1. Introduction
Auditory processing disorder (APD) and specific language impairment (SLI) are developmental communication disorders that clinicians and researchers have investigated for decades (Jerger, 2009; Leonard, 1998). Auditory processing disorder (APD) is defined as “difficulties in the processing of auditory information in the central nervous system” (American Speech-Language-Hearing Association [ASHA], 2005, p. 1). The diagnosis is given when functional listening difficulties are observed in the presence of normal peripheral hearing and the child demonstrates deficits in one or more auditory skill areas that include discrimination, pattern recognition, temporal integration and ordering, dichotic listening, and the perception of degraded stimuli (ASHA, 2005). Children with APD often have difficulties with reading, spelling, and expressive and receptive language (ASHA, 2005; Dawes, Bishop, Sirimanna, & Bamiou, 2008; Jerger & Musiek, 2000; Sharma, Purdy & Kelly, 2009).
Difficulties with reading, spelling, and expressive and receptive language are also observed in children with specific language impairment (SLI; Catts & Kamhi, 2005; Leonard, 1998). A diagnosis of SLI is given to children whose language abilities are not as well developed as those of other children who are the same age, exhibit the same level of nonverbal intelligence, and have similar opportunities for learning. Language deficits may be observed for expressive language only, or for expressive and receptive language. Possible reasons for language delay, including hearing loss, oral-motor dysfunction, cognitive impairment, and social-behavioral disorders, are ruled out in SLI (Leonard, 1998).
1.1 Controversy regarding APD
One controversy is whether there is truly a “disorder” of auditory processing with underlying etiological unity that is distinct from other learning disabilities (Cacace & McFarland, 1998; Dawes & Bishop, 2009; Moore, 2006). Clinical commentaries in textbooks (Bellis, 2003; Chermak & Musiek, 1997) and consensus statements (ASHA, 2005; Jerger & Musiek, 2000) assume the validity of APD as a construct; however, researchers have noted that individuals with APD often present with language and/or reading deficits similar to those observed in individuals with SLI. Studies by Sharma et al. (2009) and Dawes et al. (2008) have documented language and reading deficits in individuals with a clinical diagnosis of APD or laboratory test performance indicative of APD. In stating that APD “may lead to or be associated with difficulties in higher order language, learning, and communication functions” (p. 2) ASHA’s (2005) technical report not only notes that APD and SLI have overlapping symptoms, but also suggests that APD plays a causal role in some language impairments.
There is more consensus about the validity of SLI as a construct; however, the precise nature and etiology of the deficits in SLI remain unresolved (Leonard, 1998). One hypothesis, which continues to be controversial, is that auditory processing deficits play a causal role in SLI (Dawes & Bishop, 2009; Moore, 2006; Rosen, 2003). This hypothesis has been considered for decades (see reviews by Rees, 1973; Rosen, 2003) and there is a large literature investigating it (e.g., Banai & Kraus, 2007; Basu, Krishnan, & Weber-Fox, 2009; Bishop & McArthur, 2005; McArthur, Atkinson, & Ellis, 2009; Tallal, 2004; Tallal & Piercy, 1973a,b). Although many individuals with SLI, dyslexia, or more broadly defined language-learning problems have difficulty processing brief, rapidly presented stimuli and/or making frequency discriminations, a substantial proportion of these individuals perform within the normal range on auditory processing tasks (e.g., Banai, Nicol, Zecker, & Kraus, 2005; Bishop, Adams, Nation, & Rosen, 2005; McArthur & Bishop, 2005). These observations suggest that SLI can be present without auditory processing deficits (Bishop, Carlyon, Deeks, & Bishop, 1999).
1.2 Relationships between APD and SLI
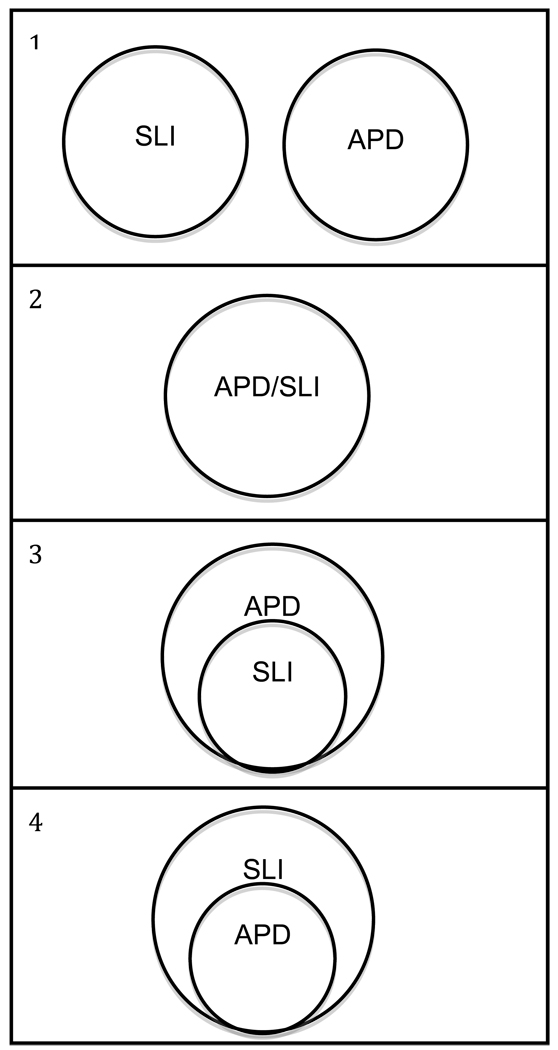
Four possible relations between APD and SLI are described below and depicted in Figure 1.
SLI and APD are distinct constructs that can be distinguished theoretically and clinically.
SLI and APD are different labels for the same construct.
SLI is a subset of APD. Some children with APD have SLI, but all children with SLI have APD.
APD is a subset of SLI. Some children with SLI have APD, but all children with APD have SLI.
Figure 1.
Four possible relationships between the constructs of specific language impairment (SLI) and auditory processing disorder (APD).
Alternative 1 is often assumed in discussions of APD and its relation to other disorders (e.g., ASHA, 2005); however, it has not been tested directly. Clinically, APD and SLI can be difficult to distinguish (ASHA, 2005; Dawes & Bishop, 2009; Jerger & Musiek, 2000; Moore, 2006), but this observation need not lead us to conclude that the two constructs are isomorphic (Alternative 2). Although investigations of Alternative 3, which start with a sample of individuals with APD, are scarce, Sharma et al. (2009), Dawes et al. (2008), and Ferguson, Hall, Riley, and Moore (2010) have documented language and reading deficits in individuals with APD. Alternative 4 has received the most research attention, frequently motivated by a desire to better understand SLI. As reviewed in the previous section, there is considerable evidence that a substantial proportion of children with SLI have auditory processing deficits (e.g., Banai et al., 2005; Basu et al., 2009; Bishop et al., 2005; Bishop & McArthur, 2005; McArthur et al., 2009; McArthur & Bishop, 2005). The exact nature of those deficits, however, remains unclear, and it is also not clear whether clinicians would consider the children with SLI who have auditory processing deficits to also have APD, because the tasks used by researchers to assess auditory processing are not the same as those used by clinicians to diagnose APD. No study has found an entire sample of children with SLI who all have auditory processing deficits, or an entire sample of children with APD who all have language deficits.
Although the current study does not attempt to distinguish among the four alternatives, it does seek to provide a thorough description of the behavioral profiles of children with APD and children with SLI. This behavioral description is a necessary first step toward determining which of the four alternatives is most accurate. To date, there is little research directly investigating the overlap in symptoms observed among children with APD and children with SLI. We wanted to know whether APD and SLI might be distinguished behaviorally in this sample.
1.3 A laboratory test-based behavioral profile
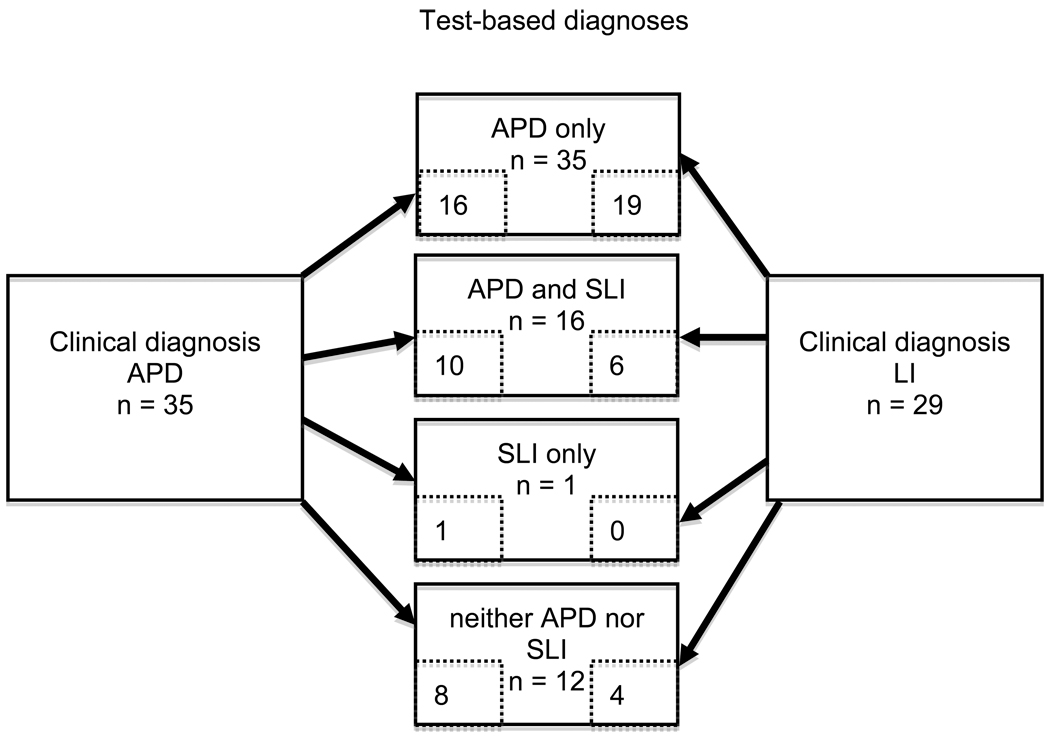
The characteristics of individuals with SLI are fairly well described for a wide range of behaviors that include spoken language comprehension and production, literacy, and memory (Leonard, 1998). Although comparatively little is known about the behavioral characteristics of individuals with APD, clinical profiles may include reading and spelling difficulties, phonological deficits, poorer verbal than performance IQ, and poor vocabulary (Bellis, 2006) -- all of which are likely to be observed in children with SLI (Leonard, 1998). For the present study, measures were chosen that would assess these different aspects of language and literacy, as well as auditory processing performance. We attempted to assemble a comprehensive battery that could be completed in a reasonable amount of time. The battery was used to classify children into two groups: those children with and without SLI, and those children with and without APD. These classifications, unlike the clinical classifications with which the children entered the study, were not mutually exclusive. In fact, as shown in Figure 2, a substantial proportion of children met the criteria for both SLI and APD. These non-mutually exclusive classifications derived from our laboratory testing are hereafter referred to as “test-based” classifications. We recognize that the clinical diagnoses that the children received from practitioners were also based on test scores.
