Abstract
BACKGROUND AND PURPOSE
Mounting evidence implicates matrix metalloproteinase (MMP) in the vascular dysfunction and remodelling associated with hypertension. We tested the hypothesis that treatment with pyrrolidine dithiocarbamate (PDTC), which interferes with NF-κB-induced MMPs gene transcription, could exert antihypertensive effects, prevent MMP-2 and MMP-9 up-regulation, and protect against the functional alterations and vascular remodelling of two-kidney, one clip (2K1C) hypertension.
EXPERIMENTAL APPROACH
Sham-operated or hypertensive rats were treated with vehicle or PDTC (100 mg·Kg−1·day−1) by gavage for 8 weeks. Systolic blood pressure (SBP) was monitored weekly. Aortic rings were isolated to assess endothelium-dependent relaxations. Quantitative morphometry of structural alterations of the aortic wall was carried out in haematoxylin/eosin sections. Formation of vascular reactive oxygen species (ROS), and inducible (i) NOS and phosphorylated-p65 NF-κB subunit expression were measured in the aortas. MMP-2 and MMP-9 aortic levels and gelatinolytic activity were determined by gelatin and in situ zymography and by immunofluorescence.
KEY RESULTS
Treatment with PDTC attenuated the increases in SBP and prevented the endothelial dysfunction associated with 2K1C hypertension. Moreover, PDTC reversed the vascular aortic remodelling, the increases in aortic ROS levels and in iNOS and phosphorylated-p65 NF-κB expression found in 2K1C rats. These effects were associated with attenuation of 2K1C up-regulation of aortic MMP-2 and MMP-9 levels and gelatinolytic activity.
CONCLUSION AND IMPLICATIONS
These findings suggest that PDTC down-regulates vascular MMPs and ameliorates vascular dysfunction and remodelling in renovascular hypertension, thus providing evidence supporting the suggestion that PDTC is probably a good candidate to be used to treat hypertension.
Keywords: pyrrolidine dithiocarbamate, 2K1C hypertension, matrix metalloproteinase, vascular remodelling, NF-κB
Introduction
Vascular dysfunction and remodelling are prominent features in hypertension and involve cell proliferation and extracellular matrix (ECM) synthesis and reorganization (Intengan and Schiffrin, 2001; Humphrey, 2008), thus leading to cardiovascular complications and target organ damage (Intengan and Schiffrin, 2001; Lemarie et al., 2010). In this respect, many recent studies implicate a group of zinc-dependent endopeptidases with a major role in the ECM remodelling, the matrix metalloproteinases (MMPs), in the pathogenic mechanisms responsible for the development of vascular alterations of hypertension (Flamant et al., 2007; Watts et al., 2007; Castro et al., 2008). Of relevance to the present study, altered MMP-2 and MMP-9 levels were shown to play a major role in the hyperplasia of vascular smooth muscle cells (VSMCs) and ECM reorganization, thus leading to vascular remodelling and dysfunction (Newby, 2006; Humphrey, 2008; Lemarie et al., 2010). Moreover, inhibiting MMPs (Castro et al., 2008; 2010) and some antihypertensive drugs that can modify MMP levels (Martinez et al., 2008; Ceron et al., 2010) were shown to ameliorate vascular dysfunction and remodelling in renovascular hypertension, probably as a result of the down-regulation of MMPs, even though only modest reductions in blood pressure were reported in these studies.
MMPs are regulated at gene expression, by post-translational activation of zymogens, and by interaction with their endogenous inhibitors [the tissue inhibitors of metalloproteinases, (TIMPs) ] (Clark et al., 2008; Murphy and Nagase, 2008). Relevant regulators of vascular MMPs expression include a variety of growth factors and cytokines, vasoactive peptides and ROS (Clark et al., 2008; Lemarie et al., 2010). The promoter regions of genes encoding some MMPs contain consensus sequences for known DNA-binding proteins, including NF-κB (Clark et al., 2008). Importantly, NF-κB is an oxidant-sensitive transcriptional factor, which plays a crucial role in the expression of many genes involved in vascular inflammation and remodelling (Van der Heiden et al., 2010), and its effects have been studied with the use of pyrrolidine dithiocarbamate (PDTC), which is an antioxidant that blocks NF-κB activation (Schreck et al., 1992). Interestingly, treatment with PDTC exerts antihypertensive effects in different models of hypertension, and attenuates VSMC proliferation and arterial hypertrophy (Muller et al., 2000; Beswick et al., 2001; Rodriguez-Iturbe et al., 2005; Lemarie et al., 2006; Humphrey, 2008; Zhou et al., 2010). Consistent with these findings, PDTC has been shown to blunt vascular shear stress-induced NF-κB activation and MMP-2 and MMP-9 expression and activity (Castier et al., 2009). However, no previous study has examined whether treatment with PDTC produces antihypertensive effects in the 2-kidney, 1-clip (2K1C) hypertension model, which has been associated with increased vascular expression and activity of MMP-2 and MMP-9, vascular dysfunction and arterial remodelling (Castro et al., 2008; 2010).
2K1C hypertension is clearly associated with the up-regulation of the renin–angiotensin system, and increased angiotensin II levels cause the structural and functional vascular alterations found in this model (Brasier et al., 2002). NF-κB is required for angiotensin II-dependent differentiation and proliferation of VSMC (Zahradka et al., 2002), and for the up-regulation of MMPs in vitro (Guo et al., 2008), therefore, in the present study, we tested the hypothesis that treatment with PDTC could exert antihypertensive effects, prevent MMP-2 and MMP-9 up-regulation and protect against the functional alterations and vascular remodelling associated with 2K1C hypertension.
