Abstract
Vertically aligned, laterally spaced nanoscale titanium nanotubes were grown on a titanium surface by anodization, and the growth of chondroprogenitors on the resulting surfaces was investigated. Surfaces bearing nanotubes of 70 to 100 nm in diameter were found to trigger the morphological transition to a cortical actin pattern and rounded cell shape (both indicative of chondrocytic differentiation), as well as the up-regulation of type II collagen and integrin β4 protein expression through the down-regulation of Erk activity. Inhibition of Erk signaling reduced stress fiber formation and induced the transition to the cortical actin pattern in cells cultured on 30-nm-diameter nanotubes, which maintained their fibroblastoid morphologies in the absence of Erk inhibition. Collectively, these results indicate that a titanium-based nanotube surface can support chondrocytic functions among chondroprogenitors, and may therefore be useful for future cartilaginous applications.
Keywords: cell differentiation, chondrocytes, integrin β4, microfilaments, nanotubes, titanium dioxide
Introduction
New developments in nanotechnology are quickly penetrating the fields of basic biology and medicine. Nanotechnology is poised to provide new tools for investigating biosystems, overcome existing challenges in biotechnology and biomedicine, and form the basis for building advanced biomaterials on new platforms. At present, titanium (Ti) and its alloys are frequently used for dental and orthopedic implants because they offer the benefits of low toxicity, superior corrosion resistance, favorable mechanical properties, and good biocompatibility. Ti is a bioinert material; once Ti-based biomedical devices are implanted into the living body, they are encapsulated by fibrous tissues that isolate them from the surrounding tissues.
It has been speculated that osteoblast (bone-forming cell) functions could be enhanced in vitro by unique nanometer-scale surface features of the culture substratum (Webster and Ejiofor, 2004). Nanoscale morphology is believed to play an important role in bone growth, which takes place in the pores. Studies have shown that nanoscale features can mimic the natural environment of bone cells, and osteoblasts on nanophase metallic implants were found to have increased adhesion and calcium/phosphorus mineral deposition (Webster and Ejiofor, 2004). However, relatively few studies have examined the interactions between bone cells and the surfaces of anodically grown TiO2 nanotubes. Here, we used an anodization process to grow nanoporous TiO2 on a Ti surface, and then evaluated the behavior of chondroprogenitors cultured on this surface.
During development, most bone forms through endochondral ossification, wherein the bones are first laid down as cartilaginous precursors (Karsenty and Wagner, 2002). This process involves a precise series of events that include the aggregation and differentiation of mesenchymal cells, and the proliferation, hypertrophy and death of chondrocytes (Delise et al., 2000). Chondrogenesis is characterized by a drastic change in cell shape, from fibroblastoid to round or polygonal (von der Mark and von der Mark, 1977). Chondrocytes display mostly cortical organization of their actin filaments, whereas precursor cells and dedifferentiated chondrocytes have more fibrillar actin organizations (Idowu et al., 2000; Langelier et al., 2000). The molecular mechanisms responsible for these transitions are largely unknown, but the reorganization of actin filaments is known to be a critical regulatory factor for chondrogenesis (Daniels and Solursh, 1991; Kim et al., 2003). Unfortunately, chondrocytes can lose their chondrogenic potential and dedifferentiate when moved from a three-dimensional architecture to two-dimensional (2D) culture.
Here, we studied the in vitro behavior, morphology and cell adhesion of chondroprogenitors cultured on vertically aligned Ti nanotubes having various diameters. The cells underwent morphological transitions to cortical actin patterns and rounded cell shape, both of which are indicative of chondrocytic differentiation. Thus, our results suggest that 2D culture on TiO2 may facilitate the use of such cells for a variety of therapeutic applications aimed at treating cartilage-degenerating diseases, including osteoarthritis.
Results and Discussion
Morphological transition is essential for the differentiation and redifferentiation of chondrocytes
The ultimate goal in tissue engineering is to recreate the native architecture to a degree capable of supporting the growth and expansion of progenitor cells (e.g., chondrocytes or mesenchymal stem cells), and facilitate their free diffusion and movement throughout the structure. Several culture methods have been devised to overcome the tendency of chondrocytes to dedifferentiate when subjected to 2D culture. For example, researchers have used polysaccharide-based hydrogels, includeing agarose, chitosan and alginates (Suh and Matthew, 2000), as culture substrates. Chitosan is a partially deacetylated product of chitin that has film-forming properties, mimics the natural environment found in the living articular cartilage matrix, and has been shown to help chondrocytes maintain their rounded cell shape when used as a culture substrate (Lahiji et al., 2000; Suh and Matthew, 2000).