Figure 2.
Numbers of participants with clinical diagnoses of auditory processing disorder (APD) and language impairment (LI) in each test-based subgroup (APD and specific language impairment [SLI]).
The position stated as alternative (1) in the previous section—SLI and APD are distinct constructs that can be distinguished theoretically and clinically—suggests that the test-based behavioral profiles of children with SLI and children with APD will differ. Specific predictions about how they might differ are stated in the following paragraphs along with the descriptions of the measures that were used. To the extent that alternative (1) is not supported by the data, we would conclude that particular behavioral characteristics may be shared between children with SLI and children with APD. More specifically, if SLI and APD were isomorphic, one would not expect to find group mean differences between children with SLI and APD.
Standardized tests frequently used in research on language disorders were chosen to sample receptive and expressive vocabulary and grammar, to the extent that it is possible to isolate modalities and domains of language. The nonword repetition test, a processing-based language measure, was also included. Poor nonword repetition has been found to be strongly associated with SLI across a number of studies and nonword repetition tasks (Graf Estes, Evans, & Else-Quest, 2007). Given the evidence for language difficulties in children with APD, we might expect that both APD and SLI groups will perform poorly on spoken language measures. However, to the extent that APD affects the comprehension of auditory input, performance on tasks that assess receptive language could be particularly weak in children with APD.
Four tests of auditory processing were chosen to represent temporal processing, frequency and duration discrimination, and dichotic listening, following the minimal battery approach of Jerger and Musiek (2000). The specific tests (described in section 2) selected were commonly used, commercially available tests that include expected scores for children in the age range of the participants in the present study. Tests with speech and non-speech stimuli were used. One would expect the performance of children with SLI to be better with non-speech stimuli relative to speech stimuli because the language deficits of children with SLI would limit their performance on the latter. Children with APD, if they have problems with all types of auditory input, could be expected to perform at least as poorly with non-speech stimuli as with speech stimuli (Dawes & Bishop, 2009; Moore, 2006). Oral reading fluency was assessed because reading problems are often associated with both APD and SLI (e.g., Catts & Kamhi, 2005; Sharma et al., 2009). A nonverbal IQ test was included, to rule out cognitive impairment in the participants and to provide a measure of general intellectual ability.
Measures of nonverbal and verbal working memory were also included in the test battery. The Competing Language Processing Test (CLPT; Gaulin & Campbell, 1994) is often used to assess verbal working memory in studies of children with SLI (e.g., Karasinski & Ellis Weismer, 2010; Montgomery & Evans, 2009; Thordardottir, 2008). The Spatial Working Memory Test (SWMT; Ellis Weismer, 2008) is a visual span task that was chosen because its structure is parallel to that of the CLPT. Verbal working memory is known to be a weakness for children with SLI (Montgomery, 2003). It may also be a weakness for children with APD, although the evidence regarding APD is equivocal. In their sample of children with diagnosed or suspected APD, 76% of whom also met criteria for LI, Sharma et al. (2009) found that 59% of the entire sample had poor forward digit span scores. The mean backward digit span score for the sample was within 1 SD of the mean. Ferguson et al. (2010) found that children with SLI were significantly poorer on a combined forward and backward digit span score compared to the control group, whereas children with APD did not differ from controls. The evidence is mixed as to whether children with SLI perform poorly on tasks that tap nonverbal working memory (Archibald & Gathercole, 2007; Bavin, Wilson, Maruff, & Sleeman, 2005; Ellis Weismer, 2008; Hoffman & Gillam, 2004). Nonverbal working memory is not expected to be depressed in children with APD.
A test of motor speed was included in the present study because children with SLI often demonstrate motor difficulties (Hill, 2001). There have been no reports of such difficulties in children with APD. A tapping task used by Bishop (2002) was selected because it has been shown to discriminate between children with SLI and controls and is quick and easy to administer. Finally, a parental checklist of attentional behaviors was included because APD and attention deficit/hyperactivity disorder often co-occur and have some common symptoms (Chermak, Tucker, & Seikel, 2002; Dawes & Bishop, 2009; Riccio, Hynd, Cohen, Hall, & Molt, 1994).
The measures used in this study vary in the extent to which their psychometric properties have been documented. Some are standardized tests that have documented validity and reliability with a limited number of populations. However, their sensitivity and specificity for identifying a disorder may be less well established (Spaulding, Plante, & Farinella, 2006). Other measures are published tests with suggested cutoff scores for different age bands, but without normative data. Still others are experimental measures that have not been used systematically in a normative fashion. Although the purpose of the study was not to critique specific instruments, in section 4 we consider some implications of the varying extent to which the psychometric properties of the measures we used are well known.
1.4 Purpose and research questions
The overall purpose of the present study was to determine whether behavioral profiles associated with APD and SLI could be clearly distinguished in a single sample of children with clinical histories of these disorders, when the children were grouped according to clinical history and according to laboratory testing. The data generated will contribute to better understanding of the constructs of SLI and APD, and how to assess them. The specific goals of the current study were, first, to describe the behavioral profiles of children with APD, and the behavioral profiles of children with SLI; and second, to document similarities and differences between these profiles. We grouped our sample in three ways to address the following three questions.
Did the test-based behavioral profile of children with a clinical diagnosis of APD differ from the profile of children with language impairment (LI) who were not diagnosed with APD?
Did measures of spoken language, auditory processing, reading fluency, memory, motor speed, and nonverbal cognitive abilities serve to distinguish children who were classified as having APD (or SLI) from children who were not classified as having APD (or SLI) with our test battery?
Did the test-based classifications addressed in question 2 agree with the clinical diagnoses with which children entered the study?
2. Method
2.1. Participants
2.1.1. Recruitment
Participants were recruited by asking school- and clinic-based speech-language pathologists (SLPs) and clinic-based audiologists to distribute invitation letters to the parents of children who met investigator-provided inclusionary and exclusionary criteria. Participants were also recruited through two private schools serving children with language-related learning disabilities. Brochures about the study were distributed through schools and parent groups. To protect participants’ confidentiality, clinicians did not identify to the investigators the children that they had referred. Potential participants were not known to the investigators unless their parents initiated contact.
The two inclusionary criteria given to professionals and listed in the brochure were that children must be monolingual English speakers, and that they have a clinical diagnosis of APD or receive services for language difficulties beyond speech-sound disorders. The five exclusionary criteria included: no hearing impairment; no concerns with overall cognitive development; not known to have a psychiatric disorder, pervasive developmental disorder, neurological damage or disease; no uncorrected visual impairment; and no frank motor impairment. Attention-deficit/hyperactivity disorder (ADHD) was not an exclusionary criterion, as we expected that excluding children with ADHD would render the sample less representative of children who received APD and language disorder diagnoses.
As children were enrolled, the investigators obtained a parent’s report of a diagnosis of APD or LI and a parent’s report of services received by the child from a school, agency, or professional in private practice. These parent reports were used to classify each child into one of two groups: children who had a diagnosis of APD and children who had LI. Any child who, according to parent report, had been diagnosed by an audiologist as having APD was classified in the APD group, even if the initial referral came from the child’s school SLP. Whenever possible, the parent’s report of diagnosis was confirmed with professional documentation, such as a report from an audiologist, psychologist, or SLP, or an Individualized Education Plan (IEP). Evaluations by professionals included standardized testing and observation of behavior and academic performance. None of the professional evaluations used a battery of auditory processing or language tests identical to the ones used in the present study. Some of the professionals used tests that were employed in the present study. In particular, the SSW, one of the most commonly used tests of auditory processing (Emanuel, 2002), was often used in APD evaluations. Steps taken to avoid repetition of specific tests are noted in section 2.7. Professional documentation was provided for 45 participants. Table 1 provides descriptive data for the sample that includes a breakdown by diagnostic category.
Table 1.
Mean, standard deviations, sample sizes, and effect sizes for all variables by diagnosis group
| Variable | Entire sample (n = 64) |
Entered study with APD diagnosis (n = 35) |
Entered study with LI diagnosis (n = 29) |
f̂ (APD vs. LI) |
Typically-developing sample (n = 20) |
|---|---|---|---|---|---|
| Gender (M:F) | 40:24 Mean (SD) |
25:10 Mean (SD) |
15:14 Mean (SD) |
10:10 Mean (SD) |
|
| Age (years) | 10.1 (1.1) | 10.3 (1.2) | 10.0 (0.9) | -- | 10.3 (1.5) |
| Full scale UNIT scorea | 96.5 (10.8) | 97.3 (11.4) | 95.6 (10.1) | -- | -- |
| UNIT Symbolic Memory*b | 8.5 (2.4) n = 63 |
8.8 (2.3) n = 34 |
8.14 (2.5) | 0.07 | -- |
| UNIT Cube Design* | 10.4 (2.4) | 10.3 (2.6) | 10.4 (2.2) | 0.00 | -- |
| CELF-4 Concepts & Following Directions* b | 7.3 (3.0) | 7.1 (3.2) | 7.6 (2.8) | 0.00 | -- |
| CELF-4 Formulated Sentences* b | 9.5 (2.8) | 9.4 (2.8) | 9.6 (2.9) | 0.00 | -- |
| Expressive vocabulary (EVT or W-J III)* a | 92.7 (11.9) n = 63 |
92.6 (11.1) n = 34 |
92.7 (12.9) | 0.00 | -- |
| Expressive Vocabulary Testa | 91.4 (11.3) n = 43 |
91.5 (11.5) n = 28 |
91.4 (11.4) n = 15 |
-- | -- |
| Woodcock-Johnson III Picture Vocabularya | 95.3 (12.9) n = 20 |
98.2 (7.2) n = 6 |
94.1 (14.7) n = 14 |
-- | -- |
| Peabody Picture Vocabulary Test* a | 98.6 (11.9) | 99.2 (10.9) | 97.8 (13.1) | 0.00 | -- |
| Nonword Repetition Test* d | 0.79 (0.08) | 0.78 (0.08) | 0.79 (0.09) | 0.00 | -- |
| Frequency Pattern Test* d | 0.53 (0.27) n = 63 |
0.59 (0.28) | 0.45 (0.24) n = 28 |
0.23 | 0.75 (0.19) |
| Duration Pattern Test* d | 0.38 (0.23) | 0.42 (0.23) | 0.34 (0.24) | 0.11 | 0.58 (0.14) |
| Dichotic Digits-Right* d | 0.88 (0.09) | 0.89 (0.09) | 0.88 (0.09) | 0.00 | 0.97 (0.03) |
| Dichotic Digits-Left* d | 0.84 (0.13) | 0.85 (0.14) | 0.83 (0.12) | 0.00 | 0.93 (0.05) |
| SSW (left competing)* | 9.9 (3.7) | 9.4 (4.1) | 10.4 (3.2) | 0.05 | 7.1 (4.0) |
| SSW (total errors)* | 20.9 (9.3) | 20.1 (9.8) | 21.8 (8.6) | 0.00 | 14.0 (5.7) |
| SWMT recall* d | 0.65 (0.16) | 0.67 (0.16) | 0.63 (0.16) | 0.04 | 0.83 (0.07) |
| CLPT recall* d | 0.53 (0.13) | 0.53 (0.13) | 0.53 (0.14) | 0.00 | 0.71 (0.13) |
| GORT-4 Fluency* b | 6.2 (3.0) | 6.8 (2.8) | 5.5 (3.1) | 0.18 | -- |
| Motor speed (taps/30 s with dominant hand)* | 99.5 (15.6) | 101.3 (13.1) | 97.4 (18.1) | 0.00 | 115.0 (15.3) |
| CADS-P Total scale* c | 61.9 (12.4) | 61.2 (11.9) | 62.7 (13.1) | 0.00 | -- |
Note:
Variable included in omnibus group comparison; APD = auditory processing disorder; LI = language impairment; UNIT = Universal Nonverbal Intelligence Test; CELF-4 = Clinical Evaluation of Language Fundamentals 4th edition; SSW = Staggered Spondaic Word Test; SWMT = Spatial Working Memory Test; CLPT = Competing Language Processing Test; GORT-4 = Gray Oral Reading Tests 4th edition; CADS-P = Conners’ Attention Deficit Scale-Parent;
test mean 100, SD 15;
test mean 10, SD 3;
test mean 50, SD 10;
proportion correct
2.1.2. Participant characteristics
Seventy children participated in the study. The data provided by 6 children who scored 75 or less on the Universal Nonverbal Intelligence Test (UNIT; Bracken & McCallum, 1998) were dropped from all analyses, yielding an analysis sample of 64 children. This criterion was used to exclude children with cognitive impairment. The criterion of 75 or less was determined by taking a score of 70 as the cutoff for mild mental retardation (American Psychiatric Association, 2000) and adding 5.05, the standard error of measurement of the test for the age range of the participants.
