Abstract
A variety of benzylidenethiazole analogs have been demonstrated to inhibit 5-lipoxygenase (5-LOX). Here we report the anti-atherogenic potential of 5-(4-hydroxy-2,3,5-trimethylbenzylidene) thiazolidin-2,4-dione (HMB-TZD), a benzylidenethiazole analog, and its potential mechanism of action in LDL receptor-deficient (Ldlr-/-) mice. HMB-TZD Treatment reduced leukotriene B4 (LTB4) production significantly in RAW264.7 macrophages and SVEC4-10 endothelial cells. Macrophages or endothelial cells pre-incubated with HMB-TZD for 2 h and then stimulated with lipopolysaccharide or tumor necrosis factor-alpha (TNF-α) displayed reduced cytokine production. Also, HMB-TZD reduced cell migration and adhesion in accordance with decreased proinflammatory molecule production in vitro and ex vivo. HMB-TZD treatment of 8-week-old male Ldlr-/- mice resulted in significantly reduced atherosclerotic lesions without a change to plasma lipid profiles. Moreover, aortic expression of pro-atherogenic molecules involved in the recruitment of monocytes to the aortic wall, including TNF-α , MCP-1, and VCAM-1, was downregulated. HMB-TZD also reduced macrophage infiltration into atherosclerotic lesions. In conclusion, HMB-TZD ameliorates atherosclerotic lesion formation possibly by reducing the expression of proinflammatory molecules and monocyte/macrophage recruitment to the lesion. These results suggest that HMB-TZD, and benzylidenethiazole analogs in general, may have therapeutic potential as treatments for atherosclerosis.
Keywords: antioxidants, arachidonate 5-lipoxygenase, atherosclerosis, endothelial cells, macrophages
Introduction
Cyclooxygenase (COX) and 5-lipoxygenase (5-LOX) produce prostaglandins, thromboxanes, and leukotrienes (LTs) from arachidonic acid. These metabolites have been shown to play important roles in inflammation, thrombosis, allergy, and cancer (Vila, 2004). 5-LOX is a key enzyme involved in the production of proinflammatory LTs and has been linked to the pathogenesis of various inflammatory disorders (Vila, 2004). LTs are produced by cells critical to the inflammatory response, namely macrophages, neutrophils, eosinophils, mast cells, and endothelial cells (Lewis et al., 1990; Zou et al., 2009; Kim et al., 2010). In response to a diversity of immune and inflammatory stimuli, LTs are secreted into the extracellular space where they bind receptors, including BLT1, BLT2, CysLT1, and CysLT2, and induce inflammation (Yokomizo et al., 1997, 2000; Vila, 2004; Lötzer et al., 2007; Sánchez-Galán et al., 2009).
Atherosclerosis is a chronic inflammatory vascular disease involving inflammatory mediators, monocytes, endothelial cells, and smooth muscle cells (Ross, 1999). In atherosclerotic lesions, the number of 5-LOX-expressing cells, which are primarily macrophages, is markedly increased (Spanbroek et al., 2003). In fact, a polymorphism in the 5-LOX promoter has been linked to atherosclerosis (Dwyer et al., 2004). Therefore, inhibition or genetic ablation of the 5-LOX pathway has been reported to decrease the size of atherosclerotic lesions by reducing the production of inflammatory arachidonic acid metabolites such as LTB4 (Mehrabian et al., 2002; Heller et al., 2005).
Due to their strong anti-inflammatory properties, modifiers of the 5-LOX pathway have been considered potential therapeutics for treating cardiovascular disease (Funk, 2005). Many multi-substituted benzylidenethiazole analogs derived from 2,6-di-tert-butylphenol have been found to reduce inflammatory processes in vivo and in vitro by inhibiting both COX and 5-LOX (Unangst et al., 1994). Previously, we reported that one such analog, 5-(3,5-di-tert-butyl-4-hydroxybenzylidene) thiazolidin-2,4-dione (BHB-TZD), also possesses strong anti-atherogenic activity (Choi et al., 2010). In this study, we examined the anti-atherogenic activity of another benzylidenethiazole analog, 5- (4-hydroxy-2,3,5-trimethylbenzylidene) thiazolidin- 2,4-dione (HMB-TZD). Interestingly, although the structure of HMB-TZD is highly similar to that of BHB-TZD, HMB-TZD inhibits 5-LOX strongly but blocks COX weakly. Moreover, HMB-TZD exhibits anti-oxidative activity, preventing oxidative modification of low-density lipoprotein (LDL) (Jeong et al., 2004). These characteristics of HMB-TZD prompted us to investigate the effect of this analog on atherogenesis and its mechanism of action in Ldlr-/- mice.