Methods
Animals and treatments
All animal care and experimental procedures complied with the Guidelines published by the National Institutes of Health (NIH). Experimental protocols followed the standards and policies of the University of Sao Paulo's Animal Care and Use Committee. Renovascular hypertension was induced in rats as previously described (Castro et al., 2008). Briefly, ketamine 100 mg·kg−1 and xylazine 10 mg·kg−1 i.p. were used to anaesthetize male Wistar rats (approximately 180 g) and, after a midline laparotomy, a silver clip with an internal diameter (ID) of 0.20 mm was placed around the left renal artery. Sham-operated rats underwent the same surgical procedure, except for the placement of the renal artery clip. The rats were maintained on a 12 h light/dark cycle and kept at room temperature (22–25°C) with free access to standard rat chow and water.
The animals were randomly divided into four experimental groups as follows: 2K1C hypertension group that received water (2K1C vehicle), 2K1C hypertension group that received PDTC (2K1C PDTC), sham-operated group that received water (sham vehicle) and sham-operated group that received PDTC (sham PDTC). PDTC was given by gavage (100 mg·kg−1·day−1). Treatment with PDTC was started 2 weeks after 2K1C hypertension was induced and maintained for eight additional weeks. The dose of PDTC used in the present study was chosen based on previous findings showing antihypertensive effects in spontaneously hypertensive rats (SHR) associated with suppression of NF-κB activation (Rodriguez-Iturbe et al., 2005). Tail systolic blood pressure (SBP) was assessed weekly by tail-cuff plethysmography, and rats were considered to be hypertensive when SBP was higher than 160 mmHg 2 weeks after the surgery.
Vascular reactivity
After 8 weeks of treatment with PDTC or vehicle, the animals were killed by decapitation, and their thoracic aortas were isolated and cleaned of connective tissue and fat. Aortic rings, 4 mm in length, were cut and mounted for isometric tension recording (Castro et al., 2008; Martinez et al., 2008). The rings were placed in bath chambers (5 mL) for isolated organs containing modified Krebs salt solution with the following composition (mM): NaCl 130, CaCl2 1.6, MgSO4 1.2, KH2PO4 1.2, KCl 4.7, NaHCO3 14.9, glucose 5.5, which was maintained at 37°C and pH 7.4, and bubbled with 95% O2 and 5% CO2. The system was connected to an isometric force displacement transducer (TRI201, Panlab, Barcelona Spain) and the responses were recorded on a computer system using the Chart Pro 5 (PowerLab, ADInstruments, Sydney, Australia). The aortic rings were subjected to a basal tension of 1.5 g during 60 min equilibration period with the bath fluid being changed every 15–20 min. Endothelial integrity was assessed qualitatively by the degree of relaxation caused by ACh (10−6 M) in the presence of contractile tone induced by phenylephrine (10−7 M). The ring was discarded if relaxation with ACh was not 70% or greater. The aortic rings were pre-contracted with phenylephrine (10−7 M) and when the contraction reached a plateau, ACh (10−10 to 10−5 M) was added cumulatively to endothelium intact rings. The magnitude of contraction induced by phenylephrine did not differ among the experimental groups at 10−7 M. Relaxation was expressed as a percentage change from phenylephrine-contracted levels. Concentration–response curves were fitted using a non-linear interactive fitting program (Graph Pad Prism 5.0; GraphPad Software Inc., La Jolla, CA, USA). Agonist potency was expressed as pD2 (negative logarithm of the molar concentration of agonist producing 50% of the maximum response), and maximum response was expressed as Emax (maximum effect elicited by the agonist).
Morphometric analysis of the vascular wall
After 8 weeks of treatment with PDTC or vehicle, the rats were killed by decapitation. Their thoracic aorta was harvested, cleaned of connective tissue, immediately fixed in 4% phosphate-buffered paraformaldehyde at pH 7.4 and embedded in paraffin blocks. Four micrometre-thick slices were stained with haematoxylin and eosin. The morphometric parameters were calculated as described before (Dao et al., 2001; Castro et al., 2008). Media cross-sectional area (CSA) was calculated by subtracting the lumen internal area (Ai) from the external area (Ae), which was measured in each tissue section (×250). The external diameter (ED) and the ID were calculated as the square root of 4Ae/π and 4Ai/π, respectively. Media thickness (M) was calculated as (ED − ID)/2. Finally, M-to-lumen diameter (M/L) was also calculated. The number of VSMCs in the aortic wall was measured by the tridimensional dissector method on two consecutive sections (Castro et al., 2008). This method is independent of nuclei orientation, form and size. Stained sections were examined with light microscopy (DMLB; Leica, Bensheim, Germany) and the image was captured at ×400 (Dao et al., 2001). These structural analyses in the media were evaluated by using ImageJ Program (NIH, Bethesda, MD, USA).
Assessment of vascular ROS
Dihydroethidium (DHE), a sensitive superoxide probe, was used to evaluate in situ production of ROS (Castro et al., 2009; Ceron et al., 2010). Briefly, aortic tissues were embedded vertically in Tissue-tek® (Sakura Finetek, Torrance, CA, USA) and then frozen and cut in serial 4 µm sections. Unfixed cryosections were incubated at room temperature, in the dark, with 10 µL of DHE (10 µM) for 30 min. Sections were examined by fluorescence microscopy (Leica Imaging Systems Ltd, Cambridge, UK) and the image was captured at ×400. Red fluorescence reflected superoxide production and was evaluated by using the ImageJ program (NIH). We measured red fluorescence from 20 fields randomly selected throughout the vessel circumference, and the arithmetic mean of the fluorescence from the 20 fields was calculated for each slide.