During cartilaginous development, mesenchymal cells differentiate into chondrocytes and express cartilage-specific marker molecules, such as type II collagen and proteoglycans (Benya and Shaffer, 1982; Cancedda et al., 1995). However, when grown in monolayers either serially or for prolonged periods, chondrocytes become flattened and fibroblastic in morphology, and synthesize type I collagen instead of type II collagen (Benya and Shaffer, 1982; Bonaventure et al., 1994; Loty et al., 1995; Martin et al., 1999; Schulze-Tanzil et al., 2002). These dedifferentiated cells can recover their expression of cartilage-specific molecules when cultured in three- dimensional matrices such as agarose (Benya and Shaffer, 1982), collagen (Thenet et al., 1992), and alginate (Bonaventure et al., 1994; Binette et al., 1998; Lemare et al., 1998; Liu et al., 1998).
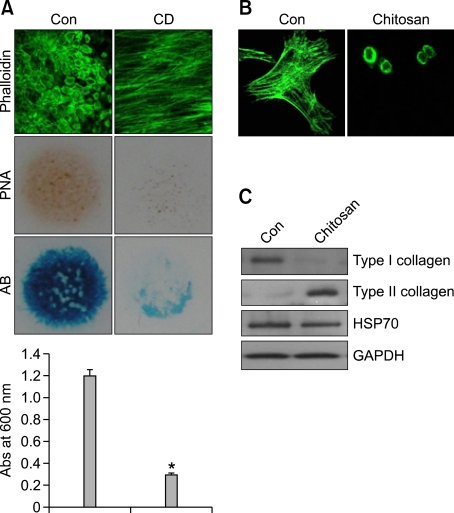
To examine the importance of actin cytoskeletal rearrangement, mesenchymal cells were treated with cytochalasin D (CD) which causes disruption of the actin cytoskeleton. Treatment with CD, which causes disruption of the actin cytoskeleton, inhibited precartilage condensation and chondrogenic differentiation, as assessed by PNA staining and alcian blue staining of sulfated proteoglycans, respectively (Figure 1A) indicating that modulation of actin cytoskeleton is vital for chondrogenesis.
Figure 1.
Morphological transition to a cortical actin pattern is required for chondrogenic differentiation of limb mesenchymal cells. (A) Differentiation-competent mesenchymal cells were prepared as described in the Methods section. Cells were grown on coverslips, treated with cytochalasin D (CD), stained with PNA on day 3 of culture, immunostained for F-actin with Alexa488-phalloidin on day 4 of culture, or stained with Alcian blue on day 5 of culture. Dedifferentiated chondrocytes were cultured on a chitosan membrane. (B) Cells were stained with Alexa-488-conjugated phalloidin. (C) Changes in the protein levels of type I and type II collagen were examined by Western blotting. The data shown are representative of at least four independent experiments. *, statistically different from control cells (P < 0.001).
Differentiated chondrocytes were obtained from day-7 micromass cultures, subcultured to monolayer, and passaged every 2-3 days until passage 4 (P4). During this time, we monitored the progressive loss of their differentiated phenotypes. We then transferred the P4 cells to a chitosan membrane and examined their redifferentiation. After 5 days of culture on the chitosan membrane, the dedifferentiated chondrocytes had regained their differentiated phenotypes. Furthermore, immunocytochemistry and Western blot analysis showed that these cells harbored increased expression of type II collagen, a marker for differentiation, and decreased expression of type I collagen, a marker for dedifferentiation (Figures 1B and 1C).