Results
A potent binding mode of HMB-TZD in the catalytic channel of 5-LOX
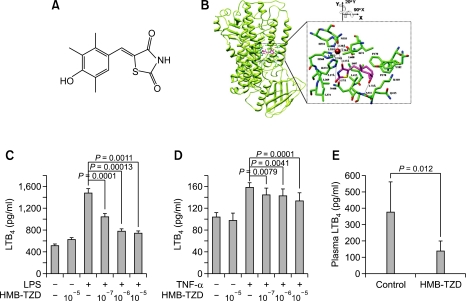
To elucidate a potential mode of binding of HMB-TZD to the active site of mouse 5-LOX, an in silico docking experiment was performed. Since a crystal structure of mouse 5-LOX is unavailable, we modeled this enzyme on the crystal structure of 8R-LOX from P. homomalla (Neau et al., 2009) using MODELLER. The root-mean-square deviation of 577 Cα-atom pairs between the final model of mouse 5-LOX and 8R-LOX is 0.340 Å, suggesting that these structures are similar. Our model of the structure of mouse 5-LOX consists of an N-terminal C2-like domain and a larger C-terminal catalytic domain composed mostly of α-helices. Within the center of the catalytic domain is a putative arachidonate binding channel connected to the catalytic iron as found in other lipoxygenases (Neau et al., 2009). Our docking experiment reveals that HMB-TZD binds to the catalytic channel of 5-LOX (Figures 1A and 1B). The hydrophobic HMB moiety of HMB-TZD is positioned in the hydrophobic portion of the channel lined by Phe170, Phe178, Tyr182, Ile407, Ala411, Leu415, and Leu608. In addition, one oxygen atom of the HMB moiety forms a hydrogen bond with the nitrogen atom of the Ala411 backbone. The polar TZD moiety is located adjacent to the catalytic iron and is stabilized by hydrogen bonding between one of its oxygen atoms and His373 and Asn408. The catalytic iron is coordinated by the side chains of four amino acids (i.e., His368, His373, His551, and Asn555) and the main carboxylate of I674 located in the C-terminus. Interestingly, these results demonstrate that one oxygen atom of the TZD moiety occupies the sixth position of the catalytic iron with an approximate distance of 3.17 Å. In the purple oxygenase structure, the peroxide crucial for the catalytic activity of the enzyme occupies the sixth ligand position in the iron coordination sphere (Skrzypczak-Jankun et al., 2001). Structural comparison of several soybean 3-LOX complex structures with inhibitors such as 4-nitrocatechol (1NO3), 4-hydroperoxy-2-methoxyphenol (1HU9), protocatechuic acid (1N8Q), and epigallocathechin (1JNQ) showed a similar mode of binding between their analogous oxygen atom to the catalytic iron. Therefore, the mechanism underlying HMB-TZD inhibition of 5-LOX may be similar to these soybean 3-LOX inhibitors.
Figure 1.
HMB-TZD reduces LTB4 production in vivo and in vitro. (A) Structure of HMB-TZD. (B) HMB-TZD embedded in the catalytic channel of 5-LOX. The best position for HMB-TZD within the 5-LOX catalytic channel as modeled from the docking experiment was drawn using Chimera. The zoomed image shows surrounding residues interacting with HMB-TZD. 5-LOX is colored in green and HMB-TZD in pink. The residues are represented by ball-and-stick models with yellow representing sulfur, blue for nitrogen, and red for oxygen. (C and D) The concentrations of LTB4 in RAW264.7 macrophage (C) and SVEC4-10 endothelial cell (D) culture supernatant were measured by ELISA. RAW264.7 macrophages and SVEC4-10 endothelial cells were pre-incubated with HMB-TZD (10-5, 10-6, or 10-7 M) for 2 h, and then stimulated with LPS (1 µg/ml) or TNF-α (20 ng/ml) for 24 h, respectively. (E) Effect of HMB-TZD treatment on the plasma LTB4 level in Ldlr-/- mice.
