Abstract
During the acute phase of infection, T. cruzi replicates extensively and releases immunomodulatory molecules that delay parasite-specific responses mediated by effector T cells. This mechanism of evasion allows the parasite to spread in the host. Parasite molecules that regulate the host immune response during Chagas'disease have not been fully identified. GPI-anchored mucins, glycoinositolphospholipids, and glycoproteins comprise some of the most abundant T. cruzi surface molecules. IL-10 IFN-γ-secreting CD4+ T cells are activated during chronic infections and are responsible for prolonged persistence of parasite and for host protection against severe inflammatory responses. In this work we evaluated the role of rMBP::SSP4 protein of T. cruzi, a recombinant protein derived from a GPI anchored antigen, SSP4, as an immunomodulator molecule, finding that it was able to induce high concentrations of IL-10 and IFN-γ both in vivo and in vitro; during this last condition, both cytokines were produced by IL-10-IFN-γ-secreting CD4+ T cells.
Keywords: Trypanosoma cruzi, IL-10, IFN-γ, CD4+ T cells
Introduction
Chagas' disease is caused by the protozoan parasite Trypanosoma cruzi 1. It is estimated that 14-16 million people in Latin America and 1 million in the US are infected with this parasite 2. The metacyclic trypomastigote infects host cells and inside them, differentiate into the amastigote forms that are responsible for parasite replication. 3. During infection with T. cruzi, the activation of the immune system is likely to be involved in at least two aspects of Chagas' disease pathophysiology, which are, the control of parasite replication and spread in the host tissues, and the inflammatory reaction in infected host tissues. At the onset of infection, T. cruzi activates cells from macrophage lineage to produce high levels of chemokines and proinflammatory cytokines. Most notably, cells exposed to protozoan products will produce IL-12 which is responsible for initiation of IFN-γ synthesis 4, 5. On the other hand, IL-10 has also been associated with susceptibility to T. cruzi infection. IL-10 inhibits macrophages functions including the activation by IFN-γ to kill intracellular T. cruzi. Thus it seems that these two cytokines, IL-10 and IFN-γ are opposed in critical aspects of immunity in response to T. cruzi infection 6-8.
Little is known about parasites molecules that trigger the innate immune system during early stages of protozoan infections. In the last few years, the identification and structural characterization of parasite molecules that initiates the synthesis of different cytokines and other molecules by immune cells has been the focus of many studies. Experimental evidence strongly suggests that GPI anchors derived from Plasmodium falciparum, Trypanosoma brucei and T. cruzi play an important role on the activation of the innate immune system during protozoan infection 9-12.
There are several reports describing CD4+ T cell clones producing IFN-γ and IL-10; these were first obtained in 1999 from bronchoalveolar lavage fluid in acute tuberculosis patients 13. IL-10 and IFN-γ-secreting CD4+ T cells have been found during chronic infections, such as TB, Leishmania, Lyme disease, a T. gondii mouse model, and L. major. These cells are now considered able to prevent collateral immune damage, as well as being used by pathogens to prevent their elimination 14. Under normal conditions there is no substantial co-expression of IL-10 and IFN-γ cells in IL-10 reporter mice, even in the intestine, where most IL-10-secreting CD4+ cells reside. It is also known that a threshold of activation and repeated stimulation of the immune system are required for the generation of these cells. Parasite molecules that may regulate host responses have not been fully explored 15. In the present work, we investigated the role of rMBP::SSP4, a recombinant protein derived from Trypanosoma cruzi (a major surface glycoprotein (SSP4) that is bound to the plasma membrane by a GPI anchor) on the immune system as a result of immunization. Results showed that this antigen was able to induce a broad range of cytokines such as IL-4, IL-10, IL-12, and TNF-α, and IFN-γ both in vivo and in vitro, and that the two major cytokines produced, IL-10 and IFN-γ, were produced by CD4+ T cells, a regulatory population previously described and named IL-10-IFN-γ-secreting CD4+ T cells.
Materials and Methods
Mice
C57BL/129 mice were housed in a controlled microenvironment at the animal facility at Ohio State University and managed according to institutional animal care guidelines.
