Abstract
Cardiac lymphatic system in the remodeling after acute myocardial infarction (AMI) has been overlooked. We wanted to investigate the role of bone marrow-derived endothelial progenitor cells (EPCs) and their contribution to lymphatic distribution in myocardial remodeling after AMI. Mouse (C57bl/6J) MI models were created by ligation of the left anterior descending coronary artery and were treated with phosphate buffered saline (PBS) or EPCs. Real-time RT-PCR with 2- to 4-week myocardial tissue samples revealed that lymphangiogenetic factors such as vascular endothelial growth factor (VEGF)-C (8.5 fold, P < 0.05), VEGF-D (6.1 fold, P < 0.05), Lyve-1 (15 fold, P < 0.05), and Prox-1 (11 fold, P < 0.05) were expressed at significantly higher levels in the PBS group than the EPC group. The PBS group also showed a significantly higher density of lymphatic vessels in the peri-infarction area. Echocardiography showed that from 2 weeks after the treatment, left ventricle (LV) dimensions at both systole and diastole were significantly smaller in the EPC group than in the PBS group (P < 0.01) and LV fractional shortening was higher in the EPC group accordingly (P < 0.01). Lymphangiogenic markers increased in a mouse MI model. EPC transplantation decreased lymphangiogenesis and adverse ventricular remodeling after AMI. These novel findings suggest that new lymphatic vessels may be formed in severely damaged myocardium, and may be involved in adverse myocardial remodeling after AMI.
Keywords: endothelial cells, lymphangiogenesis, myocardial infarction, stem cells, ventricular remodeling
Introduction
Acute myocardial infarction (AMI) and subsequent ischemic heart failure are well known worldwide health problems and leading causes of morbidity and mortality. Heart failure is an unfavorable consequence of myocardial remodelling after AMI (Sun, 2009). Cardiac structural remodelling is associated with inflammatory reactions, followed by scar formation in the infarcted area as well as changes in the non-infarcted myocardium, including interstitial fibrosis and vascular remodeling (Sun et al., 2000).
The lymphatic system of the heart has largely been overlooked (Miller et al., 1964). Miller et al. reported that chronic impairment of cardiac lymph flow resulted in a significant increase in fibrous and elastic tissue in the ventricular endocardium (Miller et al., 1963). Ventricular subendocardial hemorrhages were frequently noted early after lymphatic obstruction. The hemorrhagic areas could lead to fibrosis in the presence of continued lymphatic impairment. Previously, Ishikawa et al. investigated cardiac lymphatics in the various phases of healing after AMI (Ishikawa et al., 2007). They found that newly formed lymphatic vessels first appeared in the early stages of granulation, subsequently increased in the late stages, and remained up to the scar phase. In another experimental study, lymphatic flow was increased three hours after the occlusion of the left anterior descending coronary artery (LAD) (Feola and Lefer, 1977). The enhancement of the cardiac lymphatic system after AMI suggests a role in myocardial remodeling after AMI. However, lymphangiogenesis has been poorly examined in the myocardial remodeling process after AMI.
Endothelial progenitor cells (EPCs) exist in bone marrow (BM) and peripheral blood. Flk-1 and CD34 antigens are used to detect putative EPCs. In vitro, EPCs can be differentiated into endothelial lineage cells, and in animal models of ischemia, heterologous, homologous, and autologous EPCs are shown to incorporate into sites of active neovascularization (Young et al., 2007). Recently, the tranplantation of EPCs has been associated with favorable effects on myocardial remodeling after AMI (Cho et al., 2007).
We therefore investigated cardiac lymphangiogenesis after MI and whether local transplantation of BM-derived EPCs could contribute to lymphangiogenesis during the myocardial remodeling process in mouse models of AMI.