Growth on TiO2 nanotubes causes apparent chondrocytic differentiation of chondroprogenitors
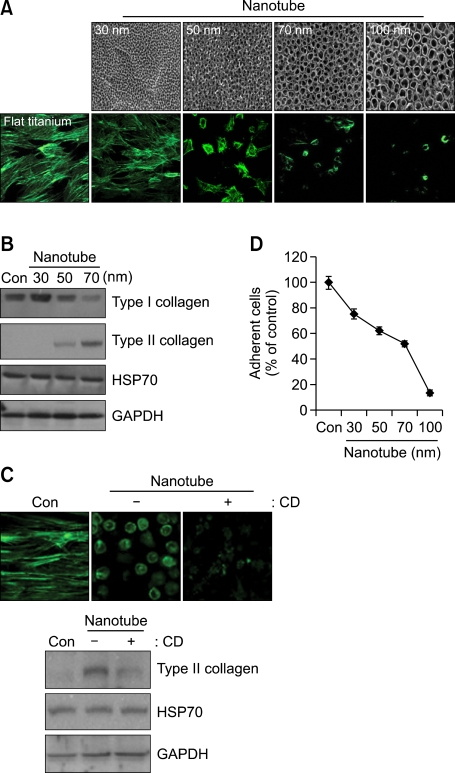
Several recent studies showed that a nanotube-bearing surface allows dynamic and coordinated changes in cytoskeletal organization, locomotion, and cell-to-cell communication in a great variety of cell systems, including dendritic cells, monocytes, human embryonic kidney 293T cells, normal rat kidney cells, primary macrophages, T cells, activated B cells, and human glioblastoma cells (Ryu et al., 2005; Dumortier et al., 2006; Brammer et al., 2008; Gurke et al., 2008; Kaiser et al., 2008; Williams et al., 2008; Park et al., 2009). Since morphological transition via reorganization of the actin cytoskeleton is essential for chondrocytic differentiation, we hypothesized that a nanotube-bearing surface could be a useful culture substrate for maintaining chondrocytic phenotypes and allowing chondrocytes to differentiate. However, no previous study has examined chondrocytic differentiation on a nanotube-bearing surface. In the present study, we examined the behavior of chondroprogenitors on a Ti surface that had been oxidized by anodization in 0.5% HF at 20 V for 20 min. Scanning electron microscopic (SEM) images indicated that the inner diameters of the nanotube-like structures ranged from 30 to 100 nm (Figure 2A, upper panel). Chondroprogenitors cultured on control Ti surfaces showed elongated and flattened morphologies. In contrast, cells cultured on nanotubes having inner diameters of 70 and 100 nm showed the rounded cells shape and cortical actin organizations characteristic of chondrocytes (Figure 2A, lower panel). Western blotting confirmed that chondroprogenitors grown on the nanotube surfaces showed increased expression of type II collagen (Figure 2B). Notably, this apparent nanotube-induced chondrogenic differentiation was inhibited by cytochalasin D-induced disruption of the actin cytoskeleton (Figure 2C).
Figure 2.
Culture on a TiO2 nanotube surface affects the cell morphology and actin cytoskeleton of chondroprogenitors. Wing limb mesenchymal cells were cultured on Ti-control (flat titanium) and TiO2 nanotube surfaces having different inner diameters (SEM images). (A) Immunocytochemistry with Alexa-488-conjugated phalloidin. (B) Changes in the protein levels of Type I and type II collagen were examined by Western blotting. (C) Cells were cultured on Ti-control (flat titanium) and 70-nm-diameter TiO2 nanotube surfaces in the absence or presence of cytochalasin D (CD). Cells were stained with Alexa-488-conjugated phalloidin, and changes in the protein level of type II collagen were examined by Western blotting. (D) The number of adhered cells was counted. The data shown are representative of at least four independent experiments. The mean is plotted and the error bars represent 95% CI (lower/upper limit).
The unique tube-like structures of nanotubes have been shown to provide added surface area and reactive sites for protein interactions, and to promote osteoblast adhesion (Webster and Ejifor, 2004). In contrast, we herein observed that the attachment of chondroprogenitors culture appeared to be decreased on TiO2 nanotubes versus the control Ti surface (Figure 2D). Compared to diameters of 70 nm and 100 nm of nanotube relatively few chondroprogenitors attaching, in 50 nm nanotube, more than 60% of cells are strongly attached with chondrocyte-specific morphology. This could be a positive promising in cartilage engineering since most of culture substrates such as agarose, chitosan and alginates are facing a limited cell adhesion.