HMB-TZD reduced LTB4 production in vitro and in vivo
To determine the effect of HMB-TZD on arachidonic acid metabolite production, we first assessed the effect of the analog on the production of LTB4, a representative proatherogenic arachidonic acid metabolite produced by the 5-LOX pathway. In RAW264.7 macrophages and SVEC4-10 endothelial cells, lipopolysaccharide (LPS) or tumor necrosis factor-alpha (TNF-α) stimulated LTB4 production was reduced significantly by HMB-TZD treatment in a dose-dependent manner, respectively (Figures 1C and 1D). To examine the effect of HMB-TZD on plasma LTB4 levels, Ldlr knockout (Ldlr-/-) mice were fed a western diet supplemented with 1% (w/w) HMB-TZD. After 8 weeks, the LTB4 level was 63% lower in HMB-TZD-treated animals than in control mice (Figure 1E). Since many derivatives of 2,6-di-tert-butylphenol have been shown to have an inhibitory effect on COX (Unangst et al., 1994), we measured plasma prostaglandin E2 (PGE2) levels to assess whether HMB-TZD also inhibits the COX pathway in vivo. We found no significant difference between the control and HMB-TZDtreated groups (311 ± 36 vs. 325 ± 21 pg/ml, respectively). Taken together, our data demonstrate that HMB-TZD reduces LTB4 production effectively in Ldlr-/- mice.
HMB-TZD reduced atherosclerotic lesion formation in Ldlr-/- mice
Initially, to investigate the pharmacokinetics of HMB-TZD, Ldlr-/- mice were administered 3 mg of HMB-TZD once orally. This dose was equivalent to the daily amount ingested from the diet used in this study (i.e., 1% w/w HMB-TZD as mentioned above). The plasma HMB-TZD level in the treated animals increased up to 1.9 × 10-6 M at 2 h and then decreased dramatically, indicating that HMB-TZD is metabolized and excreted normally.
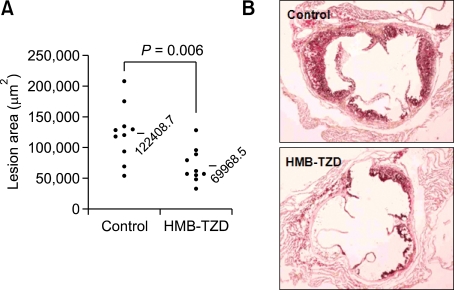
To determine the effect of HMB-TZD on the development of atherosclerosis, we analyzed atherosclerotic lesions in aortic vessels from animals that were fed the western diet with and without HMB-TZD for 8 weeks. HMB-TZD supplementation did not affect plasma concentrations of total cholesterol, triglyceride, HDL-cholesterol, and LDL-cholesterol (Supplemental Data Figure S1). Necropsy and histopathological examinations did not reveal notable lesions in the parenchymal organs of both groups of mice (data not shown). In addition, daily food intake and body weight gain was similar between the groups (data not shown). Measurement of the lesion size by computer-associated morphometry revealed that the mean lesion area of the aortic sinus in HMB-TZD-treated mice was smaller than that of control animals by 43% (Figures 2A and 2B).
Figure 2.
HMB-TZD treatment reduced fatty streak lesions in Ldlr-/- mice. (A) The mean size of fatty streak lesions in HMB-TZD-treated (n = 10) and control (n = 10) mice. (B) Representative oil red O staining of atherosclerotic lesions in control and HMB-TZD-treated mice.
Reactive oxygen species play an important role in atherogenesis through multiple mechanisms, including LDL oxidation. The lipid peroxidation product, malondialdehyde (MDA), may also be critical to atherogenesis. Previously, HMB-TZD had been reported to reduce LDL oxidation (Jeong et al., 2004). To determine the antioxidative activity of HMB-TZD, the levels of plasma lipid peroxide and lesional superoxide were measured. Although not statistically significant, the average lipid peroxide and lesional superoxide levels were lower in the HMB-TZD-treated group (Supplemental Data Figure S2).
HMB-TZD reduced the expression of vascular proatherogenic genes in Ldlr-/- mice
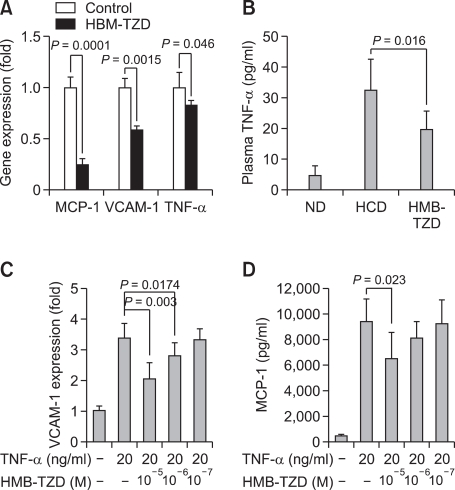
To understand the mechanism underlying the anti-atherogenic activity of HMB-TZD, we measured the expression of atherosclerosis-related genes, including MCP-1, VCAM-1, and TNF-α, in the aortas of HMB-TZD-treated and control mice. Using real-time reverse transcription-polymerase chain reaction (RT-PCR), we found that MCP-1 and VCAM-1 mRNAs were decreased by 75% and 41%, respectively, in the HMB-TZD-treated group compared to control animals (Figure 3A). In addition, TNF-α mRNA and plasma TNF-α levels were also reduced significantly by HMB-TZD administration (Figures 3A and 3B).