Measurement of aortic phosphorylated p65 subunit of NF-κB andiNOS levels by Western blot
To investigate the activation of NF-κB in the vessel wall in this model of hypertension, we measured the expression of the phosphorylated-p65 (Ser532) subunit of NF-κB (Zhang et al., 2005; Zhou et al., 2010) and iNOS levels as a marker of NF-κB-regulated inflammatory gene expression (Hong et al., 2000; Muller et al., 2000). Briefly, aortic extracts, normalized for protein concentration, were separated by SDS-PAGE and then transferred onto nitrocellulose membranes (GE Healthcare, Madison, WI, USA). After being blocked in 5% non-fat milk, membranes were incubated overnight with specific mouse antibody against the phosphorylated-p65 (Ser 536) subunit of NF-κB (Cell Signaling, Beverly, MA, USA) or iNOS (Transduction Laboratories, Lexington, KY, USA) and also (after being stripped) were incubated with monoclonal anti-β-actin antibody (Millipore, Billerica, MA, USA) for 1 h at room temperature. Membranes were incubated with secondary horseradish peroxidase conjugated antibody (Chemicon, Tremecula, CA, USA) for 1 h at room temperature and were then developed by chemiluminescence with the ECL Millipore system. Band intensities were quantified by densitometric scanning using the ImageJ software (NIH).
Measurement of aortic MMP-2 and MMP-9 levels by gelatin zymography
Gelatin zymography was performed as previously described (Castro et al., 2008; Martinez et al., 2008). Briefly, tissues were homogenized in buffer containing 50 mM Tris–HCl, pH 7.4, 10 mM CaCl2, 1 mM 1,10-phenanthroline, 1 mM phenylmethylsulphonylfluoride (PMSF) and 1 mM NEM (N-ethylmaleimide). These extracts, normalized for protein concentration, were subjected to electrophoresis on 12% SDS-PAGE co-polymerized with gelatin (0.1%). After electrophoresis, the gel was incubated for 1 h at room temperature in a 2% Triton X-100 solution (VETEC, Vila Olímpia, Sao Paulo, Brazil), followed by further incubation at 37°C for 16 h in Tris–HCl buffer, pH 7.4, containing 10 mM CaCl2. The gels were fixed with 30% methanol and 10% acetic acid, stained with 0.05% Coomassie brilliant blue G-250 (USB Corporation, Cleveland, OH, USA), and then destained with 30% methanol and 10% acetic acid. Gelatinolytic activities were detected as unstained bands that were quantified by densitometry using a Kodak Electrophoresis Documentation and Analysis System 290 (Kodak, Rochester, NY, USA). Inter-gel analysis was possible after normalization of the gelatinolytic activity with an internal standard (fetal calf serum).
In situ zymography and immunofluorescence for MMP-2 and MMP-9
The activity of MMPs in the media and intima of frozen thoracic aorta was measured by in situ zymography using a gelatinolytic fluorogenic substrate in the presence or absence of specific inhibitors (Castro et al., 2010). Briefly, aortic tissues were embedded vertically in Tissue-tek and then frozen and cut in serial 4 µm sections. Vessel sections were incubated in dark humidified chambers with 1.0 µg·mL−1 dye-quenched (DQ) gelatin in Tris–HCl buffer, pH 7.4, containing 10 mM CaCl2 and 1 µM ZnCl2 for 1 h. The sections were examined by fluorescent microscopy (Leica Imaging Systems Ltd) and the image was captured at ×400. The proteolytic activity was detected as bright green fluorescence, which indicates substrate breakdown, and was evaluated by using the ImageJ Program (NIH). Assessment of the gelatinolytic activity was carried out by quantifying the intensity of green fluorescence from 20 fields selected around the vessel circumference. The arithmetic mean of the fluorescence from the 20 fields was calculated for each slide. This number of fields per slide corresponds to approximately 20–30% of the total aortic area being studied and has led to inter-assay coefficients of variations of less than 3%. Phenanthroline at 1 mM and PMSF at 1 mM were used to confirm the activity of MMPs in the aorta sections. While phenanthroline fully inhibited MMP activity, PMSF produced no significant effects (data not shown).
To co-localize MMP-2 or MMP-9 with in situ gelatinolytic activity, tissue sections were briefly fixed in 4% phosphate-buffered paraformaldehyde, pH 7.4, after incubation with DQ gelatin. Thereafter, sections were incubated for 1 h with either a mouse anti-MMP-2 MAb (1:1000 dilution, MAB3308, Chemicon) or a mouse anti-MMP-9 MAb (1:1000 dilution, MAB3309, Chemicon), which were detected with a rhodamine-conjugated secondary antibody (1:200 dilution, AP160P, Chemicon) for 1 h. To confirm the specificity of antibodies, the primary antibody was omitted and substituted with PBS + 1% BSA. Rhodamine did not bind non-specifically in tissue sections. The Photoshop program was used to co-localize the aortic photographs.
Statistical analysis
Results are expressed as means ± SEM. Between group comparisons were assessed by two-way anova (SigmaStat for Windows, Jadel Scientific, San Jose, CA, USA) or by one-way anova followed by the Tukey's test. Probability value P<0.05 was considered significant.
Results
Treatment with PDTC reduces 2K1C-induced hypertension
The baseline SBP and body weight were similar in the four experimental groups before surgery, and no significant changes in SBP were seen in sham groups (Figure 1A and B; P > 0.05). Significant increases in SBP were found 2 weeks after surgery in 2K1C groups. However, lower SBP levels were found in hypertensive animals treated with PDTC compared with those found in hypertensive animals treated with vehicle, and this difference was significant from the third week until the end of treatment (Figure 1A; P < 0.05). Significantly lower weight gain was found in hypertensive groups compared with the sham-operated groups (Figure 1B; P < 0.05).
Figure 1.