Mice Immunization
Female C57BL/129 mice, aged 10-12 weeks were immunized by intraperitoneal injection with rMBP::SSP4 protein emulsified in complete Freund's adjuvant (CFA) at the first time and incomplete Freund's adjuvant (IFA) the following times (Sigma). Each mouse was injected with 10 µg of rMBP::SSP4 in 100 µL of PBS mixed with 100 µL of CFA or IFA, on days 1, 7, and 14. Control mice were injected with 100 µL of PBS plus CFA/IFA following the same procedure.
Antibody Isotyping by ELISA
Fourteen days after the last immunization mice were sacrificed and bleed and sera was used to determine the isotype of produced antibodies. Immunoglobulin (Ig) G1 and IgG2a were evaluated by ELISA. Plates were coated with 5 µg of rMBP::SSP4 in carbonate buffer, pH 9.0 and incubated at 4 ºC overnight. Plates were washed and blocked with 200 µL per well of 5 % non-fat milk in PBS for 1 h at 37 ºC. Plates were washed and incubated with immune serum (anti-rMBP::SSP4) for 2 h at 37 ºC. After washing, 100 µL per well peroxidase-labelled rat anti-mouse IgG subclass antibodies (1:5000 dilution) were added (Pharmingen), diluted in PBS-25% FBS and incubated for 2 h at 37 ºC. After incubation, N.N.-Dimethylformamide plus ABTS (2,2'-azino-di-(3-ethylbenzthiazoline-6-sulfonate) were added as substrate, 10 min later, the reaction was stopped with 50 µL of 5% phosphoric acid and plates were read immediately at 450 nm.
In vitro splenocytes culture
Spleen cells from immunized or control C57BL/129 mice, were incubated with DMEM containing 10% FCS at 37 ºC in 5% CO2 in 24-well plates (2 x106 cells/well). Spleen cells were cultured separately in the presence of 10 µg/mL rMBP::SSP4 protein, 4 µg/mL Concanavalin A (ConA) or medium alone in 24-well plates (Costar). Culture supernatants were collected after 72 h and cytokine concentrations were determined. All experiments were controlled for stimulation with MBP alone (data not shown).
Determination of Cytokine pattern by ELISA
Interleukin 4 (IL-4), IL-6, IL-10, IL-12, TNF-α, and IFN-γ were quantified by ELISA in culture supernatants of spleen cells under different conditions of re-stimulation, and in sera, according to the manufacturer's protocol. Briefly, 96 well flat bottom plates were coated over night with capture antibody at a final antibody concentration of 2 μg/mL, and then plates were blocked with 10% PBS-FCS, washed three times and incubated with the antigen overnight at 4 ºC. After washing, plates were incubated with biotinylated anti-cytokine Ab at 1 μg/mL for 1 h in the dark. Plates were washed and streptavidin-Alkaline Phosphatase at 1:2000 was added for 30 min in the dark, then plates were washed and 100 µL of p-Nitro Phenyl Phosphate (pNPP) substrate were added to each well and incubated until the color changed (approximately 1 h). Plates were read at 405 nm.
Flow Cytometry Analysis and Intracellular Staining
Spleen cells from immunized and non immunized mice, cultured for 72 h, were stained with PE anti-mouse-CD4 or APC anti-mouse-CD8 according to the manufacture's protocol. Briefly, nonspecific staining was blocked with anti-CD16/CD32 mAb (Fc block). Cells were incubated with appropriate antibodies for 30 min on ice, and washed twice with PBS containing 2% FCS. Working solutions of (1X) Fix/Perm buffer and Perm buffer were prepared prior to intracellular staining. Once prepared, 1 mL of 1X Fix/Perm solution was added to each tube, vortexed and incubated at room temperature in the dark for 20 min, then cells were spin down and the supernatant was removed. Cells were washed and re-suspended in 1 mL 1X Perm buffer, incubated at room temperature in the dark for 15 min, spin down and the supernatant was discarded. Then, the respective antibodies were added: APC anti-mouse-IL-10 antibody or PE-anti-mouse IFN-γ antibody to the appropriate tubes, and incubated at room temperature in the dark for 30 min. Finally, samples were washed twice with cell staining buffer and re-suspended in 0.5 mL cell staining buffer. Samples were read in a FACS Calibur.
Statistical analysis
Analyses were performed using GraphPad Prism version 5.0 software. Differences were considered significant when a p value of less than 0.05 was obtained.
