Abstract
Objectives
Since 2003, U.S. organizations have recommended universal screening, rather than targeted screening, of HIV-infected persons for gonorrhea (NG) and Chlamydia (CT). Our objective was to determine whether wider testing resulting from these guidelines would produce an increase in NG/CT diagnoses.
Methods
We studied 3,283 patients receiving HIV care 1999–2007 in the Johns Hopkins Hospital HIV clinic. The two primary outcomes were: 1) the occurrence of any NG/CT testing in each year of care and 2) the occurrence of any positive result(s) in years of testing. The proportion of all patients in care who were diagnosed with NG/CT was defined as the number of patients with positive results divided by the number of patients in care. Trends were analyzed with repeated measures logistic regression.
Results
The proportion of patients tested for NG/CT increased steadily from 0.12 in 1999 to 0.33 in 2007 (OR per year for being tested, 1.17 [1.15, 1.19]). The proportion positive among those tested decreased significantly after 2003 (OR per year 0.67 [0.55, 0.81]). The proportion of all patients in care diagnosed with NG/CT therefore remained generally stable 1999–2007 (OR per year 0.97 [0.91, 1.04]).
Conclusions
Universal annual screening, as implemented, did not increase the proportion of all patients in care who were diagnosed with NG/CT. Similarly low implementation rates have been reported in cross-sectional studies. If future efforts to enhance implementation do not yield increases in diagnoses, then guidelines focusing on targeted screening of high risk groups rather than universal screening may be warranted.
Keywords: HIV prevention, health service research, Neisseria gonorrhoeae, Chlamydia trachomatis, screening, guidelines
INTRODUCTION
Regularly screening persons living with HIV (PLWH) for Neisseria gonorrhoeae (NG) and Chlamydia trachomatis (CT) is expected to decrease HIV transmission and reduce the sequelae of pelvic inflammatory disease. Prompting interventions to change high risk sexual behavior and reducing genital fluid HIV levels are mechanisms by which NG/CT detection and treatment may reduce HIV transmission.[1–5] In diverse locations, the prevalence of NG and/or CT among patients attending HIV clinics has ranged from 2.5 to 11%.[6–17] The incidence may be as high as 15/100 person-years.[7] The majority of NG/CT infections among PLWH are asymptomatic.[6–8, 18]
In 2003, the US Centers for Disease Control and Prevention (CDC) and other health and professional organizations strengthened earlier recommendations to screen all sexually active PLWH for NG/CT upon enrollment into care and at least annually thereafter.[19, 20] Oral and rectal (extragenital) sites should be included based upon sexual history. Among men, we have previously demonstrated an increase after 2003 in the level of NG/CT screening upon enrollment into HIV care.[21]
We hypothesized that due to the 2003 guidelines, the proportion of HIV patients diagnosed with NG/CT would have increased during 1999–2007. This hypothesis presupposed that the proportion of patients tested annually would have increased after 2003 and that the proportion found positive among those tested would have remained relatively constant. To test our hypothesis, we determined trends in proportion of patients tested annually, proportion positive among those tested, and the resulting proportion diagnosed with NG/CT among all patients in care within a large HIV clinic in Baltimore, Maryland.
METHODS
Since 1989, the Johns Hopkins HIV Clinical Cohort (JHHCC) study has offered enrollment to all patients who initiate longitudinal care at the Johns Hopkins Hospital HIV clinic. Over 99% of patients have enrolled. As described elsewhere, [22] technicians abstract comprehensive demographic, clinical, and laboratory data from medical records, health system databases, and outside facilities. Use of the JHHCC for this analysis was approved by the Johns Hopkins School of Medicine Institutional Review Board (NA_00028824).
For each participant, we identified active calendar years as those with at least one outpatient visit and at least one measured CD4 count. Because the 2003 guidelines call for NG/CT screening upon enrollment into HIV care as well as annually thereafter, the year of enrollment was included if enrollment occurred at least 90 days prior to December 31st. For subjects exiting the cohort, the exit year was included if exit occurred after July 1st.
The first outcome, the occurrence of NG/CT testing, was a binary variable determined for each year of active follow-up and was considered positive if the patient had at least one completed test (either culture or nucleic acid-based (NA)) of the genital, rectal, or oral areas for NG and/or CT. At the population level, this variable was defined as the proportion tested. The second outcome, the occurrence of NG/CT positivity if tested, was a binary variable determined for each year in which testing occurred; it was considered positive if the patient had at least one positive result for NG, CT, or both. At the population level, this variable was defined as the proportion positive among those tested. The occurrence of being diagnosed with NG/CT was defined as having at least one positive result during a year of active clinic follow-up regardless of being tested or not. At the population level, this variable was termed the proportion diagnosed with NG/CT among all those in care.