Figure 3.
Effect of HMB-TZD on inflammatory gene expression in the aorta and endothelial cells. Total RNA was isolated from five pooled thoracic aortas from control and HMB-TZD-treated mice. (A) The expression of MCP-1, VCAM-1, and TNF-α mRNA was quantitated by real- time RT-PCR and normalized to the expression of GAPDH. Each PCR reaction was performed in triplicate, and gene expression levels and standard errors were determined. (B) Reduction in plasma TNF-α level in HMB-TZD-treated animals. (C, D) The effect of HMB-TZD on VCAM-1 expression and MCP-1 production in HUVECs stimulated with TNF-α. HUVECs were pre-treated with HMB-TZD at the indicated concentration for 2 h, and then stimulated with 20 ng/ml of TNF-α for 24 h.
Since HMB-TZD treatment reduces VCAM-1 expression in the aortic wall, we investigated whether this compound affects cultured endothelial cells. Our data demonstrate that HMB-TZD reduced VCAM-1 expression significantly in TNF-α-stimulated endothelial cells in a dose-dependent manner. Moreover, MCP-1 production was decreased (Figures 3C and 3D), indicating that HMB-TZD reduced pro-inflammatory gene expression directly in endothelial cells.
HMB-TZD inhibited monocyte adhesion to endothelium and transmigration
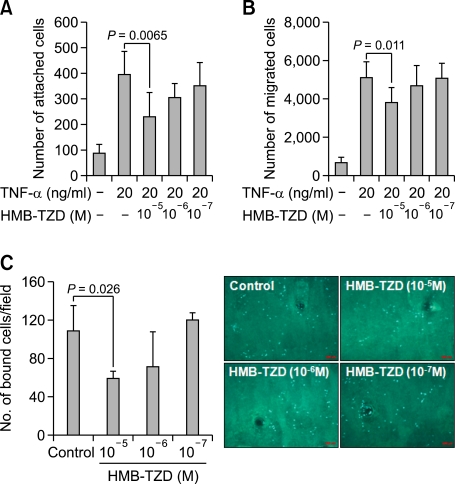
Since HMB-TZD reduced the production of inflammatory molecules in cytokine-stimulated endothelial cells directly, we performed in vitro cell migration and adhesion assays, as well as ex vivo adhesion assays using aortic organ culture. These studies revealed that monocyte adhesion and migration diminished significantly with HMB-TZD treatment in vitro (Figures 4A and 4B). Furthermore, monocyte attachment to the aortic lumen after TNF-α treatment was also reduced by HMB-TZD (Figure 4C).
Figure 4.
HMB-TZD reduced macrophage accumulation in atherosclerotic lesions. (A) Monocyte adhesion assay. HUVECs were pre-incubated with HMB-TZD (10-5, 10-6, or 10-7 M) for 2 h, and then stimulated with TNF-α for 12 h. Next THP-1 monocytes were added to the culture. After 20 min, unbound cells were washed and the remaining attached cells were counted in five randomly selected optical fields per well. (B) Monocyte migration assay. THP-1 monocytes were loaded into the upper chamber of Transwell plates and the lower chambers were filled with conditioned medium from HUVECs treated with HMB-TZD (10-5, 10-6, or 10-7 M) or left untreated for 2 h and subsequently activated with 20 ng/ml TNF-α for 12 h. (C) Monocyte adhesion assay in aortic organ culture. Aortas were pre-treated for 2 h with 0.1% dimethyl sulfoxide or HMB-TZD (10-5, 10-6, or 10-7 M) prior to incubation with 20 ng/ml TNF-α for 12 h. After washing, aortas were incubated for 30 min with 1 × 106 CD11b+GFP+ cells, and fluorescence microscopy was used to count the bound cells.
