Abstract
This chapter reviews evidence for functional connections of the somatosensory and auditory systems at the very lowest levels of the nervous system. Neural inputs from the dosal root and trigeminal ganglia, as well as their brain stem nuclei, cuneate, gracillis and trigeminal, terminate in the cochlear nuclei. Terminations are primarily in the shell regions surrounding the cochlear nuclei but some terminals are found in the magnocellular regions of cochlear nucleus. The effects of stimulating these inputs on multisensory integration are shown as short and long-term, both suppressive and enhancing. Evidence that these projections are glutamatergic and are altered after cochlear damage is provided in the light of probable influences on the modulation and generation of tinnitus.
1.1 Introduction
Input from the auditory system can modulate or even determine touch sensation: In a simple experiment Jousmäki and Hari (1998) recorded the sounds made when subjects rubbed their hands. Increasing the high-frequency content of the same sounds played back to the subjects made the skin on their palms feel dry as parchment paper. This so called ‘parchment-skin illusion’ is an impressive example of auditory-somatosensory integration. Proprioceptive and tactile input can similarly influence sound-source lateralization, sound-level discrimination and speech perception (Lewald et al., 1999, Caclin et al., 2002, Schurmann et al., 2004, Ito et al., 2009). However, these multisensory effects are perhaps exemplified by the observation that more than half of people with tinnitus are able to alter the loudness and pitch of their tinnitus via somatic maneuvers such as jaw clenching or tensing their neck muscles (Pinchoff et al., 1998; Levine, 1999). This has has tened the search for neural connections between the two systems that could explain these phenomena (Shore, 2005b, Shore et al., 2007, Dehmel et al., 2008a). Evidence that tinnitus can arise from somatosensory insults makes determination of these connections and their functions all the more important (Rubinstein et al, 1990; Levine, 1999). Bimodal interactions between the somatosensory and auditory systems occur as a result of their extensive anatomical connections at all levels of the auditory pathway (Dehmel et al., 2008b).
1.2 Anatomy of somatosensory inputs to the CN
Anatomical tract-tracing (Shore et al., 2000, Haenggeli et al., 2005, Zhan et al., 2006, Zhou and Shore, 2006) and physiological studies (Kanold and Young, 2001, Shore, 2005) demonstrate auditory connections with the dorsal column and trigeminal systems at the very lowest levels of each sensory system, where cells in the dorsal root- and trigeminal ganglia send axons to terminate in the cochlear nucleus (CN; Fig. 1). These projections, as well as those from the brainstem somatosensory nuclei (cuneate, gracillis and spinal trigeminal) terminate as mossy fibers and en-passant endings primarily in the granule cell domain of the CN that surrounds the VCN and extends into the second layer of the DCN (Zhou and Shore, 2004, Haenggeli et al., 2005, Zhou et al., 2007) (Fig. 2). En-passant endings are also found in magnocellular regions of the VCN and deep DCN. Both mossy-fiber and en-passant endings have been shown to be glutamatergic using vesicular glutamate transporters (VGLUTs)(Herzog et al., 2001, Takamori et al., 2001, Kaneko et al., 2002, Fremeau et al., 2004, Zhou et al., 2007). The two subtypes, VGLUT1 and VGLUT2, show different distributions in the CN (Zhou et al., 2007). VGLUT1 is expressed primarily in the magnocellular regions of the VCN, the deep layer of the DCN and the molecular layer of the DCN, whereas the most intense VGLUT2 labeling is found in the GCD. While auditory nerve fibers exclusively colabel with VGLUT1 (Fig. 3)(Zhou et al., 2007, Zeng et al., 2009), somatosensory inputs from cuneate nucleus and Sp5 colabel primarily with VGLUT2 (Zeng et al., 2011). Both Sp5 and cuneate nucleus terminals concentrate in the GCD with more than 90% of the mossy fiber synapses terminating in the GCD and colabeling with VGLUT2. In contrast to the cuneate projection, many Sp5 small-bouton terminals are also located outside the GCD. These results suggest two functionally-distinct somatosensory pathways to the CN; first, a fast, precisely timed, VGLUT2 positive pathway from Sp5 and cuneate nucleus that activates granule cells consistent with the transmission characteristics of mossy fibers (McBain, 2008); second, a slower pathway that is characterized by small-bouton endings and is widely distributed in the CN. Less than half of Sp5 inputs of this type co-label with VGLUT2, suggesting the existence of non-glutamatergic, excitatory neurotransmitters of this slower pathway. The distinct associations of VGLUT1 with auditory-nerve, and VGLUT2 with non-auditory projections in the CN raises the possibility of functional differences in their roles in synaptic excitation (Gras et al., 2002, Varoqui et al., 2002, Fremeau et al., 2004). Animal models show correlations between changes in VGLUT2 expression and disorders characterized by hyperexcitability such as neuropathic pain and epilepsy (Wallen-Mackenzie et al., 2010).
Figure 1.
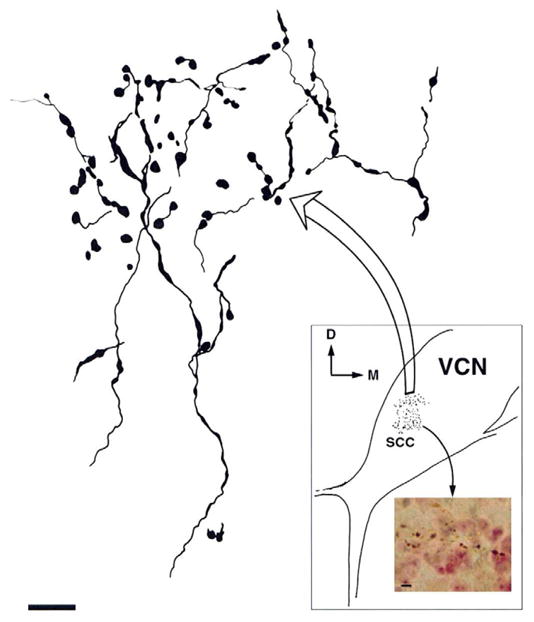
Terminals from thin axons of the trigeminal ganglion end in the small cell cap (SCC) region of the anteroventral cochlear nucleus (AVCN). A transverse section (inset) of the ventral cochlear nucleus (VCN) is drawn, indicating the location of terminals by stippling. Large arrow points to an expanded drawing of some of these terminals at upper left. Small arrow points to photomicrograph at lower right, showing an expanded view of some of the terminals. The axons typically form boutons de passage. D, dorsal; M, medial. Large scale bar = 5 μm; small scale bar = 10 μm. From Shore et al., 2000.