Results
Increased cardiac lymphangiogenesis after AMI
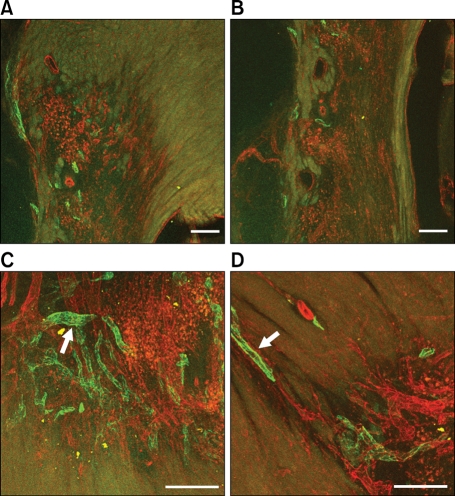
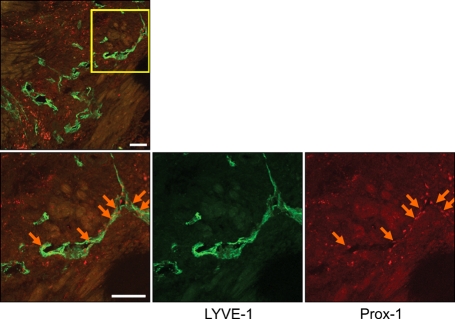
To investigate whether lymphatic vessels developed in the myocardium after AMI, we induced AMI in C57BL/6 mice by ligation of the LAD. Mice were sacrificed at 14 days after AMI. Cross sectional images showed abundant newly formed LYVE-1 positive lymphatic vessels in the peri-infarction areas (arrow), but lymphatic vessels were rarely found in the non-infarct (Figure 1A) or infarcted areas (Figure 1B). Because activating blood vessels sometimes express LYVE-1, we the confirmed lymphatics in the perinecrotic areas by double staining of LYVE-1 and Prox-1 (Figure 2).
Figure 1.
Cross sectional image of the heart 2 weeks after AMI (Red fluorescence represents PECAM for blood vessels, and green fluorescence represents LYVE-1 for lymphatic vessels). (A) Lymphatic vessels were rarely seen in the noninfarct area. (B) Lymphatic vessels were rarely seen in the infarct area. However, lower panels demonstrate newly formed lymphatic vessels in the peri-infarction area (arrow, C, D). Bars, 100 µm.
Figure 2.
Cross sectional image of the heart 2 weeks after AMI (Green fluorescence represents LYVE-1 and red fluorescence represents Prox-1 for lymphatic vessels). The lymphatics in the peri-infarction areas were confirmed by double staining of LYVE-1 and Prox-1 (arrows). Bars, 100 µm.
Transplantation of EPCs attenuates cardiac lymphangiogenesis and fibrosis after AMI
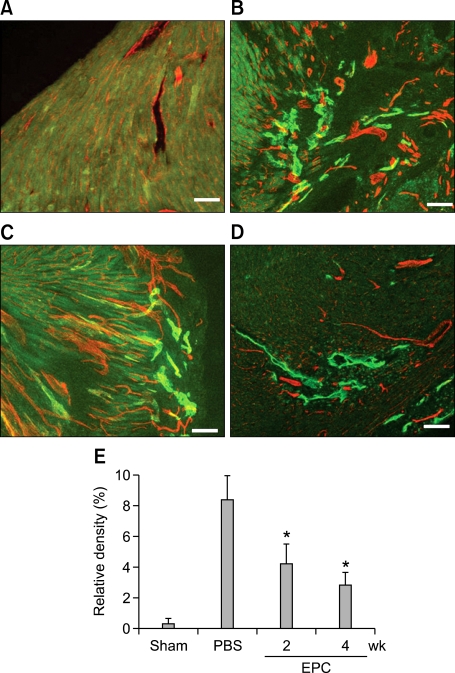
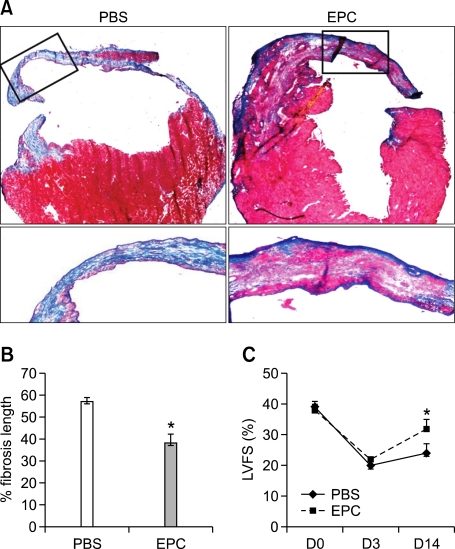
To investigate whether local transplantation of BM derived EPCs could contribute to cardiac lymphangiogenesis, we randomly assigned C57BL/6 mice to two groups, EPC or PBS, and induced MI by coronary ligation. We then transplanted 5 × 105 mouse cultured mouse EPCs, or PBS as a control, directly into the ventricular wall of the peri-infarction areas. Immunohistochemical analysis was done at 2 and 4 weeks after the experiment. Lymphatic vessels were seldom found in the sham operation group (Figure 3A). The newly formed lymphatic vessels developed peri-infarction areas in the PBS group (Figure 3B). However, the EPC treatment group showed a lesser degree of lymphatics at 2 (Figure 3C) and 4 weeks after AMI (Figure 3D). Relative density of lymphatic vessels was significantly lower in the EPC treated group (Figure 3E). Also, EPC injection decreased fibrotic scar size compared with PBS injection (Figure 4). Echocardiography showed that from 2 weeks after treatment, LV systolic function significantly improved in the EPC group (LV fractional shortening: 32 vs 24%, P < 0.01, Figure 4C).
