Abstract
Objective
Antibodies towards the citrullinated form of the synovial antigen vimentin are specific for rheumatoid arthritis (RA) and associated with HLADRB1*0401. This suggests that T cells specific for peptides derived from citrullinated-vimentin presented in the context of HLA-DRB1*0401 may contribute to the etiopathogenesis of RA. Here, we aimed to identify immunodominant epitopes from citrullinated-vimentin presented by HLADRB1*0401 and characterize the resulting T cell responses.
Methods
We first predicted an HLA-binding T cell epitope from citrullinated vimentin based on the binding motif of DRB1*0401, and then confirmed its affinity. An MHC class-II tetramer loaded with cit-vim59-78 was constructed and used to screen for specific T cells in DRB1*0401 transgenic mice, RA patients and healthy controls. Additionally, the cytokine output following cit-vim59-78 challenge was analyzed in patients and healthy subjects by multicolor flow cytometry and luminex-based analysis.
Results
The citrullinated form of vim59-78 bound to HLA-DRB1*0401 while the native version could not. Subsequently, cit-vim59-78-specific T cells were detected in immunized mice and in the periphery of both HLA-DR*0401 healthy controls and RA subjects, using MHC class-II tetramers, CD154 upregulation and intracellular cytokine measurements. Cell culture supernatants demonstrated an enhanced production of cytokines, most prominent of which was IFNγ from RA-derived cells in response to cit-vim59-78 in comparison to healthy controls.
Conclusions
Here, we describe a posttranslational modification of an RA candidate autoantigen towards which DRB1*0401-restricted, T cells can be found in both RA patients and healthy controls, but for which a proinflammatory response is uniquely found among subjects with RA.
INTRODUCTION
A role for CD4 T cells in rheumatoid arthritis (RA) is supported by the accumulation of activated CD4 T cells in the inflamed joint and by adoptive transfer experiments of T cells in animal models of RA (1, 2). A profound genetic association with several HLA-DRB1 alleles, and to a lesser extent other genes related to adaptive immunity, also supports this notion (3, 4). All RA-associated HLA-DRB1 alleles contain a similar 5aa sequence in the peptide binding groove of the third hyper-variable region of the DRB1 molecule, known as the “shared epitope” (SE) (5). Accordingly, autoreactive-CD4 T cells recognizing similar antigens in the context of MHC class-II on DRB1*SE+ molecules may initiate and maintain autoimmune mechanisms involved in RA pathogenesis.
Citrullination is the posttranslational modification of a positively charged arginine residues to a neutrally charged citrulline. The peptidyl arginine deiminase (PAD) enzymes responsible for this are upregulated under inflammatory conditions (6, 7). Antibodies generated against citrullinated proteins (anti-citrullinated protein antibodies (ACPAs)) are present in 60-70% of RA patients’ sera (8, 9), can be detected up to ten years before disease onset (10, 11), and their production is tightly linked with the HLA-SE alleles (12, 13). DRB1*0401 and DRB1*0404, in particular, are associated with distinctly high levels of antibodies toward multiple citrullinated synovial antigens (14, 15). While the pathophysiological nature of anti-citrulline immunity is still poorly understood, the greater preponderance of ACPAs detected in synovial fluid in comparison to sera from the same patients raises the possibility of local production of ACPAs at the inflammatory site (15). Taken as a whole, these data suggest a possible pathogenic role for ACPA in RA.
Since ACPAs undergo isotype switching (16), their production is expected to be T cell dependent. Indeed, T cells can recognize citrullinated antigens in the context of MHC class-II (17, 18). Additionally, Hill et al. elegantly demonstrated that the posttranslational modification of an arginine to a citrulline allows citrullinated synovial antigens to preferentially bind the DRB1*SE molecules DRB1*0101, DRB1*0401 and DRB1*0404 (18). While the positive charge of pocket 4 effectively excludes positively charged arginine, the neutrally charged citrulline can be accommodated into pocket 4 of the MHC binding groove. It is plausible that central tolerance to posttranslational modified citrullinated peptides is incomplete with respect to thymic selection of T cells. Thus, citrullinated proteins could be taken up by antigen-presenting cells in inflamed tissues, where citrullination occurs, and presented by DRB1*SE molecules to CD4 T cells. These cells could then provide help to B cells, resulting in the production of ACPA antibodies in secondary lymphoid organs or directly at the site of inflammation.
CD4 T cell reactivity to several citrullinated-vimentin epitopes have previously been described in DRB1*0401 transgenic mice (18) and recently also in DRB1*0401 RA patients (19). Here we identified a novel T cell epitope from citrullinated-vimentin (cit-vim59-78) and examined its binding to DRB1*0401 molecules. We then developed an MHC class-II tetramer for detection of cit-vim59-78-specific T cells and studied T cell responses against citrullinated-vimentin in DRB1*0401 transgenic mice. Lastly, we demonstrated the presence of cit-vim59-78-reactive CD4 T cells in both DRB1*0401 RA patients and healthy subjects and further show that production of proinflammatory cytokines in response to this epitope is unique to RA.
MATERIAL AND METHODS
Human Subjects
In total 28 HLA-DRB1*0401 subjects were recruited under the auspices of either the Benaroya Research Institute (BRI) rheumatic disease registry, the BRI immune-mediated disease registry, or the Karolinska Hospital/Karolinska Institutet arthritis research program. Informed consent was obtained from all subjects under protocols approved by the IRB at Benaroya Research Institute or the Karolinska Hospital Ethical Review Board. All patients were diagnosed as having RA by a rheumatologist in accordance with the 1987 American College of Rheumatology criteria (20). The patients recruited at Karolinska institute were used for analyzing cytokine production, and all of them had at least one copy of the HLA-DRB1*0401 allele and 21/22 had antibodies against CCP2 (Euro-diagnostica) and 19/22 had serum-antibodies for citullinated-vimentin aa60-75 (15). Control participants from BRI and Karolinska Institute were selected based on lack of personal or family history of autoimmunity or asthma. PBMC were obtained from heparinized blood by centrifugation over Ficoll-Hypaque gradients. For frozen samples PBMCs were cryopreserved in liquid nitrogen in 10% DMSO and 90% heat-inactivated FBS.