During the study period, culture was available for all sites and was the only accepted method for the mouth and rectum. From 1999–2001, NA testing was performed using PACE 2 (Gen-Probe, San Diego, CA, USA) and was available for cervical and male urethral swabs. From 2001–2007, NA testing was performed using AMPLICOR CT/NG (Roche Molecular Diagnostics, Branchburg, NJ, USA), and male urine specimens were accepted.
We measured several exposure variables that may have served to confound our assessment of trends. Age, number of clinic visits, CD4 cell count, HIV-1 RNA level, and the occurrence of syphilis testing were time-varying exposures specific to each calendar year. For CD4 and HIV-1 RNA data, mean values were created from all results within each year. Syphilis testing was defined as having at least one rapid plasma reagin test within a year. For purposes of analysis, race/ethnicity was classified as African American and white/other. HIV risk factor was classified as sexual risk (including any same sex male contact (MSM) or high risk heterosexual exposure, even if in conjunction with another risk factor) and non-sexual risk.
Analysis was performed using repeated-measures logistic regression using generalized estimating equations. After exploration of the within-subject correlation of outcomes over time, exchangeable working correlation structures with robust variance estimators were selected. Because of the a priori hypothesis that the proportion tested annually would change in 2003, calendar time was explored both as a simple linear variable and as a linear spline term with an inflection point in 2003. The quasi-likelihood under the independence model criterion (QIC) value was used to select between models.[23] To explore whether the data better fit a model with a different change point, we constructed additional models, each with the inflection point at a different calendar year. The final models selected were those with the lowest QIC values. Final multivariate models included all exposure variables for which the bivariate P was <0.2. A two-sided type I error of 5% was considered statistically significant. Stata 11.0 (StataCorp LP, College Station, TX, USA) was used for all analyses.[24]
Three planned sub-analyses were also performed: 1) results stratified by gender, 2) results among MSM only, and 3) NG positivity and CT positivity analyzed as separate outcomes among the full study cohort.
RESULTS
In total, 3,283 patients contributed 13,188 person years of active follow-up during 1999–2007. The cohort was 65% male, 79% African American, and 24% MSM (Table 1), and had a median age of 40 years (interquartile range (IQR), 35–46) during the first year of study time (either 1999 or the year of clinic enrollment if later). The median yearly number of outpatient visits to HIV and/or gynecologic providers was 4 (3–6). During each calendar year, approximately 1465 patients (range 1331–1534) were in active care. The median number of years of active care per patient was 3 (2–6).
Table 1. Demographic and clinical characteristics.
| Full Cohort | Subjects Ever-Tested for NG/CT | Subjects Ever Positive on NG/CT Testing | |
|---|---|---|---|
| N = 3283 | N = 1434 | N = 80 | |
| Gender | |||
| Male | 2123 (65) | 685 (48) | 53 (66) |
| Female | 1160 (35) | 749 (52) | 27 (34) |
| Age in 1st year of study time (years) | |||
| Median (IQR) | 40 (35–46) | 39 (33–44) | 33 (27–39) |
| 18–29 | 358 (11) | 220 (15) | 28 (35) |
| 30–39 | 1258 (38) | 591 (41) | 35 (44) |
| 40–49 | 1247 (38) | 510 (36) | 16 (20) |
| ≥50 | 420 (13) | 113 (8) | 1 (1) |
| Race/Ethnicity | |||
| African American | 2579 (79) | 1200 (84) | 71 (89) |
| White | 627 (19) | 205 (14) | 9 (11) |
| Hispanic (non-African American) | 50 (2) | 21 (1) | 0 |
| Other/Unknown | 27 (1) | 8 (1) | 0 |
| HIV Risk Factors | |||
| IDU | 687 (21) | 261 (18) | 9 (11) |
| IDU – Heterosexual | 621 (19) | 294 (21) | 11 (14) |
| IDU – MSM | 138 (4) | 46 (3) | 4 (5) |
| Heterosexual | 1002 (31) | 537 (37) | 20 (25) |
| MSM | 659 (20) | 224 (16) | 32 (40) |
| Unknown/Other | 176 (5) | 72 (5) | 4 (5) |
| Mean Visits/Year over all years in care* | |||
| Median (IQR) | 4 (3–6) | 5 (4–6) | 4 (3–6) |
| <3 | 693 (21) | 219 (15) | 16 (20) |
| 3–6 | 1848 (56) | 862 (60) | 53 (66) |
| >6 | 742 (23) | 353 (25) | 11 (14) |
| Mean CD4 count in 1st year of study time (cells/mm3) | |||
| Median (IQR) | 308 (150–501) | 333 (170–516) | 369 (263–568) |
| ≤200 | 1096 (33) | 418 (29) | 15 (19) |
| 201–350 | 751 (23) | 336 (23) | 21 (26) |
| >350 | 1436 (44) | 680 (47) | 44 (55) |
| Mean HIV-1 RNA in 1st year of study time | |||
| Median (IQR) (log10copies/mL) | 4.1 (2.8–4.8) | 4.1 (2.9–4.8) | 4.0 (3.0–4.6) |
| ≤400 copies/mL | 718 (22) | 297 (21) | 12 (15) |
| 401–9,999 copies/mL | 804 (24) | 375 (26) | 29 (36) |
| ≥10,000 copies/mL | 1722 (52) | 748 (52) | 39 (49) |
| Missing | 39 (1) | 14 (1) | 0 |
| Syphilis Testing | |||
| Ever Tested | 2487 (76) | 1196 (83) | 66 (83) |
| Never Tested | 796 (24) | 238 (17) | 14 (18) |
Values are number (percent) unless specified. Percentages may exceed 100 due to rounding.