The study sample consisted of 64 children (24 girls) between the ages of 8;5 (years;months) and 12;7, with a mean age of 10.1 years (SD 1.1). The parent or guardian of each child signed an informed consent form, and each child signed an assent form. These forms were approved by the Institutional Review Board of the Pennsylvania State University, which also approved the study.
Parents of 16 participants reported that their child had been diagnosed with attention deficit disorder (ADD) or ADHD; however, in two cases, a formal diagnosis had not been made by a qualified professional. Although these two children were not receiving medication for attention disorders, 10 of the 16 children were on medication for attention disorders at the time of participation and their parents were asked to administer medication as they would for school during the period of their child’s participation.
2.1.3. Typically-developing participants
When the study was designed, a control group of typically-developing (TD) children was not included, because the purpose of the study was to compare the behavioral profiles of children with APD and children with SLI. However, 20 TD children participated in a subset of the tasks. Their data were used to interpret scores on those tests that did not have extensive normative data. The TD children were recruited from volunteer research participant pools. Their ages were approximately evenly distributed across the range from 7;9 to 13;0, and there were equal numbers of males and females. The TD children were administered a hearing screening, auditory processing tests, and verbal working memory, spatial working memory, and motor speed tasks. The protocols used to administer and score these tasks were conducted by trained research assistants and were the same as those used for the larger sample, with the exception of the hearing screening. Pure-tone thresholds were obtained in each ear for 0.5, 1, and 2 kHz only. For 4 kHz, we confirmed that the child could detect a 20 dB tone bilaterally.
Each child’s parent provided a brief educational history. No parent reported a history of developmental problems or that their child was currently receiving special education services. Parents of two children reported that their children had previously received therapy for articulation of sounds; one parent reported that one child had received language therapy starting at the age of 15 months for a period of a few months; and another parent reported that one child had received math support in school. The means and standard deviations for the group of TD children are displayed in Table 1.
2.2 Assessment Procedures
2.2.1. Audiological assessment
Pure-tone thresholds were obtained in each ear for 0.25, 0.5, 1, 2, and 4 kHz, and all participants had thresholds of 25 dB HL or better; this criterion was chosen because most assessments were not conducted in a sound-attenuated room. Tympanograms and distortion-product otoacoustic emissions (OAEs) were obtained for each ear. Tympanograms were missing in one or both ears for 18 children due to equipment malfunction or examiner error; in each case, OAEs were normal. For 6 children, OAEs were not obtained due to equipment malfunction or examiner error. For an additional 3 participants, 2 or more of the 4 test frequencies were not passed in one or both ears. In each of these 3 cases, pure-tone thresholds were normal and the abnormal OAE result was attributable to a temporary condition such as congestion or the presence of cerumen. A word recognition performance intensity function was conducted using the Auditec W-22 word list, by measuring word recognition accuracy at 40 dB HL and at 10 dB below the individually-determined uncomfortable listening level. All participants performed within normal limits on the performance intensity function. According to parent report, 44 children had experienced at least one ear infection (23 APD, 21 LI).
2.2.2. Oral mechanism screening
An oral mechanism screening was conducted to rule out frank oral-motor impairment, in accordance with the procedure described by Miccio (2002). All participants appeared to have normal oral-motor structure and function.
2.2.3. Language tests
2.2.3.1. Syntax
Two subtests of the Clinical Evaluation of Language Fundamentals-4th edition (CELF-4; Semel, Wiig, & Secord, 2003) were administered. The Formulating Sentences subtest was used to assess expressive syntax. The Concepts and Following Directions subtest was used to assess receptive syntax. Scaled scores were determined (mean of 10 and standard deviation of 3) using the provided norms.
2.2.3.2. Vocabulary
Receptive vocabulary was assessed with the Peabody Picture Vocabulary Test-3rd edition (PPVT-3; Dunn & Dunn, 1997. Expressive vocabulary was assessed with one of two tests: either the Expressive Vocabulary Test (EVT; Williams, 1997) or the Picture Vocabulary subtest of the Woodcock-Johnson Tests of Achievement-3rd edition (PV; Woodcock, McGrew, & Mather, 2001). In the EVT, the child was presented with a picture and a word, and asked to produce a synonym of the word. The PV required the child to name pictures without a cue from the examiner. The PV was administered in 20 cases for which time was limited. All vocabulary measures yielded standard scores (mean of 100, standard deviation of 15). A single variable, expressive vocabulary, was created using scores from either the PV or EVT.
2.2.4. Auditory processing tests
2.2.4.1. Frequency Pattern Test (FPT)
The FPT (Musiek, 1994) was used to assess frequency discrimination and temporal sequencing (Bellis, 2003). A compact disc recording produced by Audiology Illustrated was used. On each trial, three tones were presented. Each tone was “high” (1122 Hz) or “low” (880 Hz). In each triplet, one tone was different from the other two and the different tone could occur in the first, second, or third position. Children were required to verbalize the sequence of tones following each triplet (e.g., “high low high”). As per test instructions, intensity of the tones was individually determined by presenting the tones at 50 to 60 dB above each participant’s 1 kHz pure tone threshold. Standard presentation was 30 trials to the right ear and 30 to the left ear, but if the child answered the first 14 out of 15 correctly or incorrectly, testing in that ear was discontinued, as directed by the administration instructions. Scores were reported as percent correct for both ears combined.
2.2.4.2. Duration Pattern Test (DPT)
The DPT (Musiek, 1994) is similar to the FPT, but the tones differ in duration rather than frequency. A compact disc recording produced by Audiology Illustrated was used. The test assesses duration discrimination and temporal sequencing (Bellis, 2003). “Short” tones were 250 ms and “long” tones were 500 ms, with a 300-ms interval between tones. Children were asked to verbalize the sequence of tones following each triplet (e.g., “short short long”). As per test instructions, intensity of the tones was individually determined by presenting the tones at 50 dB above the 1 kHz pure tone threshold. Again, standard presentation was 30 trials to each ear, but if the child scored 14 or 15 correct, or incorrect, in the first 15 trials, testing in that ear was discontinued. Scores were reported as percent correct for both ears combined.
2.2.4.3. Dichotic Digits Test (DDT)
Dichotic listening tasks such as the DDT (Musiek, Gollegly, Kibbe, & Verkest-Lenz, 1991) measure the ability to separate differing signals simultaneously delivered to the left and right ears. The DDT also requires immediate recall of auditory input. A compact disc recording produced by Audiology Illustrated was used. On each of 40 trials, the child was presented with two spoken digits (the digits 1 through 9 excluding 7 are used) simultaneously, one in each ear, and then another two digits. The child was asked to repeat the 4 digits in any order. Following test instructions, intensity of the stimuli was individually determined by presenting the digits at 50 dB above each child’s pure tone average for the 0.5, 1, and 2 kHz thresholds. Scores were reported as percent correct for each ear separately (Dichotic Digits Right (DDR) and Dichotic Digits Left (DDL)).
2.2.4.4. Staggered Spondaic Word Test (SSW)
The SSW (Katz, 2001) is a dichotic listening task that is widely used for assessment of APD. The child is presented with 4 words that can also be construed as 2 two-syllable compound words, or spondees. The first word is presented to one ear, then the next two words are presented simultaneously in the left and right ears, and the last word is presented to the other ear. For example, a trial may begin with “up” in the left ear, “stairs” and “down” in the left and right ears respectively, and “town” in the right ear. Half of the trials begin with the right ear and half begin with the left. As directed in the administration instructions, the stimuli were presented at a comfortable listening level of 50–60 dB HL. The reported scores are total number of errors out of 40 trials.
2.2.5. Other measures
2.2.5.1. Phonological memory
The Nonword Repetition Test (NRT; Dollaghan & Campbell, 1998) was also used to measure phonological memory, which is thought to be an important contributor to language ability (Coady & Evans, 2008). The NRT consists of 16 nonwords, 4 each of one-, two-, three-, and four-syllable items. A cassette recording of the stimuli made by the first author of the NRT was copied to minidisc. Performance was scored on a phoneme-by-phoneme basis, yielding an overall percent phonemes correct (PPC).
2.2.5.2. Nonverbal ability
The UNIT (Bracken & McCallum, 1998) was used to estimate nonverbal IQ. The Abbreviated Battery of the UNIT consists of two subtests. In the Symbolic Memory subtest, children were presented with sequences of black or green stick figures of a man, a woman, a boy, a girl, and a baby. A sequence was exposed for 5 s and the child had to recreate the sequence from a set of tiles. The response was not timed. The Cube Design subtest required the child to recreate three-dimensional designs using patterned blocks. The target design was exposed throughout each trial. The response had to be completed within a time limit and bonus points were awarded for rapid, correct completion. Scaled scores were computed for each subtest and an IQ standard score was also computed. For one participant, the Spatial Memory subtest was substituted for Symbolic Memory, per the procedure for spoiled subtests given in the UNIT examiner’s manual.
