Abstract
Although it has been shown that up-regulation of hypoxia-inducible factor (HIF)-1α is protective in acute ischemic renal injury, long-term over-activation of HIF-1α is implicated to be injurious in chronic kidney diseases. Angiotensin II (ANG II) is a well-known pathogenic factor producing chronic renal injury and has also been shown to increase HIF-1α. However, the contribution of HIF-1α to ANG II-induced renal injury has not been evidenced. The present study tested the hypothesis that HIF-1α mediates ANG II-induced renal injury in Sprague-Dawley rats. Chronic renal injury was induced by ANG II infusion (200ng/kg/min) for 2 weeks in uninephrectomized rats. Transfection of vectors expressing HIF-1α shRNA into the kidneys knocked down HIF-1α gene expression by 70%, blocked ANG II-induced HIF-1α activation and significantly attenuated ANG II-induced albuminuria, which was accompanied by inhibition of ANG II-induced vascular endothelial growth factor, a known glomerular permeability factor, in glomeruli. HIF-1α shRNA also significantly improved the glomerular morphological damage induced by ANG II. Furthermore, HIF-1α shRNA blocked ANG II-induced upregulation of collagen and α-smooth muscle actin in tubulointerstitial region. There was no difference in creatinine clearance and ANG II-induced increase in blood pressure. HIF-1α shRNA had no effect on ANG II-induced reduction in renal blood flow and hypoxia in the kidneys. These data suggested that over-activation of HIF-1α-mediated gene regulation in the kidney is a pathogenic pathway mediating ANG II-induced chronic renal injuries and normalization of over-activated HIF-1α may be used as a treatment strategy for chronic kidney damages associated with excessive ANG II.
Keywords: glomerular sclerosis, tubulointerstitial, fibrosis, albuminuria, renal blood flow
Hypoxia inducible factor (HIF)-1α is a transcription factor that has been recently associated with the progression of chronic renal injuries 1–4. HIF-1α is up-regulated in different chronic kidney diseases 1–3, 5–6. HIF-1α is also shown to stimulate collagen accumulation by activating fibrogenic factors such as plasminogen activator inhibitor (PAI) and tissue inhibitor of metalloproteinase (TIMP) 6–9. Therefore, although up-regulation of HIF-1α has been shown to be protective in acute ischemic injury 5, 10–11, there are evidences indicating that long-term activation of HIF-1α may be injurious in chronic kidney diseases 2–5, 12–15. Angiotensin II (ANG II), a major pathogenic factor producing renal injury in different chronic kidney diseases 1, 13–14, 16–17, has been shown to stimulate HIF-1α accumulation 18–19. However, the contribution of HIF-1α to ANG II-induced renal injury has not been determined. We recently showed that silencing of the HIF-1α gene blocked the profibrotic action of angiotensin II in cultured renal cells.20 The present study was designed to test the hypothesis that HIF-1α accumulation by ANG II is a critical mediator in ANG II-induced renal injury.
We utilized HIF-1α small hairpin RNA (shRNA) to silence the gene expression of HIF-1α and evaluated the contributing role of HIF-1α in ANG II-induced renal injuries in animals chronically infused with ANG II for 2 weeks. To our knowledge, the present study provides the first direct evidence that HIF-1α-mediated gene regulation contributes to ANG II-induced renal injuries.
Materials and Methods
Animal
Experiments were performed in male Sprague-Dawley rats (250–350 g, Harlan, Madison, WI) with free access to food and water throughout the study. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
Plasmids expressing rat HIF-1α shRNA
Predesigned rat HIF-1α siRNA was purchased from Sigma-Aldrich. Sequences of HIF-1α siRNA were: sense, GGA AAG AGA CUC AUA GAA A; antisense, UUU CUA UGA CUC UCU UUC C. After confirmation of effective knocking down of HIF-1α genes by these siRNAs in cultured rat renal medullary interstitial cells, the sequences were constructed into a pRNA-CMV3.2 vector (Genscript, Piscataway, NJ) to produce shRNA. Vectors expressing scrambled shRNA used as control were purchased from Genscript. The effective gene silencing of renal HIF-1α by shRNA in vivo was also verified in preliminary experiments.
Transfection of DNA into the kidney
Rats were uninephrectomized one week before. Plasmids (50μg) mixed in 25 % of microbubble (Optison, GE HealthCare) in saline (0.6 ml) was injected into the remaining left kidney via renal artery followed by ultrasound irritation (Sonitron 2000, Rich-Mar) as described preciously by us and others 21–26. Three groups of animals were included: Vehicle infusion + control plasmids (Ctrl), ANG II infusion + control plasmids (ANG II), and ANG II infusion + HIF-1α shRNA plasmids (ANG II + HIF-1α shRNA).
Chronic infusion of ANG II, monitoring of blood pressure, assay of urinary albumin, measurement of plasma and urinary creatinine and harvest of kidney
ANG II (Sigma-Aldrich, 200 ng/kg/min) was infused for two weeks using ALZET mini-osmotic pumps (Model 2002) implanted intraperitoneally in the surgery above. Mean arterial blood pressure (MAP) were recorded daily for three hours using a telemetry system (Data Sciences International) as we described previously 27. On the last day of experiment, twenty-four-hour urines were collected using metabolic cages. Urinary albumin concentrations were measured using a rat albumin ELISA kit (Bethyl Laboratories, Montgomery, TX). After urine collection, blood samples were collected and kidneys removed. Creatinine concentrations in plasma and urine were measured by Analysis Core Laboratory. The kidneys were cut longitudinally. Half of the kidney was fixed in 10% neutral buffered formalin and the other half dissected into cortex and medulla. A small piece of fresh cortex was used for isolation of glomeruli using differential sieving as described previously 28–29 and the rest of tissues were frozen in liquid N2 and stored in −80°C.
Measurement of renal blood flow using Doppler ultrasound
Animals were treated as described above. Before the end of experiment, rats were anesthetized with ketamine (80 mg/kg, ip) and xylazine (6 mg/kg, ip) and then renal artery blood flow velocity was measured by ultrasound imaging (Vevo 770 system, VisualSonics, Toronto, ON, Canada) 30–32 using pulse-wave Doppler mode with a dedicated 16MHz probe. The average velocity of blood flow during 1 minute was determined by multiplying Velocity Time Integral by Heart Rate 33. Vascular resistance index was also calculated.
Detection of hypoxia in the kidneys using pimonidazole staining
Renal tissue hypoxia was detected using a Hypoxyprobe™ -1 Kit (HPI, Inc. Burlington, MA) following the manufacturer’s instruction. Briefly, pimonidazole hydrochloride was injected (60 mg/kg ip) 2 h before rats were sacrificed. Immunostaining were performed as we described before 34 using antibody against pimonidazole (1:200, rabbit antisera from the same kit). The percentage of positive staining area was calculated using a computer program (Image-Pro Plus) as described previously 35.