Only visits to HIV and/or gynecologic providers included.
A total of 3437 testing episodes occurred over the study period with 44% of patients tested at least once. NG and CT were tested simultaneously in 94% of episodes, and NA testing (instead of, or in addition to, culture) was used in 94% of episodes.
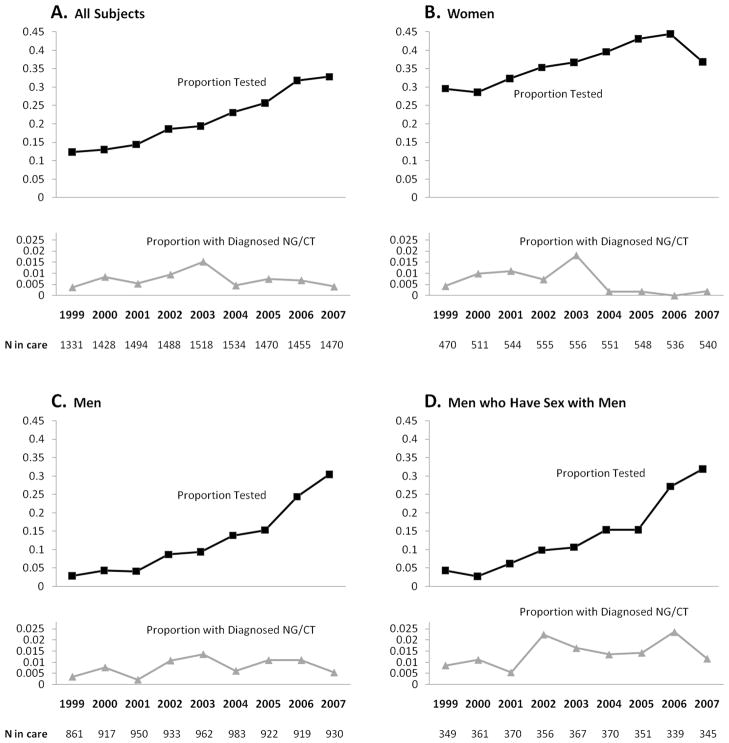
Among all patients in care, the proportion tested for NG/CT rose from 0.12 in 1999 to 0.33 in 2007 (Figure 1A). In a univariate regression, the trend was most efficiently modeled with time as a linear variable with no inflection point. The odds of being tested were estimated to increase by 17% per year (OR per year [95% confidence interval], 1.17 [1.15, 1.19]).
Figure 1.
Proportion tested and proportion diagnosed with NG/CT among all those in care. A, all subjects; B, women; C, men; and D, men who have sex with men.
Eighty subjects (6% of subjects ever tested) had at least one positive result during the study interval. Among these 80 subjects, there were 103 positive tests including 60 NG-only, 38 CT-only, and 5 concomitant NG/CT infections. Nine subjects had 2 positive tests and five subjects had 3 or more positive tests. For patients with multiple positive tests, the median time between repeat positives was 277 days (IQR, 111–765).
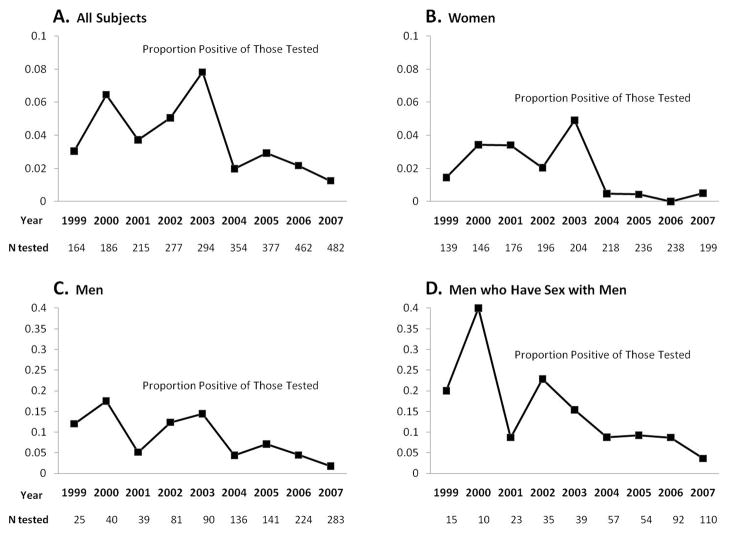
Among all subjects, the proportion positive among those tested varied from a minimum of 0.01 in 2007 to a maximum of 0.08 in 2003 (Figure 2A). A univariate regression with year modeled as a linear spline term detected no significant change (OR per year 1.07 [0.89, 1.28]) from 1999–2003 and then a significant decrease (OR per year 0.67 [0.55, 0.81]) from 2003–2007.