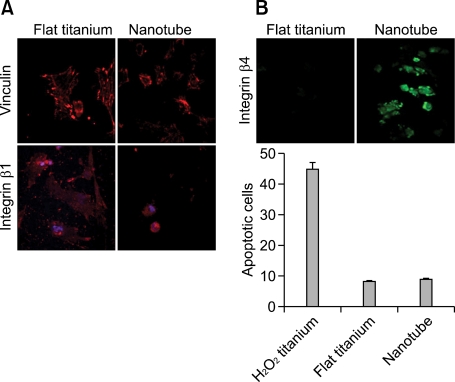
Cell adhesion is a crucial prerequisite for certain cell functions, including the synthesis of extracellular matrix proteins, the formation of focal adhesion complexes, and proliferation. Precartilage condensation, an essential step for chondrogenic differentiation, is also associated with increased cell-to-cell contacts and interactions via cell-cell adhesion molecules such as N-cadherin (Oberlender and Tuan, 1994; Delise and Tuan, 2002) and N-CAM (Widelitz et al., 1993; Leckband et al., 2006). Adhesive interactions on synthetic substrates such as nanotubes are mediated by the interactions between cell-surface integrins and extracellular proteins such as fibronectin, collage, and laminin that have been secreted to the nanotube surface (Felsenfeld et al., 1999). To further investigate the effects of a nanotube-bearing culture surface on chondrocytic differentiation, we examined the expression levels of vinculin and integrin β1, two proteins that are necessary for the binding of cell-surface integrin receptors to the extracellular matrix adhesion molecules, and form a major part of the focal adhesion complexes. As shown in Figure 3A, the focal contacts were not large dash adhesions on flat Ti, and rather focal adhesions to be more pronounced on flat control surfaces than the nanotube surfaces. However, integrin β4 expression was up-regulated in cells cultured on the nanotube surfaces (Figure 3B, upper panel). Our laboratory previously showed that downregulation of integrin β4 by blockade of gap-junction communication, an essential prerequisite for cell-cell contact and communication, induced apoptotic cell death (Jin et al., 2007). Therefore, we believe that the nanotube-induced down-regulation of focal adhesion activity observed in the present study is unlikely to be due to changes in cell survival or apoptosis (Figure 3B, lower panel).
Figure 3.
Growth on a nanotube surface modulates the expression of vinculin and integrin β4. Wing limb mesenchymal cells were cultured on Ti-control (flat titanium) and 50-nm-diameter TiO2 nanotube surfaces. (A) Cells were immunolabeled with antibodies against vinculin and integrin β1. (B) Cells were immunolabeled with antibodies against integrin β4, and the percentages of apoptotic cells were quantified by flow cytometry analysis on day 3 of culture. H2O2 were treated to induce apoptotic cell death. The data shown are representative of at least four independent experiments. The mean is plotted and the error bars represent 95% CI (lower/upper limit).
Erk signaling is involved in the apparent chondrocytic differentiation of nanotube-cultured chondroprogenitors
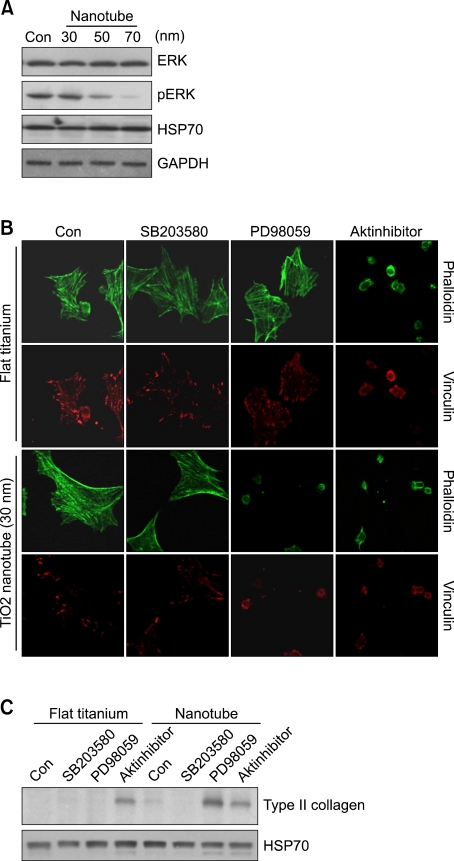
We previously showed that Erk and p38 MAPK regulate mesenchymal cell chondrogenesis by modulating the expression of various cell adhesion molecules, including N-cadherin, fibronectin, and integrins (Choi et al., 1995; Oh et al., 2000). Here, we observed that the phosphorylation level of Erk decreased as the diameter of the Ti-nanotubes on the culture substrates increased (Figure 4A). Similar to cells grown on control Ti surface, Erk was strongly activated in cells grown on 30-nm-diameter nanotubes, which did not experience the morphological transition to the chondrocytic phenotype. In contrast, Erk activity appeared to be inhibited on 50- and 70-nm-diameter nanotube-grown cultures, in which the chondrocytic phenotype was observed.
Figure 4.