Figure 3.
Cross sectional images of the heart 2 and 4 weeks after AMI (Red fluorescence represents PECAM for blood vessels, and green fluorescence represents LYVE 1 for lymphatic vessels). (A) Lymphatic vessels were seldom found in the sham operation group. (B) Newly formed lymphatic vessels were developed in the peri-infarction area in the PBS group. However, the EPC treatment group shows a lesser degree of lymphatics at 2 (C) and 4 weeks (D) after AMI. Relative density of lymphatic vessels was significantly lower in the EPC treatment group (E). Bars, 100 µm, *P < 0.05.
Figure 4.
Cross-sectional image at 4 weeks after AMI. (A) Representative Masson's trichrome staining of hearts at 4 weeks. (B) The blue color indicates fibrosis or scar. EPC transplantation decreased fibrotic scar size compared with PBS. (C) Left ventricular systolic function calculated by fractional shortening (LVFS) was significantly increased in the EPC group at 14 days after AMI. *P < 0.01.
EPC transplantation decreased the expression of the lymphangiogenetic factors
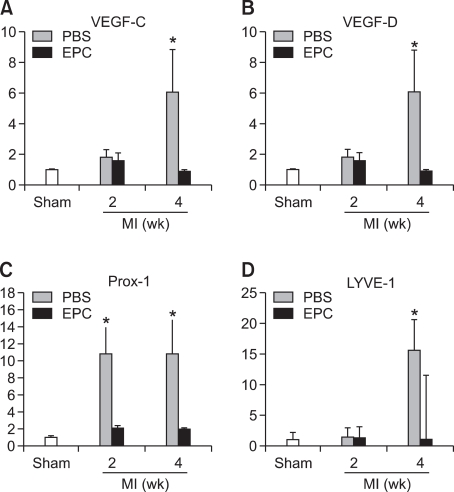
To determine whether multiple paracrine factors are associated with cardiac lymphangiogenesis after AMI, we harvested cardiac tissues in the peri-infarction areas. Real-time RT-PCR revealed that lymphangiogenetic factors were expressed at significantly higher levels of VEGF-C (8.5 fold, P < 0.05), VEGF-D (6.1 fold, P < 0.05), Lyve-1 (15 fold, P < 0.05), Prox-1 (11 fold, P < 0.05), podoplanin (1.7 fold, P = ns) in the PBS group compared to the EPC group (Figure 5).
Figure 5.
Real time RT-PCR data. Powerful lymphagiogenetic factors, VEGF-C and D were significantly increased in the PBS group but not in the EPC group after AMI (A, B). The injection of EPCs decreased Prox-1, a homeobox gene product which plays a key role in lymph vessel development and differentiation, which was significantly increased in the PBS group but not in the EPC group after AMI (C). Lyve-1, a selective lymphatic endothelial cell marker, was significantly increased in the PBS group but not in the EPC group 4 weeks after AMI (D). *P < 0.05.