Figure 2.
Proportion positive among those tested for NG/CT. A, all subjects; B, women; C, men; and D, men who have sex with men.
Among all patients in care, the proportion diagnosed with NG/CT ranged from 0.004 to 0.015 and did not show a consistent trend across time (Figure 1A). From 1999–2003, the proportion diagnosed with NG/CT increased (OR per year 1.23 [1.04, 1.47]), and from 2003–2007 it decreased (OR per year 0.77 [0.63, 0.93]). When time was modeled as a simple linear term 1999–2007, the proportion of all subjects in care who were diagnosed with NG/CT appeared stable (OR per year 0.97 [0.91, 1.04]).
In multivariate analysis of the proportion tested annually, the calendar time trend was most parsimoniously fit with an inflection point in 2001, with an estimated steeper annual increase following 2001 (OR per year 1.13 [1.04, 1.24] from 1999–2001 and OR per year 1.28 [1.25, 1.31] for 2001–2007, Table 2). Other factors significantly associated with the occurrence of NG/CT testing included female gender, younger age, African American race, more visits per year, CD4 count >350 cells/mm3, and being tested for syphilis. In multivariate analysis of the occurrence of NG/CT positivity if tested, earlier calendar year, male gender, younger age, and CD4 count >350 were significantly associated (Table 3).
Table 2. Factors associated with the occurrence of NG/CT testing among 3283 subjects in care.
| Odds Ratio (95% CI) for Being Tested | ||
|---|---|---|
| Univariate
|
Multivariate
|
|
| Calendar time† | ||
| Linear parameter | ||
| per year, 1999–2007 | 1.17 (1.15, 1.19) | |
| Linear spline | ||
| per year, 1999–2001 | 1.13 (1.04, 1.24) | |
| per year, 2001–2007 | 1.28 (1.25, 1.31) | |
| Gender | ||
| Male | 1.00 (Ref) | 1.00 (Ref) |
| Female | 4.12 (3.68, 4.61) | 3.61 (3.23, 4.04) |
| Age (years) | ||
| 18–29 | 3.12 (2.52, 3.87) | 4.71 (3.75, 5.92) |
| 30–39 | 1.61 (1.37, 1.90) | 2.39 (2.00, 2.87) |
| 40–49 | 1.36 (1.16, 1.59) | 1.66 (1.40, 1.96) |
| ≥50 | 1.00 (Ref) | 1.00 (Ref) |
| Race/Ethnicity | ||
| African American | 1.73 (1.47, 2.02) | 1.54 (1.32, 1.80) |
| White/Other | 1.00 (Ref) | 1.00 (Ref) |
| HIV risk | ||
| MSM or HET reported | 1.33 (1.16, 1.52) | 1.11 (0.97, 1.27) |
| No MSM or HET reported | 1.00 (Ref) | 1.00 (Ref) |
| Visits within the calendar year* | ||
| <3 | 1.00 (Ref) | 1.00 (Ref) |
| 3–6 | 1.63 (1.45, 1.85) | 1.69 (1.49, 1.93) |
| ≥7 | 2.04 (1.78, 2.35) | 2.38 (2.05, 2.76) |
| Mean annual CD4 count (cells/mm3) | ||
| ≤200 | 1.00 (Ref) | 1.00 (Ref) |
| 201–350 | 1.12 (0.98, 1.29) | 1.09 (0.94, 1.25) |
| >350 | 1.32 (1.17, 1.50) | 1.25 (1.10, 1.42) |
| Mean annual HIV-1 RNA (copies/mL) | ||
| ≤400 | 1.00 (Ref) | |
| 401–9,999 | 1.06 (0.91, 1.15) | |
| ≥10,000 or missing | 0.94 (0.85, 1.04) | |
| Syphilis Testing within the calendar year | ||
| Tested | 1.67 (1.52, 1.83) | 1.80 (1.62, 1.99) |
| Not-Tested | 1.00 (Ref) | 1.00 (Ref) |
Visits included only those to an HIV and/or gynecologic provider
Time modeled linearly in univariate analysis and as a linear spline in multivariate analysis
Table 3. Factors associated with the occurrence of positive results among 1434 subjects ever-tested.