Peptides
All vimentin peptides used in these studies were synthesized and purified by the manufacturer (BIO S&T). Vimentin epitopes were predicted based on favorable anchor residues at pockets 1, 6, and 9, as reported in the SYFPEITHI database (21), where arginine would naturally occur in pocket 4. Influenza hemagglutinin antigen (HA) and collagen II (CII) peptides were selected based on previous reports (22, 23) and generated in house on an Applied Biosystems 432A peptide synthesizer.
For peptide binding assays increasing concentrations of each non-biotinylated test peptide were incubated in competition with 0.01 μM biotinylated HA306-318 peptide in wells coated with HLA-DR*0401 protein as previously described (24). Europium-conjugated streptavidin (PerkinElmer) was used to measure the amount of biotin labeled peptide bound to the MHC and was quantified using a Victor2 multilabel time resolved-fluorometer (PerkinElmer). Peptide binding curves were fitted by non-linear regression with a sigmoidal dose response curve model using Prism software (Version 4.03, GraphPad Software Inc.).
Tetramer
Recombinant DRB1*0401 protein was produced as previously described (22). Briefly, soluble DR0401 was purified from insect cell culture supernatants and then biotinylated at a sequence-specific site using biotin ligase (Avidity) prior to dialysis into phosphate storage buffer. The biotinylated monomer was loaded with 0.2 mg/ml of either cit-vim59-78, HA306-318, or CII255-274 by incubating at 37°C for 72 hours in the presence of 2.5 mg/ml n-octyl-β-D-glucopyranoside and 1 mM Pefabloc SC (Sigma-Aldrich). Peptide loaded monomers were then conjugated to tetramers using R-PE streptavidin (Invitrogen) at a molar ratio of 8 to 1.
Mice
Class II deficient C57Bl/6 (I-Abo/o) mice transgenically expressing a chimeric class II containing the α1β1 domains of human DRA1*0101-B1*0401 on a mouse IE-d backbone (DR0401-IE mice) were obtained from Taconic and were housed under specific pathogen-free conditions. Mice were immunized with 100 μg of peptide in 100 ml of 50% CFA/PBS, subcutaneously at the base of the tail. On day 10 spleen and draining lymph nodes were harvested for in vitro assays. For long term studies of the in vivo response to citrullinated-vimentin peptides, mice were immunized as above and then boosted on day 21 with 100 μg of peptide in 100 ml of 50% IFA/PBS. Mice were monitored for changes in body weight weekly and paws were monitored for signs of arthritis 3x per week using a standardized grading scale (25). All animal work was approved by the BRI Animal Care and Use Committee (ACUC) and animals were housed in the BRI AAALAC-accredited animal facility.
Murine Assays
Spleens and lymph nodes were brought to a single cell suspension and any remaining red blood cells were lysed with hemolytic buffer (0.15 M NH4Cl, 0.1 mM Na2EDTA & 10 mM KHCO3). For proliferation assays cells were plated at 0.5 × 106 cells/well in 96-well round bottom plates in complete DMEM (DMEM,10% FBS (ATLAS), 100 μg/ml Penicillin, 100 U/ml Streptomycin, 50 μM β-mercaptoethanol, 2mM glutamine and 1mM sodium pyruvate) either alone, with anti-CD3/antiCD28 (2.0/0.2 mg/ml) (BioLegend), or with peptide for 96 hrs. At 72 hrs 3H-thymidine (Moravek Biochemicals) was added at 1 μCi/well. Cells were harvested to glass fiber membranes and radioactivity was quantified with a MicroBeta TriLux 1450 LSC & luminescence counter (PerkinElmer). For tetramer assays, 5.0 × 106 cells were resuspended in 200 μl of complete DMEM and stained with peptide-loaded tetramers for 60 min at 37°C. Subsequently, cells were placed on ice for 10 min with the addition of Fc-block (BD Pharmingen) before being stained with CD3 APC, CD4 PerCP, and B220 FITC (all BioLegend) for an additional 30 min on ice and then analyzed by flow cytometry.
Detection of vim59-78-specific T cell by MHC class-II tetramers in human subjects
For tetramer assays PBMCs were cultured at 6 × 106 total cells/well within a 24 well plate in RPMI-1640 + 10% pooled human serum with 10 μg/ml of either HA306-318, vim59-78 or cit-vim59-78 peptide. IL-2 (Novartis) was added at 325 IU/ml on day 6. After 14 days cells were stained for expression of CD25 FITC (BD), CD4 PerCP (BioLegend), CD45RO APC (BD), and tetramer before being ran on a FACSCalibur.
Functional cellular assay
PBMCs were cultured at a concentration of 1.2 × 106 total cells/well within a 96 flat-bottom plate in RPMI-1640 supplemented with 10% pooled human sera for 5 days in the absence or the presence of HA306-318, vim59-78 or cit-vim59-78 at a concentration of 10 μg/ml. On day 5 cells were restimulated with the same peptides for 7 hours, with 5 μg/ml brefeldin A (Sigma-Aldrich) added for the last 5 hours. For positive control, staphylococcal enterotoxin B (SEB) was added on day 5 to a separate cell culture at 1 μg/ml (Sigma-Aldrich). Following restimulation, cells were treated with LIVE/DEAD Fixable Green Dead Cell Stain (Invitrogen) and then stained for surface expression of CD3 (BioLegend), CD4 and CD14 (BD Bioscience). Cells were then washed and permeabilized using Cytofix/Cytoperm fixation/permeabilization solution kit (BD Biosciences) before being stained for the expression of TNFα, INFγ, IL-17A (BioLegend) and CD154 (BD Bioscience). Samples were run on a CyAn ADP Analyzer (Dako, Glostrup, Denmark) and the data was analyzed by FlowJo software version 8.6.36 (Tree star, Ashland, OR, USA).