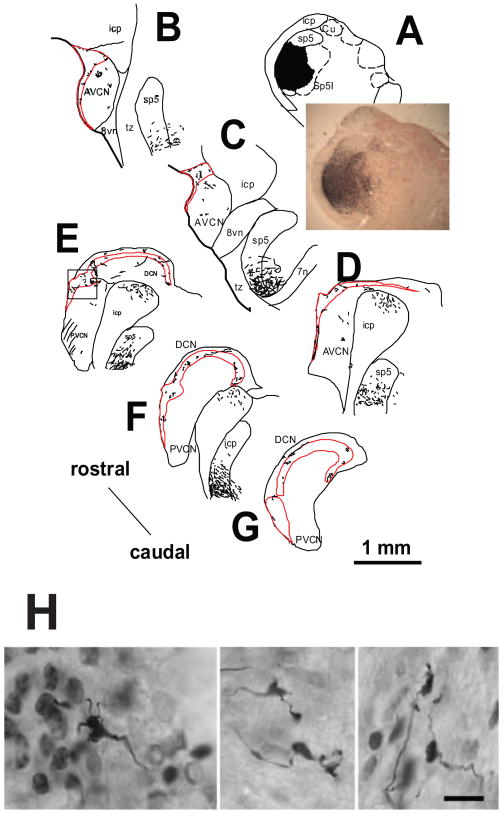
Figure 2.
Reconstruction of anterograde terminal labeling in guinea pig CN.A: BDA injection site in pars interpolaris of Sp5 (Sp5I). B–G: Drawings of transverse sections from the rostral to caudal ends of CN. Each dot represents one or more terminal endings. Thin lines represent labeled fibers. Marginal area and granular cell layers of CN are outlined by redlines. Boxed area in E represents the area of photomicrograph displayed in Figure 3. CN, cochlear nucleus; Cu, cuneate nucleus; AVCN, anteroventral CN; PVCN, posteroventral CN; DCN, dorsal CN; DAS, dorsal acoustic striae; Gr, gracile nucleus; IAS, intermediate acoustic striae; icp, inferior cerebellar peduncle; Sp5, Spinal trigeminal nucleus; sp5, spinal trigeminal tract; Sp5I, pars interpolaris of Sp5; tz, trapezoid body; 7n, facial nerve; 8vn, vestibular nerve.
H. Photomicrographs of labeled rat mossy fibers that are distinguished from terminal boutons by their larger size and lobulated appearance. Filiform appendages that terminate in small swellings are also evident. Mossy fibers from the spinal trigeminal nucleus are found restricted to the GCD. Scale bar = 10 μm. A–G from Zhou and Shore, 2000. H. From Haenggelli et al., 2005.
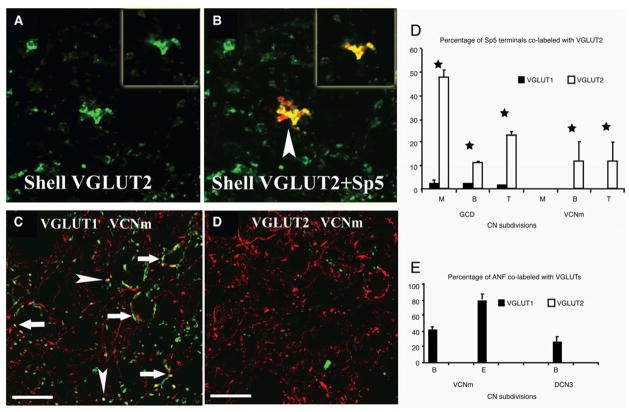
Figure 3.
High-magnification confocal images (×63) showing colocalization of anterogradely labeled Sp5 terminal endings with VGLUT2-ir in the CN small cell cap (SCC). Green, VGLUT-ir; red, Sp5 labeling; yellow, double-labeled terminals. A–D were obtained from Z projections of stacks of serial 1-μm confocal images and each show a single, 1-μm confocal image. Insets in AB show a single 1-μm confocal image. A–B: MFs are labeled with VGLUT2 (A) and BDA from Sp5. Colocalization of Sp5 MFs with VGLUT2-ir is indicated by arrowhead in B. C–D: High-magnification confocal images showing colocalization of anterogradely labeled VIII th nerve terminal endings with VGLUT1-ir in AVCNm. VIII th nerve terminals are labeled with BDA injected into the cochlea. Colocalization of VIII th nerve terminals with VGLUT1-ir is indicated by arrows in in C. Note no co-localization of VIII th nerve terminals with VGLUT2 (D). Scale bar = 10 μm. Green, VGLUT, yellow, double-labeled terminals. E–F: Percentages of Sp5 and ANF terminals labeled with VGLUTs. Stars represent significant differences (p < .1; bars represent standard errors of the mean). From Zhou et al., 2007.