Morphological and immunohistochemical analysis
The fixed kidneys were paraffin-embedded and cut into 4-μm sections. For morphological analysis, the tissue sections were stained with PAS staining. Glomerular damage was morphologically evaluated by two independent examiners who were blinded as to animal groups and semiquantitatively scored based on the degree of glomerular damage as described previously 36–37. In brief, a minimum of 20 glomeruli in each specimen were examined and the severity of lesions were graded from 0 to 4 according to the percentage of glomerular involvement. Thus, 0 = normal; 1 = <25% of glomerular area involved; 2 = 25 to 50%; 3 = 50 to 75%; and 4 = >75% of tuft area involved. The averaged scores from counted glomeruli were used as the glomerular damage index for each animal. Immunostaining was performed as we described previously 34 using antibodies against rat vascular endothelial growth factor (VEGF, monoclonal, Millipore, 1:300) and α-smooth muscle actin (rabbit polyclonal, Abcam, 1:200). Collagen I/III was stained using picro sirius red and the percentage of positive staining area was calculated using a computer program (Image-Pro Plus) as described previously 35.
RNA extraction and quantitative RT-PCR analysis of HIF-1α mRNA levels in renal cortex and VEGF mRNA levels in isolated glomeruli
Total RNA from the renal cortex and isolated glomeruli was extracted using TRIzol solution (Life Technologies, Inc. Rockville MD) and then reverse-transcribed (RT) (cDNA Synthesis Kit, Bio-Rad, Hercules, CA). The RT products were amplified using TaqMan Gene Expression Assays kits (Applied Biosystems). The level of 18S ribosomal RNA was used as an endogenous control. The relative gene expressions were calculated in accordance with the ΔΔCt method. Relative mRNA levels were expressed by the values of 2−ΔΔCt.
Preparation of tissue homogenate and nuclear extracts and Western blot analyses for protein levels of HIF-1α, collagen I/III and α-smooth muscle actin
Renal tissue homogenates, nuclear protein preparations, and Western blot analyses were performed as described previously 34. Primary antibodies used in the present study included anti-rat HIF-1α (monoclonal, Novus Biologicals, 1:300 dilution), collagen I/III (rabbit polyclonal, Calbiochem, 1:300) and α-smooth muscle actin (rabbit polyclonal, Abcam, 1:1000). The intensities of the blots were determined using an imaging analysis program (ImageJ, free download from http://rsbweb.nih.gov/ij/).
Statistics
Data are presented as means ± SE. The significance of differences in mean values within and between multiple groups was evaluated using an ANOVA followed by a Duncan’s multiple range test. Student’s t-test was used to evaluate statistical significance of differences between two groups. P<0.05 was considered statistically significant.
RESULTS
Effects of HIF-1α shRNA on ANG II-induced activation of HIF-1α
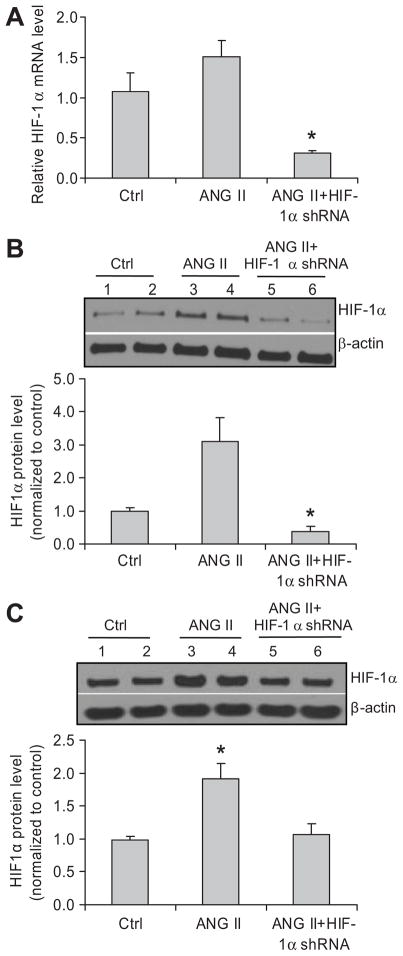
HIF-1α mRNA levels in renal cortex were knocked down by >70% in shRNA treated rats (figure 1A), indicating a successful silencing of HIF-1α gene expression in the kidneys. Renal HIF-1α protein levels were significantly increased in ANG II-infused rats, which is consistent with previous reports 19. Intra-renal transfection of HIF-1α shRNA plasmids abolished ANG II-induced increase in HIF-1α proteins in both renal cortical and medullary areas (figure 1B and C), which further confirmed successful knock-down of HIF-1α gene in the kidneys.
Figure 1. Effect of HIF-1α shRNA on ANG II-induced activation of HIF-1α in the kidneys.
Panel A: HIF-1α mRNA levels in the renal cortex by Real-time RT-PCR analysis; Panel B (cortex) and C (medulla): representative ECL gel documents of Western blot analyses depicting the protein levels of HIF-1α and summarized intensities of HIF-1α blots. *P < 0.05 vs. all other groups (n=6).
Effects of HIF-1α shRNA on creatinine clearance, ANG II-induced increases in urinary albumin excretion, and arterial pressure
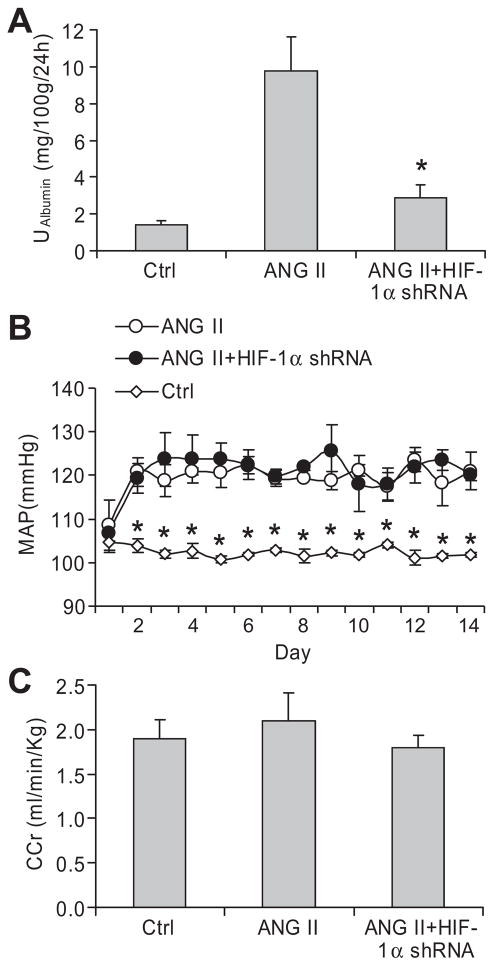
ANG II infusion caused considerable increases in urinary albumin levels, which were significantly attenuated in animals treated with HIF-1α shRNA (figure 2A), suggesting that activation of HIF-1α is involved in ANG II-induced glomerular injury. There was no difference in ANG II-induced increases in mean arterial pressure (MAP) between rats treated with ANG II + control plasmids and ANG II + HIF-1α shRNA plasmids (figure 2B), indicating that the effect of HIF-1α shRNA was not through the alteration of blood pressure. There was no difference in creatinine clearances among the three animal groups (Figure 2C). There was also no statistical difference in animal body weight at the end of the experiment, although there was a tendency toward lower body weight in ANG II group compared with control group. The body weights were 332.8 ± 21.6 g, 304.8 ± 7.4 g, and 318.7 ± 4.6 g in Control, ANG II, and ANG II+HIF-1α shRNA groups, respectively.