2.2.5.3. Reading fluency
The Gray Oral Reading Tests-4th edition (GORT-4; Wiederholt & Bryant, 2001) was administered. The child read passages of increasing length and difficulty, and the child’s response was scored for speed and accuracy; these scores were combined to yield a Fluency score. Scaled Fluency scores were computed, with a mean of 10 and a standard deviation of 3.
2.2.5.4. Motor speed
Speed of motor processing, which is often compromised in children with SLI (Hill, 2001), was assessed using a tapping task described by Bishop (2002). Children were instructed to press the button of a tally counter as many times as possible in 30 s. Two trials were completed for each hand, and the mean number of taps was recorded for each hand.
2.2.5.5. Verbal working memory
The Competing Language Processing Test (CLPT; Gaulin & Campbell, 1994) was used to assess verbal working memory. The CLPT is a listening span test that requires the child to listen to sets of simple sentences (e.g., Sugar is sweet; Apples are square). The child judged the truth of each sentence by responding “yes” or “no,” and then recalled the last word of each sentence in the set. The sets increased in size from 1 to 6. Accuracy of truth judgments and recall of words were both scored as percent correct. The stimuli were recorded by a female speaker on a Marantz PMD650 minidisc recorder using a head-mounted microphone.
2.2.5.6. Visual-spatial working memory
The Spatial Working Memory Test (SWMT; Ellis Weismer, 2008) is a visual span task that is structured in a manner parallel to the CLPT. Columns of three boxes appeared on a computer screen. A shape appeared in each box, and one of the shapes was different than the other two. The child had to point to the odd shape. The boxes then disappeared and were replaced by blank boxes. The child was required to point to the locations where the odd shapes were. The number of columns in each set increased from 2 to 6. Accuracy of identifying the odd shapes and recall of their locations were both scored as percent correct.
2.2.5.7. Attention
The Conners’ ADHD/DSM-IV-Parent version (CADS-P; Conners, 1999) is a questionnaire used to assess ADHD symptoms. Parents were asked to rate the frequency of behaviors by their child in the preceding month. The items were combined in different ways to yield 4 scales: Conners’ ADHD Index; DSM-IV: Inattentive; DSM-IV: Hyperactive-Impulsive; and DSM-IV: Total. For the analyses reported here, the Total scale was used. Results were reported as T-scores, with a mean of 50 and a standard deviation of 10.
2.3. Equipment
A portable digital audiometer (Interacoustics AD229e) or a Welch-Allyn GSI 61 Clinic Audiometer was used to obtain hearing thresholds and to present the stimuli for the auditory processing tests and the word recognition performance intensity function. Auditory input was delivered using TDH-39P (for AD229e) or TDH-50P (for GSI 61) headphones, or EARTone 5A insert phones for the few participants who preferred them (insert phones were used only with the AD229e which was separately calibrated for supra-aural and insert phones). A tympanometer (Grason-Stadler GSI 38 Auto Tymp or Welch-Allyn GSI 33 Middle-ear Analyzer) was used to assess middle-ear pressure. A Biologics AuDX was used to measure DPOAEs.
The SWMT test was presented on a laptop computer. Stimuli for the CLPT and NRT were presented with the built-in speakers of a Marantz PMD650 minidisc player at a comfortable listening level. Participant responses for the CLPT, NRT, and CELF-4 Formulated Sentences were recorded for later confirmation of scoring, using a cassette recorder or a digital recorder and a dynamic lavalier microphone clipped to the participant’s clothing.
2.4. Test-based classification of children as affected or unaffected by APD
Upon entry into the study, children were classified into one of two groups: those with and without an APD diagnosis. However, when data collected for the present study were used to group participants, it was not assumed that the categories of APD and SLI were mutually exclusive. Thus, the test data obtained for the study were used to classify each child as affected or unaffected by APD, and as affected or unaffected by SLI. A criterion for affectedness for APD was established by using cutoffs for normal performance that were provided by the test authors of the FPT, DPT, DDT, and SSW. The authors of the FPT, DPT, and DDT provide minimum expected scores for several age groups, but descriptions of the normative sample are not provided. The SSW manual includes normative data from a sample of 120 children and adults with means and standard deviations for total errors by age; however, a description of the norming sample is not given (Katz, 2001). A cutoff of 2 SD below the age-appropriate mean was used for the SSW. Because the FPT, DPT, and DDT tests do not provide SDs, the age-based cutoffs provided with the tests were used instead. ASHA (2005) recommends that scores 2 or more SDs below the mean on at least two tests, or 3 or more SDs below the mean on one test, be required for a diagnosis of APD, but because SDs are not available for the FPT, DPT, and DDT, the following criteria were used. In order to be considered as affected by APD, a child had to score below cutoffs on at least two of the four APD tests: FPT (measured as total percent correct), DPT (total percent correct), DDR (right ear percent correct), and SSW (total errors). Based on their performance on these 4 tests, 51 children were classified as affected and 13 classified as unaffected by APD.
2.5. Test-based classification of children as affected or unaffected by SLI
Although clinicians and researchers agree on the essential criteria for SLI—lower than expected language performance in the presence of adequate sensory and cognitive abilities (see Leonard, 1998)—there is far less agreement about how to operationalize “low” language performance. Tomblin, Records, and Zhang’s (1996) EpiSLI system provides the most empirically-based set of criteria. With this system, tests are combined to form composite scores representing the receptive and expressive modalities and the linguistic domains of vocabulary, grammar, and narrative. The diagnostic accuracy of the combination of tests used in the present study is not known, because the tests are not those used by Tomblin et al.; however, the principle of creating composites is the same. Tomblin et al. used local norms to determine cutoffs for the composite scores. In the absence of local norms, the present study used cutoffs based on participants’ scores relative to the test norms. As Spaulding et al. (2006) demonstrated, the cutoffs that best differentiate between children with and without language impairment vary from test to test. In the present study, we followed the EpiSLI approach by creating receptive, expressive, vocabulary, and grammar composites and requiring low performance on at least two of them. Low performance was set at –1 SD, recognizing that this might be slightly conservative for the PPVT–III and EVT and slightly liberal for the CELF-4 subtests (Spaulding et al., 2006).
The composites were created by first subtracting the test or subtest mean from each individual’s score and dividing that deviation score by the test or subtest SD. The quotients resulting from these calculations were averaged in different combinations to create four composite scores. A grammar composite combined the Formulated Sentences and Concepts & Following Directions subtests. A vocabulary composite combined the PPVT-III and the expressive vocabulary score (either EVT or WJ-III Picture Vocabulary). An expressive language composite combined the Formulated Sentences subtest and expressive vocabulary measure. A receptive language composite combined the Concepts & Following Directions subtest score and the PPVT-III score. A child was classified as affected by SLI if the child’s scores on two or more composite scores were less than or equal to 1 SD below the mean. Based on their performance on these 4 composites, 47 children were classified as unaffected and 17 were classified as affected by SLI.
2.6. General Procedure
Participants were assessed in the investigator’s laboratory, in the university’s audiology clinic, at the participant’s home or school, or in a classroom at a Penn State campus in the Philadelphia area. Every effort was made to secure a quiet location with minimal disturbance. Although the ambient noise level varied, televisions, radios, stereos, and the like were always turned off.
Audiological assessments were usually conducted first, followed by the oral-mechanism screening, and the CELF-4 and UNIT subtests. Auditory processing tests were alternated with other tests to minimize fatigue. For 29 participants, testing was conducted in 3 sessions of about 90 minutes each on different days. Four sessions were needed for 3 participants and 5 sessions for 2 participants. In cases where the availability of the participant was limited, testing was conducted in longer sessions on 1 day (n = 13) or 2 days (n = 17), with adequate rest breaks provided.
2.7. Scoring and reliability
Testing was conducted by the first author or by one of a group of trained research assistants who were undergraduate or graduate students in communication sciences and disorders. Tests were scored by the person who administered the test, and scored again by a second scorer. Any scoring discrepancies were resolved by discussion. For 15 participants, scores from within the past year on one or more tests used in the present study were available from a reliable source (audiologist, speech-language pathologist, or another laboratory in the first author’s academic department) and were used to minimize multiple exposures to the same test.
Scoring of the nonword repetition task requires broad phonetic transcription of responses. Two trained research assistants who had taken an undergraduate course in clinical phonetics transcribed the data. Both assistants transcribed the data for 10 participants to establish inter-rater reliability. Phoneme-by-phoneme raw agreement was 94%. Reliability of the percent phonemes correct (PPC) was determined by comparing the word-by-word scores of the first author to that of one of the research assistants, who both transcribed and scored responses, for 10 participants (selected independently from the transcription reliability). The scorers agreed on 96% of items; all disagreements (n = 7) were by 1 phoneme. Audio recordings of the NRT were not available for 6 participants; scores for these participants were determined from the examiner’s written record of their responses.
2.8. Statistical Analyses
We used the same three steps to compare the scores of the children with a clinical diagnosis of APD to the scores of the children who received services for language difficulties but were not diagnosed with APD (research question 1), and to compare the scores of the children classified by laboratory testing as affected (by APD or by SLI) to the scores of those who were unaffected (research question 2). For Step 1, we used Hotelling’s T2 to compare the two groups (e.g., APD, not APD) with respect to the variables used to determine group membership. Hotelling’s T2 is the multivariate analog of Student’s t-test for independent group means (Everitt & Dunn, 2001). For Step 2, we followed each statistically significant omnibus T2 test with the Holm-Bonferroni multiple comparison procedure (MCP; Holm, 1979). The latter is a sequential MCP with greater power than the traditional Bonferroni procedure (Aickin & Gensler, 1996). Our use of this procedure allowed us to control the familywise Type I error rate at 0.05 and identify the group mean differences that contributed to the rejection of the omnibus test. For Step 3, we repeated the above sequence for the remaining dependent variables that were relevant, but not used to classify participants as affected or unaffected. As with tests conducted to verify that an experimental manipulation functioned as intended, the statistical tests conducted in support of Steps 1 and 2 served to verify that our groups differed in known ways.
Finally, we used Cohen’s f̂ (Cohen, 1988) to report effect sizes associated with our tests of group mean differences. In this context, Cohen’s where F is the test statistic used to assess the group mean difference and N equals the number of study participants providing data. Cohen suggested that researchers consider f̂ = 0.10 as a small effect, f̂ = 0.25 as a medium effect, and f̂ ≥ 0.40 as a large effect. By reporting effect sizes, we offer the reader the opportunity to compare the magnitudes of group differences that were and were not statistically significant. All statistical analyses were performed using Stata version 10 (StataCorp, 2007).
3. Results
Our analyses use the available data obtained from 64 children on 18 variables. Summary statistics are presented in Tables 1, 2, and 3. Three cases had missing data on a total of three variables: FPT, UNIT Symbolic Memory, and expressive vocabulary. One participant refused to complete the FPT. The expressive vocabulary score for one participant and the UNIT Symbolic Memory subtest score for one other participant were not available due to experimenter error. Because each multivariate test was based on the number of cases that provided complete data for the set of variables used, we report the sample sizes for each analysis.