1.2 Physiology of somatosensory inputs to the CN
Stimulating the trigeminal ganglion in the absence of sound produces primarily excitation of VCN neurons (Shore et al., 2003) and both excitation and inhibition in DCN neurons, with inhibition presumably arising from cartwheel cells (Davis et al., 1996; Shore, 2005). The locations and response patterns of units responding to trigeminal stimulation are consistent with those of fusiform (Shore, 2005a, Kanold et al., 2011) or giant cells (Hackney et al., 1990) in the DCN, and bushy or stellate cells in the VCN (Shore et al., 2003). Importantly, these studies show that preceding an acoustic stimulus with somatosensory stimulation can modulate both the firing rates and temporal response patterns to the sound (Shore, 2005a, Koehler et al., 2011). Preceding sound stimulation with electrical stimulation of the TG resulted in bimodal suppression in the majority of units, and bimodal enhancement in fewer units (Shore, 2005a), similar to that seen with MSN stimulation (Fig. 4). Similarly, as seen with MSN stimulation, the bimodal integration lasted throughout the sound-evoked response (Fig. 4). Sp5 stimulation (Koehler et al., 2011), on the other hand, resulted in approximately equal numbers of units with bimodal suppression and enhancement that were segregated according to their sound-evoked response type: chopper units showed bimodal enhancement whereas buildup units showed bimodal suppression.
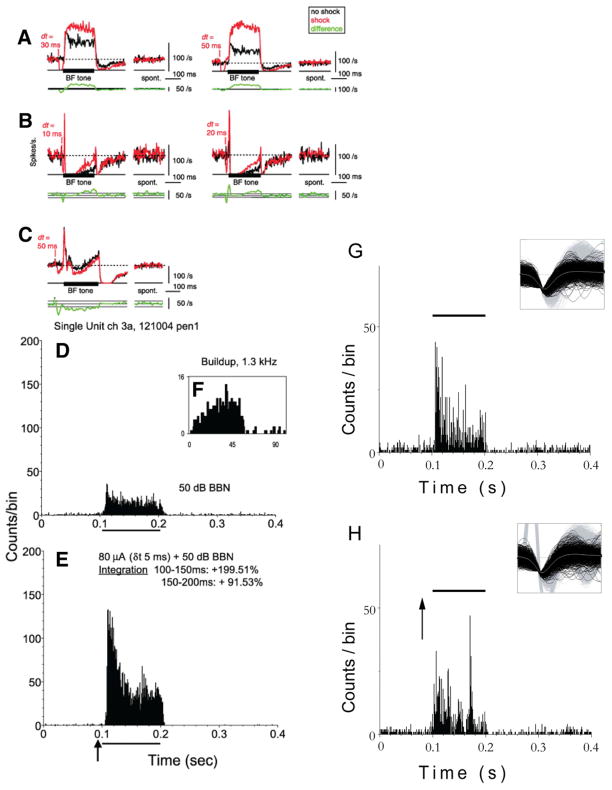
Figure 4.
Long-term changes in the tone-evoked firing rate after dorsal column(DCoN) stimulation. Examples from 3 neurons in which the effects of a DCoN stimulus pulse lasted for the duration of the 200 ms acoustic stimulus. A:DCoN stimulation at 2 delta-t values increased the tone-evoked firing rate of a tonic neuron by 41.9 and 108.1%, respectively (top traces); compare the black control PSTHs with the red with-shock PSTHs. Small or no differences occurred in the spontaneous rates (“spont”). To quantify the significance of rate changes, the rate differences (with-shock minus no-shock) are plotted below (bottom green traces, smoothed with 5-bin triangular filter) together with horizontal lines showing ±1 SD of the spontaneous rate from the PSTHs. Significant rate changes are present where the rate differences are outside the ±1 SD area. Note that large rate differences persist for the duration of the stimulus. B: Apauser neuron in which DCoN stimulation also increased the tone-evoked firing rate by 125 and 146.5%, respectively. Also note the increase in the onset peak at dt = 20 ms. C: pauser response in which the long-term effect was a decrease in discharge rate. For this case, the shock and no-shock trials were interleaved on alternating trials, as opposed to A and B where they were interleaved with other stimuli in 100-repetition sets. PSTHs (except rate differences) were not smoothed. D-E: Bimodal enhancement in a single unit from the DCN of aguinea pig: Responses of a single unit to broadband noise (BBN) alone, and preceded by trigeminal ganglion stimulation are shown in D and E, respectively. The response to combined trigeminal and acoustic stimulation is larger than the response to sound stimulation alone, indicating enhancing bimodal integration. F–G: Bimodal suppression: Responses of a single unit to broadband noise (BBN) alone, and preceded by trigeminal ganglion stimulation are shown in F and G, respectively. The response to combined trigeminal and acoustic stimulation in this case is smaller than the response to sound stimulation alone, indicating suppressive bimodal integration that lasted throughout the duration of the sound-evoked response. Responses of both units to BF tones were buildup. BBN level, 50 dB SPL (30 dB SL); current, 80 μA. Trigeminal stimulus precedes BBN by 5 ms. Solid bar above graphs shows the onset and duration of the BBN; arrow indicates onset of the bipolar trigeminal pulse (100 μs/phase). Bin width, 1 ms. An example of the magnitude of this effect is shown as percent integration in E. Insets to panels G and H represent the unit waveforms for these two units. From Shore, 2005. From: Kanold et al., 2011 (A–C); Shore 2005 (D–F).
For both TG and Sp5 stimulation the number of units showing bimodal integration was greater than the number of units showing unimodal responses: 41% of units responding to TG stimulation versus 78% of units with bimodal integration (Shore, 2005a) and 69% of units responding to Sp5 stimulation versus 79% of units with bimodal integration (Koehler et al., 2011). This is indicative of a subthreshold somatosensory response to the somatosensory stimulation that modifies sound-evoked responses and can be detected only during bimodal stimulation. Even though the effect of Sp5 stimulation alone was predominantly excitatory, the effects during bimodal integration were equally suppressive and enhancing (Shore, 2005a, Koehler et al., 2011). In those units that showed bimodal suppression, subthreshold activation of inhibitory interneurons would be necessary for the observed effects, as well as for the bimodal suppression after dorsal column stimulation (Kanold et al., 2010).