Cytokine detection in supernatants
Bead-base multiplexed cytokine assay was custom-designed and produced by BioLegend for a Luminex platform for simultaneous detection of the following cytokines: IL-1b, IL-2, IL-3, IL-4, IL-6, IL-7, IL-9, IL-10, IL-13, IL-17A, IL-17F, IL-21, IL-22, IL-23, TNFα, and IFNγ. Supernatants were collected at day 5 from cultures that were assigned for functional studies and stored at -80°C until used. Supernatants from RA patients and healthy controls were included on the same plate and analyzed according to the manufacturer’s instructions and read on Luminex100™.
Statistics
In murine studies significance was determined by Student’s t-test using Prism software version 4.03 (GraphPad Software Inc., La Jolla, CA, USA). Paired t-test (Wilcoxon singed rank test) was used in human studies for comparison between vim and cit-vim stimulated cells. Ps < 0.05 were considered statistically significant.
RESULTS
Identification of a T cell epitope from citrullinated vimentin
We recently demonstrated that antibodies against citrullinated-vimentin have the strongest association with HLA-DRB1*0401 compared to other ACPAs in synovial fluid (15). It is also known that a conversion of arginine (arg) to citrulline (cit) can transform a non-binding epitope to a binding epitope in the context of HLA-DRB1*0401, specifically when this conversion takes place in the pocket 4 position of an epitope (18, 26). Thus, we scanned vimentin for potential DRB1*0401-binding epitopes with anchor residues at pockets 1, 6, and 9, as reported in the SYFPEITHI database (21), where arg would naturally occur in pocket 4. We identified an area of vimentin, aa59-78, where citrullination could occur at three residues, R64, R69 and R71, and result in a peptide that places Cit in pocket 4 of two potential binding motifs (figure 1A). Upon synthesis of both the native and citrullinated peptides corresponding to aa 59-78, binding assays revealed that cit-vim59-78 bound HLA-DRBI*0401 while vim59-78 did not (figure 1B). Also, the two shorter sets of peptides (figure. 1A), cit-vim59-71 and cit-vim66-78, bound HLA-DRB1*0401 (figure. 1C), confirming the possibility of overlapping binding motifs within the larger cit-vim59-78 peptide.
Figure 1. Vimentin peptides and relative binding affinity to HLA-DRB1*0401.
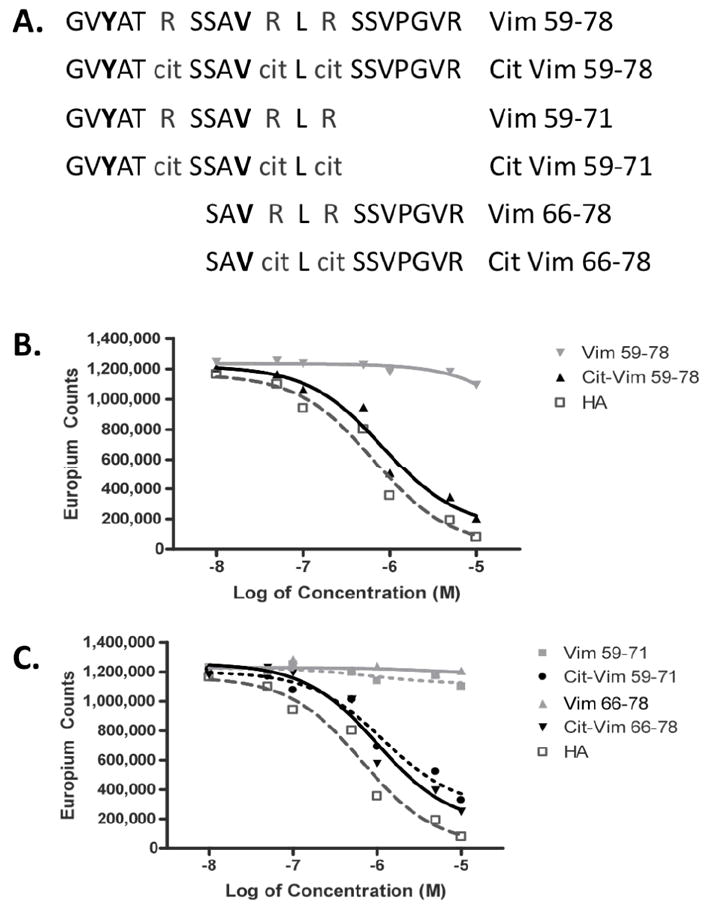
(A) Peptides synthesized from vimentin in their native and citrullinated forms. Residues in bold represent proposed P1 anchors. Binding affinity to DRB1*0401 was determined for either the long version of the peptides (B) or shorter versions, limited to one positional binding frame (C), by europium based peptide competition assay. Non-citrullinated vim peptides are shown in light gray and citrullinated in black. For comparison, the high affinity HA306-318 peptide is shown in dark gray.
Validation of cit-vim59-78 in DR0401-IE mice
Knowing that cit-vim59-78 binds well to DRB1*0401, we wished to further examine whether it could induce T cell responses in DR0401-IE transgenic mice. Mice were immunized with either vim59-78 or cit-vim59-78 and spleens along with draining lymph nodes were collected on day 10. As shown in Figure 2A, a dose dependent T cell proliferation from mice immunized with cit-vim59-78 was detected following in vitro restimulation with cit-vim59-78 but not with vim59-78. In contrast, and as could be predicted by the lack of DRB1*0401-binding capacity, T cells from mice immunized with vim59-78 did not proliferate in response to either vim59-78 or cit-vim59-78 (figure 2B). To determine if the immunogenic response to cit-vim59-78 was further restricted to one immunodominant region, in vitro restimulations were performed with cit-vim59-71 and cit-vim66-78 (figure 2C). These assays revealed cit-vim59-71 to be the immunologically dominant region for the response mounted in the cit-vim59-78 immunized DR0401-IE mice. To further elucidate the possible impact of the two binding regions within cit-vim59-78, mice were immunized with either cit-vim59-71 or cit-vim66-78. In vitro recall responses of splenocytes from mice immunized with cit-vim59-71 showed a robust response to both cit-vim59-71 along with the longer peptide, while mice immunized with cit-vim66-78 failed to mount a significant recall response (figures 2D, E).
Figure 2. Cit- vim59-78, but not vim59-78, is antigenic in DR0401-IE mice.
