TO THE EDITOR
Despite consensus that intense, intermittent ultraviolet exposure is a major melanoma risk factor, the role of DNA repair in melanoma pathogenesis remains controversial. UV-characteristic cyclobutane–pyrimidine dimers and pyrimidine–pyrimidone 6,4-photoproducts are absent in most melanomas, and many frequently encountered mutations in melanoma, such as BRAF c.1799 T->A, are not characteristic UV signature mutations(Davies et al., 2002).
We set out to explore the role of DNA repair in melanoma via microarray analysis of expression of DNA repair genes in melanoma and non-melanoma skin cancer (NMSC). Our study population included 16 primary cutaneous melanomas (PCMs), 11 squamous cell carcinomas (SCCs), and 15 basal cell carcinomas (BCCs). PCMs included 2 melanomas in situ (MIS), 2 thin (<1 mm Breslow depth, measured from top of stratum granulosum to deepest portion of tumor), 3 intermediate thickness (1–4 mm), and 9 thick melanomas (>4 mm). 40 metastatic melanoma (MM) samples were also analyzed (Supplemental Table S1).
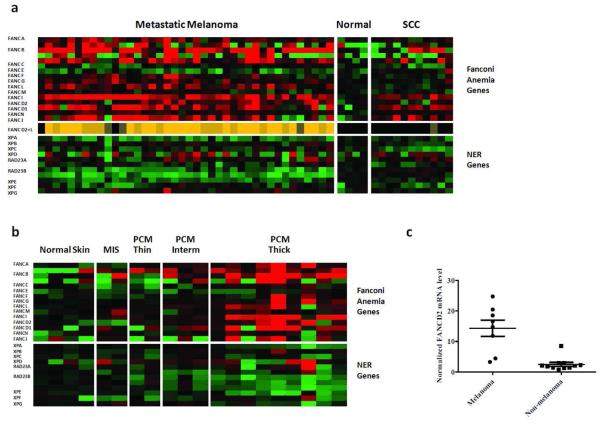
The Fanconi Anemia (FA) repair pathway is the primary cellular mechanism for addressing DNA damage related to cross-links, alkylation, and stalled replication forks(Kee and D'Andrea, 2010). We found that expression of FA genes is significantly elevated in MM compared to normal skin. The most melanoma-specific signature was provided by the aggregate expression of FANCL and FANCD2. Combined FANCD2-FANCL expression was at least 50% greater than normal skin in 35 of 40 MM, but in none of the BCCs or SCCs (Figure 1a, Supplemental Table S3). In contrast, no nucleotide excision repair (NER) genes were significantly upregulated in MM. Expression of XPA, XPE, XPG, and RAD23B were significantly decreased in MM (Figure 1a, Supplemental Tables S2, S4). This observation is consistent with the high incidence of melanoma in xeroderma pigmentosum patients with defects in the NER pathway.
Figure 1. Fanconi anemia, but not NER, genes are transcriptionally upregulated in melanoma compared to normal skin and NMSC.
(a) Microarray analysis of Fanconi anemia and NER gene expression in MM and SCC. Green and red represent decreased and increased expression relative to control, respectively. Color intensity is proportional to expression level up to a 4-fold difference from control. Middle row shows combined expression of FANCD2 + FANCL relative to normal skin; dark brown, medium tan, and orange denote 25-50% greater, 50-100% greater, and more than 100% greater than normal, respectively. (b) DNA microarray analysis of FA and NER genes in PCM. (c) qRT-PCR analysis of FANCD2 expression in PCM vs NMSC. FANCD2 expression levels were normalized to β-actin mRNA levels. Vertical bars represent standard error.
We next examined expression levels of DNA repair genes in PCM. When segregated according to Breslow depth, a stepwise increase in FA gene expression was noted with increasing melanoma thickness (Figure 1b, Supplemental Tables S5 and S6). In MIS, only one FA gene, FANCN, was over-expressed; in intermediate thickness melanomas, three genes (FANCB, G, L) were over-expressed. In thick melanomas, nine FA genes were broadly over-expressed, including FANCA, B, C, D1, D2, G, I, L, N. NER expression was not significantly increased in PCM.
We then sought to confirm our DNA microarray findings via quantitative RT-PCR (qRT-PCR) analysis of randomly selected PCM and NMSC. Consistent with the microarray results, normalized mRNA levels of the key downstream mediator FANCD2 were significantly higher in 8 PCM than in 9 NMSC (p=.0001, Figure 1c). Furthermore, there was a trend toward association between Breslow depth and FANCD2 expression level detected by qRT-PCR (data not shown).