Figure 2. Effect of HIF-1α shRNA on ANG II-induced increases in urinary albumin excretion and mean arterial pressure.
A: 24 hour albumin excretion in urine; B: mean arterial blood pressure (MAP); C: Creatinine clearance (CCr). *P < 0.05 vs. all other groups (n=6).
Effects of HIF-1α shRNA on ANG II-induced histological changes of glomeruli
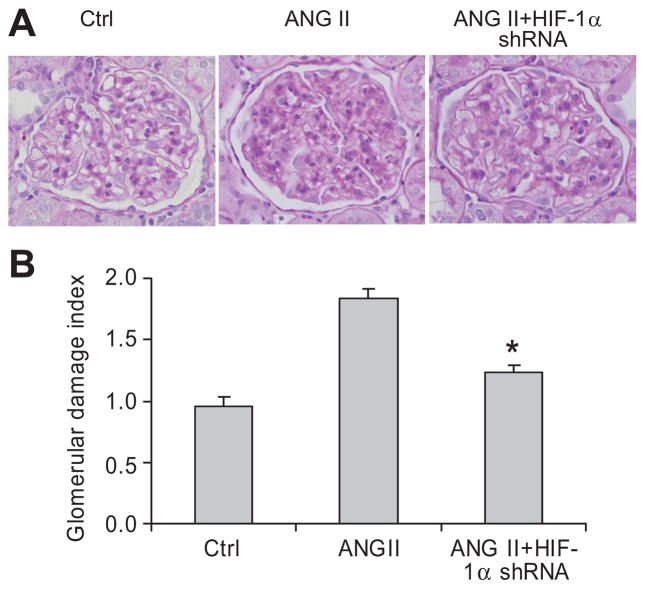
Morphological analysis showed that ANG II produced glomerular sclerotic damages as indicated by glomerular mesangial expansion with hypercellularity, capillary collapse, and fibrous deposition in glomeruli (figure 3A). The glomerular damage index was substantially higher in ANG II-treated rats (figure 3B). In HIF-1α shRNA-transfected rats, however, ANG II-induced glomerular damage was significantly diminished (figure 3A&B). These results were consistent with the data in urinary albumin excretion and further suggested that activation of HIF-1α mediates ANG II-induced glomerular injury.
Figure 3. Effect of HIF-1α shRNA on ANG II-induced morphological changes in the glomeruli.
A: Representative photomicrographs showing glomerular structures (PAS staining, 400X); B: Summarized glomerular damage index by semiquantitation of scores in different groups. * P < 0.05 vs. other two groups (n = 6).
Effects of HIF-1α shRNA on ANG II-induced increase in VEGF in glomeruli
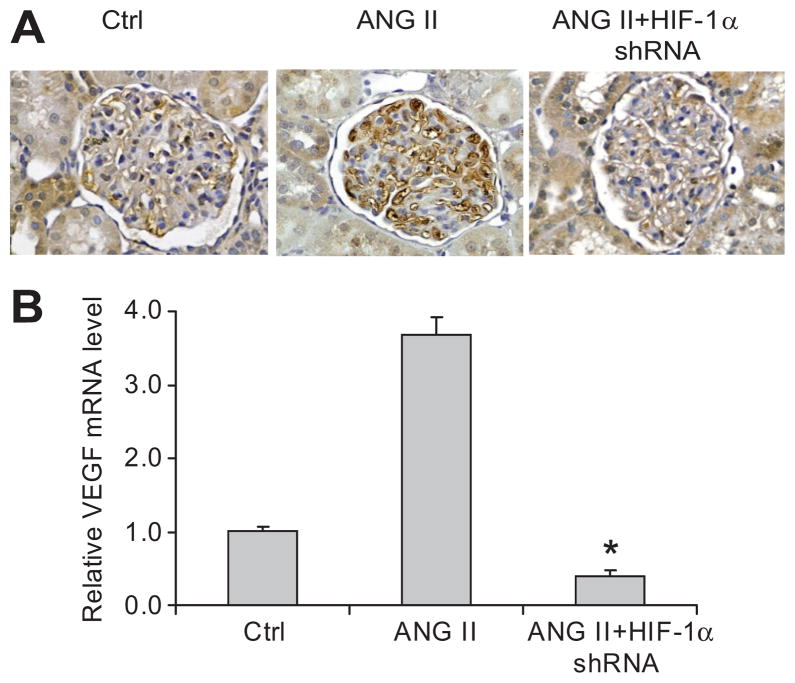
Both immunostaining and Real-Time RT-PCR showed that ANG II significantly increased VEGF expression in the glomeruli and that HIF-1α shRNA blocked ANG II-induced increase in VEGF (figure 4). These data indicated that HIF-1α-mediated activation of VEGF was involved in ANG II-induced glomerular injury.
Figure 4. Effect of HIF-1α shRNA on ANG II-induced increases of vascular endothelial growth factor (VEGF) in glomeruli.
A: Representative photomicrographs showing immunostaining of VEGF in glomeruli (brown color); B: HIF-1α mRNA levels in isolated glomeruli by Real-time RT-PCR analysis. * P < 0.05 vs. other two groups (n = 6).