Sp5 and TG stimulation can also alter the timing of sound-evoked responses (Koehler et al., 2011). A preceding Sp5 stimulus can increase or decrease the regularity of firing, which manifests as a change in the amount of chopping and the consistency of the first interspike interval (Fig. 5). First spike latencies increased in units that showed bimodal suppression, consistent with inhibition by an interneuron. The changes in temporal patterns after bimodalSp5 and sound stimulation might be caused by alterations in K+ currents, as in vitro experiments show that small depolarizing or hyperpolarizing currents before a depolarizing pulse can alter the regularity and latency of pyramidal cells’ responses (Kanold and Manis, 1999, 2001, 2005). In addition to alteration of spike timing following somatosensory stimulation (Koehler et al., 2011) changes in synchrony of firing between neurons in the DCN can occur (O’Donahue et al., 2010), an additional proposed correlate of tinnitus (Eggermont, 2005)
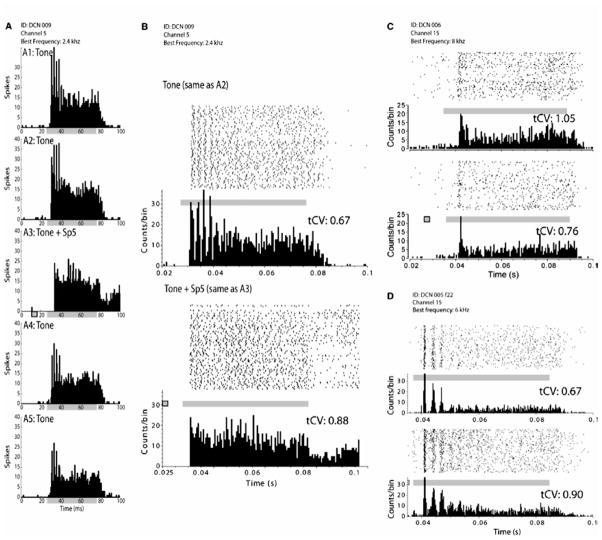
Figure 5.
Spinal trigeminal nucleus (Sp5) stimulation changes firing rate and regularity in dorsal cochlear nucleus (DCN) presumed pyramidal cells. Firing rate is suppressed and regularity of the acoustic response is decreased when sound is preceded by Sp5 stimulation. (A, A1 and A2) Identical responses of a chopper unit response to BF tones are shown prior to bimodal stimulation. (A3) Bimodal response showing suppressive integration. (A4 and A5) Partially recovered acoustic responses at 5 and 10 min following the collection of bimodal responses. (B) Raster plot and PSTH of a chopper unit response to BF tones (top, same as A2) and BF tones preceded by Sp5 stimulation (bottom, same as A3). (C) Raster plot and PSTH of a pauser unit response to BF tones (top) and BF tones preceded by Sp5 stimulation (bottom). (D) Raster plot and PSTH of a chopper unit response to BF tones (top) and BF tones preceded by Sp5 stimulation (bottom). Each PSTH is composed of 200 trials. In each raster plot, each point represents a spike and each row represents a single stimulus trial. The bottom row is the first trial. Solid gray bars indicate the duration of the acoustic stimulus. Gray bars with black borders indicate the duration of electrical stimulation of Sp5. The average value of the transient CV (tCV) is indicated above each response in (B), (C) and (D). From Koehler et al., 2011.
The bimodal integration evident in CN neurons is replicated in neurons of the IC (Jain and Shore, 2006), which receive converging inputs from both the DCN and somatosensory nuclei (Zhou and Shore, 2006). Somatosensory stimulation can affect both sound-driven and spontaneous rates for long periods of time (up to an hour) following cessation of the stimulation, a phenomenon that may be due to long term potentiation or depression (Tzounopoulos et al., 2007)..
2.1 Role of the DCN in Tinnitus
Cochlear damage, known to induce tinnitus, induces increased spontaneous firing rates in neurons in both the dorsal and ventral cochlear nucleus (DCN; VCN) (Kaltenbach and McCaslin, 1996, Bledsoe et al., 2009). Increased spontaneous rates in the DCN are observed primarily in fusiform cells, the principal output neurons (Brozoski et al., 2002, Shore et al., 2008, Finlayson and Kaltenbach, 2009), but may also be found in the inhibitory interneurons, cartwheel cells. Elevated spontaneous rates that follow hearing damage are usually confined to a restricted region of the tonotopic axis related to the region of cochlear damage and are usually maximal at frequencies above the traumatizing frequency (Kaltenbach and Godfrey, 2008). This parallels results of psychophysical studies in humans in which the tinnitus frequency is correlated with the edge frequency of the audiogram, the frequency of greatest threshold shift or the frequency range of the hearing loss (Loeb and Smith, 1967, Lockwood et al., 2002, Norena et al., 2002, Eggermont and Roberts, 2004, Weisz et al., 2006, Schaette and Kempter, 2009, Moore et al., 2010). The profile of increased spontaneous rate is wider than the profile for the response to sound, consistent with a narrow or wideband, rather than a pure tone tinnitus percept. Increases in spontaneous rates develop at different times in specific auditory structures (for example, more rapidly in VCN than in DCN) (van Heusden and Smoorenburg, 1983, Kaltenbach and Afman, 2000, Bledsoe et al., 2009)and may reflect mechanisms that change over time, since elevations in the DCN spontaneous rates survive cochlear ablation (Zacharek et al., 2002) but those in IC do not (Mulders and Robertson, 2009).
Increased burst-firing has been identified In DCN following noise exposure that could account for about 50% of SRF increases seen in this structure (Finlayson and Kaltenbach, 2009). While this suggests increased spontaneous rates in cartwheel cells, which fire complex spikes under normal conditions, it is not clear whether the increased bursting corresponds to increased cartwheel cell activity or increased burst firing in fusiform cells.