BM derived EPCs were not incorporated during lymphangiogenesis
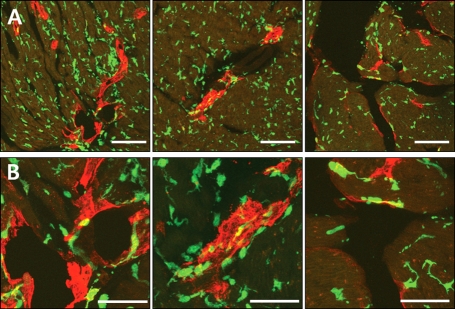
To determine whether BM-derived cells contribute to lymphangiogenesis, we used a mouse BMT model in which wild-type mice were lethally irradiated and transplanted with BM cells of eGFP transgenic mice. BM of BMT mouse was reconstituted with GFP-expressing cells to track the fate of BM-derived cells in the infarcted myocardium. We first performed BMT, then made AMI with the BMT mice at least 4 weeks later. We counted GFP positive cells incorporated into lymphatic vessels in the myocardium at 2, and 4 weeks after AMI. Fluorescent immunohistochemistry demonstrated that the GFP positive cells were abundant in cardiac tissues, which indicate cells derived from transplanted BM. But GFP positive BM-derived cells were not incorporated into newly formed lymphatic vessels in the peri-infarction areas (Figure 6). These data suggest that BM derived cells were not transdifferentiate into lymphatic endothelial cells.
Figure 6.
Cross sectional image of the heart 4 weeks after AMI (Green fluorescence represents GFP for BM-derived cells and red fluorescence represents Lyve-1 for lymphatic vessels). GFP positive BM-derived cells were not incorporated into newly formed lymphatic vessels (A: Bars, 100 µm; B: Bars, 50 µm).
Discussion
In this study, we demonstrated that lymphatic vessels were not detected in lesions with necrosis or in the non-infarction area, and most newly formed lymphatics appeared in the peri-infarction areas 2 weeks after AMI. Second, newly developed lymphatics were significantly lower in the EPC transplantation group which showed improved LV systolic function. Third, lymphangiogenetic factors VEGF-C, VEGF-D, Lyve-1, and Prox-1 were expressed at significantly higher levels in the PBS groups compared to the EPC groups. Fourth, GFP positive BM-derived cells were not incorporated into newly formed lymphatic vessels in the peri-infarction areas.
Heart failure is a major global health problem and it appears most commonly in patients with previous AMI (Sun, 2009). Myocardial remodeling usually resulting from myocardial scar formation can be seen in both the infarcted and non-infarcted myocardium through several sequential stages of myocardial necrosis, granulation and fibrosis (Lodge-Patch, 1951). Unfavorable cardiac remodeling is recognized to be a major determinant of the development of impaired ventricular function and leads to a poor prognosis.
Recent studies showed that cardiomyocytes can be replaced through a process involving differentiation, maturation and death (Anversa and Nadal-Ginard, 2002). Moreover, turnover of cardiomyocytes in the mammalian heart has been demonstrated experimentally, and shown to be triggered and enhanced by myocardial injury (Hsieh et al., 2007). These regenerative processes involve the recruitment of endogenous progenitor cells and the differentiation of these cells into cardiomyocytes or blood vessels. They are controlled by various factors expressed from the host tissue. Therefore, replacement of progenitor cells can produce functional tissue after injection or infusion of precursor cells into the damaged part. Various cell types are being tested as potential sources of cardiac progenitors for treatment of ischemic heart diseases (Byun and Kim, 2009; Hattori and Fukuda, 2010). Diverse fetal and adult tissues have been studied as sources of progenitor cells including heart, BM, skeletal muscle, fetal apparatuses, testis and adipose tissue. Any of these other organs represent a more accessible source of precursor cells than the heart. They can also provide an abundant quantity of autologous precursor cells, avoiding the risk of immune rejection of the transplanted precursor cells (Gonzales and Pedrazzini, 2009).
EPCs from peripheral blood mononuclear cells and BM have the potential to differentiate into endothelial cells during vasculogenesis. They can also differentiate into cardiomyocytes when cocultured with neonatal rat cardiomyocytes (Badorff et al., 2003). Injection of EPCs into a model with AMI was shown to improve cardiac function by promoting angiogenesis, without their differentiation into cardiomyocytes (Narmoneva et al., 2004; Cho et al., 2007; Young et al., 2007). In this study, the injected EPCs into a myocardial infarction are associated with decreased LV fibrosis, and improved LV systolic performance as in other studies.