| Odds Ratio (95% CI) for Being Positive | ||
|---|---|---|
| Univariate
|
Multivariate
|
|
| Calendar time | ||
| Linear spline | ||
| per year, 1999 – 2003 | 1.07 (0.89, 1.28) | 1.02 (0.84, 1.23) |
| per year, 2003 – 2007 | 0.67 (0.55, 0.81) | 0.63 (0.52, 0.77) |
| Gender | ||
| Male | 1.00 (Ref) | 1.00 (Ref) |
| Female | 0.26 (0.16, 0.42) | 0.14 (0.08, 0.23) |
| Age (years) | ||
| 18–29 | 6.01 (3.23, 11.19) | 4.84 (2.42, 9.68) |
| 30–39 | 2.53 (1.46, 4.39) | 2.52 (1.38, 4.60) |
| 40–49 | 1.00 (Ref) | 1.00 (Ref) |
| ≥50 | † | † |
| Race/Ethnicity | ||
| African American | 1.23 (0.57, 2.64) | |
| White/Other | 1.00 (Ref) | |
| HIV risk | ||
| MSM or HET reported | 1.97 (1.07, 3.64) | 1.40 (0.74, 2.65) |
| No MSM or HET reported | 1.00 (Ref) | 1.00 (Ref) |
| Visits within the calendar year* | ||
| <3 | 1.00 (Ref) | |
| 3–6 | 0.79 (0.45, 1.39) | |
| ≥7 | 0.67 (0.34, 1.29) | |
| Mean annual CD4 count (cells/mm3) | ||
| ≥200 | 1.00 (Ref) | 1.00 (Ref) |
| 201–350 | 1.23 (0.61, 2.50) | 1.64 (0.77, 3.47) |
| >350 | 1.88 (1.05, 3.36) | 2.75 (1.44, 5.25) |
| Mean annual HIV-1 RNA (copies/mL) | ||
| ≤400 | 1.00 (Ref) | 1.00 (Ref) |
| 401–9,999 | 1.14 (0.60, 2.17) | 1.14 (0.59, 2.19) |
| ≥10,000 or missing | 1.53 (0.96, 2.44) | 1.55 (0.87, 2.76) |
| Syphilis Testing within the calendar year | ||
| Tested | 0.90 (0.59, 1.38) | |
| Not-Tested | 1.00 (Ref) | |
Visits included only those to an HIV and/or gynecologic provider
Since only 2 patients ≥50 years old had positive NG/CT tests, this category was combined with the 40–49 year-old category
Sub-Analyses
The first sub-analysis revealed that every year women were more likely to be tested for NG/CT than men, but this difference was almost eliminated by 2007 (Figure 1B and C). For both genders, annual testing increased significantly across the study period. The proportion positive among those tested was generally lower in women (average across all years 0.017) than men (0.062). In both genders, the univariate and multivariate time trends were similar to that seen in the full cohort with relative stability from 1999–2003 followed by significant declines from 2003–2007. Among men who were tested, MSM status was associated with having a positive result (adjusted OR 2.19 [1.03, 4.67]).
The second sub-analysis was of MSM. Approximately 350 MSM were in care each year. Similar to the cohort of all men, a steadily increasing trend in the proportion tested 1999–2007 was evident (Figure 1D), and a significant decrease in the proportion positive among those tested was evident starting in 2003 (Figure 2D). The proportion of MSM tested at extragenital sites (rectum or pharynx) was approximately 0.02 each year, and this proportion did not change significantly over time (OR per year 0.99 [0.88, 1.12].) Five of 50 (10%) extragenital site tests (2 rectal NG, 2 rectal CT, and 1 oral NG) were positive.
The third sub-analysis separately examined the proportions of positive NG and CT results among all patients tested. In both cases, relative stability was seen 1999–2003 (for NG: OR per year 1.03 [0.83, 1.29], for CT: OR per year 1.15 [0.86, 1.55]) followed by statistically significant (or nearly so) declines from 2003–2007 (for NG: OR per year 0.57 [0.43, 0.75], for CT: 0.77 per year [0.58, 1.01]).
DISCUSSION
During 1999–2007, the proportion of patients in care who were diagnosed with NG/CT did not increase. The proportion tested increased steadily from 0.12 in 1999 to 0.33 in 2007, but this increase was countered by a sharp decline in positive results among those tested. These findings are relevant to the evaluation of guidelines issued in 2003 which call for universal annual NG/CT screening among PLWH.
We suspect that the proportion of clinic patients diagnosed with NG/CT remained stable because the additional testing in later years did not occur at body sites or among patients likely to test positive. Over the study interval, there was no increase in the proportion of MSM tested at oral and rectal sites. Although the number of MSM tested at extragenital sites was too small to be conclusive, the 10% positive rate is consistent with extragenital sites being a substantial reservoir of undetected infections. Patients, MSM or otherwise, who rarely attend clinic, who avoid testing, or who are infrequently offered screening because of multiple competing medical issues may be the patients most frequently infected with NG and CT and hence could represent another reservoir of undetected infections. Although less-frequent clinic attendance was not associated with having a positive result among subjects tested, such an association may have been apparent if all or nearly-all subjects in care had been tested each year.