Changes in the neural synchrony in auditory pathways in animal models (Seki and Eggermont, 2003), as another physiological correlate of tinnitus, correspond closely to the frequency profile of hearing loss and tinnitus in humans(Norena et al., 2002, Roberts et al., 2008). Increased neural synchrony in the hearing loss regions in auditory cortex and inferior colliculus corresponds well with the tinnitus spectrum. The presence of tonotopically restricted hyperactivity, bursting activity, and elevated neural synchrony in this early brainstem nucleus indicates that the DCN could convey already-formed neural patterns representing tinnitus to higher auditory nuclei such as IC and auditory cortex.
One mechanism for the increased spontaneous rates could be a reduction in inhibitory inputs to the fusiform cells (Salvi et al., 2000), or changes in glycine receptors (see above) unmasking the excitability of the fusiform cells (Caspary et al., 1987, Wang et al., 2009). Another mechanism, however, could be an increase in excitatory inputs to the CN from the somatosensory system after noise damage (Zeng et al., 2009).
2.2 Changes in Somatosensory innervation to the CN after cochlear damage
Multisensory neurons in general have a propensity for receiving cross-modal compensation following sensory deprivation (Allman et al., 2009). This susceptibility is manifest in the CN as an increase in the number of VGLUT2-positive terminals in CN regions that receive somatosensory inputs. In contrast, the number of VGLUT1-positive terminals decreased (Fig. 6). Together, these changes signify an enhanced somatosensory influence on the CN after auditory nerve denervation of the CN (Zeng et al., 2009). This altered balance of inputs from auditory and somatosensory structures affects bimodal integration, imparting greater strength to the somatosensory inputs. One physiological consequence of the increased number of VGLUT2- positive inputs is that DCN neurons become more responsive to somatosensory stimulation following cochlear damage (Shore et al., 2008). Guinea pigs with noise-induced hearing loss demonstrated significantly lower thresholds, shorter latencies and durations, and increased response amplitudes to TG stimulation than normal animals. Furthermore, the number of units exhibiting bimodal integration, as well as the degree of integration, was also enhanced after noise damage (Shore et al., 2008). Together with a higher proportion of inhibitory unimodal responses, bimodal integration was mainly suppressive in the noise-damaged animals, suggesting that projections from the TG to the CN are increased and/or redistributed to favor inhibitory interneurons after hearing loss (Shore et al., 2008). For Sp5, this redistribution appears to favor excitatory neurons, since bimodal enhancement predominates after noise damage (Dehmel et al., 2011).
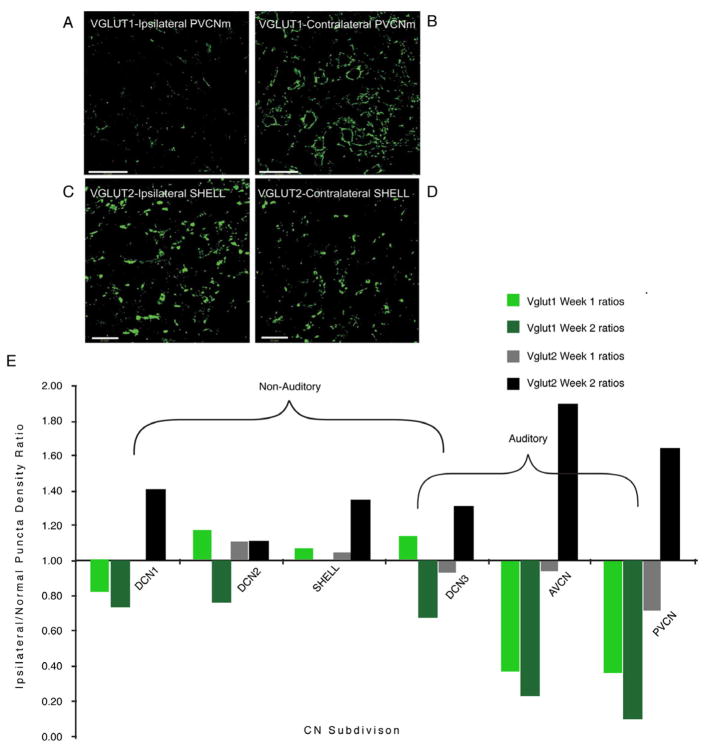
Figure 6.
VGLUT1-ir is decreased, whereas VGLUT2 is increased in the CN ipsilateral to the cochlear damage. Photomicrographs of VGLUT1-ir from the VCNm of one animal contralateral (A) and ipsilateral (B) to the cochlear damage, 2 weeks after kanamycin injections into the left cochlea. VGLUT1 is strongly expressed in PVCNm on the control side (B) as previously shown (Zhou et al., 2007) but weakly expressed ipsilateral to the deafening (A). The decrease in VGLUT1-ir reflects primarily decreased VIIIth nerve synaptic inputs after kanamycin injections into the cochlea. C,D, Photomicrographs of VGLUT2-ir from SHELL regions of CN. VGLUT2-ir in the ipsilateral SHELL region is increased ipsilateral (C) to the cochlear damage compared with the contralateral side (D). Scale bars: A–B, 50 μm; C,D20 μm. E. shows ratio plots (after deafening/before deafening) for VGLUT1 and VGLUT2 across CN regions. From Zeng et al., 2009.
The idea that an altered balance between auditory nerve and somatosensory inputs could result in tinnitus is exemplified by the finding that increased spontaneous rates after noise exposure are confined to those DCN fusiform cells that show an excitatory response to somatosensory stimulation (Shore et al., 2008). Units that did not respond to, or were inhibited by somatosensory stimulation did not show increased spontaneous rates after cochlear damage (Fig. 7). This suggests that tinnitus may be generated by a restricted group of neurons (Bauer et al., 2008). Consistent with strengthened somatosensory inputs to the CN shown by Zeng et al., (2009) and Shore et al., (2008), FMRI results showed that jaw movements evoked more activity in the CNs of tinnitus subjects than in non-tinnitus subjects (Fig. 5; (Lanting et al., 2010).