Erk signaling acts as negative regulator for the nanotube culture-induced chondrocytic transition. (A) Wing limb mesenchymal cells were cultured on Ti-control (flat titanium) and TiO2 nanotube surfaces having different inner diameters. Changes in the protein levels of Erk and pErk were examined by Western blotting. HSP70 and GAPDH were detected as a loading control. (B) Wing limb mesenchymal cells were incubated with/without 500 nM AKT inhibitor IV, 10 µM PD98059, or 10 µM SB203580 and the expression and phosphorylation levels of p38 MAPK, Erk, and AKT was examined by Western blotting (upper panel). Wing limb mesenchymal cells were cultured on Ti-control (flat titanium) and 30-nm-diameter TiO2 nanotube surfaces in the presence or absence of 500 nM AKT inhibitor IV, 10 µM PD98059, or 10 µM SB203580, and immunolabeled with Alexa-488-conjugated phalloidin and an anti-vinculin antibody (lower panel). (C) Changes in the protein levels of type II collagen were examined by Western blotting. HSP70 was detected as a loading control. The data shown are representative of at least four independent experiments.
To examine whether the morphological transition to the chondrocytic phenotype was due to physical environmental stress, we cultured chondroprogenitors on anodized amorphous 30-nm-diameter nanotube and Ti-control surfaces in the presence or absence of various signaling molecules, includeing SB203580 (a specific inhibitor of p38 MAPK), PD98059 (a specific inhibitor of Erk), and an AKT inhibitor IV (Figure 4B).
Based on our findings, we hypothesized that nanotube-modulated Erk activity could be responsible for the observed chondrocytic differentiation. From this, we further theorized that exposure of cells grown on 30-nm-diameter nanotubes (which were characterized by Erk overexpression and stress fiber accumulation) to PD98059 would cause cells to transition to the chondrocytic phenotype. As predicted, Erk inhibition induced the morphological transition of mesenchymal cells to the chondrocytic cell shape in cultures grown on 30-nm-diameter nanotubes. Consistent with our previous findings in micromass culture (Jin et al., 2007), these results suggest that Erk signaling acts as negative regulator for actin cytoskeletal reorganization.
Our laboratory previously showed that activation of AKT plays an important role in coordinating the chondrogenic phenotype independent of the physical stress arising from an increase cell density (Jin et al., 2007). However, we herein found that inhibition of AKT signaling induced chondrocytic differentiation in chondroprogenitors grown on both Ti-control and 30-nm-diameter nanotubes. This suggests that AKT signaling can induce cortical reorganization of the actin cytoskeleton and cellular rounding of chondrogenic-competent cells regardless of the presence of nanotubes. Consistent with this finding, Western blotting showed that exposure of cells to PD98059 and the AKT inhibitor IV triggered increased expression of type II collagen (Figure 4C). Thus, our present results together with the previous report suggest that the effects of a given inhibitor may differ depending on the utilized culture substratum. Future work will be required to determine the mechanism(s) underlying the substratum-specific responses of chick limb mesenchymal cells to AKT inhibitors. In this study, nanotubes of 70 to 100 nm in diameter were found to trigger the morphological transition and up-regulation of type II collagen expression which are typical markers for chondrogenic differentiation through down-regulation of Erk signaling suggesting that a titanium-based nanotube surface can support chondrocytic functions among chondroprogenitors, and may therefore be useful for future cartilaginous applications.
Methods
Cell culture and treatments
Mesenchymal cells derived from the distal tips of Hamburger-Hamilton stage 22/23 embryo leg buds of fertilized White Leghorn chicken eggs were micromass cultured as previously described (Jin et al., 2007). Briefly, the cells were suspended at a density of 2 × 107 cells/ml in Ham's F-12 medium containing 10% fetal bovine serum, 100 IU/ml penicillin and 100 µg/ml streptomycin (Gibco Invitrogen, Grand Island, NY). The cells were incubated for 1 h at 37℃ under 5% CO2.
For dedifferentiated chondrocyte, after 7 days of culture, the cells were dissociated with 0.25% trypsin/1.0 mM EDTA and collagenase and replated at a density of 1 × 105 cells/cm2. These cells were subcultured three more times at 2-4 days intervals. Then, cells were seeded at 5000 cells/cm2 onto uncoated or chitosan-coated 35 mm culture dishes. The culture medium was changed 24 h after cell seeding and every 48 h thereafter.
Cells were treated with following reagents; 10 µM PD169316 and 10 µM SB203580 (Calbiochem, San Diego, CA), AKT inhibitor IV (Chemicon, Temecula, CA), 1 µg/ml cytochalasin D (Sigma, St. Louis, MO).