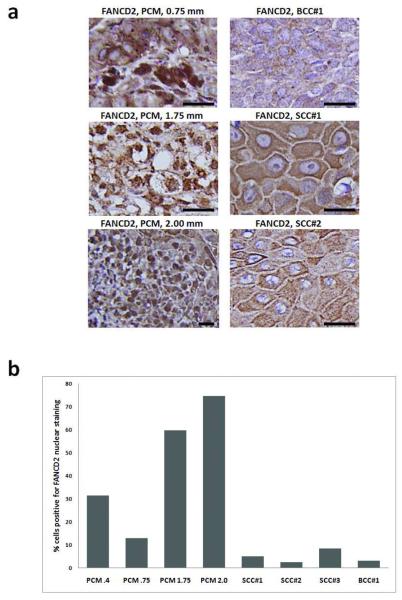
To assess changes in FA expression at the protein level, we analyzed 4 randomly selected melanomas and 4 NMSCs by immunohistochemistry for expression of FANCD2. These included two thin and two intermediate thickness PCMs, as well as 3 SCCs and 1 BCC (Supplemental Table S7). When comparing PCMs to NMSCs, 45% of melanoma cells had intense homogenous to granular nuclear staining (Figure 2), versus 4.8% in NMSC, (p=.029). When comparing PCMs of differing depth, we found increased FANCD2 staining with increased Breslow thickness. The two thin PCM displayed nuclear staining of FANCD2 in 13% and 31% of cells, respectively, compared to 60 and 75% in intermediate thickness melanomas. No significant elevation of markers of S-phase progression including Ki-67 labeling index (data not shown), or transcript levels of cyclin A, cyclin E, or histone H3, were found in melanoma compared to NMSC (Supplemental Table S8). Thus, we believe that increased FA gene expression in melanoma was not simply due to increased proliferative index.
Figure 2. Strong nuclear FANCD2 protein expression is observed in primary melanoma but not NMSC.
Paraffin-embedded tumors were immunostained with 1:50 anti FANCD2 (H-300) (sc28194, Santa Cruz Inc, Santa Cruz CA) and then biotinylated anti-rabbit antibody (Vector Laboratories, Burlingame CA). (a) Photomicrographs of FANCD2 protein expression in PCM (left panels) and NMSC (right panels). Breslow thickness is shown for each PCM. Black scale bars at lower right of each panel indicate 50 μm. (b) Percentage of PCM and NMSC cells with positive nuclear staining for FANCD2 by IHC. Numbers associated with each PCM on the horizontal axis represent Breslow thickness.
While increased gene expression does not necessarily imply FA pathway hyperactivation, three lines of evidence suggest this is likely the case. First, given that FANCD2-FANCI nuclear complexes are an established hallmark of FA pathway activation(Kee and D'Andrea, 2010), the increased homogenous and granular nuclear staining we observed for FANCD2 protein in melanoma appears consistent with FA activation as well. Second, FA pathway activity correlates closely with level of FA gene transcripts (Hoskins et al., 2008; Taniguchi et al., 2002; Vaughn et al., 1996). Finally, FA activation through over-expression of FA pathway constituents has been reported to confer cellular resistance to DNA damage(Chen et al., 2007; Hazlehurst et al., 2003).
These reports suggest that the FA pathway may function in oncogenesis in addition to its previously implicated role of tumor suppressor (Condie et al., 2002). Consistent with this hypothesis, FANCC knockout mice are deficient in STAT-1 mediated IFN-γ signaling (Pang et al., 2000), a pathway that may promote UVB-mediated melanomagenesis (Zaidi et al., 2011). Additionally, activation of the FA pathway could provide resistance to increased endogenous DNA damage typically seen in oncogenic states(Nitta et al., 2010) and confer survival advantage to melanoma cells. Further supporting this hypothesis, activation of the FA pathway has been associated with resistance to a variety of DNA damaging agents including BCNU and temozolomide (Chen et al., 2007), the tumoricidal effect of which is synergistically increased by bortezomib, a FA pathway inhibitor(Jacquemont and Taniguchi, 2007).
Recent studies have shed light on possible mechanisms of FA gene upregulation during melanoma pathogenesis. For instance, E2F activates the FANCD2 gene by binding consensus promoter response elements (Hoskins et al., 2008). Similarly, NF-κB binds and activates the FANCD2 promoter (Yarde et al., 2009) as well as mediates nuclear localization of FANCD2 (Ma et al., 2009). Finally, p53 has been shown to bind the FANCC promoter and up-regulate FANCC transcription(Liebetrau et al., 1997). Aberrant regulation of such transcription factors during melanomagenesis may account for increased FA gene transcription that is disassociated from cell-cycle dependent regulation.
In summary, we have found that FA DNA repair genes are transcriptionally upregulated in melanoma. Furthermore, increased FA gene expression correlates with increased Breslow thickness. In the context of the broader literature, our results suggest that activation of the FA pathway could contribute to melanomagenesis and resistance to chemotherapy. As such, the FA genes constitute potential targets in the development of novel melanoma therapeutics.