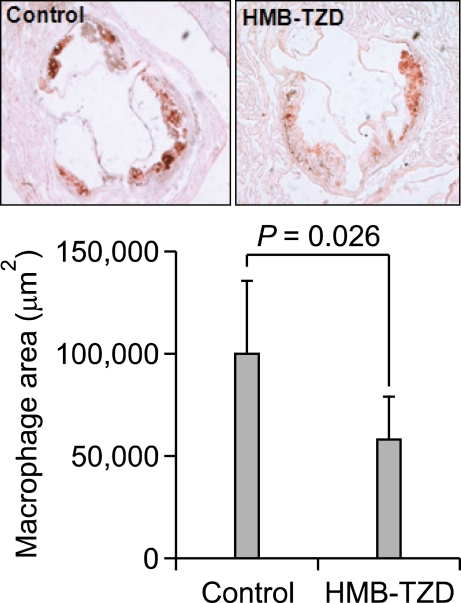
HMB-TZD reduced macrophage accumulation in atherosclerotic lesions
Finally, we measured the area occupied by macrophages in the aortic sinus to determine whether HMB-TZD treatment reduces monocyte recruitment to atherosclerotic lesions. HMB-TZD administration reduced the macrophage area significantly compared to untreated animals (Figure 5), suggesting that this compound affects macrophage infiltration in atherosclerotic lesions.
Figure 5.
HMB-TZD treatment reduced macrophage infiltration into the aortic sinus of Ldlr-/- mice. Representative immunohistochemical staining of macrophages in control and HMB-TZD-treated mice are shown (upper). The graph shows the mean size of macrophage positive areas in each group (lower).
Discussion
In this study, we show that HMB-TZD ameliorates the formation of typical atheromatous lesions in Ldlr-/- mice without changing the plasma concentrations of total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides. HMB-TZD also reduced the plasma level of LTB4, suggesting that this analog inhibits the 5-LOX pathway in vivo. Our data also demonstrate that this compound reduces the macrophage accumulation in atherosclerotic lesions. LTB4 is well-established as an inducer of several inflammatory processes, including chemotaxis, leukocyte adhesion to the vascular endothelium, and gene expression (Yokomizo et al., 1997, 2000; Vila, 2004; Sánchez-Galán et al., 2009). Reduced LTB4 production due to inhibition of 5-LOX activity can reduce macrophage adhesion and transmigration into the subintimal space, leading to a reduction in atherosclerotic lesion size (Aiello et al., 2002; Friedrich et al., 2003; Huang et al., 2004). Results from this study demonstrate that HMB-TZD may reduce macrophage accumulation in vessel walls, in part, by inhibiting the 5-LOX pathway, which results in downregulation of LTB4.
In this study, we show that HMB-TZD reduces LTB4 production effectively in RAW264.7 macrophages and SVEC4-10 endothelial cells. Moreover, HMB-TZD reduced plasma LTB4 levels significantly. A previous study demonstrated that benzylidenethiazole analogs derived from 2,6-di-tert-butylphenol could also inhibit COX activity (Unangst et al., 1994). Therefore, we tested the effect of HMB-TZD on PGE2 production in RAW264.7 macrophages. Our data show that HMB-TZD inhibition of PGE2 production was weak (data not shown). Furthermore, we also found that the plasma PGE2 level did not change with HMB-TZD supplementation. This result indicates that the attenuated atherosclerosis observed in the HMB-TZD-treated group was not mediated by COX inhibition.
In addition to anti-diabetic effects such as reducing the plasma glucose level and acting as PPARγ agonists, thiazolidinediones (TZDs) exhibit anti-atherogenic activity (Tontonoz and Spiegelman, 2008). HMB-TZD has a structure similar to TZDs and was demonstrated previously to react with PPARγ (Momose et al., 2002). Therefore, HMB-TZD is considered a PPARγ agonist. However, we found that HMB-TZD did not increase luciferase reporter activity when controlled by a PPARγ-responsive element, whereas troglitazone, which was used as a positive control, increased this activity dramatically (data not shown). Furthermore, HMB-TZD supplementation did not affect plasma glucose levels (data not shown). These results indicate that HMB-TZD is not a PPARγ agonist, and that PPARγ is not involved in the attenuation of atherosclerosis by HMB-TZD.
Inhibitors of 5-LOX have been demonstrated to attenuate airway inflammation by reducing LT production (Berger et al., 2007). 5-LOX inhibitors have been used as therapeutics for asthma patients. In addition to their potent proinflammatory properties, leukotrienes have been demonstrated recently to affect atherosclerosis. Inhibitors of the 5-LOX pathway have been shown to possess anti-atherogenic activity (Aiello et al., 2002; Jawien et al., 2006). Our data support the hypothesis that inhibiting the 5-LOX pathway is a potential therapeutic strategy for treating atherosclerosis.