The cardiac lymphatic system modulates myocardial fluid homeostasis between fluid filtration into the myocardial interstitium from blood capillaries and its removal via the lymphatic system (Mehlhorn et al., 2001). In the process of myocardial remodeling, the viable cardiomyocytes around the infarcted area produce cytoprotective proteins and cytokines which enhance the healing of the affected lesion (Ishikawa et al., 2000, 2005). In this process, VEGF is essential for angiogenesis in the healing area (Banai et al., 1994). VEGF is promptly expressed in the surviving cardiomyocytes around the infarcted lesion after the onset of AMI (Ishikawa et al., 2000) and angiogenesis in the lesion starts at 4-5 h and continues up to three months (Ishikawa et al., 2007). Although there are several studies regarding angiogenesis in the infarcted area (Asahara et al., 1999; Ren et al., 2002), lymphangiogenesis in the healing process after AMI has been rarely evaluated, previously (Ishikawa et al., 2007). Ishikawa et al. reported their data about myocardial tissues in various phases of healing after AMI from 40 autopsied hearts (Ishikawa et al., 2007). They found that lymphatic vessels were not detected in stages with coagulation necrosis. However, newly formed lymphatics firstly appeared in the early stages of granulation, subsequently increased in the late stages and remained up to the scar phase. They also found that lymphangiogenesis occurred after blood vessel angiogenesis in the healing process of AMI and lymphatics participate mainly in fibrosis maturation and scar formation through the drainage of excessive proteins and fluid. In the experimental study with dogs by Feola et al. (Feola and Lefer, 1977), lymphatic flow rate was increased about 3 h after ligation of LAD and increased coronary capillary permeability. Increased lymphatic flow rate might be associated with interstitial edema caused by LAD ligation. Moreover, lymphatic flow impairment caused by lymphatic disruption or flow resistance may occur in some diseases such as AMI and mediastinal lymphadenopathy, in which functional lymphangiogenesis (lymphatic vessel regeneration) will improve cardiac lymph flow (Rockson, 2008). In our study, potent lymphangiogenetic factors including VEGF-C and D, Prox-1, and Lyve-1 were significantly expressed in the peri-infarction areas after AMI. The results of our experiment are in agreement with the earlier studies. The use of EPCs decreased the expression of these factors and extent of lymphatic vessels. In the EPC treatment group, the extent of fibrosis was significantly lower than in the control group. From these results, it can be seen that injected EPCs enhanced the healing process after AMI, reduced interstitial edema and decreased the formation of lymphatic vessels.
In conclusion, lymphangiogenic markers increased in a mouse model of AMI. EPC transplantation improved LV function and attenuated lymphangiogenesis and adverse ventricular remodeling within 2 weeks after AMI. BM-derived cells were not incorporated into newly formed lymphatic vessels in the peri-infarction areas.
Methods
Animals and model of myocardial infarction
Wild-type C57BL/6J mice and ubiquitous eGFP-expressing transgenic mice with a C57 background (Oriental Bio, Korea), aged 6-10 weeks, were used. Mice were bred and maintained at the animal facilities at the Chungnam National University Hospital. All animal experiments and protocols were approved by the animal studies committee at Chungnam National University Hospital, and conducted in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, and all University requirements.
Mice were anesthetized with an intraperitoneal injection of avertin (0.014 mg/kg; 2,2,2-Tribromoethanol; Sigma-Aldrich), intubated with a 22G IV catheter, and artificially ventilated with a mechanical ventilator (Harvard Apparatus). After thoracotomy at the left 4th or 5th intercostal space, the LAD was ligated just distal to the bifurcation of the diagonal branch using 8.0 polypropylene sutures through a dissecting microscope (Qin et al., 2006; Cho et al., 2007). The apex of the left ventricle (LV) was observed for evidence of myocardial blanching and akinesia indicating interruption of coronary flow. After induction of MI, 5 × 105 mouse EPCs in a volume of 50 µl PBS was directly injected into the peri-infarction and apical areas (total of four sites) using a 30 G needle. In control groups, the same volume of PBS was injected in an identical fashion. At predetermined time points, mice were killed and hearts were harvested.