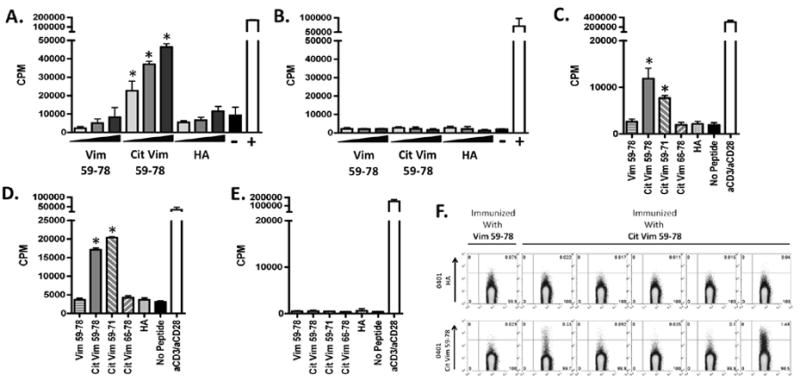
3H-thymidine incorporation from splenocytes of mice immunized with either (A) cit-vim59-78 or (B) vim59-78 after in vitro restimulation of vim59-78, cit- vim59-78, and HA306-318 peptides at 4, 20, & 100 μg/ml. Splenocytes alone served as a negative control while anti-CD3/anti-CD28 stimulation served as a positive control. Results are representative of n= 20 and n= 8, respectively. (C) Splenocytes from mice immunized with cit- vim59-78 were challenged with the different versions of the epitope (Fig 1A) (n=13). Conversely, mice were also immunized with either cit-vim59-71 (D)(n=7) or cit-vim66-78 (E)(n=5), and challenged with long and short peptides. In C, D, and E peptide challenge was done at 5 μg/ml and figures display one representative mouse out of each experiment. (F) Cit-vim specific CD4+ T cells were detected in cit- vim59-78 immunized mice by tetramer staining. Here a representative panel of mice harvested at 13-weeks is shown, where 4/5 cit-vim59-78 immunized mice are tetramer positive. Samples had to have > 4-fold background to be considered positive. 0401-HA tetramer is used as a negative control for 0401-cit-vim59-78 tetramer. * designates p values of < 0.05.
T cell specificity to cit-vim was further confirmed using MHC class-II tetramers loaded with cit-vim59-78. Tetramer positive CD4 T cells specific to cit-vim59-78 were seen in 18/21 mice immunized with cit- vim59-78, while no tetramer positive cells were found among those immunized with vim59-78.
To determine if extended immunologic activity against cit-vim59-71 resulted in gross biological effects we immunized DR0401-IE mice and monitored for signs of arthritis over a period of 13 weeks. Although we were able to show a strong proliferative recall response (supplemental fig. 1), and could detect antigen-specific T cells with DRB1*0401 tetramers loaded with cit-vim59-71 at 13 weeks (fig 2F), none of the mice (n=13) developed any signs of ankle swelling or polyarthritis (data not shown).
Identification of cit-vim59-78 reactive-CD4 T cells in DRB1*0401 RA patients and healthy controls using MHC class-II tetramers
To determine whether T cells specific for this peptide were present in humans we chose first to determine whether HLA-DRB1*0401, cit-vim59-78 specific T cells could be expanded from PBMC and identified using HLA class II tetramers. As autoreactive CD4 T cells are present at low frequencies in healthy subjects as well as in individuals with autoimmunity (27), we assessed T cell reactivity to cit-vim59-78 in both HLA-DRB1*0401+ healthy controls and RA patients. PBMC were stimulated with either vim59-78, cit-vim59-78, or HA306-318 along with IL-2, then stained with HLA-DRB1*0401 tetramers on day 14. In cultures stimulated with cit-vim59-78 we were able to detect cit-vim59-78 tetramer positive cells, while such antigen-specific T cells were not present in either the vim59-78 or HA306-318 stimulated cultures. We detected cit-vim59-78 tetramer positive CD4 T cells in 2/6 RA patients and 2/6 healthy controls. A similar percentage of DR0401-cit-vim59-78 cells were found in the RA and healthy control samples when using this approach, as shown in Figures 3A and 3B. These findings demonstrate that T cells specific for cit-vim59-78 are detectable in some HLA DRB1*0401 individuals, but this approach was unable to demonstrate differences in the number of these cells in RA patients as compared to controls.
Figure 3. Cit-vim59-78 specific CD4 T cells from both RA subjects and healthy controls can be detected by tetramer.
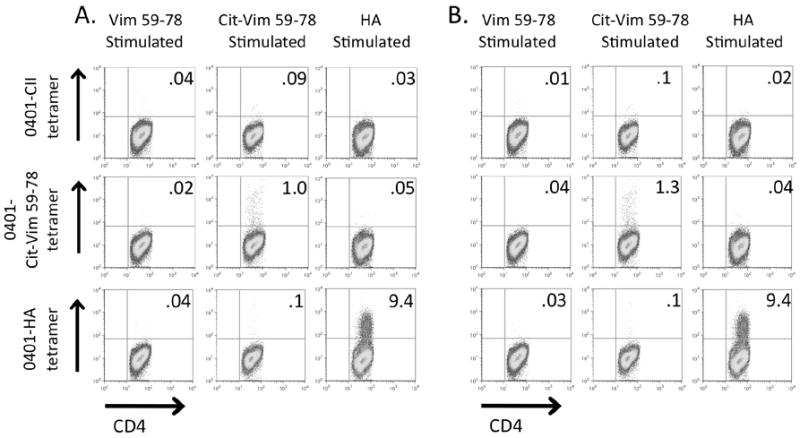
A representative cit-vim59-78 tetramer positive sample from one HLA-DRB1*0401-positive RA patient (A) and one HLA-DRB1*0401-positive healthy control subject (B). PBMCs were stimulated with either vim59-78, cit-vim59-78, or HA306-318 in the presence of IL-2. Samples were then stained for tetramer on day 14. 0401-CII is shown as a universal negative control.