In conclusion, our study demonstrates that HMB-TZD reduces monocyte adhesion and infiltration. We also show that this compound attenuates atherosclerosis in LDL receptor- deficient mice. Furthermore, using in silico docking, we demonstrated that HMB-TZD can bind to the catalytic channel of 5-LOX. By reducing the LTB4 level, HMB-TZD decreased monocyte adhesion and transmigration in vitro, thereby inhibiting monocyte recruitment into the plaque. Our findings clearly support that this group of benzylidene derivatives are potential therapeutic candidates for treating atherosclerosis.
Methods
Chemicals and cell culture
HMB-TZD (Figure 1A) was obtained from the Korea Research Institute of Bioscience and Biotechnology. RAW264.7, SVEC4-10 and THP-1 cells were maintained in Dulbecco's Modified Eagles Medium (DMEM, Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum. RAW264.7 macrophages were pre-incubated for 2 h with HMB-TZD at concentrations that did not cause cytotoxicity as determined by the MTT assay (10-5, 10-6, or 10-7 M; data not shown). Then RAW264.7 macrophages and SVEC4-10 endothelial cells were activated with 1 µg/ml LPS or 20 ng/ml TNF-α for 24 h. The level of LTB4 in tissue culture supernatants from these cultures was subsequently measured by enzyme-linked immunosorbent assay (ELISA). Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (BioWhittaker, Walkersville, MD) and maintained in EGM-2 Bullet kit media (BioWhittaker) at 37℃ and 5% CO2. All HUVECs used in this study were passaged 3 to 7 times.
Model building and evaluation
The primary sequence of mouse arachidonate 5-LOX consists of 674 amino acids. The Plexaura homomalla 8R-lipoxygenase (8R-LOX) sequence exhibiting 60% homology with 5-LOX was obtained selected using BLAST (http://blast.ncbi.nlm.nih.gov) for use as a template. The 3D structure coordinates of 8R-LOX (PDB ID: 3DY5) were obtained from the Brookhaven Protein databank. MODELLER 9v8 (Eswar et al., 2006) was used to build a computational homology model of 5-LOX that satisfies spatial restraints. The input files for MODELLER were the pdb file of the template, the alignment file of the template and target sequences, and Python command script files. Energy-minimized models were produced using CHARMM energy parameters. The most reasonable model was selected based on evaluation and quality assessment of the stereochemistry of the model by PROCHECK (Laskowski et al., 1996). ProSa (Sippl, 1993) generating energy graphs based on interactions between Cβ-Cβ pairs and Cβ-surface was also used to check strained regions in the model.
In vivo docking
To probe the preferred binding mode of HMB-TZD to the active site of 5-LOX, in silico docking studies were performed using the modeled structure of 5-LOX. To accomplish this, the docking algorithm Surflex-Dock, which is part of the SYBYL software package (version SYBYL-X 1.1, Tripos, Inc.), was used. The active site of 5-LOX was defined using the SYBYL structure preparation tool based on the complex structures of 3-LOX from soybean with 1NO3, 1HU9, 1N8Q, 13(S)-hydroperoxy-9(Z), 11(E)-octadecadienoic acid, or 1JNQ. The HMB-TZD structure drawn in PubChem Sketcher v2.4 (http://pubchem.ncbi.nlm.nih.gov) was converted to a 3D structure and energy-minimized with the Gasteiger-Huckel force field and saved in the MOL format for docking.
Ldlr-/- mice and HMB-TZD treatment
Eight-week-old male Ldlr-/- mice (n = 20) were divided randomly into two equal-sized groups, control and HMB-TZD-treated. The control group was fed a western diet (CRF-1 supplemented with 0.15% cholesterol, 20% fat, and 0.05% Na-cholate, Oriental Yeast Co. Ltd., Tokyo, Japan), while the HMB-TZD-treated mice were fed the same diet supplemented with 1% (w/w) HMB-TZD. All mice were given water and food ad libitum. After 8 weeks, the mice were sacrificed, and plasma levels of various molecules were determined using an automatic blood chemical analyzer (HITACHI, Japan). Other parenchymal organs were harvested for histopathological examination. This investigation conforms to the Guidelines for the Care and Use of Laboratory Animals published by the United States National Institutes of Health. All animal study protocols were approved by the Institutional Animal Care and Usage Committee of Ewha Womans University (Seoul, Korea).
Measurement of LTB4, PGE2, MCP-1, and TNF-α levels
The levels of LTB4, PGE2, MCP-1, and TNF-α in plasma or cell culture supernatant were quantified by ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Each measurement was performed in triplicate and three independent measurements were conducted.