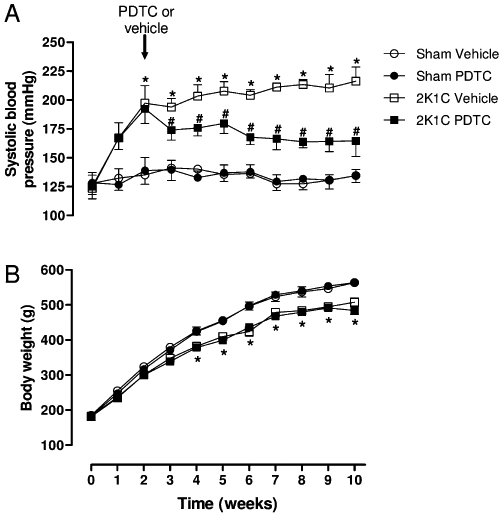
SBP measured by tail-cuff method (A) and body weight (B) in the four experimental groups along 10 weeks of study. Values are expressed as mean ± SEM (n = 12 per group). *P < 0.05 versus sham vehicle group. # P < 0.05 versus 2K1C vehicle group.
Treatment with PDTC ameliorates vascular function
To evaluate the effects of treatment with PDTC on vascular function, rat aortic rings were isolated and their functional response to ACh was assessed in organ chamber experiments. As shown in Figure 2, maximum ACh-induced relaxation was significantly reduced in aortic rings from the 2K1C vehicle group as compared with those from the sham groups (Figure 2 and Table 1; P < 0.05). The impaired response to ACh was completely reversed by treatment with PDTC (Figure 2 and Table 1; P < 0.05). However, no significant differences were found in the pD2 values obtained for ACh (Table 1; P > 0.05).
Figure 2.
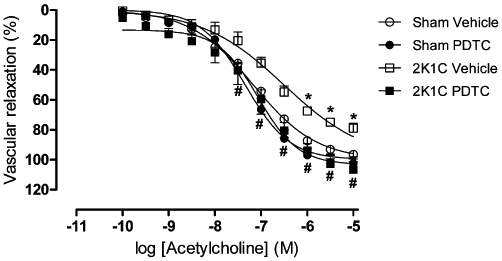
Endothelial cell-dependent vasorelaxation induced by ACh in rat aortic rings preparations. Values are expressed as mean ± SEM (n = 6 per group). *P < 0.05 versus sham vehicle group. #P < 0.05 versus 2K1C Vehicle group.
Table 1.
Values of pD2 and maximum relaxation (Emax) for endothelial cell-dependent vasorelaxation induced by ACh in rat aortic rings preparations
| Sham vehicle | Sham PDTC | 2K1C Vehicle | 2K1C PDTC | |
|---|---|---|---|---|
| pD2 ACh | 7.07 ± 0.30 | 7.22 ± 0.11 | 7.07 ± 0.19 | 7.07 ± 0.14 |
| Emax ACh | 96.4 ± 5.1 | 105.7 ± 4.7 | 78.9 ± 3.1* | 106.7 ± 7.1 |
Values are expressed as mean ± SEM (n = 5 per group).
P < 0.05 versus sham vehicle group.
Treatment with PDTC inhibits vascular remodelling associated with 2K1C hypertension
2K1C hypertension was associated with arterial wall hypertrophy, with significant increases in the number of VSMCs, increased aortic CSA and increased M/L ratio (Figure 3; P < 0.05). Treatment with PDTC prevented these structural alterations (Figure 3; P < 0.05).
Figure 3.
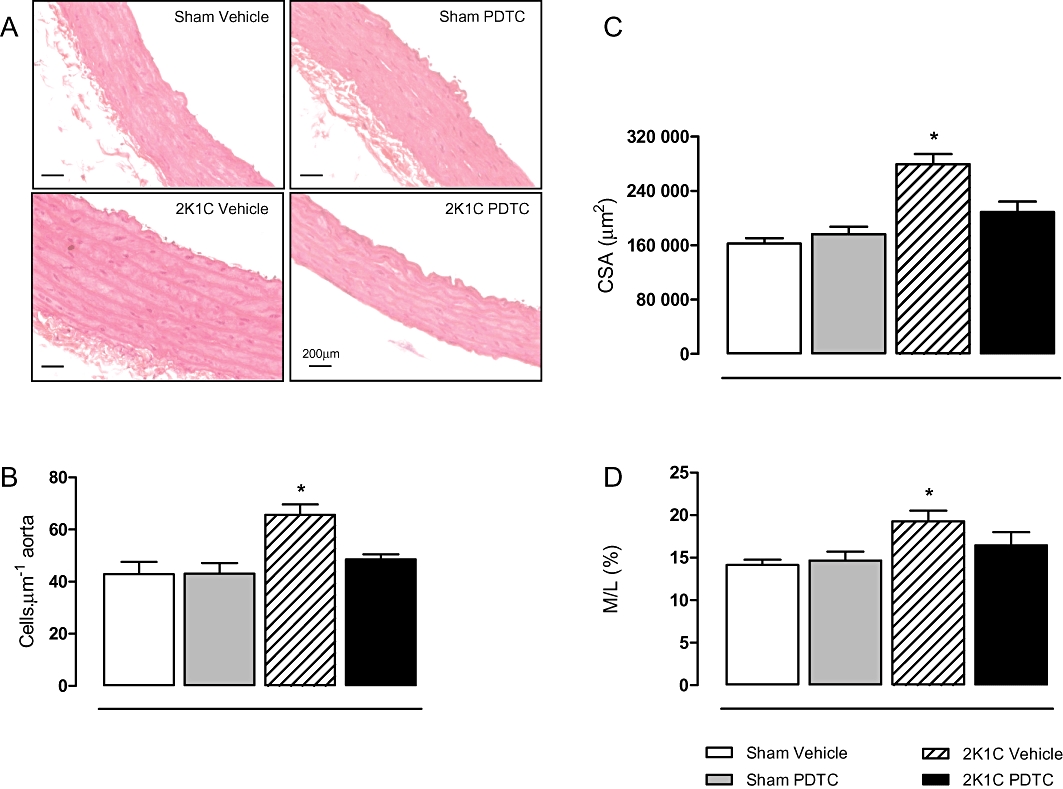
Effects of treatment with PDTC on aortic structural alterations induced by hypertension. Representative photographs of aortic samples (×400) stained by haematoxylin and eosin (A). Panels (B, C and D) show the numbers of VSMCs in the aortic media, CSA and media to lumen ratio (M/L), respectively. Values are expressed as mean ± SEM (n = 5–7 per group). *P < 0.05 versus sham vehicle group.