Phenotypic characterization of cit-vim59-78 reactive-CD4 T cells in DRB1*0401 RA patients and healthy controls
In order to increase our ability to detect cit-vim59-78 specific T cells and at the same time functionally characterize their response we assayed antigen stimulated PBMC for CD154 expression and secretion of TNFα, IFNγ and IL-17 using multicolor flow cytometry. CD154 is an early activation marker, previously described as a marker for CD4 T cells responding to a specific antigen (28-30). This allowed for detection of cit-vim reactive T cells in conjunction with expression of cytokines in response to stimulation. Supplemental figure 2 demonstrates this approach with the influenza-derived HA306-318 peptide.
To determine cit-vim specific reactivity, DRB1*0401 RA patients and healthy controls were stimulated with either vim59-78 or cit-vim59-78 and the expression of CD154, along with TNFα, IFNγ and IL-17 secretion, was analyzed. As shown in figure 4A, CD4 T cells from RA patients exposed to cit-vim59-78 expressed significantly higher levels of CD154 than those exposed to vim59-78 (p<0.001). This response was seen in 33% of healthy controls (3/9 subjects had an increase greater than 3 fold) and in 59% of RA patients (13/22). Intracellular staining revealed a significant increase in the production of the pro-inflammatory cytokines IFNγ and TNFα following stimulation with cit-vim59-78 in comparison to vim59-78. (Figure 4B, C and D) with a trend toward a higher frequency of IL-17 secreting cells in the T cells of RA subjects but not healthy controls.
Figure 4. Reactivity to cit-vim59-78 in DRB1*0401 RA patients and healthy controls.
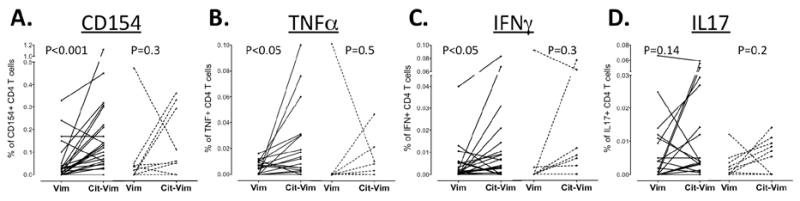
PBMCs from RA patients (n=22) and control (n=9) were stimulated with either vim59-78 or cit-vim59-78 before being stained for expression of (A) CD154 and intracellular content of (B) TNFα, (C) IFNγ and (D) IL-17A in CD4+ T cells as measured by flow cytometry. Percentages of reactive-CD4 T cells are shown following subtraction of background levels (from unstimulated cultures). RA patients’ responses are shown in solid lines, whereas dotted lines represent healthy controls.
Cytokine secretion upon cit- vim59-78 stimulation in DRB1*0401 human subjects
As monitored by the CD154 assay, both healthy controls and patients could mount a response to cit-vim59-78. To further substantiate whether the type or quantity of cytokines produced in response to vim59-78 differed between the two groups, we assessed the quantity of cytokines in supernatants taken from primary stimulation cultures by use of a multiplex panel for 16 different cytokines on RA (n=19) and healthy control (n=9) samples. Figure 5A depicts the cytokine response of the RA patient who had the most robust response of any subject tested, with INFγ, IL-1β, IL-6, IL-9, IL-10, IL-13 and IL-17F being elevated. Intercomparison within the RA cohort showed a significantly higher cytokine production in response to cit-vim59-78 in comparison to vim59-78 (p<0.05) (Fig. 5B). Such a difference could not be seen in healthy controls. Also, the overall response to cit-vim was higher in patients in comparison to healthy controls (p<0.01). Some cytokines, i.e. INFγ, IL-2 and IL-10 were significantly higher produced by patients than healthy subjects in response to cit-vim59-78. Additionally, other cytokines displayed a trend towards increased production in patients, i.e. IL-1β, IL-3, IL-6, IL-9, IL-13, IL-17F, IL-23 and TNFα. Interestingly, following stimulation with influenza antigen, i.e. HA, a similar difference in the cytokine patterns could be observed between healthy controls and RA patients (figure 5C).
Figure 5. Cytokine milieu following in vitro stimulation of PBMCs from RA patients and healthy controls.
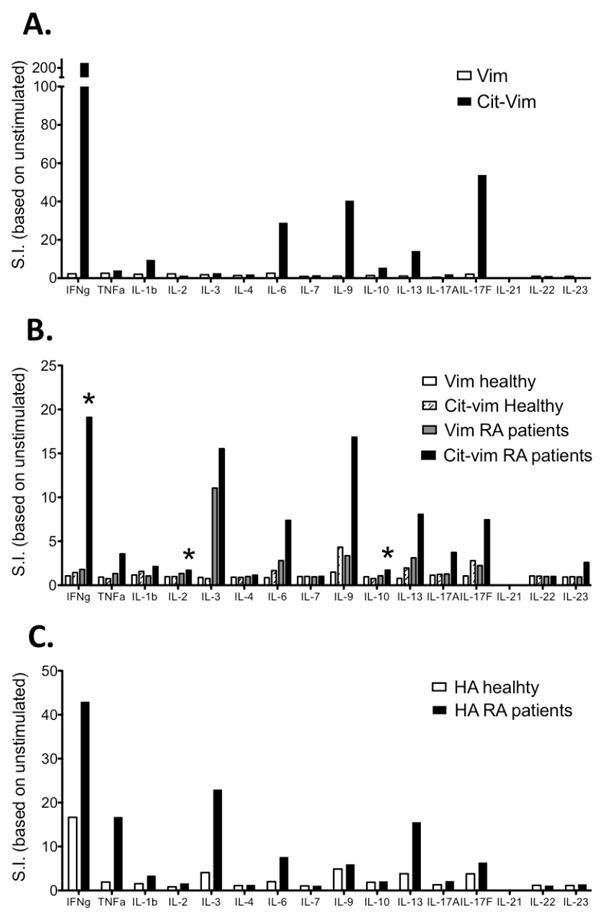
The levels of 16 different cytokines (IFNγ, TNFα, IL-1b, IL-2, IL-3, IL-4, IL-6, IL-7, IL-9, IL-10, IL-13, IL-17A, IL-17F, IL-21, IL-22 and IL-23) were measured by Luminex Multiplex array following peptide stimulation. (A) Production of inflammatory cytokines by an RA patient in response to cit-vim59-78 but not vim59-78. (B) Average cytokine response following stimulation with cit-vim59-78 or vim59-78 (in 19 RA patient and 9 healthy control cultures). (C) Cytokine production in response to HA306-318 stimulation in RA patients and healthy controls, where donors are the same as seen in 5B. Stimulation index (S.I.) was calculated as: pg of cytokine from peptide stimulated culture/pg of cytokine from an unstimulated culture.