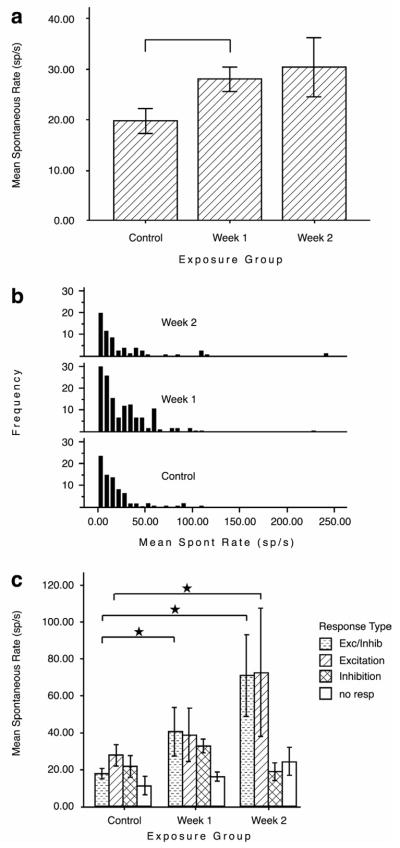
Figure 7.
(A) Mean spontaneous rates (SRs) for dorsal cochlear nucleus single units at 1 and 2 weeks after noise exposure at 120 dB SPL. SR is significantly higher at 1 week following exposure (Bonferroni-adjusted comparison; *P < 0.05). (B) Frequency distribution plots indicate that the increased SR is accounted for mostly by an increase in the number of medium SR values. (C) The distribution of SRs by responses to trigeminal stimulation indicates that only units that are activated by trigeminal stimulation (those that display excitatory and excitatory/inhibitory responses) showed increased SRs after noise exposure. Units that were inhibited by trigeminal stimulation and units that did not respond to trigeminal stimulation did not show increased SR after noise damage. Error bars are standard errors of the mean. From Shore et al., (2008).
Increased spontaneous rates in fusiform cells may also result from changes in synaptic plasticity at parallel fiber-fusiform synapses and parallel-fiber cartwheel cell synapses (Fujino and Oertel, 2003, Tzounopoulos et al., 2004, Tzounopoulos et al., 2007). Activation of the granule cell-cartwheel cell network by paired tones can lead to a plasticity-dependent reduction in the response to that sound in a DCN circuit model (Roberts et al., 2006) while pinna stimulation can lead to a reduction in DCN spontaneous firing rates for minutes (Zhang and Guan, 2008).
One consequence of increased spontaneous rate in specified groups of neurons is increased synchrony of firing between neurons (Seki and Eggermont, 2003), which has also been reported in the rat DCN after noise damage (O’Donahue et al., 2010). Synchrony in one region can be transmitted with high fidelity to other brain centers (Masuda and Kori, 2007, Takahashi et al., 2009), and may be one mechanism by which the cortical synchrony reported above occurs.
Summary
There is accumulating evidence that plastic changes triggered by insults to either the somatosensory or auditory input pathways to the DCN lead to compensatory shifts in the balance of excitation and inhibition. This imbalance is reflected in the upregulation of glutamatergic inputs from somatosensory pathways after deafening, the increased sensitivity of DCN neurons to somatosensory stimuli and, as a consequence, the increased spontaneous rates of a restricted group of neurons that are excited by those somatosensory inputs. Accompanying downregulation of glycinergic transmission would further shift this balance towards excitation. The increased sensitivity to somatosensory inputs has also been verified in an imaging study with tinnitus patients.
The notion of increased spontaneous rate as a neuronal correlate of tinnitus derives from the correlation between increased spontaneous rate in animal models after cochlear damage that causes tinnitus. However, the correspondence in animal and human models between behaviorally verified tinnitus spectra and auditory insults, with the sites of increased spontaneous rates, need further exploration. Tinnitus correlates of changes in temporal firing properties such as synchrony have been less extensively studied in the DCN; however, this might be an important tinnitus correlate that effectively triggers increased activity to higher stages of the auditory pathway. In this context, studies showing that somatosensory inputs alter the timing of sound-evoked responses as well as firing rates of DCN neurons are important. How the somatosensory influence on spike timing is altered in animals with behaviorally confirmed tinnitus will provide insight into the importance of DCN spike-timing in tinnitus. The somatosensory influence on spike rate and timing shown in animal experiments is mirrored in the ability of patients to modify the loudness as well as the pitch of their tinnitus by somatic maneuvers.
Research Highlights.
The cochlear nucleus receives non-auditory as well as auditory inputs
Many of these inputs arise in the somatosensory system
Inputs to the cochlear nucleus from the somatosensory system arise in both trigeminal and dorsal column systems
Somatosensory inputs to the cochlear nucleus are glutamatergic
Somatosensory inputs to the cochlear nucleus are enhanced after cochlear damage and may lead to hyperactivity that could lead to tinnitus
Acknowledgments
This work was supported by NIH Grants NIDCD R01 004825 and P30 DC 05188 and the Tinnitus Research Consortium and the Tinnitus Research Initiative. We thank Ben Yates for expert graphical assistance.