TiO2 nanotube preparation
The preparation procedure of TiO2 nanotube layer on Ti substrate was reported previously (Oh et al., 2000; Brammer et al., 2008). Briefly describing, Ti sheet thickness: 250 µm, purity: 99.5% (Alfa-Aesar, Ward Hill, MA) was cleaned by acetone, 70% ethanol, and water in this order. Cleaned specimens were anodized by hydrofluoric acid (0.5 w/v% purity: 48%, EM-Science, Gardena, CA) at 20 V for 30 min. The prepared TiO2 nanotube specimens were washed by running water for 30 s, dried at 80℃ overnight, and heat-treated at 500℃ for 2 h. Heat-treated specimens were cut into 1.27 × 1.27 cm2, then sterilized by autoclave.
Chitosan coating
One gram of chitosan powder (Sigma, St. Louis, MO) was dissolved in 2% (v/v) acetic acid solution to give a final concentration of 0.1% (w/v). The solution was stirred overnight and autoclaved for 10 min, until the material was completely dissolved. Two milliliters of sterile chitosan solution was used to evenly coat the surface of each 35 mm culture dish, then evaporated to dryness for at least 24 h at room temperature in a laminar clean bench. The coated dishes were neutralized by addition of 1 ml of 0.5 M NaOH followed by several washings with Hanks' balanced salt solution.
Apoptosis assay
Apoptosis were analyzed by a flow cytometer (FACS calibure, Becton-Dickinson, France). To detect extent of propidium iodide, cells were excited at 488 nm and emission was observed at 585 nm.
Western-blot analysis
Proteins (30 µg) or conditioned media were separated by 10% polyacrylamide gel electrophoresis containing 0.1% SDS and transferred to nitrocellulose membrane (Schleicher and Schuell, Germany). The membranes were incubated for 1 h at room temperature in blocking buffer (20 mM Tris-HCl, 137 mM NaCl, pH 8.0, containing 0.1% Tween and 3% non-fat dry milk), and probed with antibodies against Type I and II collagen and GAPDH (Santa Cruz Biotech, Santa Cruz, CA), and HSP70 (Stressgene, San Diego, CA). The blots were developed with a peroxidase-conjugated secondary antibody and reacted proteins were visualized using the electrochemiluminescence system (Pierce Biotechnology Inc., Rockford, MN).
Immunocytochemistry
Cells grown on cover slips were washed 3 times with phosphate-buffered saline (PBS), then fixed and permeabilized as described above. For actin staining, each culture was stained with Alexa488-phalloidin (Molecular Probes, Eugene, OR) prepared in PBS containing 1% (v/v) bovine serum albumin for 1 h at room temperature in a lightproof box. For immunocytochemistry, cultured cells were stained with antibody against vinculin (Santa Cruz Biotechnology, Santa Cruz, CA) and integrin β1 and β4 (Santa Cruz Biotechnology, Santa Cruz, CA), washed 3 times with PBS, and incubated with secondary antibody conjugated with either TRITC (for vinculin and integrin β1) or FITC (for integrin β4) for 1 h at room temperature. Cultures were then washed 3 times with water and mounted with Gel/Mount (Biomedia, Foster City, CA). The slides were examined using a confocal microscope (MRC 1024/ES, Bio-Rad Laboratory, CA).
Acknowledgements
This works was supported by National Research Foundation of Korea Grant funded by the Korean Government (2010-0002709).