Results and Discussion
Parasite persistence depends on a combination of factors, including release of molecules that interfere with the immune response. Early work demonstrated that T. cruzi suppresses lymphocyte activation, an effect that depends on the density of parasites 16. Therefore, suppression induced by parasite molecules is more relevant at the acute phase, when the concentration of such molecules can be fairly high. The best characterized parasite molecules are surface glycosylphosphatidylinositol (GPI)-anchors, to which both pro- and anti-inflammatory effects have been ascribed 17. TcSSP4, the gene that codifies for a T. cruzi amastigote-specific surface GPI anchored antigen which is released to the culture medium by phospholipase C activity, was cloned in the EcoR1 site of the expression vector pMAL-C2, resulting in the plasmid pMAL-TcSSP4. This plasmid was used to transform E. coli DH5-α, to obtain the recombinant protein MBP::SSP4 (rMBP::SSP4).
Ramos-Ligonio et al., (2004) demonstrated that rMBP::SSP4 is a modulator of humoral and cellular immune responses, inducing low levels of IgA, IgM and IgG3, but high levels of IgG1, IgG2a and IgG2b isotypes; moreover, rMBP::SSP4 induced the expression of iNOS and the production of NO by macrophages. rMBP::SSP4 was also able to induce the mRNA for IL-1α, IL-6, IL-12, IFN-γ and TNF-α cytokines in control mice and IL-10 in immunized mice, suggesting that TcSSP4 might exert a regulatory influence on macrophages during the immune response against T. cruzi.
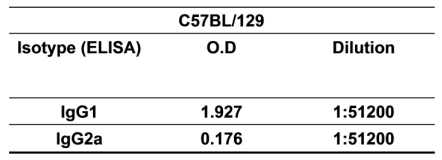
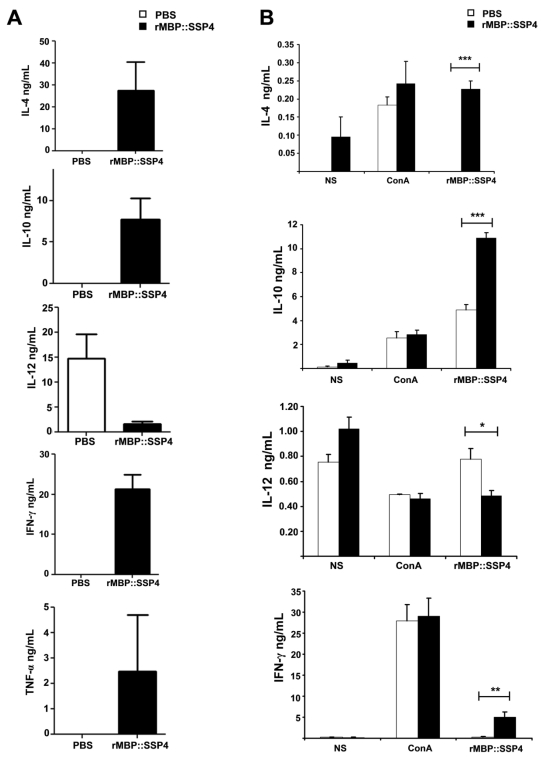
Based on this knowledge, we assessed the antigen-specific humoral and cellular immune responses induced by rMBP::SSP4 in C57BL/129 mice, a resistant strain model of T. cruzi infection. Results showed that the isotype pattern was maintained after immunization with rMBP::SSP4, showing high levels of IgG1 and IgG2a, confirming the immunogenicity of SSP4 (Table 1). We then examined the serum cytokine profile from immunized and non-immunized mice in response to antigen stimulation. Results showed that this antigen induces the secretion of several cytokines such as IL-4, IL-10, IFN-γ, and TNF-α in considerable amounts, whereas IL-12 was produced at a very low level, suggesting that SSP4 is an immunomodulator molecule of T. cruzi (Fig. 1A). To assess whether local cytokine profile in the spleens of immunized and non-immunized mice was consistent with systemic cytokine levels in vivo, we determined the profile of cytokines in the supernatants of spleen cells of both groups under re-stimulation conditions (Fig. 1B). Results confirmed that the same cytokine profile is present in vitro; IL-4, IL-10, IL-12, and IFN-γ were detected in the supernatants of immunized mice. However, IL-12 production by cells from immunized mice was lower than that produced by non-immunized cells when they were re-stimulated in vitro with rMBP::SSP4. These results clearly showed that the immune response induced in vivo is maintained in vitro. However, higher concentrations of cytokines were found in the serum in comparison with those present in the supernatants from spleen cells (Fig. 1); either IL-5 nor IL-6 were detected in serum or culture supernatants even under re-stimulation (data not shown). Supernatants of inguinal lymph nodes were also analyzed finding only IL-10 (data not shown). It is important to mention that the two major cytokines found were IL-10 and IFN-γ, which have been broadly studied during T. cruzi infection and have been shown that play an important role during Chagas disease.