Table 2.
Means, standard deviations, multiple comparison test results, and effect sizes by test-based APD classification
| Variable | APD affected | APD unaffected | Holm test results | f̂ | |
|---|---|---|---|---|---|
| Gender (M:F) | 30:21 Mean (SD) |
10:3 Mean (SD) |
-- F |
p |
|
| Age (years) | 10.2 (1.0) | 9.7 (1.2) | -- | -- | |
| UNIT Symbolic Memoryb | 8.5 (2.5) | 8.5 (1.9) | 0.03 | .8586 | 0.00 |
| UNIT Cube Designb | 9.8 (2.1) | 12.5 (2.4) | 14.16 | .0050 | 0.47 |
| CELF-4 Concepts & Following Directionsb | 7.0 (3.1) | 8.5 (2.4) | 2.40 | .6326 | 0.16 |
| CELF-4 Formulated Sentencesb | 9.2 (2.9) | 10.8 (2.0) | 3.10 | .4990 | 0.20 |
| Expressive vocabulary (EVT or W-J III)a | 91.2 (11.9) | 98.2 (10.4) | 3.59 | .5657 | 0.21 |
| Peabody Picture Vocabulary Test a | 97.1 (12.1) | 104.3 (9.4) | 3.70 | .6503 | 0.22 |
| Nonword Repetition Testd | 0.78 (0.08) | 0.83 (0.06) | 3.68 | .5997 | 0.22 |
| Dichotic Digits-Leftd | 0.82 (0.14) | 0.90 (0.05) | 3.47 | .4729 | 0.21 |
| SWMT recalld | 0.64 (0.17) | 0.69 (0.12) | 1.27 | 1.000 | 0.00 |
| CLPT recalld | 0.53 (0.14) | 0.56 (0.11) | 0.68 | 1.000 | 0.00 |
| GORT-4 Fluencyb | 5.5 (2.8) | 8.7 (2.5) | 13.15 | .0071 | 0.45 |
| Motor speed (taps/30s with dominant hand) | 99.3 (15.8) | 100.6 (15.0) | 0.05 | 1.000 | 0.00 |
| CADS-P Total scalec | 63.3 (12.2) | 56.3 (11.8) | 3.52 | .5255 | 0.19 |
Note: APD = auditory processing disorder; UNIT = Universal Nonverbal Intelligence Test; CELF-4 = Clinical Evaluation of Language Fundamentals 4th edition; EVT = Expressive Vocabulary Test; WJ-III = Woodcock-Johnson III Picture Vocabulary subtest; SWMT = Spatial Working Memory Test; CLPT = Competing Language Processing Test; GORT-4 = Gray Oral Reading Tests 4th edition; CADS-P = Conners’ Attention Deficit Scale-Parent;
test mean 100, SD 15;
test mean 10, SD 3;
test mean 50, SD 10;
proportion correct
Table 3.
Means, standard deviations, multiple comparison test results, and effect sizes by test-based SLI classification
| Variable | SLI affected | SLI unaffected | Holm test results | f̂ | |
|---|---|---|---|---|---|
| Gender (M:F) | 11:6 Mean (SD) |
29:18 Mean (SD) |
F |
p |
|
| Age (years) | 10.2 (1.0) | 10.1 (1.1) | -- | -- | |
| Nonword Repetition Test | 0.72 (0.09) | 0.81 (0.06) | 21.45 | .0002 | 0.58 |
| UNIT Symbolic Memory a | 7.4 (2.2) | 8.9 (2.3) | 4.99 | .1756 | 0.26 |
| UNIT Cube Design a | 9.6 (2.2) | 10.7 (2.5) | 2.34 | .6583 | 0.15 |
| Frequency Pattern Test c | 0.39 (0.24) | 0.58 (0.26) | 6.04 | .1349 | 0.31 |
| Duration Pattern Test c | 0.36 (0.27) | 0.39 (0.22) | 0.11 | 1.0 | 0.00 |
| Dichotic Digits-Right c | 0.84 (0.09) | 0.90 (0.08) | 6.95 | .0961 | 0.27 |
| Dichotic Digits-Left c | 0.74 (0.18) | 0.88 (0.08) | 16.55 | .0015 | 0.53 |
| SSW (total errors) | 29.2 (8.1) | 17.9 (7.7) | 23.65 | .0001 | 0.63 |
| SWMT recall c | 0.55 (0.19) | 0.69 (0.14) | 10.49 | .0196 | 0.37 |
| CLPT recall c | 0.47 (0.15) | 0.55 (0.12) | 6.03 | .1191 | 0.26 |
| GORT-4 Fluency a | 5.2 (2.5) | 6.5 (3.1) | 2.31 | .5337 | 0.15 |
| Motor speed (taps/30s with dominant hand) | 99.4 (18.0) | 99.6 (14.8) | 0.05 | .8296 | 0.00 |
| CADS-P Total scale b | 62.5 (11.3) | 61.6 (12.8) | 0.09 | 1.0 | 0.00 |
Note: SLI = specific language impairment; UNIT = Universal Nonverbal Intelligence Test; SSW = Staggered Spondaic Word Test; SWMT = Spatial Working Memory Test; CLPT = Competing Language Processing Test; GORT-4 = Gray Oral Reading Tests 4th edition; CADS-P = Conners’ Attention Deficit Scale-Parent;
test mean 10, SD 3;
test mean 50, SD 10;
proportion correct
3.1. Comparisons of diagnostic groups
Children who entered the study with a clinical diagnosis of APD and children who entered the study without that diagnosis were compared on measures of nonverbal ability, language, auditory processing, working memory, reading fluency, motor speed, and attention. The respective group means and standard deviations for the 18 variables are shown in Table 1. Complete data were available for 33 children in the APD group and 28 in the LI group. The test for equality of the two mean vectors indicated that the group means were not significantly different (Wilks’ Λ = 0.75, F (18,42) = 0.787, p = 0.703).
3.2. Comparison of children affected and unaffected with APD
Hotelling’s T2 was used to compare the means of the children who were classified as affected or unaffected with APD according to the FPT, DPT, DDR, and SSW tests. Because 1 case had missing data on 1 of these variables, the test was based on the data provided by 50 affected and 13 unaffected cases. The omnibus test indicated that, as expected, there were statistically significant group mean differences [Wilks’ Λ = 0.63, F (4, 58) = 8.34, p < .0001]; the Holm-Bonferroni MCP indicated that there were statistically significant group mean differences on each variable. The effect sizes as measured by Cohen’s f̂ (Cohen, 1988) ranged from 0.24 to 0.57. Hotelling’s T2 was also used to compare the means of the children who were classified as affected or unaffected with APD on the 13 variables that were not used to classify participants. Because 2 cases had missing data on 2 of these variables, the test was based on the data provided by 62 cases, 49 affected and 13 unaffected. The omnibus test indicated that there were statistically significant group mean differences [Wilks’ Λ = 0.63, F (13, 48) = 2.21, p < .03]; and the Holm-Bonferroni MCP indicated that the groups differed significantly on two variables: Cube Design and reading fluency. Table 2 displays the means, SDs, Holm test results and effect sizes for each variable. To summarize, the performance of children affected with APD differed significantly from the performance of those unaffected with APD on the four variables used to classify APD, as well as Cube Design and reading fluency.
3.3. Comparison of children affected and unaffected with SLI
When children’s scores on the 4 variables used to form the composites were compared, 1 child was lost to missing data on 1 of the variables. Thus, the group classified as having SLI numbered 17 children; the group classified as not having SLI numbered 46 children. Hotelling’s T2 and the Holm-Bonferroni MCP indicated that, as expected, the group means were significantly different on each of the four variables (CELF-4 Concepts & Following Directions, CELF -4 Formulated Sentences, EVT, PPVT-III) used to classify children as having or not having SLI [Wilks’ Λ = 0.42, F (4, 58) = 19.72, p < .0001]. Cohen’s f̂ (Cohen, 1988) ranged from 0.46 to 0.83. The two groups (46 unaffected, 16 affected) were also compared on the 13 variables that were not used to classify participants as affected or not affected with SLI. Hotelling’s T2 showed that there were statistically significant group mean differences [Wilks’ Λ = 0.47, F (13, 48) = 4.11, p < .01] and the Holm-Bonferroni MCP showed that the group means were significantly different with respect to four variables: NRT, DDL, SSW, and Spatial Working Memory recall. Table 3 displays means, SDs, Holm test results (df = 1, 60), and effect sizes for each variable. To summarize, the performance of children affected with SLI differed significantly from the performance of those unaffected with SLI on the four variables used to classify SLI, as well as nonword repetition, Dichotic Digits Left, the SSW test, and Spatial Working Memory.
3.4. Test-based classifications vs. clinical diagnoses
Table 4 presents a cross-classification of the 64 study participants by the two test-based classifications (APD/not APD and SLI/not SLI) and the clinical diagnosis that each child had upon entry to the study. Figure 2 shows how the participants in the clinical diagnosis groups were distributed among the groups derived from test-based classifications. Descriptive data on the language, auditory processing, and other variables for the cross-classified groups are shown in Table 5, except for those cells with frequencies of 1 or 0.
Table 4.
Cross-classification of the two test-based classifications and the clinical diagnosis classification
| Clinical diagnosis |
Test-based classifications | |||
|---|---|---|---|---|
| APD unaffected | APD affected | |||
| SLI unaffected | SLI affected | SLI unaffected | SLI affected | |
| APD | 8 | 1 | 16 | 10 |
| LI | 4 | 0 | 19 | 6 |
Note: APD = auditory processing disorder; LI = language impairment; SLI = specific language impairment
Table 5.