Supplementary Material
Acknowledgements
The authors would like to thank the following dermatologists for referring participants to this study: Lymarie Aguila, MD, Norma Alonso, MD, Coty Benmaman, MD, Moises Benmaman, MD, Olivia Benmaman, MD, Alma Cruz, MD, Laureano Giraldez, MD, José A. Hernández, MD, David Latoni, MD, Luis J. Ortiz, MD, Aida Quintero, MD, Edgardo Rodríguez, MD, Hiram Ruiz Santiago, MD, Rafael Vélez, MD. Additional thanks go to Mr. Bob Ritchie of the RCMI Publications Office (Grant #G12 RR003050) for editing this manuscript. Clark C. Chen is supported by the Doris Duke Charitable Foundation Clinical Scientist Development Award, the Sontag Foundation Distinguished Scientist Award, the Burroughs Wellcome Fund Career Awards for Medical Sciences, the Forbeck Scholar Award, and the Kimmel Foundation for Cancer Research Scholar Award.
Grant support: National Functional Genomics Center at the H. Lee Moffitt Cancer and Research Institute [JM and AR], [NCRR RCMI-NIH Program Grant, 2-G12-RR03050-20 JM].
Footnotes
Conflicts of interest: the authors have no conflicts of interest to declare
References
- Chen CC, Taniguchi T, D'Andrea A. The Fanconi anemia (FA) pathway confers glioma resistance to DNA alkylating agents. Journal of Molecular Medicine. 2007;85:497–509. doi: 10.1007/s00109-006-0153-2. [DOI] [PubMed] [Google Scholar]
- Condie A, Powles RL, Hudson CD, Shepherd V, Bevan S, Yuille MR, et al. Analysis of the Fanconi anaemia complementation group A gene in acute myeloid leukaemia. Leukemia & Lymphoma. 2002;43:1849–1853. doi: 10.1080/1042819021000009274. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Hazlehurst LA, Enkemann SA, Beam CA, Argilagos RF, Painter J, Shain KH, et al. Genotypic and phenotypic comparisons of de novo and acquired melphalan resistance in an isogenic multiple myeloma cell line model. Cancer Research. 2003;63:7900–7906. [PubMed] [Google Scholar]
- Hoskins EE, Gunawardena RW, Habash KB, Wise-Draper TM, Jansen M, Knudsen ES, et al. Coordinate regulation of Fanconi anemia gene expression occurs through the Rb/E2F pathway. Oncogene. 2008;27:4798–4808. doi: 10.1038/onc.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont C, Taniguchi T. Proteasome function is required for DNA damage response and fanconi anemia pathway activation. Cancer Research. 2007;67:7395–7405. doi: 10.1158/0008-5472.CAN-07-1015. [DOI] [PubMed] [Google Scholar]
- Kee Y, D'Andrea AD. Expanded roles of the Fanconi anemia pathway in preserving genomic stability. Genes & Development. 2010;24:1680–1694. doi: 10.1101/gad.1955310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetrau W, Budde A, Savoia A, Grummt F, Hoehn H. p53 activates Fanconi anemia group C gene expression. Human Molecular Genetics. 1997;6:277–283. doi: 10.1093/hmg/6.2.277. [DOI] [PubMed] [Google Scholar]
- Ma DJ, Li SJ, Wang LS, Dai J, Zhao SL, Zeng R. Temporal and spatial profiling of nuclei-associated proteins upon TNF-alpha/NF-kappaB signaling. Cell Research. 2009;19:651–664. doi: 10.1038/cr.2009.46. [DOI] [PubMed] [Google Scholar]
- Nitta M, Kozono D, Kennedy R, Stommel J, Ng K, Zinn PO, et al. Targeting EGFR Induced Oxidative Stress by PARP1 Inhibition in Glioblastoma Therapy. PLoS ONE. 2010;5:e10767. doi: 10.1371/journal.pone.0010767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Q, Fagerlie S, Christianson TA, Keeble W, Faulkner G, Diaz J, et al. The Fanconi anemia protein FANCC binds to and facilitates the activation of STAT1 by gamma interferon and hematopoietic growth factors. Molecular and Cellular Biology. 2000;20:4724–4735. doi: 10.1128/mcb.20.13.4724-4735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Garcia-Higuera I, Andreassen PR, Gregory RC, Grompe M, D'Andrea AD. S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood. 2002;100:2414–2420. doi: 10.1182/blood-2002-01-0278. [DOI] [PubMed] [Google Scholar]
- Vaughn JP, Cirisano FD, Huper G, Berchuck A, Futreal PA, Marks JR, et al. Cell cycle control of BRCA2. Cancer Research. 1996;56:4590–4594. [PubMed] [Google Scholar]
- Yarde DN, Oliveira V, Mathews L, Wang X, Villagra A, Boulware D, et al. Targeting the Fanconi anemia/BRCA pathway circumvents drug resistance in multiple myeloma. Cancer Research. 2009;69:9367–9375. doi: 10.1158/0008-5472.CAN-09-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, et al. Interferon-gamma links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469:548–553. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.