Statistical analysis
Statistical significance between two groups was determined with the two-tailed Student's t test. Analysis of variance followed by Fisher's Protected Least Significant Difference was used for comparisons within a test group. All data in this study are expressed as the mean ± standard deviation. Values of P < 0.05 were considered significant.
Supplemental data
Supplementary data were included with methods for establishing the pharmacokinetics of HMB-TZD in Ldlr-/- mice, measurement of plasma lipid peroxide, measurement of superoxide in atherosclerotic lesions, histopathology, quantitative real-time RT-PCR, macrophage adhesion assay, mouse aorta isolation, and ex vivo adhesion assay and cell migration. Supplemental Data include two figures and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-43-8-05.pdf.
Acknowledgements
This study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (A090264).
Abbreviations
- 5-LOX
5-lipoxygenase
- BHB-TZD
5-(3,5-di-tert-butyl-4-hydroxybenzylidene) thiazolidin-2,4-dione
- COX
cyclooxygenase
- HMB-TZD
5-(4-hydroxy-2,3,5-trimethylbenzylidene) thiazolidin-2,4-dione
- ICAM-1
intercellular adhesion molecule-1
- Ldlr
low density lipoprotein receptor
- TNF-α
tumor necrosis factor-alpha
- VCAM-1
vascular cell adhesion molecule-1
Supplementary Material
Supplemental Data
References
- 1.Aiello RJ, Bourassa PA, Lindsey S, Weng W, Freeman A, Showell HJ. Leukotriene B4 receptor antagonism reduces monocytic foam cells in mice. Arterioscler Thromb Vasc Biol. 2002;22:443–449. doi: 10.1161/hq0302.105593. [DOI] [PubMed] [Google Scholar]
- 2.Berger W, De Chandt MT, Cairns CB. Zileuton: clinical implications of 5-Lipoxygenase inhibition in severe airway disease. Int J Clin Pract. 2007;61:663–676. doi: 10.1111/j.1742-1241.2007.01320.x. [DOI] [PubMed] [Google Scholar]
- 3.Choi JH, Jeon HJ, Park JG, Sonn SK, Lee MR, Lee MN, You HJ, Kim GY, Kim JH, Lee MH, Kwon OS, Nam KH, Kim HC, Jeong TS, Lee WS, Oh GT. Anti-atherogenic effect of BHB-TZD having inhibitory activities on cyclooxygenase and 5-lipoxygenase in hyperlipidemic mice. Atherosclerosis. 2010;212:146–152. doi: 10.1016/j.atherosclerosis.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, Lusis AJ, Mehrabian M. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 5.Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen MY, Pieper U, Sali A. Comparative protein structure modeling using Modeller. Curr Protoc Bioinformatics. 2006 doi: 10.1002/0471250953.bi0506s15. Chapter 5:Unit 5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedrich EB, Tager AM, Liu E, Pettersson A, Owman C, Munn L, Luster AD, Gerszten RE. Mechanisms of leukotriene B4--triggered monocyte adhesion. Arterioscler Thromb Vasc Biol. 2003;23:1761–1767. doi: 10.1161/01.ATV.0000092941.77774.3C. [DOI] [PubMed] [Google Scholar]
- 7.Funk CD. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat Rev Drug Discov. 2005;4:664–672. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- 8.Heller EA, Liu E, Tager AM, Sinha S, Roberts JD, Koehn SL, Libby P, Aikawa ER, Chen JQ, Huang P, Freeman MW, Moore KJ, Luster AD, Gerszten RE. Inhibition of atherogenesis in BLT1-deficient mice reveals a role for LTB4 and BLT1 in smooth muscle cell recruitment. Circulation. 2005;112:578–586. doi: 10.1161/CIRCULATIONAHA.105.545616. [DOI] [PubMed] [Google Scholar]
- 9.Huang L, Zhao A, Wong F, Ayala JM, Struthers M, Ujjainwalla F, Wright SD, Springer MS, Evans J, Cui J. Leukotriene B4 strongly increases monocyte chemoattractant protein-1 in human monocytes. Arterioscler Thromb Vasc Biol. 2004;24:1783–1788. doi: 10.1161/01.ATV.0000140063.06341.09. [DOI] [PubMed] [Google Scholar]
- 10.Jawien J, Gajda M, Rudling M, Mateuszuk L, Olszanecki R, Guzik TJ, Cichocki T, Chlopicki S, Korbut R. Inhibition of five lipoxygenase activating protein (FLAP) by MK-886 decreases atherosclerosis in apoE/LDLR-double knockout mice. Eur J Clin Invest. 2006;36:141–146. doi: 10.1111/j.1365-2362.2006.01606.x. [DOI] [PubMed] [Google Scholar]
