuals with HbA1c <7.0%, the mean adjusted wound area healed was 0.197 cm2 per day (95% CI: 0.066, 0.329). For individuals with HbA1c between 7.0 and 8.0%, the mean adjusted wound area healed was 0.157 cm2 per day (95% CI: 0.003, 0.312) and for individuals with HbA1c ≥8.0%, the mean adjusted wound area healed was 0.028 cm2 per day (−0.051, 0.107). Overall, HbA1c categories at baseline were inversely associated with mean daily change in wound area (p-trend = 0.03). These results demonstrate an inverse correlation between HgA1C levels and rate of healing in diabetic wounds.
Figure 2.
Baseline HbA1c is inversely associated with adjusted mean wound area healed per day (cm2/day). Model is adjusted for the demographic variables of age, gender, and race/ethnicity.
DISCUSSION
In this sample of diabetic individuals seen at the Johns Hopkins Wound Clinic, only elevated HbA1c was significantly independently associated with wound-area healing rate. This relationship was stronger for the wounds located on the foot, which were insensate neuropathic wounds (approximately 60% of all wounds). When our analysis was restricted to these foot wounds, the association with HbA1c remained significant, but when restricted to wounds at all other locations, the association was no longer significant. This relationship was also stronger among participants with peripheral artery disease. Our results suggest that HbA1c is an important clinical predictor of wound healing rate, particularly in those with neuropathic foot wounds and in those with peripheral artery disease.
To our knowledge, a strong association between HbA1c and wound healing over time in a large clinic population of diabetic individuals is previously unreported. Previous studies have found either no association (Apelqvist and Agardh, 1992; Margolis et al., 2000) or have reported a trend, but have had small sample size and did not statistically analyze data (Markuson et al., 2009). Previous studies have used need for amputation (Apelqvist and Agardh, 1992) or total time to complete healing (Margolis et al., 2000; Markuson et al., 2009) as the outcome of interest. The innovative use of a quantitative endpoint (healed area per day) might contribute to our success in demonstrating the correlation between HbA1c and wound healing. Although our endpoint differs from endpoints used in previous studies, all are assessing the same underlying wound healing process.
There are many physiologic factors that are thought to contribute to poor wound healing in diabetic individuals, including decreased or impaired keratinocyte and fibroblast migration and proliferation, cytokine and growth factor function, and angiogenic response, and response to infection among others (Brem and Tomic-Canic, 2007). Many of these hypothesized mechanisms for poor wound healing in diabetic individuals involve hyperglycemia. Hyperglycemia reduces keratinocyte migration and proliferation in vitro (Lan et al., 2008; Terashi et al., 2005). Additionally, hyperglycemia can also add to the oxidative stress with the production of reactive oxygen species (Guo and Dipietro, 2010). The formation of advanced glycation end products have also been associated with impaired wound healing in diabetic mice (Guo and Dipietro, 2010; Peppa and Vlassara, 2005).
We did not find an association between any other demographic, clinical, and laboratory variables and wound-area-healing rate per day. Reports in the literature about an association between sex and wound healing have been mixed. Marston et al. report female patients as being more likely to heal (Marston, 2006), but Margolis et al., Golinko et al., Ince et al. and Oyibo et al. (Golinko et al., 2009; Ince et al., 2007; Margolis et al., 2000; Oyibo et al., 2001) did not find an association. Margolis et al report that foot ulcers are more likely to heal in nonwhite individuals (odds ration: 0.64, 95% CI: 0.43, 0.96) (Margolis et al., 2000). The literature on age is also mixed, Apelqvist and Agardh (Apelqvist and Agardh, 1992) reported a higher risk of amputation with increasing age, but Ince et al, Golinko et al., and Margolis et al. (Golinko et al., 2009; Ince et al., 2007; Margolis et al., 2000) reported no association with age. Our results are similar to Golinko et al. (Golinko et al., 2009) who reported no association with cholesterol or white blood cell count between foot amputee patients and non-amputee patients. Although the associations between demographic, clinical, laboratory variables and healing in the literature are not consistent, our study is largely in agreement with existing reports where the majority of clinical data are not associated with wound healing.
Previous reports associated faster wound healing with smaller wound size (Margolis et al., 2002, 2003; Margolis et al., 2000). While our study showed a similar trend, HbA1c better predicted healing rates than baseline wound size. Several previous studies have not fully adjusted for HbA1c (Margolis et al., 2000) or did not include HbA1c in their model (Margolis et al., 2003; Margolis et al., 2008) when exploring the association between healing rates and wound size.
There are a number of limitations that are important to consider in the interpretation of our results. First, this study was limited by the retrospective nature of design. Accordingly, the evaluation of risk factors and confounding factors was limited to variables accurately present the WoundMatrix database and the electronic medical record. We were unable to evaluate duration of diabetes, wound duration, or patient compliance. We were also limited by a smaller sample size for our subgroup analyses. Additionally, since our participants were drawn from patients attending a specialized wound clinic, the generalizability of these results may be limited.