Effects of HIF-1α shRNA on ANG II-induced interstitial injuries
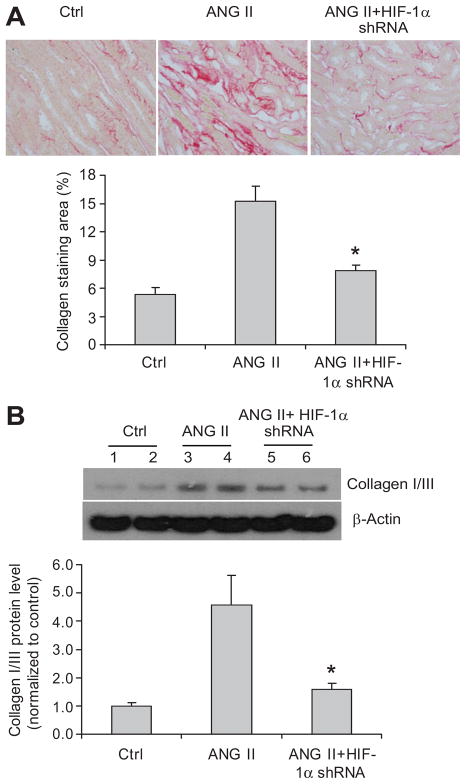
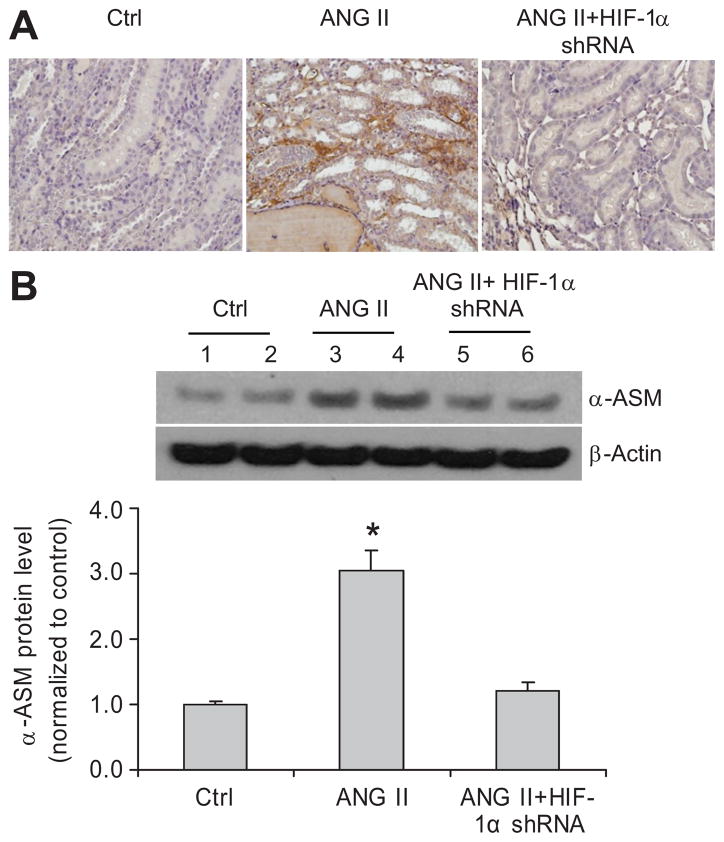
The positive staining of collagens and α-smooth muscle actin (SMA) in the outer medulla was used as the index of interstitial injuries. ANG II infusion significantly increased the positive staining area of collagens and α-SMA (figure 5A and 6A), which is consistent with previous reports 38–39. In rats treated with HIF-1α shRNA, ANG II-induced increases in the positive staining of collagens were significantly attenuated (figure 5A). Further quantitation of collagen I/III expression in the cortex by Western blot analyses also showed that ANG II increased the protein levels of collagens and that HIF-1α shRNA blocked the effect of ANG II on collagens (figure 5B), both of which are consistent with the results of collagen staining (figure 5A). The analysis of α-SMA expression showed the same pattern as that of collagens. ANG II increased the levels of α-SMA and HIF-1α shRNA blocked the effect of ANG II on α-SMA, as shown by immunostaining and immunoblotting analyses of α-SMA (figure 6). These data demonstrated that HIF-1α activation participated in the fibrotic effect of ANG II in the renal tubulointerstitial area.
Figure 5. Effect of HIF-1α shRNA on ANG II-induced increases of collagen I/III in the kidneys.
A: Representative photomicrographs showing staining of collagens in outer medulla (red color) and calculated percentage of positively stained area. B: representative ECL gel documents of Western blot analyses depicting the protein levels of collagens in renal cortex and summarized intensities of collagen blots. * P<0.05 vs. other two groups (n=6).
Figure 6. Effect of HIF-1α shRNA on ANG II-induced increases of α-smooth muscle actin (SMA) in the kidneys.
A: Representative photomicrographs showing staining of α-SMA in outer medulla (brown color). B: representative ECL gel documents of Western blot analyses depicting the protein levels of α-SMA in renal cortex and summarized intensities of α-SMA blots. * P<0.05 vs. other two groups (n=6).
Effects of HIF-1α shRNA on ANG II-induced ischemia/hypoxia in the kidneys
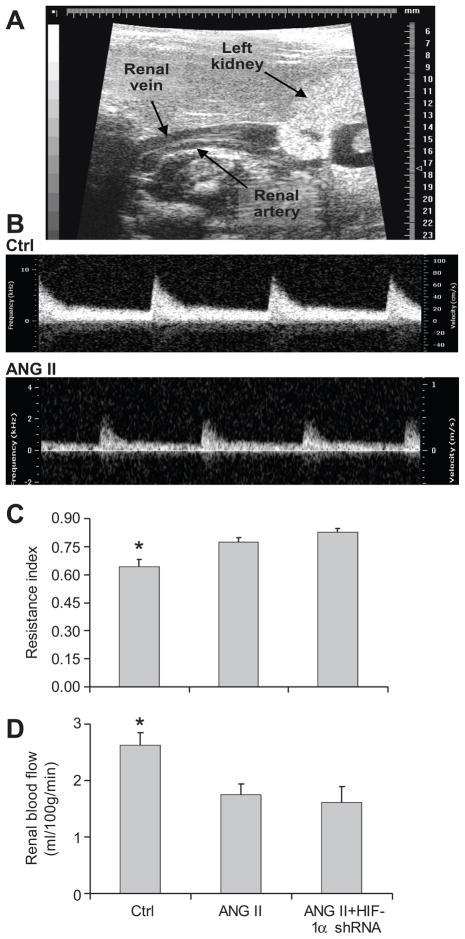
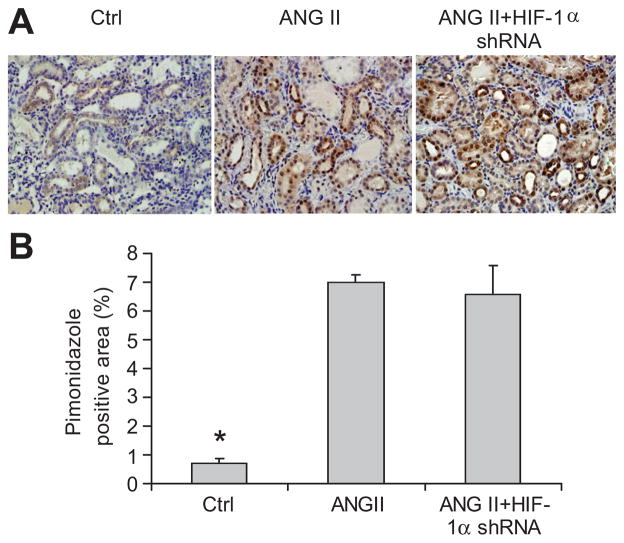
ANG II infusion significantly increased renal vascular resistance and reduced renal blood flow compared with control animals (figure 7). There was no significant difference in renal vascular resistance and blood flow between animals treated with ANG II+HIF-1 α shRNA and ANG II + control plasmids. Similarly, the areas of positive staining of hypoxia probe were largely increased in rats treated with ANG II compared with control rats, which exhibited weak and less positive staining, while HIF-1α shRNA did not affect ANG II-induced changes in the staining of hypoxia probe (figure 8). These data showed that ANG II caused ischemia/hypoxia in the kidneys, which was not influenced by HIF-1α shRNA.