Publicly reported sexually transmitted infection data for Baltimore 1999–2007 do not reveal evidence of a strong declining trend in the number of NG/CT diagnoses per capita. Comparing 2004–2007 to 1999–2003, combined NG and CT diagnoses per capita in Baltimore exhibited a 5% decrease, [25] which is far less than the 59% decrease in proportion of tests positive seen in our study between these time periods. While we cannot rule out a tripling of the proportion of the city-wide population tested (such as occurred in our clinic), we have no reason to believe such an uptake in testing happened during 1999–2007 as there were no large-scale city NG/CT campaigns. Also, we do not believe that detection remained stable because providers were effectively capturing most incident cases prior to 2003. This would imply <1% point prevalence in our clinic population, which is inconsistent with literature predicting a 2.5–11% point prevalence[6–17] and with the overall high rates of NG/CT in Baltimore.[26]
Kahle et al. evaluated trends in proportion tested for NG/CT and proportion diagnosed among 1720 HIV-infected persons in Seattle before and after publication of local guidelines for annual screening in 2001.[27] The proportion tested for NG/CT increased from approximately 0.1 prior to the guidelines to 0.2 afterward (reported data extrapolated to yearly intervals). Similar to our results, the proportion diagnosed with CT among all subjects in care remained unchanged. In contrast, the proportion diagnosed with NG increased.
Contrary to the hypothesized large uptake after 2003, annual NG/CT testing in our clinic increased uniformly across the study period. The uniform rise may be explained by providers gradually adopting new behavior as supporting literature accumulated.[1–3, 6, 8, 20, 28] The multivariate analysis estimated a small upward inflection in 2001; a contributing factor may be the introduction of urine sampling (replacing urethral swabs) for NA testing in men in 2001.
Similarly low proportions of patients tested for NG/CT (ranging from 0.15 to 0.33) have been reported in multiple U.S. and international HIV clinics despite local guidelines.[6, 15, 16, 27, 29, 30] These collective findings suggest that implementing universal NG/CT screening is difficult. Remembering and finding time to broach sexual health and convincing patients to submit non-blood samples may represent substantial challenges. The very low level of extragenital testing among MSM probably reflects these challenges. Additional evidence that obtaining non-blood samples may be a major barrier comes from the comparison of NG/CT testing to syphilis testing in our subjects. During 1999–2007, the proportion of patients tested annually for syphilis (data not shown) ranged from 0.53 (2007) to 0.64 (2001), and hence was substantially higher than for NG/CT in all years.
Our increase in the proportion of patients tested for NG/CT without a resultant rise in detection signifies a need for additional studies on the feasibility and cost-effectiveness of a universal screening approach. Our study is the first that we know of to compare the proportion of patients in care diagnosed with NG/CT before and after the 2003 guidelines. Before strong conclusions about the effectiveness of a universal approach can be made, studies from additional centers are needed. Also, our clinic’s implementation fell too far short of standards to be able to make strong conclusions about a universal approach. Better implementation has been reported.[15, 30, 31] During 2005, an HIV clinic in Melbourne, Australia performed complete genital and extragenital NG/CT testing on 41% of MSM.[15] Further research is needed to determine if high (e.g. >50%) adherence to universal screening with appropriate coverage of extragenital sites can be routinely implemented in HIV clinics and if such an approach is optimal for case detection.
An alternative to universal screening which deserves implementation and cost-effectiveness research is targeted screening of high risk groups. Targeted screening offers the advantage of requiring far fewer tests, but it may be inconvenient for providers to repeatedly reassess risk status. Many studies, including the present, have examined predictors of positive results among those tested, and have therefore shed some light on defining high risk groups.[6, 7, 11, 27] It should be noted, however, that our findings of factors associated with NG/CT positivity are open to bias given that we could only examine the 44% of patients who were ever tested.
Urethral NG in men contrasts with other sites of infection in both men and women and with CT at all sites because it is more likely to be symptomatic.[32] This should be considered when determining whether a population of men should undergo screening, as opposed to diagnostic (symptom-prompted) testing, at the urethral site. In previous studies of HIV-infected MSM, 80–100% of NG (and, interestingly, nearly the same proportion of CT) found during screening (i.e. all patients were asymptomatic at the time) were from extragenital sites.[7–9] Hence, another scenario to consider from health, feasibility, and cost standpoints is whether screening in HIV-infected MSM should be limited to extragenital sites.