Abbreviations
- CN
cochlear nucleus
- VCN
ventralcochlear nucleus
- DCN
dorsalcochlear nucleus
- GCD
Granule cell domain
- VGLUTs
vesicular glutamate transporters
- VGLUT2
vesicular glutamate transporters 2
- VGLUT1
vesicular glutamate transporter 1
- Sp5
Spinal trigeminal nucleus
- IC
Inferior colliculus
- DCoN
dorsal column
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allman BL, Keniston LP, Meredith MA. Adult deafness induces somatosensory conversion of ferret auditory cortex. Proc Natl Acad Sci U S A. 2009;106:5925–5930. doi: 10.1073/pnas.0809483106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008 doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe SC, Jr, Koehler S, Tucci DL, Zhou J, Le Prell C, Shore SE. Ventral cochlear nucleus responses to contralateral sound are mediated by commissural and olivocochlear pathways. J Neurophysiol. 2009;102:886–900. doi: 10.1152/jn.91003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caclin A, Soto-Faraco S, Kingstone A, Spence C. Tactile “capture” of audition. Percept Psychophys. 2002;64:616–630. doi: 10.3758/bf03194730. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Pazara KE, Kossl M, Faingold CL. Strychnine alters the fusiform cell output from the dorsal cochlear nucleus. Brain Res. 1987:273–82. 417. doi: 10.1016/0006-8993(87)90452-5. [DOI] [PubMed] [Google Scholar]
- Dehmel S, Cui Y, Shore S. Cross-modal Interactions of auditory and somatic inputs in the brainstem and midbrain and their imbalance in tinnitus and deafness. American Journal of Audiology. 2008a;17:193–209. doi: 10.1044/1059-0889(2008/07-0045). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmel S, Cui YL, Shore SE. Cross-modal interactions of auditory and somatic inputs in the brainstem and midbrain and their imbalance in tinnitus and deafness. Am J Audiol. 2008b;17:S193–209. doi: 10.1044/1059-0889(2008/07-0045). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmel S, Pradhan S, Parikh M, Anderson K, Shore SE. Long term effects of somatosensory inputs on neuronal discharges in the dorsal cochlear nucleus of neormal and noise-exposed guinea pigs. Abstracts of the association for research in otolaryngology. 2011;34:437. [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Finlayson PG, Kaltenbach JA. Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hear Res. 2009;256:104–117. doi: 10.1016/j.heares.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proc Natl Acad Sci U S A. 2003;100:265–270. doi: 10.1073/pnas.0135345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney CM, Osen KK, Kolston J. Anatomy of the cochlear nuclear complex of guinea pig. Anat Embryol (Berl) 1990;182:123–149. doi: 10.1007/BF00174013. [DOI] [PubMed] [Google Scholar]
- Haenggeli CA, Pongstaporn T, Doucet JR, Ryugo DK. Projections from the spinal trigeminal nucleus to the cochlear nucleus in the rat. J Comp Neurol. 2005;484:191–205. doi: 10.1002/cne.20466. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Tiede M, Ostry DJ. Somatosensory function in speech perception. Proc Natl Acad Sci U S A. 2009;106:1245–1248. doi: 10.1073/pnas.0810063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Shore S. External inferior colliculus integrates trigeminal and acoustic information: unit responses to trigeminal nucleus and acoustic stimulation in the guinea pig. Neurosci Lett. 2006;395:71–75. doi: 10.1016/j.neulet.2005.10.077. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000;140:165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA. Dorsal cochlear nucleus hyperactivity and tinnitus: are they related? Am J Audiol. 2008;17:S148–161. doi: 10.1044/1059-0889(2008/08-0004). [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, McCaslin DL. Increases in spontaneous activity in the dorsal cochlear nucleus following exposure to high intensity sound: A possible neural correlate of tinnitus. Audit Neurosci. 1996;3:57–78. [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Davis KA, Young ED. Somatosensory context alters auditory responses in the cochlear nucleus. J Neurophysiol. 2010 doi: 10.1152/jn.00807.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Davis KA, Young ED. Somatosensory context alters auditory responses in the cochlear nucleus. J Neurophysiol. 2011 doi: 10.1152/jn.00807.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Manis PB. Transient potassium currents regulate the discharge patterns of dorsal cochlear nucleus pyramidal cells. J Neurosci. 1999;19:2195–2208. doi: 10.1523/JNEUROSCI.19-06-02195.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Manis PB. A physiologically based model of discharge pattern regulation by transient K+ currents in cochlear nucleus pyramidal cells. J Neurophysiol. 2001;85:523–538. doi: 10.1152/jn.2001.85.2.523. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Manis PB. Encoding the timing of inhibitory inputs. J Neurophysiol. 2005;93:2887–2897. doi: 10.1152/jn.00910.2004. [DOI] [PubMed] [Google Scholar]
- Koehler SD, Pradhan S, Manis PB, Shore SE. Somatosensory inputs modify auditory spike timing in dorsal cochlear nucleus principal cells. Eur J Neurosci. 2011;33:409–420. doi: 10.1111/j.1460-9568.2010.07547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting CP, de Kleine E, Eppinga RN, van Dijk P. Neural correlates of human somatosensory integration in tinnitus. Hear Res. 2010;267:78–88. doi: 10.1016/j.heares.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Lewald J, Karnath HO, Ehrenstein WH. Neck-proprioceptive influence on auditory lateralization. Exp Brain Res. 1999;125:389–396. doi: 10.1007/s002210050695. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med. 2002;347:904–910. doi: 10.1056/NEJMra013395. [DOI] [PubMed] [Google Scholar]
- Loeb M, Smith RP. Relation of induced tinnitus to physical characteristics of the inducing stimuli. J Acoust Soc Am. 1967;42:453–455. doi: 10.1121/1.1910600. [DOI] [PubMed] [Google Scholar]
- Masuda N, Kori H. Formation of feedforward networks and frequency synchrony by spike-timing-dependent plasticity. J Comput Neurosci. 2007;22:327–345. doi: 10.1007/s10827-007-0022-1. [DOI] [PubMed] [Google Scholar]