Figure 7. Effect of HIF-1α shRNA on ANG II-induced decreases in renal blood flow.
A: Representative image showing renal artery. B: Representative images showing renal blood flow velocity Doppler waveform. C: Calculated renal vascular resistance index (RI). RI = (Vs−Vd)/Vs, Vs = peak systolic velocity, Vd = end-diastolic velocity. D: Summarized renal blood flow velocity. Velocity time integral (VTI) is the area under the curve of Doppler waveform. Multiplying the VTI by cross sectional area of renal artery (π × r2) and heart rate (HR) provides an estimate of the average velocity of blood flow (cm3) during 1 minute. * P<0.05 vs. other two groups (n=4–6).
Figure 8. Effect of HIF-1α shRNA on ANG II-induced hypoxia in the kidneys.
A: Representative photomicrographs showing the staining of hypoxia probe pimonidazole in outer medulla (brown color). B: Calculated percentage of positively stained area. * P<0.05 vs. other two groups (n=5–6).
Discussion
The present study showed that chronic infusion of ANG II increased HIF-1α levels in the kidneys and gene silencing of HIF-1α in the kidneys significantly attenuated ANG II-induced elevation of urinary albumin excretion, glomerular morphological changes, increase of glomerular VEGF expression, upregulation of α-SMA, and collagen accumulation. It is suggested that over-activation of HIF-1α in the kidney is a crucial mediator in ANG II-induced chronic renal injury.
ANG II is an important pathogenic factor involved in many different chronic kidney diseases1, 13–14, 16–17. The mechanisms by which ANG II produces renal injuries are not fully understood. Inhibition of urinary albumin excretion and glomerulosclerosis by HIF-1α shRNA in the present study demonstrated that HIF-1α-mediated gene activation participated in ANG II-induced glomerular damage. The effects of HIF-1α shRNA on HIF-1α levels were different in cortex and medulla. These differences may be attributable to regional differences in vascular architecture/distribution between the renal cortex and medulla, which bring 90% of the blood supply to the renal cortex and 10% to the renal medulla40–41. As a result, tissue blood perfusion in the medulla is about 30% of that in the cortex41. Therefore, the injected DNA via renal artery would be delivered into cortex more than medulla and consequently knocked down HIF-1α more in cortex than in medulla.
Many target genes of HIF-1α have been implicated in chronic renal injury, such as plasminogen activator inhibitor (PAI) and tissue inhibitor of metalloproteinase (TIMP) 6–9. VEGF is one of HIF-1α target genes and ANG II-stimulated podocyte-derived VEGF, as a glomerular permeability factor, has been suggested to be a major cause for the development of proteinuria in diabetic nephropathy 42–46. We therefore detected whether HIF-1α-mediated alteration of VEGF was involved in ANG II-induced glomerular damages. Our results showed that improvement of ANG II-induced glomerular damages was associated with inhibition of VEGF by HIF-1α shRNA, further suggesting that HIF-1α-mediated gene activation participates in ANG II-induced glomerular damage.
In addition, ANG II-induced renal tubulointerstitial damage contributes to the progression of chronic renal injury 12, 47–50. The present study also demonstrated that ANG II-induced tubulointerstitial damage was mediated by HIF-1α as indicated by the inhibition of collagen I/III and α-SMA accumulation in HIF-1α shRNA-treated animals. An interesting finding in the present study was that HIF-1α shRNA blocked ANG II-induced α-SMA. α-SMA is a well known marker of epithelial-mesenchymal transition (EMT). ANG II-induced EMT has been indicated as an important mechanism for the progression of chronic kidney diseases 12, 51. Although both HIF-1α 3, 9, 52 and ANG II 49, 53 have been shown to promote EMT, the interaction between HIF-1α and ANG II in the process of EMT remains unclear. We recently showed that HIF-1α mediated ANG II-induced cell transdifferentiation in cultured renal cells20. The present in vivo data further suggest that HIF-1α may participate in ANG II-induced EMT in the kidneys. Overall, results from the present study suggest that HIF-1α-mediated gene activation may represent a novel mechanistic pathway in ANG II-induced renal injury.
It has been demonstrated that hypertensive renal injury is largely dependent on renal perfusion pressure (RPP) 38, 54–55. The present study showed that HIF-1α shRNA remarkably attenuated renal injury without altering ANG II-induced hypertension, indicating that HIF-1α may be also involved in kidney damage caused by increased RPP. Whether increased RPP stimulates HIF-1α and its detailed role in renal injury need to be clarified in future investigations.
Because ischemia/hypoxia is involved in ANG II-induced renal injury 56–57, we then assessed whether possible changes in the status of hypoxia/oxygenation contributed to the beneficial effects of HIF-1α shRNA. Our results showed that the attenuation of renal injury by HIF-1α shRNA was not through the improvement in renal blood perfusion/oxygenation. Therefore, the beneficial effect of HIF-1α shRNA would be probably through inhibition of HIF-1α-mediated gene activations associated with renal injuries.
The present study demonstrated that accumulation of HIF-1α by ANG II is a critical mediator in ANG II-induced kidney damage. How ANG II activates HIF-1α requires further clarification. In this regard, a direct stimulating effect by ANG II and ANG II-induced ischemia/hypoxia may have contributed to HIF-1α activation in the present study. Consistent with our results, previous reports have also demonstrated that ANG II reduces renal blood perfusion 56–57 and produces hypoxia in the kidneys 56. Meanwhile, ANG II has been shown to stimulate HIF-1α under normoxia as well18–20. Therefore, activation of HIF-1α may be through both direct effect and ischemic effect of ANG II. One possible pathway mediating ANG II-induced HIF-1α activation would be HIF-prolyl hydroxylases, the enzymes that promote the degradation of HIF-1α 58–59. The activities of HIF-prolyl hydroxylases is inhibited by low oxygen tension 58–59 and also regulated by mechanisms independent of oxygen levels 60–63, such as redox signals 20, 60–61. These oxygen-dependent and -independent mechanisms may be involved in ANG II-induced HIF-1α activation in the kidneys. ANG II may inhibit HIF-prolyl hydroxylase to consequently activate HIF-1α, which needs to be elucidated in future.