Table 1.
Isotyping of anti-rMBP::SSP4 antibodies
Fig 1.
Cytokines profiles induced in vivo (A) and in vitro (B) after rMBP::SSP4 immunization. Mice were bleed two weeks after the third immunization and cytokines were measured in sera and culture supernatants from spleen cells by ELISA. Spleen cells were harvested after 72 h. A) IL-4, IL-10, IL-12, IFN-γ and TNF-α in serum from both groups of mice, immunized (full bars) and non-immunized (open bars). B) IL-4, IL-10, IL-12 and IFN-γ production by spleen cells under different conditions of re-stimulation: NS, (non-stimulated), ConA and rMBP::SSP4 for immunized (full bars) and non immunized mice (open bars). Histograms show values in ng/mL (mean±SD) of three experiments run in duplicate.
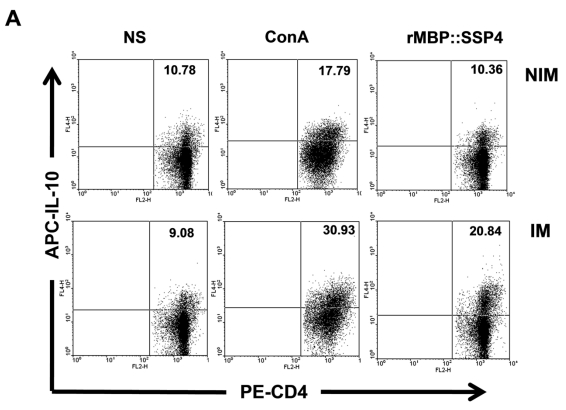
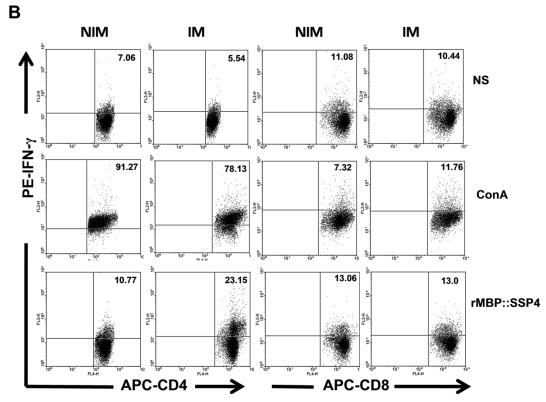
We then proceeded to define the T cell subpopulation(s) responsible of IL-10 and IFN-γ production. Spleen cells from C57BL/129 mice were stained for CD4 surface marker and then for intracellular IL-10. Results showed that IL-10 was indeed produced by CD4+ T cells after immunization and re-stimulation conditions, finding a clear difference between immunized and non-immunized mice (20.84% versus 10.36%) (Fig. 2A). T cells, both CD4+ and CD8+, and NK cells are among the major producers of IFN-γ. Because high levels of this cytokine were also produced after rMBP::SSP4 immunization, we also looked for its cell source. The presence of IFN-γ was analyzed in spleen cells after re-stimulation in CD4 and CD8 T cells. In immunized mice, this cytokine was produced by CD4+ T cells, similar to the production of IL-10, finding that 23.15% of CD4 T cells were IFN-γ producers in comparison with only 10.77% of CD4+ T cells from non-immunized mice under rMBP::SSP4 stimulation (Fig. 2B). CD8+ T cells didn't produce IFN-γ in considerable amounts, neither from immunized or non-immunized mice, even under re-stimulation conditions.
Fig 2.
