The incidence of obesity in Western countries has dramatically increased along with its associated co-morbidities (type 2 diabetes, hypertension, heart disease, cancer…) and diminished life expectancy 1. Attempts at controlling this with diet have proven relatively ineffective. Consequently, obese patients (especially with co-morbidities) are increasingly referred for bariatric surgery. Within that domain, laparoscopic adjustable gastric banding (LAGB) has become more popular as it is associated with fewer short term complications than gastric bypass procedures 2. However, as exemplified in the report by Khan et al, LAGB may induce esophageal motility disorders 3.
In this issue of Journal of Clinical Gastroenterology, Khan et al. reported six cases of esophageal dilatation and severe esophageal dysmotility following LAGB placement 3. All of the patients except one were symptomatic with dysphagia and/or regurgitation. Esophageal manometry performed with a conventional technique (pressure sensors spaced at 5 cm intervals) revealed absent peristalsis in all cases. Esophageal dilatation was present on barium swallow. These findings were consistent with pseudoachalasia. After liquid removal from the implant, symptoms improved in all but one patient. The band was then removed in that patient and symptoms resolved. Esophageal dilatation improved in all patients. Manometric findings were variable: one patient exhibited a return of normal peristalsis, two had partial return of peristalsis and two had persistent absent peristalsis. These results emphasized the potential reversibility of pseudo-achalasia in patients with LAGB. The implications for the clinician are important; patients with dysphagia and/or regurgitation in the context of LAGB should be evaluated for pseudoachalasia. Since pseudoachalasia in this setting may be reversible, band deflation or band removal should be advised in such patients.
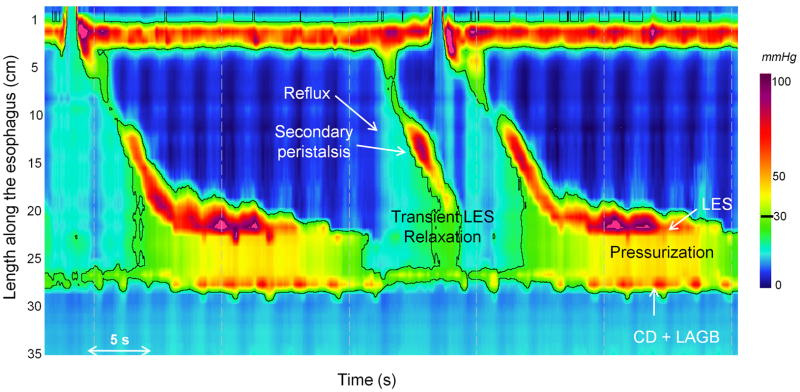
Esophageal manometry is the most sensitive procedure for the diagnosis of achalasia 4. The manometric criteria for achalasia are impaired esophago-gastric junction (EGJ) relaxation and absent peristalsis. High resolution manometry with esophageal pressure topography (EPT) exhibits increased sensitivity for detecting achalasia because of better metrics for characterizing EGJ relaxation and distal esophageal contractility 5. EPT also allows for the differentiation of three clinically-relevant achalasia subtypes 6. High resolution manometry with EPT can also be useful in distinguishing some forms of secondary achalasia from primary idiopathic achalasia. For example, in the case of LAGB, the site of the banding may be identified as a high pressure zone distal from the lower esophageal sphincter (LES), sometimes with the crural diaphragm superimposed 7 (Figure 1). The distinction between localizing the outflow obstruction at the LES or the Lapband is crucial in cases of suspected achalasia. Moreover, bolus clearance can also be inferred by the pattern of pressurization 8. A recent study using high resolution manometry in 22 symptomatic Lapband patients revealed the presence of esophageal dysmotility in 90% and pressurization (compartmentalized or pan-esophageal) in 68% 9. The pressurization pattern in the gastric pouch seems to be quite characteristic of an obstruction induced by LAGB (Figure 1) and is a useful sign for differentiating pseudo-achalasia induced by the band from primary idiopathic achalasia with obstruction at the level of the LES.
Figure 1.
EPT of a dysphagic patient with after LAGB exhibiting a pseudo-achalasia pattern. Although the patient exhibits EJG outflow obstruction with bolus pressurization well above 30 mmHg, the site of the outflow obstruction is distal to the LES, instead localized to the site of the LAGB/crural diaphragm (CD) combination. Some peristalsis appears preserved, but this pattern of bolus pressurization is easily misinterpreted as a simultaneous contraction using conventional manometry techniques. Note also the occurrence of a transient LES relaxation with reflux followed by secondary peristalsis.