PDTC abolished 2K1C-induced increases in oxidative stress
We used the fluorescent superoxide indicator DHE to study the effects of PDTC on 2K1C hypertension-induced increases in ROS production in aortic tissues. Figure 4 shows that 2K1C hypertension increased vascular ROS levels significantly (P < 0.05), and treatment of hypertensive animals with PDTC abolished 2K1C-induced increases in oxidative stress (Figure 4; P < 0.05).
Figure 4.
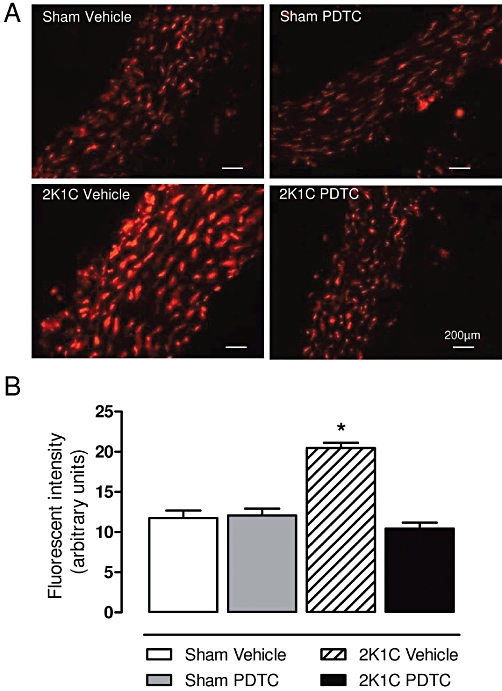
Effects of treatment with PDTC on vascular ROS production. Representative photomicrographs (×400) with red fluorescence in DHE aortic samples (A) and the quantification of aortic fluorescence (B). Values are expressed as mean ± SEM (n = 5 per group). *P < 0.05 versus sham vehicle group.
Effects of treatment with PDTC on vascular NF-κB activation and iNOS expression
Phosphorylation of p65 at serine 536 increases transactivation of NF-κB and target genes expression. As Figure 5A and B shows, protein expression of phopho-p65 NF-κB was significantly increased in the aortas of 2K1C hypertensive rats (P < 0.05). PDTC treatment prevented the increases in phophorylation of p65 NF-κB (P < 0.05). As the transcription and expression of inflammatory genes such as iNOS is typically elicited by NF-κB activation, we investigated the effects of PDTC on vascular iNOS expression to gain information on the extent of NF-κB inhibition by PDTC. Figure 5A and B clearly shows that 2K1C hypertension is associated with increased vascular iNOS expression (P < 0.05), and that treatment with PDTC suppressed this alteration in hypertensive animals (P < 0.05).
Figure 5.
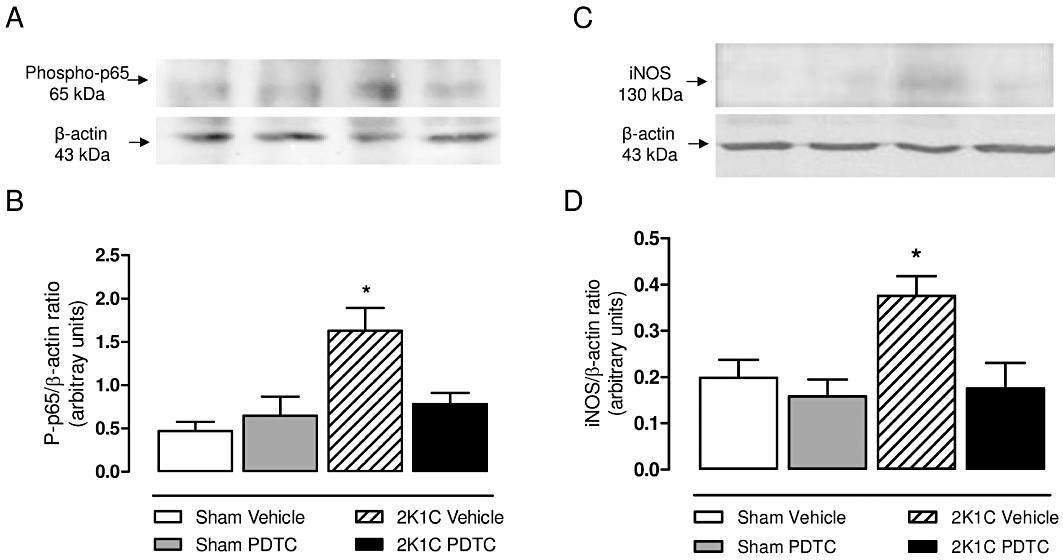
Effects of treatment with PDTC on the levels of phosphorylated p65 NF-κB (A and B), and iNOS expression (C and D) in aortic tissue. Representative Western blots and densitometric analysis for phosphorylated-p65 (Ser 532) NF-κB subunit (A and B), and iNOS (C and D). Values are expressed as mean ± SEM (n = 4 per group). *P < 0.05 versus sham vehicle group.