Our study is limited by our inability to distinguish diagnostic testing from screening. We suspect the majority of increased testing in later years represented screening. A steady yearly increase in symptomatic complaints among patients in care seems unlikely without a rise in the proportion diagnosed with NG/CT. Although our clinic provides comprehensive HIV and primary care, some patients may have been treated for NG/CT at other locations such as emergency rooms. We do not suspect that a large rise in such treatment occurred after 2003. Our data come from a single clinic with a large population of African-Americans and IDU. Nonetheless, our findings may be applicable to many urban HIV clinics.
Our study has found that an increase in the proportion of patients undergoing annual testing for NG/CT during 1999–2007 was not associated with an increase in the proportion of patients found to be infected. Our findings indicate a need to re-evaluate the clinical effectiveness and cost-effectiveness of a universal screening approach for NG/CT case detection compared to alternatives such as targeted screening of high risk groups. Until clear evidence exists, annual NG/CT screening according to guidelines should continue being promoted. Better implementation of universal screening may be widely feasible and may increase case detection.
KEY MESSAGES.
Widening testing for Gonorrhea and Chlamydia did not increase detection of these STIs in an HIV clinic population.
Screening oral and rectal sites among MSM may be especially important to NG/CT case detection in HIV clinics.
If efforts to improve implementation of universal NG/CT screening do not increase detection, targeted screening of high risk persons living with HIV may be preferable.
Acknowledgments
We are grateful to all the patients, physicians, and staff involved in the Johns Hopkins HIV Clinical Cohort.
Funding: National Institutes of Health K23AI084854, R01 AG026250, R01 DA011602, R01 AA16893, K24 DA00432 and National Center for Research Resources KL2RR025006-01
Footnotes
Conference Presentation: The findings herein were presented, in part, at the 47th Annual Meeting of the Infectious Diseases Society of America, November 1, 2009, Oral Abstract #1258.
Author Contributions: S.A.B, K.G.G., and K.A.G. contributed to the study design, analysis, and writing and revisions of the manuscript. R.D.M. contributed to the data collection, analysis, and revisions of the manuscript. S.J.G. contributed to the analysis and revisions of the manuscript. C.L.T. and K.R.P. contributed to the study design and revisions of the manuscript. All authors had full access to the data in the study and can take responsibility for the integrity of the data and of the analysis.
Potential Conflicts of Interest: R.D.M has been a consultant for Bristol-Myers Squibb and has received research funding from Merck, Pfizer, and Gilead. K.A.G. has been a consultant and received research funding from Tibotec. All other authors: no conflicts.
References
- 1.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–73. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 2.Sadiq ST, Taylor S, Copas AJ, et al. The effects of urethritis on seminal plasma HIV-1 RNA loads in homosexual men not receiving antiretroviral therapy. Sex Transm Infect. 2005;81:120–3. doi: 10.1136/sti.2004.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClelland RS, Wang CC, Mandaliya K, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS. 2001;15:105–10. doi: 10.1097/00002030-200101050-00015. [DOI] [PubMed] [Google Scholar]
- 4.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis. 2008;35:946–59. doi: 10.1097/OLQ.0b013e3181812d15. [DOI] [PubMed] [Google Scholar]
- 5.Fisher JD, Fisher WA, Cornman DH, et al. Clinician-delivered intervention during routine clinical care reduces unprotected sexual behavior among HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41:44–52. doi: 10.1097/01.qai.0000192000.15777.5c. [DOI] [PubMed] [Google Scholar]
- 6.Farley TA, Cohen DA, Wu SY, et al. The value of screening for sexually transmitted diseases in an HIV clinic. J Acquir Immune Defic Syndr. 2003;33:642–8. doi: 10.1097/00126334-200308150-00014. [DOI] [PubMed] [Google Scholar]
- 7.Rieg G, Lewis RJ, Miller LG, et al. Asymptomatic sexually transmitted infections in HIV-infected men who have sex with men: prevalence, incidence, predictors, and screening strategies. AIDS Patient Care STDS. 2008;22:947–54. doi: 10.1089/apc.2007.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phipps W, Stanley H, Kohn R, et al. Syphilis, chlamydia, and gonorrhea screening in HIV-infected patients in primary care, San Francisco, California, 2003. AIDS Patient Care STDS. 2005;19:495–8. doi: 10.1089/apc.2005.19.495. [DOI] [PubMed] [Google Scholar]