- 11.Jeong TS, Kim JR, Kim KS, Cho KH, Bae KH, Lee WS. Inhibitory effects of multi-substituted benzylidenethiazolidine- 2,4-diones on LDL oxidation. Bioorg Med Chem. 2004;12:4017–4023. doi: 10.1016/j.bmc.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Kim JM, Lee EK, Park G, Kim MK, Yokozawa T, Yu BP, Chung HY. Morin modulates the oxidative stress-induced NF-kappaB pathway through its anti-oxidant activity. Free Radic Res. 2010;44:454–461. doi: 10.3109/10715761003610737. [DOI] [PubMed] [Google Scholar]
- 13.Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 14.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 15.Lötzer K, Jahn S, Kramer C, Hildner M, Nüsing R, Funk CD, Habenicht AJ. 5-Lipoxygenase/cyclooxygenase-2 cross-talk through cysteinyl leukotriene receptor 2 in endothelial cells. Prostaglandins Other Lipid Mediat. 2007;84:108–115. doi: 10.1016/j.prostaglandins.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Mehrabian M, Allayee H, Wong J, Shi W, Wang XP, Shaposhnik Z, Funk CD, Lusis AJ. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res. 2002;91:120–126. doi: 10.1161/01.res.0000028008.99774.7f. [DOI] [PubMed] [Google Scholar]
- 17.Momose Y, Maekawa T, Yamano T, Kawada M, Odaka H, Ikeda H, Sohda T. Novel 5-substituted 2,4-thiazolidinedione and 2,4-oxazolidinedione derivatives as insulin sensitizers with antidiabetic activities. J Med Chem. 2002;45:1518–1534. doi: 10.1021/jm010490l. [DOI] [PubMed] [Google Scholar]
- 18.Neau DB, Gilbert NC, Bartlett SG, Boeglin W, Brash AR, Newcomer ME. The 1.85 A structure of an 8R-lipoxygenase suggests a general model for lipoxygenase product specificity. Biochemistry. 2009;48:7906–7915. doi: 10.1021/bi900084m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Galán E, Gómez-Hernández A, Vidal C, Martn-Ventura JL, Blanco-Colio LM, Muñoz-García B, Ortega L, Egido J, Tuñón J. Leukotriene B4 enhances the activity of nuclear factor-kappaB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc Res. 2009;81:216–225. doi: 10.1093/cvr/cvn277. [DOI] [PubMed] [Google Scholar]
- 21.Sippl MJ. Recognition of errors in three-dimensional structures of proteins. Proteins. 1993;17:355–362. doi: 10.1002/prot.340170404. [DOI] [PubMed] [Google Scholar]
- 22.Skrzypczak-Jankun E, Bross RA, Carroll RT, Dunham WR, Funk MO., Jr Three-dimensional structure of a purple lipoxygenase. J Am Chem Soc. 2001;123:10814–10820. doi: 10.1021/ja011759t. [DOI] [PubMed] [Google Scholar]
- 23.Spanbroek R, Grabner R, Lotzer K, Hildner M, Urbach A, Ruhling K, Moos MP, Kaiser B, Cohnert TU, Wahlers T, Zieske A, Plenz G, Robenek H, Salbach P, Kuhn H, Radmark O, Samuelsson B, Habenicht AJ. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci USA. 2003;100:1238–1243. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 25.Unangst PC, Connor DT, Cetenko WA, Sorenson RJ, Kostlan CR, Sircar JC, Wright CD, Schrier DJ, Dyer RD. Synthesisand biological evaluation of 5-[[3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl]methylene]oxazoles, -thiazoles, and -imidazoles: novel dual 5-lipoxygenase and cyclooxygenase inhibitors with antiinflammatory activity. J Med Chem. 1994;37:322–328. doi: 10.1021/jm00028a017. [DOI] [PubMed] [Google Scholar]
- 26.Vila L. Cyclooxygenase and 5-lipoxygenase pathways in the vessel wall: role in atherosclerosis. Med Res Rev. 2004;24:399–424. doi: 10.1002/med.10065. [DOI] [PubMed] [Google Scholar]
- 27.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 28.Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med. 2000;192:421–432. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou Y, Kim DH, Jung KJ, Heo HS, Kim CH, Baik HS, Yu BP, Yokozawa T, Chung HY. Lysophosphatidylcholine enhances oxidative stress via the 5-lipoxygenase pathway in rat aorta during aging. Rejuvenation Res. 2009;12:15–24. doi: 10.1089/rej.2008.0807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data