Abbreviations
- CD
cytochalasin D
- Erk
extracellular-signal regulated kinases
- HSP70
heat shock protein 70
- p38 MAPK
p38 mitogen-activated protein kinases
- Ti
titanium
References
- 1.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 2.Binette F, McQuaid DP, Haudenschild DR, Yaeger PC, McPherson JM, Tubo R. Expression of a stable articular cartilage phenotype without evidence of hypertrophy by adult human articular chondrocytes in vitro. J Orthop Res. 1998;16:207–216. doi: 10.1002/jor.1100160208. [DOI] [PubMed] [Google Scholar]
- 3.Bonaventure J, Kadhom N, Cohen-Solal L, Ng KH, Bourguignon J, Lasselin C, Freisinger P. Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res. 1994;212:97–104. doi: 10.1006/excr.1994.1123. [DOI] [PubMed] [Google Scholar]
- 4.Brammer KS, Oh S, Gallagher JO, Jin S. Enhanced cellular mobility guided by TiO2 nanotube surfaces. Nano Lett. 2008;8:786–793. doi: 10.1021/nl072572o. [DOI] [PubMed] [Google Scholar]
- 5.Cancedda R, Descalzi Cancedda F, Castagnola P. Chondrocyte differentiation. Int Rev Cytol. 1995;159:265–358. doi: 10.1016/s0074-7696(08)62109-9. [DOI] [PubMed] [Google Scholar]
- 6.Choi B, Chun JS, Lee YS, Sonn JK, Kang SS. Expression of protein kinase C isozymes that are required for chondrogenesis of chick limb bud mesenchymal cells. Biochem Biophys Res Commun. 1995;216:1034–1040. doi: 10.1006/bbrc.1995.2724. [DOI] [PubMed] [Google Scholar]
- 7.Daniels K, Solursh M. Modulation of chondrogenesis by the cytoskeleton and extracellular matrix. J Cell Sci. 1991;100:249–254. doi: 10.1242/jcs.100.2.249. [DOI] [PubMed] [Google Scholar]
- 8.Delise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8:309–334. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 9.Delise AM, Tuan RS. Analysis of N-cadherin function in limb mesenchymal chondrogenesis in vitro. Dev Dyn. 2002;225:195–204. doi: 10.1002/dvdy.10151. [DOI] [PubMed] [Google Scholar]
- 10.Dumortier H, Lacotte S, Pastorin G, Marega R, Wu W, Bonifazi D, Briand JP, Prato M, Muller S, Bianco A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006;6:1522–1528. doi: 10.1021/nl061160x. [DOI] [PubMed] [Google Scholar]
- 11.Felsenfeld DP, Schwartzberg PL, Venegas A, Tse R, Sheetz MP. Selective regulation of integrin--cytoskeleton interactions by the tyrosine kinase Src. Nat Cell Biol. 1999;1:200–206. doi: 10.1038/12021. [DOI] [PubMed] [Google Scholar]
- 12.Gurke S, Barroso JF, Hodneland E, Bukoreshtliev NV, Schlicker O, Gerdes HH. Tunneling nanotube (TNT)-like structures facilitate a constitutive, actomyosin-dependent exchange of endocytic organelles between normal rat kidney cells. Exp Cell Res. 2008;314:3669–3683. doi: 10.1016/j.yexcr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Idowu BD, Knight MM, Bader DL, Lee DA. Confocal analysis of cytoskeletal organisation within isolated chondrocyte sub-populations cultured in agarose. Histochem J. 2000;32:165–174. doi: 10.1023/a:1004095207330. [DOI] [PubMed] [Google Scholar]
- 14.Jin EJ, Park KS, Bang OS, Kang SS. AKT signaling regulates actin organization via modulation of MMP-2 activity during chondrogenesis of chick wing limb bud mesenchymal cells. J Cell Biochem. 2007;102:252–261. doi: 10.1002/jcb.21430. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser JP, Wick P, Manser P, Spohn P, Bruinink A. Single walled carbon nanotubes (SWCNT) affect cell physiology and cell architecture. J Mater Sci Mater Med. 2008;19:1523–1527. doi: 10.1007/s10856-007-3296-y. [DOI] [PubMed] [Google Scholar]
- 16.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Kim JH, Akaike T. Regulation of cell adhesion signaling by synthetic glycopolymer matrix in primary cultured hepatocyte. FEBS Lett. 2003;553:433–439. doi: 10.1016/s0014-5793(03)01047-0. [DOI] [PubMed] [Google Scholar]
- 18.Lahiji A, Sohrabi A, Hungerford DS, Frondoza CG. Chitosan supports the expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J Biomed Mater Res. 2000;51:586–595. doi: 10.1002/1097-4636(20000915)51:4<586::aid-jbm6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 19.Langelier E, Suetterlin R, Hoemann CD, Aebi U, Buschmann MD. The chondrocyte cytoskeleton in mature articular cartilage: structure and distribution of actin, tubulin, and vimentin filaments. J Histochem Cytochem. 2000;48:1307–1320. doi: 10.1177/002215540004801002. [DOI] [PubMed] [Google Scholar]