Our study also has important strengths. First, the use of the specialized wound healing electronic medical record databases allowed for precise measuring of wound size via high-resolution digital photography at two separate time points (Quan et al., 2007). Additionally, while many previous studies of wound healing in diabetes have focused only on wounds of the lower extremity or foot, our study includes all types and locations of wounds found in our diabetic population. Consistent with a recent consensus statement which states that wound healing outcomes should be the rate of total wound closure, we used daily wound healing rate as our outcome (Armstrong et al., 2009).
In summary, of the clinical, laboratory and demographic variables commonly measured in diabetic patients in wound clinics such as the Johns Hopkins Wound Clinic, only elevated HbA1c was significantly associated with poor wound-area healing rate per day. Our study suggests that hyperglycemia, as assessed by HbA1c is associated with slower wound healing in patients with diabetes, particularly for neuropathic foot wounds and in patients with peripheral artery disease. Future prospective studies should assess the effect of tight glycemic control to decrease HbA1c levels in wound healing.
MATERIALS AND METHODS
STUDY POPULATION
Participants were selected from the Johns Hopkins Wound Clinic database, WoundMatrix, which contains information on individuals seen in the clinic between 2004 and 2010. Participants with diabetes were identified using the following ICD-9 codes: 250.0, 250.00, 250.01, 250.02, 250.03, 250.8, 250.80, 250.81, 250.82, and 250.83. Criteria for inclusion in the study were good quality digital photographs of a wound at baseline and at a later follow-up visit as well as complete information on baseline demographic and clinical variables. Participants who were excluded from our analysis were similar to those included on demographic and clinical variables. MEASUREMENT OF EXPOSURES During visits to the Johns Hopkins Wound Clinic, blood pressure, pulse, and temperature were measured with the individual in a sitting position using standard clinical procedure by the nursing staff. A physician assessed neuropathy at each clinic visit using a Semmes Weinstein filament. All wound treatment was documented and was found to be homogeneous for all patients with diabetic wounds. Standard treatment for diabetic wounds includes removal of non-viable tissue, local dressing (antimicrobial dressings with silver), control of blood glucose levels, offloading with proper shoes if the wound is on the lower extremity, and antibiotic treatment if infection if present. Laboratory test results (HbA1c, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, and white blood cell count) and body mass index, smoking status, and peripheral artery disease data were doubly abstracted from individual’s electronic medical record and any differences reconciled. All values abstracted were obtained at the date closest to the date of the baseline visit. Values of HbA1c, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, and body mass index were obtained a mean of 60 days before the first clinic visit and values of white blood cell count were obtained an average of 4 days before the clinic visit. We modeled the single baseline measurement of HbA1c continuously (per 1.0% point) and as a categorical variable using clinically relevant cut-points of <7.0%, 7.0–8.0%, and ≥8.0% (American Diabetes Association, 2000).
MEASURMENT OF WOUND DIMENSIONS
We determined the location and area of wounds in diabetic patients both at baseline and a second visit a median of 1 month later. On the day of each visit to the Johns Hopkins Wound Clinic, a high-resolution digital photograph of each wound with a ruler (in cm) underneath was captured. Measurements were calibrated to 3 cm using the ruler in the photograph. The area of wound and largest width and largest length were traced digitally to obtain the wound dimensions using the WoundMatrix software. Digital tracing of wounds with WoundMatrix software is reproducible and precise with acceptable variation among readers (Quan et al., 2007).
STATISTICAL ANALYSIS
The change in wound area in cm2 per day was the outcome for our primary analysis. The change in wound area per day was calculated as the difference between wound area at visit 1 (baseline) and at a subsequent visit divided by the number of days between the two visits (median of 32 days, interquartile range: 18–61). We estimated the change in wound area (cm2 per day) using a multiple linear regression model with robust standard errors and adjusted for clustering of wounds within individuals. Model discrimination was assessed with the use of AIC values. All continuous covariates were expressed in standard deviation units, with the exception of HbA1c, which was expressed per 1% point, for clinical interpretability. Inferences were the same when we log transformed HbA1c to make the variable more normally distributed, so HbA1c was left untransformed for clinical interpretability. Variables of interest included age, gender, race/ethnicity, pulse, systolic and diastolic blood pressures, temperature, body mass index, HbA1c, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, neuropathy, peripheral artery disease, smoking, wound number, wound location, and white blood cell count. We tested for trends across the medians of HbA1c categories.
To assess the possibility of differential healing rates between neuropathic foot wounds, and other wounds in diabetic patients (mainly large surgical non-healing wounds), we performed a sensitivity analysis stratified by wound location (neuropathic foot wounds versus all other wounds). Neuropathic foot wounds were defined as wounds occurring on weight bearing portions of the foot in individuals with documented peripheral neuropathy. Additionally, we performed a sensitivity analysis stratified by peripheral artery disease status.
All reported p-values are two-sided and p<0.05 was considered statistically significant. Analyses were performed using Stata Version 11 (Stata, 2009). This protocol was approved by the Johns Hopkins Institutional review Board (NA_00035916) and was done in adherence to the Helsinki Guidelines. Patient consent was not required because our study was retrospective and used deidentified data.