It should be noted that there are controversial reports regarding the role of HIF-1α in chronic renal injury. It was previously reported that genetic ablation of renal epithelial HIF-1α inhibited the development of renal tubulointerstitial fibrosis in unilateral ureteral obstruction rats 9. Conversely, it was shown that induction of HIF-1α by CoCl2 ameliorated the renal injuries in progressive Thy1 nephritis rat model 64 and in obese, hypertensive type 2 diabetes rat model 65. More puzzling evidence on this topic came from reports that used the same animal model and obtained conflicting results regarding the role of HIF-1α in chronic renal injury. One study demonstrated that stable expression of HIF-1α in tubular epithelial cells promoted interstitial fibrosis in 5/6 nephrectomy mice 15, while two other reports showed that upregulation of HIF-1α by either CoCl2 or dimethyloxalylglycine protected tubulointerstitium in 5/6 nephrectomy rats 66–67. These discrepancies might be attributed to the differences in disease models, disease stages, and approaches to manipulate HIF-1α.
By comparing the above controversial studies, those that employed genetic approaches to manipulate HIF-1α levels, including upregulation and downregulation, showed that HIF-1α was an injurious mediator, while all the studies using pharmacological approaches to increase HIF-1α levels showed that HIF-1α was a protective factor. The difference between gene manipulation and pharmacological intervention may be the specificity of targeting HIF-1α. Nonspecific actions in addition to stimulating HIF-1α by pharmacological interventions can not be totally ruled out. In this regard, it has been shown that the reagents used to up-regulate HIF-1α levels execute some other actions independent of HIF-1α induction 68–69. Commonly used hypoxia mimetic agents such as cobalt, nickel, and desferrioxamine interfere with iron metabolism and may interrupt many other iron-dependent enzymes 68–69. Dimethyloxalylglycine is an analog of 2-oxoglutarate and may act as inhibitors of many other oxoglutarate-dependent enzymes in addition to HIF prolyl-hydroxylases 69–70. Therefore, the protective role of HIF-1α in those studies using pharmacological reagents may not be firmly concluded until it could be shown that blockade of HIF-1α upregulation eliminates the renal protective effect by those pharmacological reagents. Apparently, more detailed investigations are required regarding the role of HIF-1α pathway in chronic kidney diseases under different situations. There may be possibilities that activation of HIF-1α is injurious under certain conditions and protective in some other situations, which needs to be clarified in future studies. Nonetheless, our results provided strong evidence that long-term over-activation of HIF-1α mediated ANG II-induced renal damages in the model used in the present study.
Perspectives
The present study demonstrated that blockade of HIF-1α accumulation attenuated ANG II-induced renal injury. It is suggested that over-activation of HIF-1α-mediated gene regulation in the kidney may constitute a new pathogenic pathway mediating renal injury under various pathological conditions associated with excessive ANG II and that normalization of over-activated HIF-1α may be a useful strategy in the treatment of chronic renal injury with elevated levels of ANG II.
Acknowledgments
Source of Funding
National Institutes of Health Grant HL-89563 and DK-54927
Footnotes
Conflict of Interest
None
References
- 1.Nangaku M, Fujita T. Activation of the renin-angiotensin system and chronic hypoxia of the kidney. Hypertens Res. 2008;31:175–184. doi: 10.1291/hypres.31.175. [DOI] [PubMed] [Google Scholar]
- 2.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 3.Higgins DF, Kimura K, Iwano M, Haase VH. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 2008;7:1128–1132. doi: 10.4161/cc.7.9.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haase VH. Pathophysiological Consequences of HIF Activation. Annals of the New York Academy of Sciences. 2009;1177:57–65. doi: 10.1111/j.1749-6632.2009.05030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nangaku M, Eckardt KU. Hypoxia and the HIF system in kidney disease. J Mol Med. 2007;85:1325–1330. doi: 10.1007/s00109-007-0278-y. [DOI] [PubMed] [Google Scholar]
- 6.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 7.Norman JT, Orphanides C, Garcia P, Fine LG. Hypoxia-induced changes in extracellular matrix metabolism in renal cells. Exp Nephrol. 1999;7:463–469. doi: 10.1159/000020625. [DOI] [PubMed] [Google Scholar]
- 8.Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- 9.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberger C, Rosen S, Shina A, Frei U, Eckardt K-U, Flippin LA, Arend M, Klaus SJ, Heyman SN. Activation of hypoxia-inducible factors ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrol Dial Transplant. 2008;23:3472–3478. doi: 10.1093/ndt/gfn276. [DOI] [PubMed] [Google Scholar]
- 11.Hill P, Shukla D, Tran MGB, Aragones J, Cook HT, Carmeliet P, Maxwell PH. Inhibition of Hypoxia Inducible Factor Hydroxylases Protects Against Renal Ischemia-Reperfusion Injury. J Am Soc Nephrol. 2008;19:39–46. doi: 10.1681/ASN.2006090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwano M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens. 2004;13:279–284. doi: 10.1097/00041552-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Klahr S, Morrissey J. Progression of chronic renal disease. Am J Kidney Dis. 2003;41:S3–7. doi: 10.1053/ajkd.2003.50074. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell MP. Renal tubulointerstitial fibrosis. New thoughts on its development and progression. Postgrad Med. 2000;108:159–162. 165, 171–152. doi: 10.3810/pgm.2000.07.1155. [DOI] [PubMed] [Google Scholar]