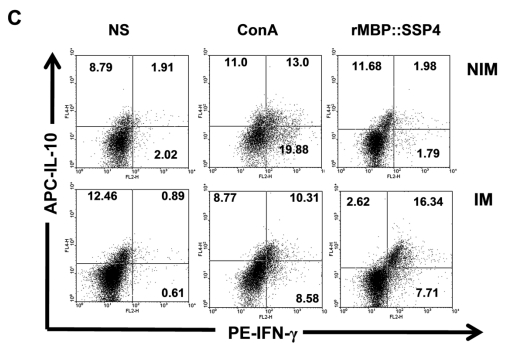
CD4+ T cells produce both IL-10 and IFN-γ as a result of rMBP::SSP4 immunization and re-stimulation. Spleen cells from non-immunized (NIM) and immunized (IM) mice were cultured under different conditions of re-stimulation: NS, ConA and rMBP::SSP4 for 72 h. A) Dot blots graphs showing the production of intracellular IL-10 in gated CD4+ cells. B) Cells were stained for surface CD4 or CD8. Subsequently cells were stained for intracellular IFN-γ. Live cells were gated and CD4+ cells and CD8+ as well were selected. Density plots show the production of intracellular IFN-γ. The percentage of IFN-γ positive cells is indicated in each panel. C) Spleen cells suspensions were cultured under different conditions of re-stimulation as above, and CD4+ cells were selected and subsequently stained for intracellular IFN-γ and IL-10. Density plots show the production of both intracellular cytokines in CD4+ cells. The percentage of double cytokine positive cells is indicated in each panel. Data are representative of three independent experiments.
After finding that IL-10 and IFN-γ were produced by CD4+ T cells, and having knowledge that after repeated stimulation with some antigens, IL-10-IFN-γ-secreting T cells can be induced, we were interested in looking for double producer cells in this model. Results showed that IL-10 and IFN-γ were simultaneously produced by CD4+ T cells; a clear difference was observed between spleen cells from immunized mice (16.34%) and non-immunized mice (1.98%) under re-stimulation condition with rMBP::SSP4 (Fig. 2C). No differences between groups were observed in the condition where cells didn't receive stimulus or were stimulated with ConA.
Therefore, based on the above results, we demonstrate that MBP::SSP4, a recombinant protein derived of T. cruzi, induces IL-4, IL-10, IL-12, and IFN-γ in vitro and IL-4, IL-10, IFN-γ and TNF-α in vivo as a result of immunization, and that the two major cytokines produced in vitro were IL-10 and IFN-γ both of which were produced by IL-10-IFN-γ-secreting CD4+T cells (double producers). Studies done with other T. cruzi derived recombinant protein (rMBP::TcHsP70 administered with or without adjuvant) and in one case with the same immunization scheme, did not induce the same pattern of cytokines production (unpublished data). Therefore, we can conclude that the immune response induced by SSP4 is specific for this particular antigen.
Regulatory T cells are groups of T cells that maintain lymphocyte homeostasis and immune tolerance and down-regulate immune responses to prevent excessive immune responses against pathogens 19. Tregs include naturally occurring thymus-derived Foxp3+ CD25+ T cells, induced Foxp3+ CD4+ T cells, and Foxp3+/- IL-10 secreting CD4+ T cells. Although IL-10-IFN-γ-secreting CD4 T cells have been recognized for many years, it was only discovered recently that the IL-10 IFN-γ-secreting T bet+ T cells in a T. gondii infection mouse model were required to prevent early mortality caused by excessive inflammation 15. Similar results were obtained in a cutaneous L. major infection model 20. These cells are required for maintaining a chronic, no resolving infection status. There are evidences that these IL-10 IFN-γ-secreting CD4+ T cells are also suppressive, data that can explain the low proliferation observed in spleen cells proliferation assays. IL-10 IFN-γ-secreting CD4+ T cells are antigen-induced; these double producers' cells are usually generated in a strong immune-stimulating environment and they mediate the suppressive function through IL-10 with the help of IFN-γ 21. These cells are now considered able to prevent collateral immune damage, and also it has been described that these cells could be used by pathogens to prevent their elimination; this is one more useful data to explain the long persistence of T. cruzi on its host.
In conclusion, IL-10 IFN-γ-secreting CD4+ T cells are suppressive cells that play an important role in regulating immune responses. Thus, activation of these cells may result in curable autoimmune diseases. However, their modulation for infectious diseases treatment could be more complicated, as inhibition of these cells would also lead to over immune responses. Investigating how to balance IL-10 and IFN-γ secretion by these cells, might provide better management for those infectious diseases, where IL-10-IFN-γ-secreting cells play a role in infection control.