Means and standard deviations by cross-classified group
| Variable | Cross-classified group | |||||
|---|---|---|---|---|---|---|
| Clinical APD + Test-based APD (n = 16) |
Clinical APD + Test-based APD & SLI (n = 10) |
Clinical APD + neither (n = 8) |
Clinical LI + Test-based APD (n = 19) |
Clinical LI + Test-based APD & SLI (n = 6) |
Clinical LI + neither (n = 4) |
|
| Full scale UNIT score a | 96.6 (11.6) | 92.8 (9.9) | 102.1 (10.6) | 97.6 (9.0) | 85.3 (4.7) | 101.5 (11.6) |
| CELF-4 Concepts & Following Directions b | 8.3 (2.5) | 3.9 (2.5) | 9.0 (2.6) | 8.3 (2.7) | 5.0 (2.2) | 8.0 (2.6) |
| CELF-4 Formulated Sentences b | 10.6 (1.8) | 6.3 (2.1) | 11.4 (1.7) | 10.2 (2.3) | 6.8 (3.9) | 10.8 (0.5) |
| Expressive vocabulary (EVT or W-J III) a | 93.2 (8.4) | 86.6 (12.1) | 101.3 (8.4) | 95.2 (13.0) | 81.5 (9.2) | 97.8 (9.1) |
| Peabody Picture Vocabulary Test a | 101.4 (9.0) | 90.4 (8.7) | 106.1 (11.4) | 102.2 (11.1) | 80.7 (8.1) | 102.8 (3.3) |
| Nonword Repetition Test d | 0.80 (0.07) | 0.72 (0.07) | 0.81 (0.07) | 0.81 (0.06) | 0.69 (0.11) | 0.87 (0.01) |
| Frequency Pattern Test d | 0.58 (0.28) | 0.42 (0.24) | 0.81 (0.18) | 0.44 (0.21) | 0.26 (0.15) | 0.79 (0.19) |
| Duration Pattern Test d | 0.43 (0.20) | 0.32 (0.24) | 0.47 (0.24) | 0.30 (0.19) | 0.37 (0.29) | 0.50 (0.37) |
| Dichotic Digits-Right d | 0.89 (0.09) | 0.83 (0.07) | 0.95 (0.05) | 0.88 (0.08) | 0.85 (0.11) | 0.95 (0.07) |
| Dichotic Digits-Left d | 0.88 (0.07) | 0.73 (0.20) | 0.90 (0.06) | 0.86 (0.10) | 0.70 (0.14) | 0.88 (0.02) |
| SSW (total errors) | 18.2 (8.4) | 30.2 (7.3) | 12.1 (3.8) | 20.5 (8.1) | 30.3 (7.6) | 15.5 (1.3) |
| SWMT recall d | 0.69 (0.15) | 0.60 (0.21) | 0.72 (0.12) | 0.67 (0.14) | 0.46 (0.15) | 0.66 (0.15) |
| CLPT recall d | 0.56 (0.07) | 0.44 (0.16) | 0.58 (0.11) | 0.55 (0.15) | 0.50 (0.13) | 0.50 (0.12) |
| GORT-4 Fluency b | 6.8 (2.7) | 5.2 (2.6) | 8.4 (2.6) | 5.0 (2.9) | 4.7 (2.1) | 9.3 (2.9) |
| Motor speed (taps/30 s with dominant hand) | 102.6 (15.4) | 101.9 (9.0) | 97.1 (13.8) | 96.8 (14.1) | 93.9 (28.8) | 106.0 (19.1) |
| CADS-P Total scale c | 61.5 (13.1) | 62.1 (10.5) | 60.5 (13.0) | 65.0 (12.6) | 64.7 (13.5) | 48.8 (5.7) |
Note: APD = auditory processing disorder; LI = language impairment; SLI = specific language impairment; UNIT = Universal Nonverbal Intelligence Test; CELF-4 = Clinical Evaluation of Language Fundamentals 4th edition; EVT = Expressive Vocabulary Test; WJ-III = Woodcock-Johnson III Picture Vocabulary subtest; SSW = Staggered Spondaic Word Test; SWMT = Spatial Working Memory Test; CLPT = Competing Language Processing Test; GORT-4 = Gray Oral Reading Tests 4th edition; CADS-P = Conners’ Attention Deficit Scale-Parent;
test mean 100, SD 15;
test mean 10, SD 3;
test mean 50, SD 10;
proportion correct
Because gold standards do not exist, we used 2 × 2 cross-classification tables to assess agreement between the clinical diagnoses and test-based classifications. Toward this end, we computed simple odds (the ratio of Yes/No responses) and odds ratios (ratios of two simple odds). The simple odds of a child who entered with a clinical diagnosis of APD being classified as APD on the basis of the tests was 2.9 (Yes/No= 26/9) and the simple odds of a child who entered without a clinical diagnosis of APD being classified as APD on the basis of the tests was 6.3 (Yes/No= 25/4). A test that the odds ratio of 0.46 (2.9/6.3) did not differ significantly from 1 [z = −1.16, p = .244] indicated that the odds of a test-based diagnosis of APD were no greater for those who had entered the study with a clinical diagnosis of APD than for those who had not. Then, the simple odds of a child who entered with a clinical diagnosis of LI being classified as LI on the basis of the tests was 0.26 (Yes/No= 6/23) and the simple odds of a child who entered without a clinical diagnosis of LI being classified as SLI on the basis of the tests was 0.46 (Yes/No= 11/24). A test that the odds ratio of 1.76 (0.46/0.26) was not statistically different from 1 [z = 0.96, p = .336] indicated that the odds of receiving a test-based diagnosis of LI were no greater for those who entered the study with that clinical diagnosis than they were for those who did not enter with that clinical diagnosis.
3.5. Performance of typically-developing children
Using the criterion for APD affectedness of at least two scores below cutoffs, 10 of the 20 TD children would be classified as having APD. Table 1 includes means and standard deviations for TD participants on age, auditory processing tests, CLPT, SWMT, and motor speed. All 10 of the TD participants who met criteria for APD were 9 years of age or older. All 10 were below the cutoff for the DPT; 8 of 10 were below the cutoff for the SSW; and 5 of 10 were below the cutoff for the FPT. Seven children were below cutoffs on 2 tests, and 3 were below cutoffs on 3 tests. Of the 10 children who did not meet the criteria for APD, 2 were below the SSW cutoff only and 2 were below the DPT cutoff only.
4. Discussion
4.1. Group comparisons
We found no statistically significant group mean differences on any of the 18 behavioral variables examined between children who received a diagnosis of APD from an audiologist and children who received services for language impairments but did not have an APD diagnosis. Specifically, the two groups did not differ on tasks that measured auditory processing, grammar and vocabulary, reading fluency, verbal and nonverbal working memory, motor speed, and parent-rated attentional functioning. These results suggest that there may not be a distinct cognitive-behavioral profile associated with an APD diagnosis. Although we did not have control over or complete documentation of the methods used for diagnosing APD in our sample, the lack of a distinct profile is consistent with conclusions reported by Dawes et al. (2008), as well as the oft-repeated observation that children with APD have language and reading difficulties (e.g., ASHA 2005; Jerger & Musiek, 2000). The findings are also consistent with those of Ferguson et al. (2010), who used a wide range of measures to compare the language, communication, and cognitive skills of children with SLI, children with APD, and a random sample of schoolchildren. Ferguson et al. found that the SLI and APD groups showed group mean differences on very few variables, although both clinical groups generally performed more poorly than the control group.
One approach to coping with similarity between APD and LI is to recommend thorough multi-disciplinary testing, as in the 2005 ASHA technical report. Such an approach may not solve the problem of differential diagnosis, however. A multi-disciplinary team including an audiologist, a speech-language pathologist, and a psychologist (ASHA, 2005) would be likely to administer a wide range of auditory processing, language, reading, and memory measures similar to those used in the current study. However, these tests did not differentiate the clinical diagnosis groups.
When laboratory testing was used to classify children as affected or unaffected with SLI and APD, the multivariate tests detected four group mean differences for the SLI/not SLI comparison, and two group mean differences for the APD/not APD comparison. On average, the children who met the test-based criterion for APD had lower scores on Cube Design and reading fluency than did the children who did not meet the criterion, and children who met the test-based criterion for SLI had poorer performance on nonword repetition, spatial working memory, SSW word recall, and the left ear score for Dichotic Digits than did the children who did not meet the criterion. Thus, different sets of variables distinguished the affected from unaffected groups for APD and SLI. Interpretation of the group differences, however, is not straightforward.
One finding that coincided with predictions based on theory and prior findings was that children who were classified on the basis of the tests as affected with SLI had poorer performance on the nonword repetition task. The meta-analysis of Graf Estes et al. (2007) showed that nonword repetition performance is consistently poorer in children with SLI compared to age peers. It was unexpected, and striking, to find that children classified as having or not having SLI were differentiated by a nonlinguistic task (SWMT) and two auditory processing tests, but not by language-loaded tasks such as the CLPT or the reading test. Children with APD were differentiated from those without APD by a nonverbal ability test and a reading test, but not by the receptive language measures that were predicted to show differences.
Although it is often risky to devise post-hoc explanations for why certain variables could differentiate the groups, we offer the following observations. The two auditory processing measures that differentiated between children with and without SLI both involved recall of linguistic material. In the DDL, the linguistic material (digit names) is highly practiced and has minimal semantic value, but it is speech. In the SSW, the linguistic material consists of compound words and the task appears to draw heavily on lexical knowledge to assist word recognition and recall. The other auditory processing tasks, although they require verbal report, do not use linguistic stimuli. Thus, the fact that performance on the SSW and DDL tasks differed between children with and without SLI is understandable; however, these differences on auditory processing tasks contribute little toward establishing a set of tests that differentiate APD and SLI.
It is not surprising that the performance on the SWMT of children with SLI was lower than the performance of those without SLI. Ellis Weismer (2008) found that adolescents with SLI performed more poorly than typically-developing peers on the SWMT. In the SLI literature, some studies have reported problems with nonverbal working memory (e.g., Bavin et al., 2005; Hoffman & Gillam, 2004) and others have not found a nonverbal working memory deficit (Archibald & Gathercole, 2007). In contrast, a consistent finding is that verbal working memory is a weakness for children with SLI (Archibald & Joanisse, 2009; Montgomery, 2003). However, verbal working memory as measured by the CLPT did not differ for the children affected and unaffected with SLI.
Given that auditory processing deficits are often, but not always, associated with dyslexia (Ramus, 2003), it may not be surprising to find that reading fluency was poorer among children affected with APD than children unaffected with APD. Poorer performance on Cube Design, however, is difficult to interpret. According to the authors of the UNIT, the Cube Design subtest is intended to assess nonsymbolic reasoning (Bracken & McCallum, 1998). There is no evidence or proposal in the literature for a weakness in nonsymbolic reasoning for children with APD. Children with SLI, on the other hand, have been found to show deficits in several types of nonverbal tasks (Leonard, 1998), and often score below age peers on nonverbal subscales of IQ tests (Miller & Gilbert, 2008; Swisher & Plante, 1993; Swisher, Plante, & Lowell, 1994), but such a difference was not found in the current study.
Overall, it is difficult to account for the presence of some group mean differences and the absence of others. One reason for the observed findings may be that there is no universally agreed-upon set of tests for diagnosis of SLI or APD. Because the instruments vary across studies, it is possible that not all children classified as SLI (or APD) in the present study would be so classified in another study (see Miller & Gilbert, 2008, for a discussion of this problem as it pertains to nonverbal IQ tests). Any inconsistency in classification is likely to contribute to inconsistency across studies regarding whether children with SLI (or APD) truly differ from a comparison group on a particular variable.
The presence of floor effects as a possible explanation for the lack of group mean differences on most variables can be discounted because the great majority of the sample means for the standardized tests were within normal limits. The mean for GORT-4 reading fluency was the only standardized score that fell more than 1 SD below the mean, and that variable did differentiate between children with and without APD. Therefore, overall depressed scores do not seem to account for the lack of group differences.