- 20.Leckband D, Prakasam A. Mechanism and dynamics of cadherin adhesion. Annu Rev Biomed Eng. 2006;8:259–287. doi: 10.1146/annurev.bioeng.8.061505.095753. [DOI] [PubMed] [Google Scholar]
- 21.Lemare F, Steimberg N, Le Griel C, Demignot S, Adolphe M. Dedifferentiated chondrocytes cultured in alginate beads: restoration of the differentiated phenotype and of the metabolic responses to interleukin-1beta. J Cell Physiol. 1998;176:303–313. doi: 10.1002/(SICI)1097-4652(199808)176:2<303::AID-JCP8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Lee YW, Dean MF. Re-expression of differentiated proteoglycan phenotype by dedifferentiated human chondrocytes during culture in alginate beads. Biochim Biophys Acta. 1998;1425:505–515. doi: 10.1016/s0304-4165(98)00105-6. [DOI] [PubMed] [Google Scholar]
- 23.Loty S, Forest N, Boulekbache H, Sautier JM. Cytochalasin D induces changes in cell shape and promotes in vitro chondrogenesis: a morphological study. Biol Cell. 1995;83:149–161. doi: 10.1016/0248-4900(96)81303-7. [DOI] [PubMed] [Google Scholar]
- 24.Madsen K, Moskalewski S, Thyberg J, Friberg U. Comparison of the in vitro effects of colchicine and its derivative colchiceine on chondrocyte morphology and function. Experientia. 1979;35:1572–1573. doi: 10.1007/BF01953199. [DOI] [PubMed] [Google Scholar]
- 25.Martin I, Vunjak-Novakovic G, Yang J, Langer R, Freed LE. Mammalian chondrocytes expanded in the presence of fibroblast growth factor 2 maintain the ability to differentiate and regenerate three-dimensional cartilaginous tissue. Exp Cell Res. 1999;253:681–688. doi: 10.1006/excr.1999.4708. [DOI] [PubMed] [Google Scholar]
- 26.Oberlender SA, Tuan RS. Spatiotemporal profile of N-cadherin expression in the developing limb mesenchyme. Cell Adhes Commun. 1994;2:521–537. doi: 10.3109/15419069409014216. [DOI] [PubMed] [Google Scholar]
- 27.Oh CD, Chang SH, Yoon YM, Lee SJ, Lee YS, Kang SS, Chun JS. Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J Biol Chem. 2000;275:5613–5619. doi: 10.1074/jbc.275.8.5613. [DOI] [PubMed] [Google Scholar]
- 28.Park EJ, Cho WS, Jeong J, Yi J, Choi K, Park K. Pro-inflammatory and potential allergic responses resulting from B cell activation in mice treated with multi-walled carbon nanotubes by intratracheal instillation. Toxicology. 2009;259:113–121. doi: 10.1016/j.tox.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Ryu JH, Kim MS, Lee GM, Choi CY, Kim BS. The enhancement of recombinant protein production by polymer nanospheres in cell suspension culture. Biomaterials. 2005;26:2173–2181. doi: 10.1016/j.biomaterials.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Schulze-Tanzil G, de Souza P, Villegas Castrejon H, John T, Merker HJ, Scheid A, Shakibaei M. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002;308:371–379. doi: 10.1007/s00441-002-0562-7. [DOI] [PubMed] [Google Scholar]
- 31.Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 32.Takigawa M, Takano T, Shirai E, Suzuki F. Cytoskeleton and differentiation: effects of cytochalasin B and colchicine on expression of the differentiated phenotype of rabbit costal chondrocytes in culture. Cell Differ. 1984;14:197–204. doi: 10.1016/0045-6039(84)90046-0. [DOI] [PubMed] [Google Scholar]
- 33.Thenet S, Benya PD, Demignot S, Feunteun J, Adolphe M. SV40-immortalization of rabbit articular chondrocytes: alteration of differentiated functions. J Cell Physiol. 1992;150:158–167. doi: 10.1002/jcp.1041500121. [DOI] [PubMed] [Google Scholar]
- 34.von der Mark K, von der Mark H. The role of three genetically distinct collagen types in endochondral ossification and calcification of cartilage. J Bone Joint Surg Br. 1977;59-B:458–464. doi: 10.1302/0301-620X.59B4.72756. [DOI] [PubMed] [Google Scholar]
- 35.Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25:4731–4739. doi: 10.1016/j.biomaterials.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Widelitz RB, Jiang TX, Murray BA, Chuong CM. Adhesion molecules in skeletogenesis: II. Neural cell adhesion molecules mediate precartilaginous mesenchymal condensations and enhance chondrogenesis. J Cell Physiol. 1993;156:399–411. doi: 10.1002/jcp.1041560224. [DOI] [PubMed] [Google Scholar]
- 37.Williams CM, Engler AJ, Slone RD, Galante LL, Schwarzbauer JE. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res. 2008;68:3185–3192. doi: 10.1158/0008-5472.CAN-07-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]