Acknowledgments
Yevel Flores-García was a recipient of a “Beca Mixta” from CONACYT-México (207007) to support a training period at Ohio State University as part of her PhD program. This work was partially supported by CONACYT México grant 34991 to JLR.
References
- 1.Andrews N W, Hong K S, Robbins E S. et al. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp Parasitol. 1987;64:474–484. doi: 10.1016/0014-4894(87)90062-2. [DOI] [PubMed] [Google Scholar]
- 2.de Meis J, Morrot A, Farias-de-Oliveira DA. et al. Differential regional immune response in Chagas disease. PLoS Negl Trop Dis. 2009;7:e417. doi: 10.1371/journal.pntd.0000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Chagas' disease. WHO. 1993.
- 4.Cardillo F, Voltarelli JC, Reed SG. et al. Regulation of Trypanonoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect Immun. 1996;64:128–134. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biron CA, Gazzinelli RT. Effects of IL-2 on immune response to microbial infections: a key mediator in regulating disease outcome. Curr Opin Immunol. 1995;7:485–497. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 6.Silva J S, Morrissey PJ, Grabstein KH. et al. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992;175:169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorentino DF, Bond MW, Mosmann RT. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed S G, Brownell C, Russo D. et al. IL-10 mediates susceptibility to Trypanosoma cruzi infection. J Immunol. 1994;153:3135–3141. [PubMed] [Google Scholar]
- 9.Ropert C, Gazzinelli RT. Signaling of immune system cells by glycosylphosphatidylinositol (GPI) anchor and related structures derived from parasitic protozoa. Curr Opin Microbiol. 2000;3:395–403. doi: 10.1016/s1369-5274(00)00111-9. [DOI] [PubMed] [Google Scholar]
- 10.Almeida IC, Gazzinelli RT. Proinflammatory activity of glycosylphosphatidylinositol anchors derived from Trypanosoma cruzi: structural and functional analyses. J Leukoc Biol. 2001;70:467–477. [PubMed] [Google Scholar]
- 11.Camargo MM, Almeida LC, Pereira ME. et al. Glycosylphosphatidylinositol -anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J Immunol. 1997;158:5890–5901. [PubMed] [Google Scholar]
- 12.Almeida JC, Camargo MM, Procopio DO. et al. Highly purified glycosylphosphatidylinositol from Trypanosoma cruzi are potent proinflammatory agents. EMBO J. 2000;19:1476–1485. doi: 10.1093/emboj/19.7.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerosa F, Nisii C, Righetti S. et al. CD4(+) T cell clones producing both interferon-γ and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin Immunol. 1999;92:224–234. doi: 10.1006/clim.1999.4752. [DOI] [PubMed] [Google Scholar]
- 14.Nylen S, Maurya R, Eidsmo L. et al. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+(Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med. 2007;204:805–817. doi: 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Liu XS. Development and function of IL-10 IFN-γ-secreting CD4+ T cells. J Leukoc Biol. 2009;86:1–6. doi: 10.1189/jlb.0609406. [DOI] [PubMed] [Google Scholar]
- 16.Maleckar JR, Kierszenbaum F. Inhibition of mitogen-induced proliferation of mouse T and B lymphocytes by bloodstream forms of Trypanosoma cruzi. J Immunol. 1983;130:908–911. [PubMed] [Google Scholar]
- 17.DosReis GA. Evasion of immune response by Trypanosoma cruzi, the etiological agent of Chagas disease. Braz J Med Biol Res. 2011;44:84–89. doi: 10.1590/s0100-879x2011007500005. [DOI] [PubMed] [Google Scholar]
- 18.Ramos-Ligonio A, López-Monteon A, Talamás-Rohana P. et al. Recombinant SSP4 protein from Trypanosoma cruzi amastigotes regulates nitic oxide production by macrophages. Parasite Immunol. 2004;26:409–418. doi: 10.1111/j.0141-9838.2004.00729.x. [DOI] [PubMed] [Google Scholar]
- 19.O'Garra A, Viera P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 20.Anderson CF, Oukka M, Kuchroo VJ. et al. CD4(+) CD25(-) Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu XS, Leerberg J, MacDonald K. et al. IFN-(γ) promotes generation of IL-10-secreting CD4+ T cells that suppress generation of CD8 responses in an antigen-experienced host. J Immunol. 2009;183:51–58. doi: 10.4049/jimmunol.0802047. [DOI] [PubMed] [Google Scholar]