- 9.Baker J, Plankey M, Josayma Y, et al. The prevalence of rectal, urethral, and pharyngeal Neisseria gonorrheae and Chlamydia trachomatis among asymptomatic men who have sex with men in a prospective cohort in Washington, D. C AIDS Patient Care STDS. 2009;23:585–8. doi: 10.1089/apc.2008.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tunthanathip P, Lolekha R, Bollen LJ, et al. Indicators for sexual HIV transmission risk among people in Thailand attending HIV care: the importance of positive prevention. Sex Transm Infect. 2009;85:36–41. doi: 10.1136/sti.2008.032532. [DOI] [PubMed] [Google Scholar]
- 11.Mayer K, O’Cleirigh C, Skeer M, et al. Which HIV-infected MSM in care are engaging in risky sex and acquiring sexually transmitted infections: Findings from a Boston community health center. Sex Transm Infect. 2010;86:66–70. doi: 10.1136/sti.2009.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin F, Prestage GP, Zablotska I, et al. High rates of sexually transmitted infections in HIV positive homosexual men: data from two community based cohorts. Sex Transm Infect. 2007;83:397–9. doi: 10.1136/sti.2007.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HC, Ko NY, Lee NY, et al. Trends in sexually transmitted diseases and risky behaviors among HIV-infected patients at an outpatient clinic in southern Taiwan. Sex Transm Dis. 2010;37:86–93. doi: 10.1097/OLQ.0b013e3181bd8301. [DOI] [PubMed] [Google Scholar]
- 14.Cachay ER, Sitapati A, Caperna J, et al. Denial of risk behavior does not exclude asymptomatic anorectal sexually transmitted infection in HIV-infected men. PLoS One. 2009;4:e8504. doi: 10.1371/journal.pone.0008504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teague R, Mijch A, Fairley CK, et al. Testing rates for sexually transmitted infections among HIV-infected men who have sex with men attending two different HIV services. Int J STD AIDS. 2008;19:200–2. doi: 10.1258/ijsa.2007.007131. [DOI] [PubMed] [Google Scholar]
- 16.Hoover KW, Butler M, Workowski K, et al. STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis. 2010;37:771–6. doi: 10.1097/OLQ.0b013e3181e50058. [DOI] [PubMed] [Google Scholar]
- 17.Kalichman SC, Pellowski J, Turner C. Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect. 2011;87:183–90. doi: 10.1136/sti.2010.047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asymptomatic Sexually Transmitted Infections (STI) and Risk Taking Behaviors are Commonly Detected Among HIV-Infected Patients in Care: Lessons from the SUN Study Cohort. Conference on Retroviruses and Opportunistic Infections; 2006; Denver, USA. [Google Scholar]
- 19.Incorporating HIV prevention into the medical care of persons living with HIV. Recommendations of CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2003;52:1–24. [PubMed] [Google Scholar]
- 20.HIV prevention through early detection and treatment of other sexually transmitted diseases--United States. Recommendations of the Advisory Committee for HIV and STD prevention. MMWR Recomm Rep. 1998;47:1–24. [PubMed] [Google Scholar]
- 21.Berry SA, Ghanem KG, Page KR, et al. Gonorrhoea and chlamydia testing rates of HIV-infected men: low despite guidelines. Sex Transm Infect. 2010;86:481–4. doi: 10.1136/sti.2009.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17 (Suppl 1):S38–41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 23.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–5. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 24.StataCorp. Stata Statistical Software: Release. Vol. 11. 2009. [Google Scholar]
- 25.Maryland STD Statistics. [Accessed July 7th, 2010]; Available at: http://www.edcp.org/html/stds.cfm.
- 26.Sexually Transmitted Disease Surveillance. [Accessed 01/25, 2011];2007 Available at: http://www.cdc.gov/std/stats07/toc.htm.
- 27.Kahle E, Zhang Q, Golden M, et al. Trends in evaluation for sexually transmitted infections among HIV-infected people, King County, Washington. Sex Transm Dis. 2007;34:940–6. doi: 10.1097/olq.0b013e31813e0a48. [DOI] [PubMed] [Google Scholar]
- 28.Grosskurth H, Mosha F, Todd J, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet. 1995;346:530–6. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 29.Baffi CW, Aban I, Willig JH, et al. New syphilis cases and concurrent STI screening in a southeastern U.S. HIV clinic: a call to action. AIDS Patient Care STDS. 2010;24:23–9. doi: 10.1089/apc.2009.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchinson J, Goold P, Wilson H, et al. Sexual health care of HIV-positive patients: an audit of a local service. Int J STD AIDS. 2003;14:493–6. doi: 10.1258/095646203322025821. [DOI] [PubMed] [Google Scholar]
- 31.Hamlyn E, Barrett S, Kelsey J, et al. Improvement in screening for sexually transmitted infections in HIV-positive patients following implementation of a nurse-led clinic. Int J STD AIDS. 2007;18:424–6. doi: 10.1258/095646207781024720. [DOI] [PubMed] [Google Scholar]
- 32.Gaydos CA, Kent CK, Rietmeijer CA, et al. Prevalence of Neisseria Gonorrhoeae among men screened for Chlamydia Trachomatis in four United States cities, 1999–2003. Sex Transm Dis. 2006;33:314–9. doi: 10.1097/01.olq.0000194572.51186.96. [DOI] [PubMed] [Google Scholar]