Another possible explanation for the small number of statistically significant group mean differences is insufficient statistical power. The use of observed data to compute power retrospectively is not appropriate. We can, however, use effect sizes to consider the relative magnitude of the group mean differences that were or were not significantly different from zero. Considered together, both the analytic findings and the magnitude of the effect sizes presented in Tables 2 and 3 suggest that the study assessed a sufficient number of participants to only reliably detect performance differences that were large and more likely to be replicated in future studies that used the same tests. A logical next step for future research would be to replicate and extend the current findings with a larger sample, employing additional tests as well as those used in the present study.
A final consideration for interpreting the group comparisons is the overlap shown between APD and SLI test-based classifications. Ninety-four percent of the children classified as affected with SLI were also classified as affected with APD, and 74% of the children not affected with SLI were affected with APD. Given that a larger proportion of the children affected with SLI also were affected with APD, the poorer performance of the group affected with SLI on four variables may also be related to their auditory processing abilities. Indeed, two of the four variables are thought to reflect auditory processing (SSW and DDL). When the APD classification is considered, 31% of children affected with APD were also classified as affected with SLI, whereas only 8% of children not affected with APD were also classified as affected with SLI. Thus, the relatively poor performance of the children affected with APD on Cube Design and reading fluency may be related to their language abilities. However, the overlap in classifications may be less a result of comorbidity than of the sensitivity of the APD tests, as evidenced by the number of TD children who met the criteria for APD.
4.2. Clinical vs. laboratory classifications
There was no agreement between the clinical diagnoses with which children entered the study and the test-based classifications. More specifically, we could not use our test-based criteria to predict whether a child had been identified by an audiologist as having APD or were receiving language services from a speech-language pathologist. The lack of correspondence between receipt of language services and the test-based classification of SLI may indicate that clinicians regard SLI as a broader category than researchers do. The lack of correspondence between clinical and test-based APD classifications derives in part from the fact that 80% of the children met the test-based criteria for APD whereas 55% entered the study with a clinical diagnosis of APD. One possible explanation for the high proportion of children meeting the test-based APD criterion relative to those with a clinical diagnosis is that APD was under-diagnosed in the sample; that is, a substantial number of the children referred with LI should have been diagnosed with APD. However, the data provided by the 20 typically-developing children suggest that rather than under-diagnosis by clinicians, there may have been over-diagnosis by the laboratory test battery. Half of the typically-developing children, who were not suspected to have either SLI or APD, met the study criteria for APD. These results suggest that the test battery was overly sensitive, and are broadly consistent with the findings of Angerman (2004). In that study, 5 of 16 TD children failed 2 or more out of 6 auditory processing tests, and 9 of 16 failed at least 1 test.
The present study used available tests that are consistent with recommended procedures for evaluating APD (ASHA, 2005; Jerger & Musiek, 2000) and are commonly used for that purpose (Emanuel, 2002). Clearly there is a need for further research to establish the psychometric properties of auditory processing tests, especially validity (Dawes & Bishop, 2009), and also to establish the degree to which standardized language tests tax auditory processing abilities. In order to determine if SLI and APD are distinct constructs, there must be tasks that assess auditory processing with minimal influence from language knowledge or ability, and tasks that assess language knowledge and ability with minimal influence from auditory processing.
Although these issues with the tests must be kept in mind when interpreting the mean scores of the groups formed by cross-classification of clinical and test-based diagnoses (Table 5), it is worth noting the following points. On the Dichotic Digits test, right ear scores were slightly larger than left ear scores, as is usually found, but this right ear advantage was particularly marked for children who were classified by our testing as affected with both APD and SLI. A marked difference in the scores for the right and left ears may indicate poor transfer of information between the cerebral hemispheres (Keith & Anderson, 2007). The hypothesis that individuals who perform poorly on both auditory processing and language tests have compromised interhemispheric connectivity is amenable to testing with behavioral and/or neuroimaging methods. The mean FPT score was particularly small among children with a history of language services who were affected with both APD and SLI. Their mean FPT was smaller than their mean DPT, a different pattern from that of the other groups. Similarly, the SWMT score was also smallest for the same group, and was smaller than the CLPT score only for that group. Further investigation may help explain why these tasks are especially difficult for children who combine a history of language problems noted by service providers with poor performance on both auditory processing and language tests.
4.3. Limitations
The fact that all of the children participating in the current study were clinically referred is important to keep in mind when interpreting the results. Variables that distinguish between affected and unaffected groups may not be the same variables that would distinguish between typically developing children and those affected with SLI or APD. However, the problem of identifying a child with a developmental disability (although not without its pitfalls) was not the focus of this investigation. Rather, we sought to describe and contrast the profiles of children with APD and SLI.
The procedure for participant recruitment and selection could be considered both a strength and a weakness. It is a strength because, by recruiting through schools and categorizing children according to whether they did or did not receive an APD diagnosis from an audiologist, we selected a sample that has considerable ecological validity with respect to the way children with listening, reading, and language difficulties are evaluated and labeled in school settings. However, there are risks associated with this recruitment method. We did not have control over, or in many cases, knowledge of, the instruments and criteria that were used by clinicians to evaluate LI and APD. We believe that these risks were outweighed by the value of comparing test-based classifications to the classifications that were made in the “real world.” However, in order to be directly applied to clinical practice, future research must investigate the behavioral profiles of groups created by the use of specific diagnostic procedures and of instruments with well-understood psychometric properties.
4.4. Conclusions
This study addressed three questions. First, we asked if the behavioral profile of children with a clinical diagnosis of APD differed from the profile of children who received services for language difficulties but were not diagnosed with APD. The answer was no. Group mean differences were not found between those with and without an APD diagnosis. Our second question was whether measures of spoken language, auditory processing, reading fluency, memory, motor speed, and nonverbal cognitive abilities would serve to distinguish children who were classified as having APD (or SLI) on the basis of laboratory testing from children who were not classified as having APD (or SLI). Children with and without APD differed on two variables: UNIT Cube Design and reading fluency. Children with and without SLI differed on four variables: nonword repetition, spatial working memory, SSW total errors, and the left ear score for Dichotic Digits. However, interpretation of these results is not straightforward. Further investigation into the abilities required to perform each of the tasks for which differences were found may help to clarify the meaning of these group differences.
Our third question was whether the laboratory test-based classifications of APD and SLI corresponded to the clinical diagnoses with which children entered the study. The test-based classifications did not correspond closely to the clinical diagnoses. Indeed, the two ways of categorizing cases were statistically independent.
The test battery was composed of instruments commonly used to assess APD and SLI. These instruments did not distinguish between children clinically identified with and without APD, nor did our approach to classification of APD and SLI yield interpretable group differences. If APD and SLI truly are distinct constructs, other instruments are needed to distinguish them. Behavioral measures can be difficult to interpret because of the many cognitive operations that go into any voluntary response. One approach to this problem of interpretation is to devise behavioral measures that target a specific cognitive process and minimize the influence of other factors, such as non-speech auditory processing tests proposed by Cameron et al. (2009) and Moore (2006). Another approach is to use electrophysiological methods to measure the time course of cognitive processes, and we plan to use this approach in future studies. Through carefully controlled behavioral and electrophysiological investigations, the field will come closer to explaining individual differences in auditory processing and language. Ultimately, such explanations may supersede labels such as APD and SLI, and provide refined diagnostic profiles to guide targeted intervention.
Acknowledgments
This research was supported by grant 5 R03 DC007312 from the National Institute on Deafness and Other Communication Disorders. Many thanks to the children and their families who participated, to Amanda Owen, Elina Mainela-Arnold, and Patricia Deevy for their comments on earlier drafts, to Dr. Frank Musiek for kind advice and assistance, to Dr. Elise Uhring for facilitating the research in many ways, and to research assistants Jessica Muchoney, Jessica Ter Avest, Lena Higgins, Jessica Rhen, Janelle Kisiday, Trace Poll, Kara Mekarski, Brooke Littleton, Shannan Saul, Jenna Frazzini, and Amy Ishkanian.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A preliminary report of these data was presented at the Symposium on Research in Child Language Disorders, Madison, Wisconsin, in June 2009.