DISCUSSION
In this report we studied the specificity and nature of autoreactive-CD4 T cells in RA, which is fundamental to understanding RA etiopathogenesis. We focused our studies on the candidate autoantigen vimentin in its posttranslationally modified citrullinated form, and stratified our efforts according to immunogenetic background, i.e. HLA-DRB1 alleles and specific antibody reactivity. We identified an epitope that exclusively binds to DRB1*0401 in its citrullinated but not in its native form; cit-vim59-78. Further, we have demonstrated that cit-vim59-78 propagates DRB1*0401-restricted T cell responses in both mice and humans, where the cytokine response in our RA subjects is predominantly proinflammatory.
The ability of a peptide to bind MHC class II is crucial for induction of T cell mediated responses. Here we have shown that the modification of arginine to citrulline can produce a novel epitope in vimentin. Specifically we have shown that cit-vim59-78 binds to DRB1*0401 and induces an immunologic response in both mouse and man, whereas the native form of the peptide does not. In a previous report, Hill et al. also demonstrated that a citrullinated peptide from vimentin (aa65-77) binds to the most common SE-alleles (DRB1*0101, *0401 and *0404) while its native form does not (18). Our peptide differs significantly from the Hill et al. peptide in that it contains a second binding registry from residues 61 to 69, and our data suggests it is this reading frame that plays the most critical role in inducing a DRB1*0401-restricted response (figures 2C-E). Furthermore, our epitope has an additional citrullination at position 69 and has the native leucine at position 70 rather than an alanine. We believe the differences between these peptides may account for why the Hill et al. peptide induces a response in the C57Bl/6 (I-Abo/o) transgenic DRB1*0401 mouse while our cit-vim66-78 peptide does not (fig. 2E). Similarly to Hill’s epitope, cit-vim59-78 also binds to DRB1*0404 (data not shown) indicating that it may contribute to disease pathogenesis in individuals who carry other SE alleles. Seven patients included in this work also carry an DRB1*0404 allele in addition to DRB1*0401. Thus, DRB1*0404 could contribute to cit-vim66-78 presentation to T cells in those patients. However, we cannot conclude from our data whether those particular patients responded better to cit-vim66-78.
This report is the first demonstration of a HLA-DR*0401 tetramer loaded with an autoantigenic peptide allowing the detection of autoreactive CD4 T cells in RA patients. This has been proven difficult using other RA candidate autoantigens (31), although recently James et al. successfully utilized MHC HLA-DRB1*1001 class II tetramers to demonstrate CD4 T cells specific to citrullinated peptides derived from fibrinogen α, fibrinogen β, and cartilage intermediate-layer protein in the context of HLA-DRB1*1001 (32). Our current study in conjunctions with this previous study demonstrates it is possible to use tetramer technology to detect antigen-specific T cell in the setting of RA following expansion with IL-2. To further explore the function of cit-vim59-78-reactive T cells we sat up an assay to simultaneously read out CD154, IFNγ, TNFα and IL17 to ensure a stringent detection of cit-vim59-78-reactive CD4 T cells and to further assess their inflammatory features. Using this approach we could confirm the tetramer data and that cit-vim reactive T cells are present in both RA patients and healthy controls. The use of CD154 expression may represent a more true frequency determination than the MHC class II tetramer approach, which includes a more extended period of culture and the addition of IL-2 to enhance proliferation. Functional T cell studies in RA are challenging, presumably due to the immunosuppressive therapies that the patients are taking. Indeed, it has been reported that the use of different immunosuppressive drugs reduces the frequency and the cytokine producing capacity of activated T cells (33, 34). An additional contributing factor may be TCRζ down regulation (35), which may reduce the responsiveness of T cells. In this study, T cell responses were investigated in more than 45 DRB1*0401 RA patients, yet only 22 were included in our final analysis since the others failed to mount a clear response to HA306-318 antigen, which we used as an internal positive control. In contrast and further supporting these hypotheses, an HA response could be detected in all of the healthy controls studied (9/9). These findings suggest that studies performed at an earlier point during the disease course and in the absence of therapy may yield even stronger evidence of T cell responses to cit-vim.
Multiple groups have described the presence of autoreactive T cells with similar frequencies and features in healthy subjects (27, 36-39). Using two different approaches, we have shown that cit-vim59-78-reactive T cells could be detected in both healthy control subjects as well as in RA patients. Danke et al. demonstrate that autoreactive T cells in healthy subjects are primarily found among the naïve population, whereas autoreactive T cells are present in the memory populations in the case of T1D (27). Although our assays did not differentiate between naïve and memory CD4 T cell responses our data are suggestive of a similar scenario in RA, and it would be of future interest to determine if the cit-vim reactive T cells present in RA are specific to the memory pool while such T cells in control subjects are naïve.
Overall our data supports the notion that HLA DRB1*0401 positive RA patients have an increase pool of proinflammatory cit-vim reactive T cells. Individual RA patients produced a melange of proinflammatory cytokines in response to cit-vim59-78, including IFNγ, TNFα, IL-6, IL-17A, IL-17F and IL-23. Of these INFγ in particular seems to be a key cytokine in anti-citrulline immunity as its levels were significantly higher in comparison to healthy HLA DRB1*0401 controls both intracellularly and in supernatants (measured by flow cytometry and luminex respectively).