To trace BM-derived cells, bone marrow transplantation (BMT) was performed as described previously (Ii et al., 2005). In brief, each recipient wild-type mouse was lethally irradiated with 12 Gy in two equal doses of 6 Gy delivered 3 h apart. About 1 × 106 BM-mononuclear cells harvested from femurs and tibias of donor eGFP mice in a volume of 200 µl PBS were injected into the tail vein of recipient mice.
Preparation of BM-derived EPCs and cell culture
For culture of mouse EPCs, BM-mononuclear cells were collected by density gradient centrifugation with Histopaque-1083 (Sigma-Aldrich) and were plated at a density of 0.8-1.0 × 106 cells/cm2 on rat plasma vitronectin-coated (Sigma-Aldrich) dishes with EC basal medium (EBM-2) supplemented with 5% fetal bovine serum (FBS), antibiotics, and cytokine cocktail (SingleQuots; Clonetics). Nonadherent cells were removed at day 4, and cultures were supplemented with new media. The cells were maintained through day 7 and used as an EPC-enriched population (Kawamoto et al., 2001; Cho et al., 2007).
Immunohistochemical analysis
Mice were sacrificed, and hearts were perfused retrogradely through the right carotid artery with PBS containing 4% paraformaldehyde. The hearts were fixed for 4 h in paraformaldehyde and incubated overnight in 15% sucrose solution. To identify capillaries and lymphatics, anti-CD31 antibody, an EC marker (Millipore Corp., Bedford, MA) and anti-Lyve-1 antibody (Millipore Corp.) were used as primary antibodies and fluorescent conjugated species-specific antibodies (Jackson ImmunoResearch Inc., Baltimore, PA) as secondary. To measure the cardiac circumferential fibrosis area, Masson's trichrome staining was performed.
Quantitative real-time RT-PCR
We measured mRNA levels of lymphangiogenic factors in the myocardium using quantitative real-time RT-PCR (Supplemental Data Table S1). Peri-infarction myocardial tissues were harvested and pulverized by a TissueLyserII (Qiagen, Hilden, Germany). The homogenized tissue was processed to extract total RNA following a standard phenol-chloroform protocol. Total RNA concentrations were measured with a spectrophotometer (Nano-Drop Inc, ND-1000); RNA integrity was evaluated after agarose gel electrophoresis. The extracted RNA (500 ng) was subject to cDNA synthesis with M-MLV reverse transcrptase (Invitrogen, Carlsbad, CA) in a final volume of 20 µl. Real-time PCR (Corbett Research, Roter gene 6000) results were recorded as CT values (threshold cycle), which are defined as the cycle number at which the fluorescent signal generated by the SYBR green I dye and double-stranded DNA complex is statistically higher than the background signal. To adjust for different concentrations of total cDNA in the samples, ΔCT was calculated based on the difference in CT values between the target gene and the housekeeping gene GAPDH. ΔCT is inversely proportional to the mRNA level of a given gene. In data analysis, ΔCT was converted into an expression index based on the formula: 1000 × 2-ΔCT.
Statistical analysis
All results are presented as mean ± SEM. Statistical analysis was performed by an unpaired Student's t-test for comparisons between 2 groups. Values of P < 0.05 are considered statistically significant.
Supplemental data
Supplemental data include a table and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-43-8-06.pdf.
Acknowledgements
This study was supported by a grant of the Korea Healthcare technology R&D Preject, Ministry Health & Welfare, Republic of Korea (A100588), Chungnam National University Hospital Research Fund 2008, and a grant from Novartis, Republic of Korea.