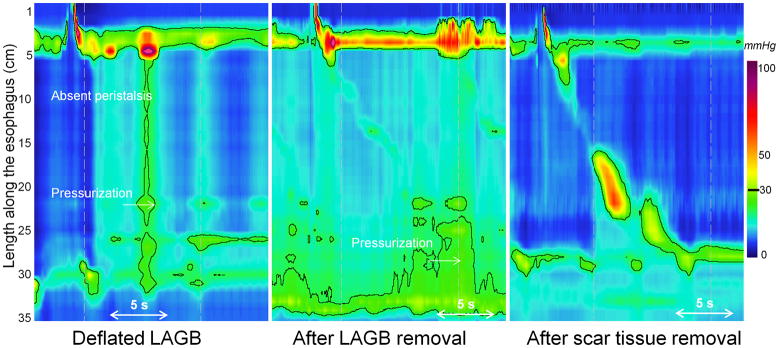
Khan et al observed some degree of reversibility to pseudoachalasia after Lapband deflation or removal. However, absent peristalsis persisted in two patients. This may be attributable to irreversible neuromuscular damage to the esophagus or it may be a manifestation of persistent obstruction ultimately attributable to fibrosis at the previous location of the Lapband. Surgically, this is recognized as encapsulation of the implant and may perpetuate an obstruction even after deflation. Illustrative of this is Figure 2 from a case report in which LAGB removal did not relieve dysphagia or normalize the EPT pattern. Only subsequent excision of the encapsulating fibrosis was associated with restoration of esophageal peristalsis in a patient presenting with pseudoachalasia from a Lapband 10. A corollary to the observation that esophageal peristalsis returns to normal after relieving EGJ obstruction is that the pathophysiology of idiopathic achalasia must also involve the distal esophagus proximal to the sphincter since recovery of peristalsis is not seen following treatment.
Figure 2.
Patient with pseudoachalasia after LABG placement. Dysphagia and obstructive pattern persisted after deflation of the implant (left) and removal of the implant (center). Only after surgical dissection to remove the encapsulating fibrosis was the obstruction relieved and peristalsis restored (right).
In conclusion, the risk of pseudoachalasia must be included among potential risks when LAGB placement is considered and pseudoachalasia should be recognized as a potential complication in the evaluation of post-procedure dysphagia and/or regurgitation. Preliminary post-operative data in morbidly obese patients suggest that esophageal dysmotility was more frequent after LAGB than after Roux-en-Y bypass procedures 11. Hence, although the magnitude of the risk is unclear, LAGB clearly can cause a syndrome that strongly mimics achalasia and, even though the disorder is potentially reversible, early recognition is likely key to insuring this. High resolution manometry is an elegant diagnostic tool in this regard.
Acknowledgments
This work was supported by R01 DK56033 (PJK) from the Public Health Service
References
- 1.Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 2.Tice JA, Karliner L, Walsh J, et al. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008;121:885–893. doi: 10.1016/j.amjmed.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 3.Khan A, Ren-Fielding C, Traube M. Potentially reversible pseudoachalasia after laparoscopic adjustable gastric banding. J Clin Gastroenterol. 2011 doi: 10.1097/MCG.0b013e318226ae14. [DOI] [PubMed] [Google Scholar]
- 4.Pandolfino JE, Kahrilas PJ American Gastroenterological A. American Gastroenterological Association medical position statement: Clinical use of esophageal manometry. Gastroenterology. 2005;128:207–208. doi: 10.1053/j.gastro.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Clouse RE, Staiano A, Alrakawi A, et al. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol. 2000;95:2720–2730. doi: 10.1111/j.1572-0241.2000.03178.x. [DOI] [PubMed] [Google Scholar]
- 6.Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: A New Clinically Relevant Classification by High-Resolution Manometry. Gastroenterology. 2008;135:1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton PR, Brown W, Laurie C, et al. The effect of laparoscopic adjustable gastric bands on esophageal motility and the gastroesophageal junction: analysis using high-resolution video manometry. Obes Surg. 2009;19:905–914. doi: 10.1007/s11695-009-9845-3. [DOI] [PubMed] [Google Scholar]
- 8.Burton PR, Brown WA, Laurie C, et al. Mechanisms of Bolus Clearance in Patients with Laparoscopic Adjustable Gastric Bands. Obes Surg. 2010;20:1265–1272. doi: 10.1007/s11695-009-0063-9. [DOI] [PubMed] [Google Scholar]
- 9.Cruiziat C, Roman S, Robert M, et al. High resolution esophageal manometry evaluation in symptomatic patients after gastric banding for morbid obesity. Dig Liver Dis. 2011;43:116–120. doi: 10.1016/j.dld.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Vallin M, Robert M, Roman S, et al. Persistent dysphagia after removal of an adjustable gastric band for morbid obesity: a rare complication. Dis Esophagus. 2010 doi: 10.1111/j.1442-2050.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 11.Merrouche M, Sabate JM, Jouet P, et al. Gastro-esophageal reflux and esophageal motility disorders in morbidly obese patients before and after bariatric surgery. Obes Surg. 2007;17:894–900. doi: 10.1007/s11695-007-9166-3. [DOI] [PubMed] [Google Scholar]