The strong genetic link between the development of ACPA positive RA and HLA-DRB1 alleles containing the shared epitope implicates the ability to these HLA alleles to present a unique set of citrullinated peptides to T cells which can drive the B cell response to these antigens. By taking a translational approach, utilizing both humanized mice and patient samples, we have identified and characterized a citrullinated T cell epitope presented in the context of DRB1*0401. However, our studies show that cit-vim specific T cells are present in healthy individuals as well as RA patients, indicating that the presence of T cells with this specificity alone is not adequate to drive the development of RA or even an antibody response to this antigen. It is likely that additional RA-associated genetic variants with immunoregulatory potential as well as environmental insults together contribute to the development of ACPA and disease pathogenesis (3, 40, 41). In summary, the proinflammatory character of the cit-vim specific T cell response observed in this study suggests that these cells play a role in citrulline autoimmunity in RA. Yet, T cell reactivity to other citrullinated antigens as well as the differences between citrulline-specific T cells from healthy controls and RA patients need to be studied in further detail.
Supplementary Material
Supplemental Figure 1. Sustained recall of cit- vim66-78 in DR0401-IE mice.
Representative plots (n=11) of DR*0401 mice immunized with cit- vim59-78 and harvested at week 13. 3H-thymidine incorporation was seen in both splenocytes (A) and lymphocytes (B) upon peptide challenge with titrations of vim59-78, cit- vim59-78, cit-vim59-71, cit-vim66-78, and HA peptide at 1, 5, & 25 μg/ml. Cells alone served as a definitive negative control while anti-CD3/anti-CD28 served as a positive control. * designates p values of < 0.05.
Supplemental Figure 2. Detection and function of HA-reactive T cells.
A comparative expression of CD154 and TNFa, IFNg and IL17 secretion in unstimulated (upper panel) and HA-stimulated (lower panel) CD4 T cells using multicolor flow cytometry.
Acknowledgments
We wish to thank the BRI Translational Research Program and Clinical Core for subject recruitment and sample processing and handling. We would like to thank Dr. Bradley Stone and Dr. Eddie James for their advice with selection of peptides and production of tetramers.
The authors would also like to thank all personal and patients at the rheumatology clinic at the Karolinska University Hospital, as well as Eva Jemseby, Gull-Britt Almgren and Julia Boström for organizing sampling, storage and administration of biomaterial.
FUNDING Work performed at BRI was supported by grants from the NIH including NIAID 5U19 AI050864, NIMS 5 R01 AR037296 and NIAMS RO1AR051394 while the studies performed at Karolinska Institutet was supported by Margaretha af Ugglas Foundation, the Swedish Research Council, the EU FP6 project, AutoCure LSHB CT-2006-018661, the EU FP7 project Masterswitch HEALTH-F2-2008-223404, the Swedish Combine program 2, the Swedish Association against Rheumatism and the King Gustaf the V:s 80 year Foundation.
Footnotes
COMPETING INTERESTS No competing interests
References
- 1.Holmdahl R, Klareskog L, Rubin K, Larsson E, Wigzell H. T lymphocytes in collagen II-induced arthritis in mice. Characterization of arthritogenic collagen II-specific T-cell lines and clones. Scand J Immunol. 1985;22(3):295–306. doi: 10.1111/j.1365-3083.1985.tb01884.x. [DOI] [PubMed] [Google Scholar]
- 2.Stanescu R, Lider O, van Eden W, Holoshitz J, Cohen IR. Histopathology of arthritis induced in rats by active immunization to mycobacterial antigens or by systemic transfer of T lymphocyte lines. A light and electron microscopic study of the articular surface using cationized ferritin. Arthritis Rheum. 1987;30(7):779–92. doi: 10.1002/art.1780300708. [DOI] [PubMed] [Google Scholar]
- 3.Mahdi H, Fisher BA, Kallberg H, Plant D, Malmstrom V, Ronnelid J, et al. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat Genet. 2009;41(12):1319–24. doi: 10.1038/ng.480. [DOI] [PubMed] [Google Scholar]
- 4.Klareskog L, Ronnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol. 2008;26:651–75. doi: 10.1146/annurev.immunol.26.021607.090244. [DOI] [PubMed] [Google Scholar]
- 5.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30(11):1205–13. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 6.Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol. 2006;38(10):1662–77. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Makrygiannakis D, af Klint E, Lundberg IE, Lofberg R, Ulfgren AK, Klareskog L, et al. Citrullination is an inflammation-dependent process. Ann Rheum Dis. 2006;65(9):1219–22. doi: 10.1136/ard.2005.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43(1):155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Avouac J, Gossec L, Dougados M. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2006;65(7):845–51. doi: 10.1136/ard.2006.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 11.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50(2):380–6. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 12.van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Huizinga TW, Toes RE, de Vries RR. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 2006;54(4):1117–21. doi: 10.1002/art.21739. [DOI] [PubMed] [Google Scholar]
- 13.Verpoort KN, Cheung K, Ioan-Facsinay A, van der Helm-van Mil AH, de Vries-Bouwstra JK, Allaart CF, et al. Fine specificity of the anti-citrullinated protein antibody response is influenced by the shared epitope alleles. Arthritis Rheum. 2007;56(12):3949–52. doi: 10.1002/art.23127. [DOI] [PubMed] [Google Scholar]
- 14.Snir O, Widhe M, von Spee C, Lindberg J, Padyukov L, Lundberg K, et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: association with HLA-DRB1 alleles. Ann Rheum Dis. 2009;68(5):736–43. doi: 10.1136/ard.2008.091355. [DOI] [PubMed] [Google Scholar]
- 15.Snir O, Widhe M, Hermansson M, von Spee C, Lindberg J, Hensen S, et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum. 62(1):44–52. doi: 10.1002/art.25036. [DOI] [PubMed] [Google Scholar]
- 16.Verpoort KN, Jol-van der Zijde CM, Papendrecht-van der Voort EA, Ioan-Facsinay A, Drijfhout JW, van Tol MJ, et al. Isotype distribution of anti-cyclic citrullinated peptide antibodies in undifferentiated arthritis and rheumatoid arthritis reflects an ongoing immune response. Arthritis Rheum. 2006;54(12):3799–808. doi: 10.1002/art.22279. [DOI] [PubMed] [Google Scholar]