- 15.Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, Haase VH, Saito Y. Stable expression of HIF-1{alpha} in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol. 2008;295:F1023–1029. doi: 10.1152/ajprenal.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-Ortega M, Ruperez M, Esteban V, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant. 2006;21:16–20. doi: 10.1093/ndt/gfi265. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Wang J, Zhou F, Wang X, Feng Z. STAT proteins mediate angiotensin II-induced production of TIMP-1 in human proximal tubular epithelial cells. Kidney Int. 2003;64:459–467. doi: 10.1046/j.1523-1755.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen TH, Wang JF, Chan P, Lee HM. Angiotensin II stimulates hypoxia-inducible factor 1alpha accumulation in glomerular mesangial cells. Ann N Y Acad Sci. 2005;1042:286–293. doi: 10.1196/annals.1338.051. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Lopez E, Lopez AF, Esteban V, Yague S, Egido J, Ruiz-Ortega MMVA-A. Angiotensin II Regulates Vascular Endothelial Growth Factor via Hypoxia-Inducible Factor-1alpha Induction and Redox Mechanisms in the Kidney. Antioxidants & Redox Signaling. 2005;7:1275–1284. doi: 10.1089/ars.2005.7.1275. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Tang L, Zhu Q, Yi F, Zhang F, Li PL, Li N. Hypoxia-inducible factor-1alpha contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int. 2011;79:300–310. doi: 10.1038/ki.2010.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan HY, Mu W, Tomita N, Huang XR, Li JH, Zhu H-J, Morishita R, Johnson RJ. Inhibition of Renal Fibrosis by Gene Transfer of Inducible Smad7 Using Ultrasound-Microbubble System in Rat UUO Model. J Am Soc Nephrol. 2003;14:1535–1548. doi: 10.1097/01.asn.0000067632.04658.b8. [DOI] [PubMed] [Google Scholar]
- 22.Hou C-C, Wang W, Huang XR, Fu P, Chen T-H, Sheikh-Hamad D, Lan HY. Ultrasound-Microbubble-Mediated Gene Transfer of Inducible Smad7 Blocks Transforming Growth Factor-{beta} Signaling and Fibrosis in Rat Remnant Kidney. Am J Pathol. 2005;166:761–771. doi: 10.1016/s0002-9440(10)62297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng Y-Y, Hou C-C, Wang W, Huang XR, Lan HY. Blockade of NF B activation and renal inflammation by ultrasound-mediated gene transfer of Smad7 in rat remnant kidney. Kidney International. 2005;67:s83–s91. doi: 10.1111/j.1523-1755.2005.09421.x. [DOI] [PubMed] [Google Scholar]
- 24.Koike H, Tomita N, Azuma H, Taniyama Y, Yamasaki K, Kunugiza Y, Tachibana K, Ogihara T, Morishita R. An efficient gene transfer method mediated by ultrasound and microbubbles into the kidney. J Gene Med. 2005;7:108–116. doi: 10.1002/jgm.632. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Chen L, Yi F, Xia M, Li P-L. Salt-Sensitive Hypertension Induced by Decoy of Transcription Factor Hypoxia-Inducible Factor-1{alpha} in the Renal Medulla. Circ Res. 2008;102:1101–1108. doi: 10.1161/CIRCRESAHA.107.169201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi F, Xia M, Li N, Zhang C, Tang L, Li P-L. Contribution of Guanine Nucleotide Exchange Factor Vav2 to Hyperhomocysteinemic Glomerulosclerosis in Rats. Hypertension. 2009;53:90–96. doi: 10.1161/HYPERTENSIONAHA.108.115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li N, Yi F, dos Santos EA, Donley DK, Li P-L. Role of Renal Medullary Heme Oxygenase in the Regulation of Pressure Natriuresis and Arterial Blood Pressure. Hypertension. 2007;49:148–154. doi: 10.1161/01.HYP.0000250086.06137.fb. [DOI] [PubMed] [Google Scholar]
- 28.Goel M, Sinkins WG, Zuo C-D, Estacion M, Schilling WP. Identification and localization of TRPC channels in the rat kidney. Am J Physiol Renal Physiol. 2006;290:F1241–1252. doi: 10.1152/ajprenal.00376.2005. [DOI] [PubMed] [Google Scholar]
- 29.Szczypka MS, Westover AJ, Clouthier SG, Ferrara JL, Humes HD. Rare Incorporation of Bone Marrow-Derived Cells Into Kidney After Folic Acid-Induced Injury. STEM CELLS. 2005;23:44–54. doi: 10.1634/stemcells.2004-0111. [DOI] [PubMed] [Google Scholar]
- 30.Cook JS, Sauder CL, Ray CA. Melatonin differentially affects vascular blood flow in humans. Am J Physiol Heart Circ Physiol. 2011;300:H670–674. doi: 10.1152/ajpheart.00710.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dieterle F, Marrer E, Suzuki E, Grenet O, Cordier A, Vonderscher J. Monitoring kidney safety in drug development: emerging technologies and their implications. Curr Opin Drug Discov Devel. 2008;11:60–71. [PubMed] [Google Scholar]
- 32.Sullivan JC, Wang B, Boesen EI, D’Angelo G, Pollock JS, Pollock DM. Novel use of ultrasound to examine regional blood flow in the mouse kidney. Am J Physiol Renal Physiol. 2009;297:F228–235. doi: 10.1152/ajprenal.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberg BB, McGahan JP. Atlas of ultrasound measurements. 2. Philadelphia, PA: Mosby Elsevier Health Sciences; 2006. [Google Scholar]
- 34.Li N, Yi F, Sundy CM, Chen L, Hilliker ML, Donley DK, Muldoon DB, Li PL. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol. 2007;292:F207–216. doi: 10.1152/ajprenal.00457.2005. [DOI] [PubMed] [Google Scholar]
- 35.Turnberg D, Lewis M, Moss J, Xu Y, Botto M, Cook HT. Complement Activation Contributes to Both Glomerular and Tubulointerstitial Damage in Adriamycin Nephropathy in Mice. J Immunol. 2006;177:4094–4102. doi: 10.4049/jimmunol.177.6.4094. [DOI] [PubMed] [Google Scholar]
- 36.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984;26:137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 37.Nangaku M, Yamada K, Gariepy CE, Miyata T, Inagi R, Kurokawa K, Yanagisawa M, Fujita T, Johnson RJ. ETB receptor protects the tubulointerstitium in experimental thrombotic microangiopathy. Kidney Int. 2002;62:922–928. doi: 10.1046/j.1523-1755.2002.00519.x. [DOI] [PubMed] [Google Scholar]
- 38.Mori T, Cowley AW., Jr Role of Pressure in Angiotensin II-Induced Renal Injury: Chronic Servo-Control of Renal Perfusion Pressure in Rats. Hypertension. 2004;43:752–759. doi: 10.1161/01.HYP.0000120971.49659.6a. [DOI] [PubMed] [Google Scholar]
- 39.Wolak T, Kim H, Ren Y, Kim J, Vaziri ND, Nicholas SB. Osteopontin modulates angiotensin II-induced inflammation, oxidative stress, and fibrosis of the kidney. Kidney Int. 2009;76:32–43. doi: 10.1038/ki.2009.90. [DOI] [PubMed] [Google Scholar]
- 40.Evans RG, Eppel GA, Anderson WP, Denton KM. Mechanisms underlying the differential control of blood flow in the renal medulla and cortex. J Hypertens. 2004;22:1439–1451. doi: 10.1097/01.hjh.0000133744.85490.9d. [DOI] [PubMed] [Google Scholar]
- 41.Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2003;284:R13–27. doi: 10.1152/ajpregu.00321.2002. [DOI] [PubMed] [Google Scholar]
- 42.Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 43.Wolf G, Ziyadeh FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol. 2007;106:p26–31. doi: 10.1159/000101797. [DOI] [PubMed] [Google Scholar]