Abbreviations
- AMI
acute myocardial infarction
- BM
bone marrow
- BMT
bone marrow transplantation
- EPCs
endothelial progenitor cells
- LAD
left anterior descending coronary artery
- LV
left ventricle
- PBS
phosphate buffered saline
- VEGF
vascular endothelial growth factor
Supplementary Material
Supplemental Data
References
- 1.Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–243. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 3.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 4.Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, Epstein SE, Unger EF. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994;89:2183–2189. doi: 10.1161/01.cir.89.5.2183. [DOI] [PubMed] [Google Scholar]
- 5.Byun KH, Kim SW. Is stem cell-based therapy going on or out for cardiac disease? Korean Circ J. 2009;39:87–92. doi: 10.4070/kcj.2009.39.3.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feola M, Lefer AM. Alterations in cardiac lymph dynamics in acute myocardial ischemia in dogs. J Surg Res. 1977;23:299–305. doi: 10.1016/0022-4804(77)90065-8. [DOI] [PubMed] [Google Scholar]
- 8.Gonzales C, Pedrazzini T. Progenitor cell therapy for heart disease. Exp Cell Res. 2009;315:3077–3085. doi: 10.1016/j.yexcr.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Hattori F, Fukuda K. Strategies for ensuring that regenerative cardiomyocytes function properly and in cooperation with the host myocardium. Exp Mol Med. 2010;42:155–165. doi: 10.3858/emm.2010.42.3.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T, Losordo DW. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via "imported" nitric oxide synthase activity. Circulation. 2005;111:1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa Y, Akasaka Y, Ishii T, Itoh K, Masuda T, Zhang L, Kiguchi H. Sequential changes in localization of repair-related proteins (heat shock protein 70, ubiquitin and vascular endothelial growth factor) in the different stages of myocardial infarction. Histopathology. 2000;37:546–554. doi: 10.1046/j.1365-2559.2000.00994.x. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa Y, Komiyama K, Masuda S, Murakami M, Akasaka Y, Ito K, Akishima-Fukasawa Y, Kimura M, Fujimoto A, Kudo I, Ishii T. Expression of type V secretory phospholipase A in myocardial remodelling after infarction. Histopathology. 2005;47:257–267. doi: 10.1111/j.1365-2559.2005.02227.x. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa Y, Akishima-Fukasawa Y, Ito K, Akasaka Y, Tanaka M, Shimokawa R, Kimura-Matsumoto M, Morita H, Sato S, Kamata I, Ishii T. Lymphangiogenesis in myocardial remodelling after infarction. Histopathology. 2007;51:345–353. doi: 10.1111/j.1365-2559.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 16.Lodge-Patch I. The ageing of cardiac infarcts, and its influence on cardiac rupture. Br Heart J. 1951;13:37–42. doi: 10.1136/hrt.13.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehlhorn U, Geissler HJ, Laine GA, Allen SJ. Myocardial fluid balance. Eur J Cardiothorac Surg. 2001;20:1220–1230. doi: 10.1016/s1010-7940(01)01031-4. [DOI] [PubMed] [Google Scholar]
- 18.Miller AJ, Pick R, Katz LN, Jones C, Rodgers J. Ventricular endomyocardial changes after impairment of cardiac lymph flow in dogs. Br Heart J. 1963;25:182–190. doi: 10.1136/hrt.25.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller AJ, Pick R, Katz LN. The importance of the lymphatics of the mammalian heart: experimental observations and some speculations. Circulation. 1964;29(Suppl):485–487. doi: 10.1161/01.cir.29.4.485. [DOI] [PubMed] [Google Scholar]
- 20.Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962–968. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin G, Ii M, Silver M, Wecker A, Bord E, Ma H, Gavin M, Goukassian DA, Yoon YS, Papayannopoulou T, Asahara T, Kearney M, Thorne T, Curry C, Eaton L, Heyd L, Dinesh D, Kishore R, Zhu Y, Losordo DW. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J Exp Med. 2006;203:153–163. doi: 10.1084/jem.20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem. 2002;50:71–79. doi: 10.1177/002215540205000108. [DOI] [PubMed] [Google Scholar]
- 23.Rockson SG. Diagnosis and management of lymphatic vascular disease. J Am Coll Cardiol. 2008;52:799–806. doi: 10.1016/j.jacc.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res. 2009;81:482–490. doi: 10.1093/cvr/cvn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, Zhang JQ, Zhang J, Lamparter S. Cardiac remodeling by fibrous tissue after infarction in rats. J Lab Clin Med. 2000;135:316–323. doi: 10.1067/mlc.2000.105971. [DOI] [PubMed] [Google Scholar]
- 26.Young PP, Vaughan DE, Hatzopoulos AK. Biologic properties of endothelial progenitor cells and their potential for cell therapy. Prog Cardiovasc Dis. 2007;49:421–429. doi: 10.1016/j.pcad.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data