PDTC inhibits 2K1C hypertension-induced vascular MMPs up-regulation and activity
Gelatin zymograms were used to assess aortic MMP levels. Figure 6A shows a representative zymogram of aortic extracts depicting three bands corresponding to three MMP-2 forms (64 KDa, 72 KDa and 75 KDa) and a 92 KDa band corresponding to MMP-9. 2K1C vehicle animals had significantly higher levels of each MMP-2 form, total MMP-2 and MMP-9 when compared with sham vehicle animals (Figure 6B and C; P < 0.05).
Figure 6.
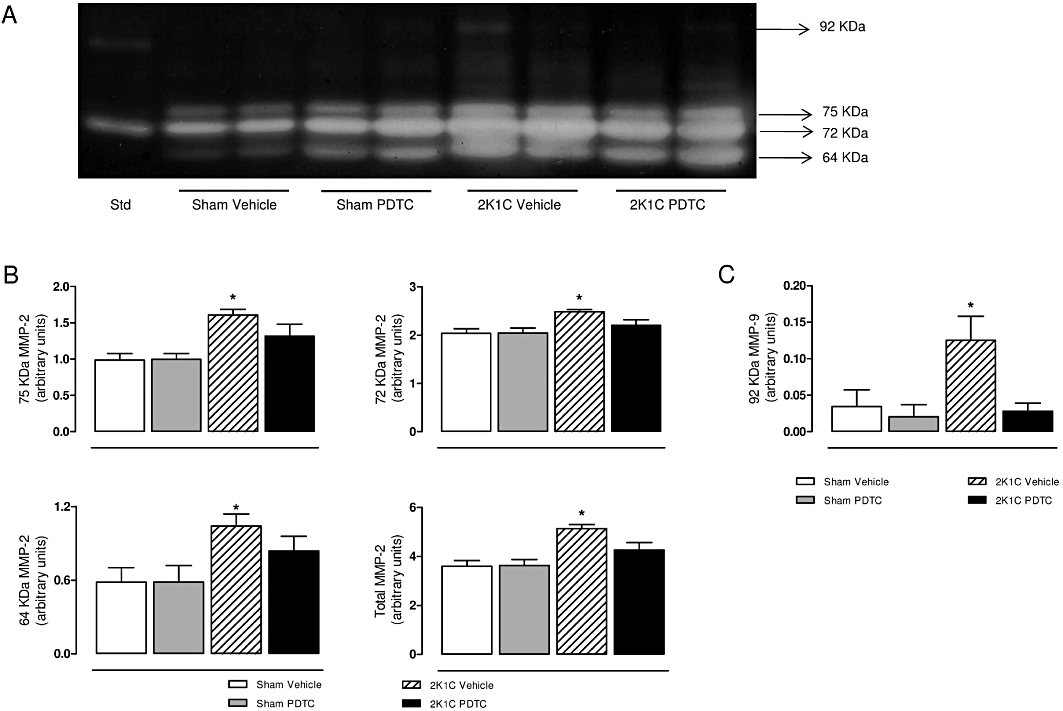
Representative SDS-PAGE gelatin zymogram of aortic samples (A). Panel (B) shows the values for each molecular weight of MMP-2 bands (75 kDa, 72 kDa, 64 kDa and total MMP-2 levels). Panel (C) shows the values for MMP-9 bands (92 kDa). Std: internal standard. Values are expressed as mean ± SEM (n = 9–11 per group). *P < 0.05 versus sham vehicle group.
We assessed the gelatinolytic activity in the aortas by in situ zymography, which provides a measure of total proteolytic activity. We found approximately 50% higher gelatinolytic activities in the aortas from 2K1C hypertensive rats compared with those found in the sham groups (Figure 7; P < 0.05). Treatment with PDTC decreased the gelatinolytic activity in hypertensive rats to similar levels as those found in the sham groups, as revealed by lower gelatinolytic activity in the 2K1C PDTC group compared with the 2K1C vehicle group (Figure 7; P < 0.05).
Figure 7.
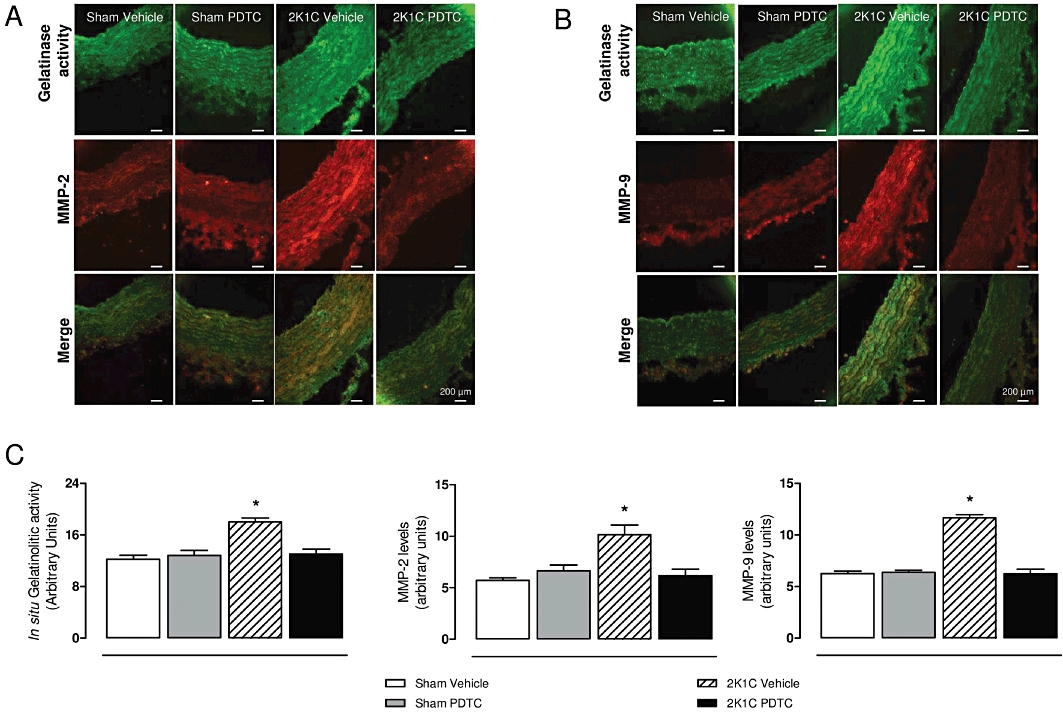
Effects of treatment with PDTC on in situ gelatinase activity and MMP-2 and -9 levels by immunofluorescence in the aortas. Representative photographs of gelatinase activity (×400), MMP-2 (A) or MMP-9 (B) staining by immunofluorescence, and their co-localization (merge) in vessels surface are shown. Panel (C) shows the quantification of percentage of vessel surface area covered by bright green fluorescence, which reflects in situ gelatinolytic activity, the quantification of percentage of vessel surface area covered by bright red fluorescence, which reflects MMP-2 levels or MMP-9 levels. Values are expressed as mean ± SEM (n = 5 per group). *P < 0.05 versus sham vehicle group.