Supplementary Material
Acknowledgments
This study was supported by the following grants: NIH/NIDDK training grant T32 DK062707 (AC), and NIH/NIAMS 1K08AR055666-01A1 (LG).
Footnotes
CONFLICTS OF INTEREST
None.
References
- Apelqvist J, Agardh CD. The association between clinical risk factors and outcome of diabetic foot ulcers. Diabetes Res Clin Pract. 1992;18:43–53. doi: 10.1016/0168-8227(92)90054-u. [DOI] [PubMed] [Google Scholar]
- Armstrong DG, Boulton AJ, Andros G, et al. Defining success in clinical trials of diabetic foot wounds: the Los Angeles DFCon consensus. Int Wound J. 2009;6:211–3. doi: 10.1111/j.1742-481X.2009.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Supplement 1. American Diabetes Association: clinical practice recommendations 2000. Diabetes Care. 2000;23(Suppl 1):S1–116. [PubMed] [Google Scholar]
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, et al. The global burden of diabetic foot disease. Lancet. 2005;366:1719–24. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–22. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–94. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golinko MS, Margolis DJ, Tal A, et al. Preliminary development of a diabetic foot ulcer database from a wound electronic medical record: a tool to decrease limb amputations. Wound Repair Regen. 2009;17:657–65. doi: 10.1111/j.1524-475X.2009.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordois A, Scuffham P, Shearer A, et al. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26:1790–5. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Sorlie P, Paulose-Ram R, et al. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591–7. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince P, Kendrick D, Game F, et al. The association between baseline characteristics and the outcome of foot lesions in a UK population with diabetes. Diabet Med. 2007;24:977–81. doi: 10.1111/j.1464-5491.2007.02189.x. [DOI] [PubMed] [Google Scholar]
- Jeffcoate WJ, Chipchase SY, Ince P, et al. Assessing the outcome of the management of diabetic foot ulcers using ulcer-related and person-related measures. Diabetes Care. 2006;29:1784–7. doi: 10.2337/dc06-0306. [DOI] [PubMed] [Google Scholar]
- Lan CC, Liu IH, Fang AH, et al. Hyperglycaemic conditions decrease cultured keratinocyte mobility: implications for impaired wound healing in patients with diabetes. Br J Dermatol. 2008;159:1103–15. doi: 10.1111/j.1365-2133.2008.08789.x. [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Allen-Taylor L, Hoffstad O, et al. Diabetic neuropathic foot ulcers: the association of wound size, wound duration, and wound grade on healing. Diabetes Care. 2002;25:1835–9. doi: 10.2337/diacare.25.10.1835. [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Allen-Taylor L, Hoffstad O, et al. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med. 2003;115:627–31. doi: 10.1016/j.amjmed.2003.06.006. [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Kantor J, Santanna J, et al. Risk factors for delayed healing of neuropathic diabetic foot ulcers: a pooled analysis. Arch Dermatol. 2000;136:1531–5. doi: 10.1001/archderm.136.12.1531. [DOI] [PubMed] [Google Scholar]
- Margolis KL, Qi L, Brzyski R, et al. Validity of diabetes self-reports in the Women’s Health Initiative: Comparison with medication inventories and fasting glucose measurements. Clinical Trials. 2008;5:240–7. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markuson M, Hanson D, Anderson J, et al. The relationship between hemoglobin A(1c) values and healing time for lower extremity ulcers in individuals with diabetes. Adv Skin Wound Care. 2009;22:365–72. doi: 10.1097/01.ASW.0000358639.45784.cd. [DOI] [PubMed] [Google Scholar]
- Marston WA. Risk factors associated with healing chronic diabetic foot ulcers: the importance of hyperglycemia. Ostomy Wound Manage. 2006;52:26–8. 30. 2 passim. [PubMed] [Google Scholar]
- Oyibo SO, Jude EB, Tarawneh I, et al. The effects of ulcer size and site, patient’s age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabet Med. 2001;18:133–8. doi: 10.1046/j.1464-5491.2001.00422.x. [DOI] [PubMed] [Google Scholar]
- Peppa M, Vlassara H. Advanced glycation end products and diabetic complications: a general overview. Hormones (Athens) 2005;4:28–37. doi: 10.14310/horm.2002.11140. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services; Atlanta, GA: 2011. [Google Scholar]
- Quan SY, Lazarus GS, Kohli AR, et al. Digital imaging of wounds: are measurements reproducible among observers? Int J Low Extrem Wounds. 2007;6:245–8. doi: 10.1177/1534734607306880. [DOI] [PubMed] [Google Scholar]
- Stata. Stata Statistical Software: Release 11. StataCorp; College Station, TX: 2009. [Google Scholar]
- Terashi H, Izumi K, Deveci M, et al. High glucose inhibits human epidermal keratinocyte proliferation for cellular studies on diabetes mellitus. Int Wound J. 2005;2:298–304. doi: 10.1111/j.1742-4801.2005.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.