- 44.Kang YS, Park YG, Kim BK, Han SY, Jee YH, Han KH, Lee MH, Song HK, Cha DR, Kang SW, Han DS. Angiotensin II stimulates the synthesis of vascular endothelial growth factor through the p38 mitogen activated protein kinase pathway in cultured mouse podocytes. J Mol Endocrinol. 2006;36:377–388. doi: 10.1677/jme.1.02033. [DOI] [PubMed] [Google Scholar]
- 45.Chen S, Lee JS, Iglesias-de la Cruz MC, Wang A, Izquierdo-Lahuerta A, Gandhi NK, Danesh FR, Wolf G, Ziyadeh FN. Angiotensin II stimulates {alpha}3(IV) collagen production in mouse podocytes via TGF-{beta} and VEGF signalling: implications for diabetic glomerulopathy. Nephrol Dial Transplant. 2005;20:1320–1328. doi: 10.1093/ndt/gfh837. [DOI] [PubMed] [Google Scholar]
- 46.Ziyadeh FN. Different roles for TGF-beta and VEGF in the pathogenesis of the cardinal features of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82 (Suppl 1):S38–41. doi: 10.1016/j.diabres.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Ruster C, Wolf G. Renin-Angiotensin-Aldosterone System and Progression of Renal Disease. J Am Soc Nephrol. 2006;17:2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- 48.Suga S, Mazzali M, Ray PE, Kang DH, Johnson RJ. Angiotensin II type 1 receptor blockade ameliorates tubulointerstitial injury induced by chronic potassium deficiency. Kidney Int. 2002;61:951–958. doi: 10.1046/j.1523-1755.2002.00208.x. [DOI] [PubMed] [Google Scholar]
- 49.Lan HY. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opin Nephrol Hypertens. 2003;12:25–29. doi: 10.1097/00041552-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 51.Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-[beta] pathway. Kidney Int. 2006;70:1914–1919. doi: 10.1038/sj.ki.5001846. [DOI] [PubMed] [Google Scholar]
- 52.Luo Y, He DL, Ning L, Shen SL, Li L, Li X. Hypoxia-inducible factor-1alpha induces the epithelial-mesenchymal transition of human prostatecancer cells. Chin Med J (Engl) 2006;119:713–718. [PubMed] [Google Scholar]
- 53.Carvajal G, Rodriguez-Vita J, Rodrigues-Diez R, Sanchez-Lopez E, Ruperez M, Cartier C, Esteban V, Ortiz A, Egido J, Mezzano SA, Ruiz-Ortega M. Angiotensin II activates the Smad pathway during epithelial mesenchymal transdifferentiation. Kidney Int. 2008;74:585–595. doi: 10.1038/ki.2008.213. [DOI] [PubMed] [Google Scholar]
- 54.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW., Jr High Perfusion Pressure Accelerates Renal Injury in Salt-Sensitive Hypertension. J Am Soc Nephrol. 2008;19:1472–1482. doi: 10.1681/ASN.2007121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia M, Li PL, Li N. Telemetric signal-driven servocontrol of renal perfusion pressure in acute and chronic rat experiments. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1494–1501. doi: 10.1152/ajpregu.90631.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujimoto S, Satoh M, Nagasu H, Horike H, Sasaki T, Kashihara N. Azelnidipine exerts renoprotective effects by improvement of renal microcirculation in angiotensin II infusion rats. Nephrol Dial Transplant. 2009;24:3651–3658. doi: 10.1093/ndt/gfp407. [DOI] [PubMed] [Google Scholar]
- 57.Thomas MC, Tikellis C, Burns WM, Bialkowski K, Cao Z, Coughlan MT, Jandeleit-Dahm K, Cooper ME, Forbes JM. Interactions between renin angiotensin system and advanced glycation in the kidney. J Am Soc Nephrol. 2005;16:2976–2984. doi: 10.1681/ASN.2005010013. [DOI] [PubMed] [Google Scholar]
- 58.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 59.Jaakkola P, Mole DR, Tian Y-M, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim Av, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 60.Callapina M, Zhou J, Schnitzer S, Metzen E, Lohr C, Deitmer JW, Brune B. Nitric oxide reverses desferrioxamine- and hypoxia-evoked HIF-1[alpha] accumulation--Implications for prolyl hydroxylase activity and iron. Experimental Cell Research. 2005;306:274–284. doi: 10.1016/j.yexcr.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 61.Page EL, Chan DA, Giaccia AJ, Levine M, Richard DE. Hypoxia-inducible Factor-1{alpha} Stabilization in Nonhypoxic Conditions: Role of Oxidation and Intracellular Ascorbate Depletion. Mol Biol Cell. 2008;19:86–94. doi: 10.1091/mbc.E07-06-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming Growth Factor beta1 Induces Hypoxia-inducible Factor-1 Stabilization through Selective Inhibition of PHD2 Expression. J Biol Chem. 2006;281:24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 63.Tug S, Reyes BD, Fandrey J, Berchner-Pfannschmidt U. Non-hypoxic activation of the negative regulatory feedback loop of prolyl-hydroxylase oxygen sensors. Biochemical and Biophysical Research Communications. 2009;384:519–523. doi: 10.1016/j.bbrc.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka T, Matsumoto M, Inagi R, Miyata T, Kojima I, Ohse T, Fujita T, Nangaku M. Induction of protective genes by cobalt ameliorates tubulointerstitial injury in the progressive Thy1 nephritis. Kidney Int. 2005;68:2714–2725. doi: 10.1111/j.1523-1755.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 65.Ohtomo S, Nangaku M, Izuhara Y, Takizawa S, Strihou CvYd, Miyata T. Cobalt ameliorates renal injury in an obese, hypertensive type 2 diabetes rat model. Nephrol Dial Transplant. 2008;23:1166–1172. doi: 10.1093/ndt/gfm715. [DOI] [PubMed] [Google Scholar]
- 66.Song YR, You SJ, Lee YM, Chin HJ, Chae DW, Oh YK, Joo KW, Han JS, Na KY. Activation of hypoxia-inducible factor attenuates renal injury in rat remnant kidney. Nephrol Dial Transplant. 2010;25:77–85. doi: 10.1093/ndt/gfp454. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka T, Kojima I, Ohse T, Ingelfinger JR, Adler S, Fujita T, Nangaku M. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest. 2005;85:1292–1307. doi: 10.1038/labinvest.3700328. [DOI] [PubMed] [Google Scholar]
- 68.Guo M, Song LP, Jiang Y, Liu W, Yu Y, Chen GQ. Hypoxia-mimetic agents desferrioxamine and cobalt chloride induce leukemic cell apoptosis through different hypoxia-inducible factor-1α independent mechanisms. Apoptosis. 2006;11:67–77. doi: 10.1007/s10495-005-3085-3. [DOI] [PubMed] [Google Scholar]
- 69.Davidson T, Salnikow K, Costa M. Hypoxia Inducible Factor-1α-Independent Suppression of Aryl Hydrocarbon Receptor-Regulated Genes by Nickel. Molecular Pharmacology. 2003;64:1485–1493. doi: 10.1124/mol.64.6.1485. [DOI] [PubMed] [Google Scholar]
- 70.Kivirikko KI, Myllyharju J. Prolyl 4-hydroxylases and their protein disulfide isomerase subunit. Matrix Biol. 1998;16:357–368. doi: 10.1016/s0945-053x(98)90009-9. [DOI] [PubMed] [Google Scholar]