To further support gelatin zymograms and in situ zymography results, we assessed MMP-2 and MMP-9 expression by immunofluorescence. We found that 2K1C hypertension was associated with increased MMP-2 and MMP-9 levels (Figure 7; P < 0.05). Treatment with PDTC notably reduced 2K1C hypertension-induced increases in aortic MMP-2 and MMP-9 levels (Figure 7; P < 0.05). Interestingly, the increased gelatinolytic activity co-localized with the increased aortic MMP-2 and MMP-9 expression (Figure 7A and B).
Discussion and conclusions
The present study demonstrates that treatment with PDTC (i) produced antihypertensive effects and prevented both functional and morphological aortic alterations; (ii) blunted vascular ROS formation and iNOS expression, which may reflect oxidative and nitrosative stress, respectively; and (iii) prevented 2K1C-induced increases in vascular MMP-2, MMP-9 and gelatinolytic activity. To our knowledge, this is the first study to show that PDTC exerts beneficial effects on 2K1C hypertension-induced arterial wall remodelling associated with the prevention of MMPs up-regulation.
The antihypertensive effects exerted by PDTC in 2K1C hypertension accord with those previously reported in other animal models of hypertension (Hong et al., 2000; Muller et al., 2000; Beswick et al., 2001; Rodriguez-Iturbe et al., 2005; Henke et al., 2007; Zhou et al., 2010) and may have resulted from improved endothelium-dependent relaxation and prevention of maladaptive vascular remodelling. Indeed, PDTC improved the vascular function in SHR (Hong et al., 2000) and prevented small vessel injury and ECM formation in the kidneys from rats with angiotensin-induced hypertension (Muller et al., 2000). Moreover, in agreement with the improved responses of 2K1C PDTC rats to acetylcholine that we found in the present study, PDTC restored the endothelium-dependent vascular relaxation and prevented aortic hypertrophy in Dahl salt-sensitive hypertensive rats (Zhou et al., 2010).
Our results confirm previous reports showing that vascular remodelling is linked to enhanced activities of MMPs in hypertension (Flamant et al., 2007; Watts et al., 2007; Castro et al., 2008). In addition to promoting VSMC proliferation and migration, thus resulting in vascular hypertrophy and hyperplasia (Castro et al., 2010), increased MMP-2 levels can degrade vasoactive peptides, thereby favouring vasoconstriction (Chow et al., 2007). Moreover, hypertension-induced increases in MMP-9 levels can promote the cleavage of a plethora of vascular mediators present on the surface of cells (Cauwe et al., 2007), thus affecting vascular homeostasis. Therefore, it is possible that many protective effects exerted by PDTC, especially in hypertension, may result from the down-regulation of MMPs.
We have previously shown that 2K1C vascular remodelling is prevented by inhibiting the formation of MMPs with doxycycline, a non-selective MMP inhibitor, or by antioxidants (Castro et al., 2008; 2009; 2010; Martinez et al., 2008; Ceron et al., 2010). Treatment with PDTC produced similar effects in the present study. These findings support those from previous studies showing that PDTC decreased the upward shift in vascular pressure–diameter relationship, which was accompanied by increased MMP-2 and MMP-9 levels in cultured arteries maintained at high flow conditions (Castier et al., 2009). Together, these findings suggest that the maladaptive vascular alterations in response to high pressure are dependent on MMPs and preventable by PDTC.
Angiotensin II plays a pivotal role in the development and maintenance of high blood pressure in this classic model of hypertension (Martinez-Maldonado, 1991). Angiotensin II can directly activate NF-κB by inducing phosphorylation of its p65 subunit at serine 536, thus increasing its transcriptional activity (Zhang et al., 2005). PDTC inhibits NF-κB activation and its subsequent translocation to the nucleus, thus impairing the transcription of target genes (Schreck et al., 1992). However, although its mechanisms of action are not completely known, they include antioxidant properties (Schreck et al., 1992) and interference with inhibitor of NF-κBα degradation, which is a protein that forms a complex with NF-κB precluding its nuclear translocation (Schreck et al., 1992; Guo et al., 2008). Indeed, PDTC normalized the levels of phopho-p65 NF-κB in aortas of Dahl salt-sensitive hypertensive rats (Zhou et al., 2010), and we found the same effects in our model, indicating that NF-κB activation was prevented by chronic treatment with PDTC. As PDTC is a useful tool for the analysis of NF-κB-regulated gene expression, including iNOS (Hong et al., 2000), the suppression of increased iNOS expression in hypertensive rats by PDTC reported in the present study is consistent with the idea that PDTC clearly inhibited NF-κB. In line with this finding, the attenuation of MMP-9 levels may reflect lower activation of NF-κB in endothelial cells from hypertensive animals, as previously shown (Henke et al., 2007).