- McBain CJ. Differential mechanisms of transmission and plasticity at mossy fiber synapses. Prog Brain Res. 2008;169:225–240. doi: 10.1016/S0079-6123(07)00013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BC, Vinay, Sandhya The relationship between tinnitus pitch and the edge frequency of the audiogram in individuals with hearing impairment and tonal tinnitus. Hear Res. 2010;261:51–56. doi: 10.1016/j.heares.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience. 2009;164:733–746. doi: 10.1016/j.neuroscience.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Norena A, Micheyl C, Chery-Croze S, Collet L. Psychoacoustic characterization of the tinnitus spectrum: implications for the underlying mechanisms of tinnitus. Audiol Neurootol. 2002;7:358–369. doi: 10.1159/000066156. [DOI] [PubMed] [Google Scholar]
- O’Donahue H, Campagnloa L, Manis PB. Spontaneous Calcium Signals in the Dorsal Cochlear nucleus after noise damage. ARO. 2010;33:240. [Google Scholar]
- Roberts LE, Moffat G, Baumann M, Ward LM, Bosnyak DJ. Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. J Assoc Res Otolaryngol. 2008;9:417–435. doi: 10.1007/s10162-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts PD, Portfors CV, Sawtell N, Felix R. Model of auditory prediction in the dorsal cochlear nucleus via spike-timing dependent plasticity. Neurocomputing. 2006;69:1191–1194. [Google Scholar]
- Schaette R, Kempter R. Predicting tinnitus pitch from patients’ audiograms with a computational model for the development of neuronal hyperactivity. J Neurophysiol. 2009;101:3042–3052. doi: 10.1152/jn.91256.2008. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Caetano G, Jousmaki V, Hari R. Hands help hearing: facilitatory audiotactile interaction at low sound-intensity levels. J Acoust Soc Am. 2004;115:830–832. doi: 10.1121/1.1639909. [DOI] [PubMed] [Google Scholar]
- Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180:28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Shore S, Zhou J, Koehler S. Neural mechanisms underlying somatic tinnitus. Prog Brain Res. 2007;166:107–123. doi: 10.1016/S0079-6123(07)66010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE. Multisensory integration in the dorsal cochlear nucleus: unit responses to acoustic and trigeminal ganglion stimulation. Eur J Neurosci. 2005a;21:3334–3348. doi: 10.1111/j.1460-9568.2005.04142.x. [DOI] [PubMed] [Google Scholar]
- Shore SE. Sensory Nuclei in Tinnitus. In: Snow J, editor. Tinnitus: Theory and Management. Hamilton, London: BC Decker Inc; 2005b. pp. 125–141. [Google Scholar]
- Shore SE, El-Kashlan HK, Lu J. Effects of trigeminal ganglion stimulation on unit activity of ventral cochlear nucleus neurons. Neuroscience. 2003;119:1085–1101. doi: 10.1016/s0306-4522(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci. 2008;27:155–168. doi: 10.1111/j.1460-9568.2007.05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Vass Z, Wys NL, Altschuler RA. Trigeminal ganglion innervates the auditory brainstem. J Comp Neurol. 2000;419:271–285. doi: 10.1002/(sici)1096-9861(20000410)419:3<271::aid-cne1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Kori H, Masuda N. Self-organization of feed-forward structure and entrainment in excitatory neural networks with spike-timing-dependent plasticity. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;79:051904. doi: 10.1103/PhysRevE.79.051904. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2) J Neurosci. 2001;21:RC182. doi: 10.1523/JNEUROSCI.21-22-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heusden E, Smoorenburg GF. Responses from AVCN units in the cat before and after inducement of an acute noise trauma. Hear Res. 1983;11:295–326. doi: 10.1016/0378-5955(83)90064-3. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schäfer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen-Mackenzie A, Wootz H, Englund H. Genetic inactivation of the vesicular glutamate transporter 2 (VGLUT2) in the mouse: what have we learnt about functional glutamatergic neurotransmission? Ups J Med Sci. 2010;115:11–20. doi: 10.3109/03009730903572073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N, Hartmann T, Dohrmann K, Schlee W, Norena A. High-frequency tinnitus without hearing loss does not mean absence of deafferentation. Hear Res. 2006;222:108–114. doi: 10.1016/j.heares.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Zacharek MA, Kaltenbach JA, Mathog TA, Zhang J. Effects of cochlear ablation on noise induced hyperactivity in the hamster dorsal cochlear nucleus: implications for the origin of noise induced tinnitus. Hear Res. 2002;172:137–143. doi: 10.1016/s0378-5955(02)00575-0. [DOI] [PubMed] [Google Scholar]
- Zeng C, Nannapaneni N, Zhou J, Hughes LF, Shore S. Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus. J Neurosci. 2009;29:4210–4217. doi: 10.1523/JNEUROSCI.0208-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Shroff H, Shore SE. Cuneate and spinal trigeminal nucleus projections to the cochlear nucleus are differentially associated with vesicular glutamate transporter-2. Neuroscience. 2011;176:142–151. doi: 10.1016/j.neuroscience.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Pongstaporn T, Ryugo DK. Projections of the second cervical dorsal root ganglion to the cochlear nucleus in rats. J Comp Neurol. 2006;496:335–348. doi: 10.1002/cne.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Guan Z. Modulatory effects of somatosensory electrical stimulation on neural activity of the dorsal cochlear nucleus of hamsters. J Neurosci Res. 2008;86:1178–1187. doi: 10.1002/jnr.21560. [DOI] [PubMed] [Google Scholar]
- Zhou J, Nannapaneni N, Shore S. Vessicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol. 2007;500:777–787. doi: 10.1002/cne.21208. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Projections from the trigeminal nuclear complex to the cochlear nuclei: a retrograde and anterograde tracing study in the guinea pig. J Neurosci Res. 2004;78:901–907. doi: 10.1002/jnr.20343. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol. 2006;495:100–112. doi: 10.1002/cne.20863. [DOI] [PubMed] [Google Scholar]