Contributor Information
Carol A. Miller, Department of Communication Sciences and Disorders, The Pennsylvania State University
David A. Wagstaff, HHD Consulting Group, College of Health and Human Development, The Pennsylvania State University
References
- Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: The Bonferroni vs Holm methods. American Journal of Public Health. 1996;86:726–728. doi: 10.2105/ajph.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revision. Washington, DC: Author; 2000. [Google Scholar]
- American Speech-Language-Hearing Association. (Central) Auditory Processing Disorders. 2005 [Technical Report]. Available from http://www.asha.org/docs/html/TR2005-00043.html. [Google Scholar]
- Angerman SK. Doctoral dissertation. 2004. Using the Stroop and Garner effects to assess central auditory function. Retrieved from ProQuest Dissertations and Theses. (Publication Number AAT 3124740) [Google Scholar]
- Archibald LMD, Gathercole SE. The complexities of complex memory span: Storage and processing deficits in specific language impairment. Journal of Memory and Language. 2007;57:177–194. [Google Scholar]
- Archibald LMD, Joanisse MF. On the sensitivity and specificity of nonword repetition and sentence recall to language and memory impairments in children. Journal of Speech, Language, and Hearing Research. 2009;52:899–914. doi: 10.1044/1092-4388(2009/08-0099). [DOI] [PubMed] [Google Scholar]
- Banai K, Kraus N. Neurobiology of (central) auditory processing disorder and language-based learning disability. In: Musiek FE, Chermak GD, editors. Handbook of (central) auditory processing disorder: Vol. I Auditory neuroscience and diagnosis. San Diego: Plural Publishing; 2007. pp. 89–116. [Google Scholar]
- Banai K, Nicol T, Zecker SG, Kraus N. Brainstem timing: implications for cortical processing and literacy. Journal of Neuroscience. 2005;25(43):9850–9857. doi: 10.1523/JNEUROSCI.2373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu M, Krishnan A, Weber-Fox C. Brainstem correlates of temporal auditory processing in children with specific language impairment. Developmental Science. 2009 doi: 10.1111/j.1467-7687.2009.00849.x. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Bavin EL, Wilson PH, Maruff P, Sleeman F. Spatio-visual memory of children with specific language impairment: evidence for generalized processing problems. International Journal of Language and Communication Disorders. 2005;40:319–332. doi: 10.1080/13682820400027750. [DOI] [PubMed] [Google Scholar]
- Bellis TJ. Assessment and management of central auditory processing disorders in the educational setting: From science to practice. 2nd ed. Clifton Park, NY: Thomson Learning; 2003. [Google Scholar]
- Bellis TJ. Interpretation of APD test results. In: Parthasarathy TK, editor. An introduction to auditory processing disorders in children. Mahwah, NJ: Erlbaum; 2006. pp. 145–160. [Google Scholar]
- Bishop DVM. Motor immaturity and specific speech and language impairment: Evidence for a common genetic basis. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2002;114:56–63. doi: 10.1002/ajmg.1630. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Adams CV, Nation K, Rosen S. Perception of transient nonspeech stimuli is normal in specific language impairment: Evidence from glide discrimination. Applied Psycholinguistics. 2005;26:175–194. [Google Scholar]
- Bishop DVM, Carlyon RP, Deeks JM, Bishop SJ. Auditory temporal processing impairment: Neither necessary nor sufficient for causing language impairment in children. Journal of Speech, Language, and Hearing Research. 1999;42:1295–1310. doi: 10.1044/jslhr.4206.1295. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, McArthur GM. Individual differences in auditory processing in specific language impairment: A follow-up study using event-related potentials and behavioural thresholds. Cortex. 2005;41:327–341. doi: 10.1016/s0010-9452(08)70270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken B, McCallum S. Universal Nonverbal Intelligence Test. Itasca, IL: Riverside; 1998. [Google Scholar]
- Cacace AT, McFarland DJ. Central auditory processing disorder in school aged children: A critical review. Journal of Speech, Language, and Hearing Research. 1998;41:355–373. doi: 10.1044/jslhr.4102.355. [DOI] [PubMed] [Google Scholar]
- Cameron S, Brown D, Keith R, Martin J, Watson C, Dillon H. Development of the North American Listening in Spatialized Noise -Sentences Test (NA LiSN-S): Sentence equivalence, normative data, and test-retest reliability studies. Journal of the American Academy of Audiology. 2009;20:128–146. doi: 10.3766/jaaa.20.2.6. [DOI] [PubMed] [Google Scholar]
- Catts HW, Kamhi AG. Language and reading disabilities. 2nd edition. Boston: Pearson; 2005. [Google Scholar]
- Chermak GD, Musiek FE. Central auditory processing disorders: New perspectives. San Diego: Singular; 1997. [Google Scholar]
- Chermak GD, Tucker E, Seikel JA. Behavioral characteristics of auditory processing disorder and attention-deficit hyperactivity disorder: predominantly inattentive type. Journal of the American Academy of Audiology. 2002;13:332–338. [PubMed] [Google Scholar]
- Coady JA, Evans JL. Uses and interpretations of non-word repetition tasks in children with and without specific language impairments (SLI) International Journal of Language and Communication Disorders. 2008;43:1–40. doi: 10.1080/13682820601116485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- Conners CK. Conners’ attention deficit scale-parent version. North Tonawanda, NY: Multi-Health Systems; 1999. [Google Scholar]
- Dawes P, Bishop DVM. Auditory processing disorder in relation to developmental disorders of language, communication and attention: a review and critique. International Journal of Language & Communication Disorders. 2009;44:440–465. doi: 10.1080/13682820902929073. [DOI] [PubMed] [Google Scholar]
- Dawes P, Bishop DVM, Sirimanna T, Bamiou D-E. Profile and aetiology of children diagnosed with auditory processing disorder (APD) International Journal of Pediatric Otorhinolaryngology. 2008;72:483–489. doi: 10.1016/j.ijporl.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Dollaghan C, Campbell TF. Nonword repetition and child language impairment. Journal of Speech, Language, and Hearing Research. 1998;41:1136–1146. doi: 10.1044/jslhr.4105.1136. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody picture vocabulary test-third edition. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Ellis Weismer S. Living on the edge of communicative competence: Processing capacity limitations. Presented at the annual meeting of the American Speech-Language-Hearing Association; Chicago, IL. 2008. Nov, [Google Scholar]
- Emanuel DC. The auditory processing battery: Survey of common practices. Journal of the American Academy of Audiology. 2002;13:93–117. [PubMed] [Google Scholar]
- Everitt BS, Dunn G. Applied multivariate data analysis. 2nd ed. London: Arnold; 2001. [Google Scholar]
- Ferguson MA, Hall RL, Riley A, Moore DR. Communication, listening, cognitive and speech perception skills in children with auditory processing disorder (APD) or specific language impairment (SLI) Journal of Speech, Language, and Hearing Research. 2010 doi: 10.1044/1092-4388(2010/09-0167). Published online. [DOI] [PubMed] [Google Scholar]
- Gaulin CA, Campbell TF. Procedure for assessing verbal working memory in normal school-age children: Some preliminary data. Perceptual and Motor Skills. 1994;79:55–64. doi: 10.2466/pms.1994.79.1.55. [DOI] [PubMed] [Google Scholar]
- Graf Estes K, Evans JL, Else-Quest NM. Differences in the nonword repetition performance of children with and without specific language impairment: A meta-analysis. Journal of Speech, Language, and Hearing Research. 2007;50:177–195. doi: 10.1044/1092-4388(2007/015). [DOI] [PubMed] [Google Scholar]
- Hill EL. Non-specific nature of specific language impairment: A review of the literature with regard to concomitant motor impairments. International Journal of Language and Communication Disorders. 2001;36:149–171. doi: 10.1080/13682820010019874. [DOI] [PubMed] [Google Scholar]
- Hoffman LM, Gillam RB. Verbal and spatial information processing constraints in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2004;47:114–125. doi: 10.1044/1092-4388(2004/011). [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Jerger J. The concept of auditory processing disorder: A brief history. In: Cacace AT, McFarland DJ, editors. Controversies in central auditory processing disorder. San Diego: Plural Publishing; 2009. pp. 1–13. [Google Scholar]
- Jerger J, Musiek F. Report of the consensus conference on the diagnosis of auditory processing disorders in school-aged children. Journal of the American Academy of Audiology. 2000;11:467–474. [PubMed] [Google Scholar]
- Karasinski C, Ellis Weismer S. Comprehension of inferences in discourse processing by adolescents with and without language impairment. Journal of Speech, Language, and Hearing Research. 2010;53:1268–1279. doi: 10.1044/1092-4388(2009/09-0006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J. Central Test Battery. Vancouver, WA: Precision Acoustics; 2001. [Google Scholar]
- Keith RW, Anderson J. Dichotic listening tests. In: Musiek FE, Chermak GD, editors. Handbook of (central) auditory processing disorder: Vol. I Auditory neuroscience and diagnosis. San Diego: Plural Publishing; 2007. pp. 207–230. [Google Scholar]
- Leonard LB. Children with specific language impairment. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- McArthur GM, Atkinson CM, Ellis D. Atypical brain responses to sounds in children with specific reading and language impairments. Developmental Science. 2009;12:768–783. doi: 10.1111/j.1467-7687.2008.00804.x. [DOI] [PubMed] [Google Scholar]
- McArthur GM, Bishop DVM. Speech and non-speech processing in people with specific language impairment: A behavioural and electrophysiological study. Brain and Language. 2005;94:260–273. doi: 10.1016/j.bandl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Miccio A. Clinical problem solving: Assessment of phonological disorders. American Journal of Speech Language Pathology. 2002;11:221–229. [Google Scholar]
- Miller CA, Gilbert E. Comparison of performance on two nonverbal intelligence tests by adolescents with and without language impairment. Journal of Communication Disorders. 2008;41:358–371. doi: 10.1016/j.jcomdis.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JW. Working memory and comprehension in children with specific language impairment: What we know so far. Journal of Communication Disorders. 2003;36:221–231. doi: 10.1016/s0021-9924(03)00021-2. [DOI] [PubMed] [Google Scholar]
- Montgomery JW, Evans JL. Complex sentence comprehension and working memory in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2009;52:269–288. doi: 10.1044/1092-4388(2008/07-0116). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR. Auditory processing disorder (APD): Definition, diagnosis, neural basis, and intervention. Audiological Medicine. 2006;4:4–11. [Google Scholar]
- Musiek FE. Frequency (pitch) and duration pattern tests. Journal of the American Academy of Audiology. 1994;5:265–268. [PubMed] [Google Scholar]
- Musiek F, Gollegly K, Kibbe K, Verkest-Lenz S. Proposed screening test for central auditory disorders: Follow-up on the Dichotic Digits Test. The American Journal of Otology. 1991;12:109–113. [PubMed] [Google Scholar]
- Ramus F. Developmental dyslexia: Specific phonological deficit or general sensorimotor dysfunction? Current Opinion in Neurobiology. 2003;13:212–218. doi: 10.1016/s0959-4388(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Rees NS. Auditory processing factors in language disorders: The view from Procrustes’ bed. Journal of Speech and Hearing Disorders. 1973;38:304–315. doi: 10.1044/jshd.3803.304. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Hynd GW, Cohen M, Hall JW, Molt L. Comorbidity of central auditory processing disorder and attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33(6):849–857. doi: 10.1097/00004583-199407000-00011. [DOI] [PubMed] [Google Scholar]
- Rosen S. Auditory processing in dyslexia and specific language impairment: Is there a deficit? What is its nature? Does it explain anything? Journal of Phonetics. 2003;31:509–527. [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals-fourth edition. San Antonio, TX: PsychCorp; 2003. [Google Scholar]
- Sharma M, Purdy SC, Kelly AS. Comorbidity of auditory processing, language, and reading disorders. Journal of Speech, Language, and Hearing Research. 2009;52:706–722. doi: 10.1044/1092-4388(2008/07-0226). [DOI] [PubMed] [Google Scholar]
- Spaulding TJ, Plante E, Farinella KA. Eligibility criteria for language impairment: Is the low end of normal always appropriate? Language, Speech, and Hearing Services in Schools. 2006;37:61–72. doi: 10.1044/0161-1461(2006/007). [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- Swisher L, Plante E. Nonverbal IQ tests reflect different relations among skills for specifically language-impaired and normal children. Journal of Communication Disorders. 1993;26:65–71. doi: 10.1016/0021-9924(93)90016-4. [DOI] [PubMed] [Google Scholar]
- Swisher L, Plante E, Lowell S. Nonlinguistic deficits of children with language disorders complicate the interpretation of their nonverbal IQ scores. Language, Speech and Hearing Services in Schools. 1994;25:235–240. [Google Scholar]
- Tallal P. Improving language and literacy is a matter of time. Nature Reviews Neuroscience. 2004;5:721–728. doi: 10.1038/nrn1499. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Defects of nonverbal auditory perception in children with developmental aphasia. Nature. 1973a;241:468–469. doi: 10.1038/241468a0. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Developmental aphasia: Impaired rate of non-verbal processing as a function of sensory modality. Neuropsychologia. 1973b;11:389–398. doi: 10.1016/0028-3932(73)90025-0. [DOI] [PubMed] [Google Scholar]
- Thordardottir E. Language-specific effects of task demands on the manifestation of specific language impairment: A comparison of English and Icelandic. Journal of Speech, Language, and Hearing Research. 2008;51:922–937. doi: 10.1044/1092-4388(2008/068). [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Records NL, Zhang X. A system for the diagnosis of specific language impairment in kindergarten children. Journal of Speech and Hearing Research. 1996;39:1284–1294. doi: 10.1044/jshr.3906.1284. [DOI] [PubMed] [Google Scholar]
- Wiederholt JL, Bryant BR. Gray oral reading tests-4th edition. Austin, TX: Pro-Ed; 2001. [Google Scholar]
- Williams KT. Expressive vocabulary test. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III tests of achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]