- 17.Ireland J, Herzog J, Unanue ER. Cutting edge: unique T cells that recognize citrullinated peptides are a feature of protein immunization. J Immunol. 2006;177(3):1421–5. doi: 10.4049/jimmunol.177.3.1421. [DOI] [PubMed] [Google Scholar]
- 18.Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171(2):538–41. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 19.Feitsma AL, van der Voort EI, Franken KL, el Bannoudi H, Elferink BG, Drijfhout JW, et al. Identification of citrullinated vimentin peptides as T cell epitopes in HLA-DR4-positive patients with rheumatoid arthritis. Arthritis Rheum. 62(1):117–25. doi: 10.1002/art.25059. [DOI] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50(3-4):213–9. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 22.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J Clin Invest. 1999;104(12):R63–7. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson EC, Hansen BE, Jacobsen H, Madsen LS, Andersen CB, Engberg J, et al. Definition of MHC and T cell receptor contacts in the HLA-DR4restricted immunodominant epitope in type II collagen and characterization of collagen-induced arthritis in HLA-DR4 and human CD4 transgenic mice. Proc Natl Acad Sci U S A. 1998;95(13):7574–9. doi: 10.1073/pnas.95.13.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill CM, Liu A, Marshall KW, Mayer J, Jorgensen B, Yuan B, et al. Exploration of requirements for peptide binding to HLA DRB1*0101 and DRB1*0401. J Immunol. 1994;152(6):2890–8. [PubMed] [Google Scholar]
- 25.Kruisbeek AM. Isolation of mouse mononuclear cells. Curr Protoc Immunol. 2001;Chapter 3(Unit 3 1) doi: 10.1002/0471142735.im0301s39. [DOI] [PubMed] [Google Scholar]
- 26.Hill JA, Bell DA, Brintnell W, Yue D, Wehrli B, Jevnikar AM, et al. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. J Exp Med. 2008;205(4):967–79. doi: 10.1084/jem.20072051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danke NA, Yang J, Greenbaum C, Kwok WW. Comparative study of GAD65-specific CD4+ T cells in healthy and type 1 diabetic subjects. J Autoimmun. 2005;25(4):303–11. doi: 10.1016/j.jaut.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005;11(10):1113–7. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 29.Chattopadhyay PK, Yu J, Roederer M. Live-cell assay to detect antigen-specific CD4+ T-cell responses by CD154 expression. Nat Protoc. 2006;1(1):1–6. doi: 10.1038/nprot.2006.1. [DOI] [PubMed] [Google Scholar]
- 30.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11(10):1118–24. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 31.Kotzin BL, Falta MT, Crawford F, Rosloniec EF, Bill J, Marrack P, et al. Use of soluble peptide-DR4 tetramers to detect synovial T cells specific for cartilage antigens in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97(1):291–6. doi: 10.1073/pnas.97.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James EA, Moustakas AK, Bui J, Papadopoulos GK, Bondinas G, Buckner JH, et al. DR1001 presents ‘altered-self’ peptides derived from joint associated proteins by accepting citrulline in three of its binding pockets. Arthritis Rheum. doi: 10.1002/art.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamdi H, Mariette X, Godot V, Weldingh K, Hamid AM, Prejean MV, et al. Inhibition of anti-tuberculosis T-lymphocyte function with tumour necrosis factor antagonists. Arthritis Res Ther. 2006;8(4):R114. doi: 10.1186/ar1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou J, Rudwaleit M, Brandt J, Thiel A, Braun J, Sieper J. Down-regulation of the nonspecific and antigen-specific T cell cytokine response in ankylosing spondylitis during treatment with infliximab. Arthritis Rheum. 2003;48(3):780–90. doi: 10.1002/art.10847. [DOI] [PubMed] [Google Scholar]
- 35.Berg L, Ronnelid J, Klareskog L, Bucht A. Down-regulation of the T cell receptor CD3 zeta chain in rheumatoid arthritis (RA) and its influence on T cell responsiveness. Clin Exp Immunol. 2000;120(1):174–82. doi: 10.1046/j.1365-2249.2000.01180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Danke NA, Berger D, Reichstetter S, Reijonen H, Greenbaum C, et al. Islet-specific glucose-6-phosphatase catalytic subunit-related protein-reactive CD4+ T cells in human subjects. J Immunol. 2006;176(5):2781–9. doi: 10.4049/jimmunol.176.5.2781. [DOI] [PubMed] [Google Scholar]
- 37.Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172(10):5967–72. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- 38.Snir O, Lavie G, Achiron A, Bank I, Ben-Aharon T, Sredni B, et al. G-CSF enhances the adhesion of encephalitogenic T cells to extracellular matrix components: a possible mechanism for exacerbation of multiple sclerosis. J Neuroimmunol. 2006;172(1-2):145–55. doi: 10.1016/j.jneuroim.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Mandel M, Achiron A, Tuller T, Barliya T, Rechavi G, Amariglio N, et al. Clone clusters in autoreactive CD4 T-cell lines from probable multiple sclerosis patients form disease-characteristic signatures. Immunology. 2009;128(2):287–300. doi: 10.1111/j.1365-2567.2009.03117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stahl EA, Raychaudhuri S, Remmers EF, Xie G, Eyre S, Thomson BP, et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet. 42(6):508–14. doi: 10.1038/ng.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lundstrom E, Kallberg H, Alfredsson L, Klareskog L, Padyukov L. Gene-environment interaction between the DRB1 shared epitope and smoking in the risk of anti-citrullinated protein antibody-positive rheumatoid arthritis: all alleles are important. Arthritis Rheum. 2009;60(6):1597–603. doi: 10.1002/art.24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Sustained recall of cit- vim66-78 in DR0401-IE mice.
Representative plots (n=11) of DR*0401 mice immunized with cit- vim59-78 and harvested at week 13. 3H-thymidine incorporation was seen in both splenocytes (A) and lymphocytes (B) upon peptide challenge with titrations of vim59-78, cit- vim59-78, cit-vim59-71, cit-vim66-78, and HA peptide at 1, 5, & 25 μg/ml. Cells alone served as a definitive negative control while anti-CD3/anti-CD28 served as a positive control. * designates p values of < 0.05.
Supplemental Figure 2. Detection and function of HA-reactive T cells.
A comparative expression of CD154 and TNFa, IFNg and IL17 secretion in unstimulated (upper panel) and HA-stimulated (lower panel) CD4 T cells using multicolor flow cytometry.