2K1C hypertension increased ROS production and iNOS expression in the present study, and treatment with PDTC blunted these alterations. It is well known that superoxide anion and excessive NO produced by iNOS react to produce peroxynitrite (Pacher et al., 2005). These species activate key pathogenic vascular mechanisms (Pacher et al., 2005; Birukov, 2009), including activation of MMPs (Pacher et al., 2005; Kandasamy et al., 2010). Moreover, peroxynitrite inactivates TIMP-1 and TIMP-4 (Kandasamy et al., 2010), which are major endogenous MMP inhibitors. Therefore, it is possible that PDTC may have exerted beneficial effects in the present study as a result of its antioxidant effects and inhibition of iNOS expression, both leading to the attenuation of the activation of MMPs in hypertension. In addition, PDTC may have blunted the activation of NF-κB by angiontensin II or by aldosterone via increased ROS levels (Brasier et al., 2002; Sun et al., 2002), as well as inhibiting NF-κB-induced increased expression of MMPs (Browatzki et al., 2005; Castier et al., 2009).
We found that PDTC reduced ROS formation and iNOS expression; therefore, a reduction in oxidative stress could be involved in the hypotensive effect exerted by PDTC. However, our results do not make it clear as to whether the reduction in oxidative stress was upstream or downstream of NF-κB. This is because PDTC has antioxidant properties (Schreck et al., 1992), and therefore, its effects are broader than selective NF-κB inhibition.
In conclusion, our findings suggest that PDTC down-regulates vascular MMPs and ameliorates vascular dysfunction and remodelling in renovascular hypertension. We speculate that NF-κB inhibition could result in reduced arterial stiffness, a process strongly correlated with clinical hypertension (Lemarie et al., 2010). It provides further evidence supporting the proposal that PDTC is a good candidate for treating hypertension.
Acknowledgments
We gratefully acknowledge the excellent technical support of Junia Ramos. This study was funded by Fundação da Amparo a Pesquisa do Estado de São Paulo (Brazil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil).
Glossary
Abbreviations
- 2K1C
two-kidney, one-clip hypertension
- CSA
media cross-sectional area
- DHE
dihydroethidium
- ECM
extracellular matrix
- M/L
media-to-lumen diameter
- MMP
matrix metalloproteinase
- PDTC
pyrrolidine dithiocarbamate
- SBP
systolic blood pressure
- SHR
spontaneously hypertensive rats
- TIMPs
tissue inhibitors of metalloproteinases
- VSMCs
vascular smooth muscle cells
Conflicts of interest
None.
References
- Beswick RA, Zhang H, Marable D, Catravas JD, Hill WD, Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension. 2001;37:781–786. doi: 10.1161/01.hyp.37.2.781. [DOI] [PubMed] [Google Scholar]
- Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid Redox Signal. 2009;11:1651–1667. doi: 10.1089/ars.2008.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier AR, Recinos A, 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22:1257–1266. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- Browatzki M, Larsen D, Pfeiffer CA, Gehrke SG, Schmidt J, Kranzhofer A, et al. Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-kappaB and activator protein 1 in a redox-sensitive manner. J Vasc Res. 2005;42:415–423. doi: 10.1159/000087451. [DOI] [PubMed] [Google Scholar]
- Castier Y, Ramkhelawon B, Riou S, Tedgui A, Lehoux S. Role of NF-kappaB in flow-induced vascular remodeling. Antioxid Redox Signal. 2009;11:1641–1649. doi: 10.1089/ars.2008.2393. [DOI] [PubMed] [Google Scholar]
- Castro MM, Rizzi E, Figueiredo-Lopes L, Fernandes K, Bendhack LM, Pitol DL, et al. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis. 2008;198:320–331. doi: 10.1016/j.atherosclerosis.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Castro MM, Rizzi E, Rodrigues GJ, Ceron CS, Bendhack LM, Gerlach RF, et al. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic Biol Med. 2009;46:1298–1307. doi: 10.1016/j.freeradbiomed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Castro MM, Rizzi E, Prado CM, Rossi MA, Tanus-Santos JE, Gerlach RF. Imbalance between matrix metalloproteinases and tissue inhibitor of metalloproteinases in hypertensive vascular remodeling. Matrix Biol. 2010;29:194–201. doi: 10.1016/j.matbio.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42:113–185. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- Ceron CS, Castro MM, Rizzi E, Montenegro MF, Fontana V, Salgado MC, et al. Spironolactone and hydrochlorothiazide exert antioxidant effects and reduce vascular matrix metalloproteinase-2 activity and expression in a model of renovascular hypertension. Br J Pharmacol. 2010;160:77–87. doi: 10.1111/j.1476-5381.2010.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow AK, Cena J, Schulz R. Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol. 2007;152:189–205. doi: 10.1038/sj.bjp.0707344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362–1378. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Dao HH, Lemay J, de Champlain J, deBlois D, Moreau P. Norepinephrine-induced aortic hyperplasia and extracellular matrix deposition are endothelin-dependent. J Hypertens. 2001;19:1965–1973. doi: 10.1097/00004872-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, et al. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 2007;50:212–218. doi: 10.1161/HYPERTENSIONAHA.107.089631. [DOI] [PubMed] [Google Scholar]
- Guo RW, Yang LX, Wang H, Liu B, Wang L. Angiotensin II induces matrix metalloproteinase-9 expression via a nuclear factor-kappaB-dependent pathway in vascular smooth muscle cells. Regul Pept. 2008;147:37–44. doi: 10.1016/j.regpep.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Henke N, Schmidt-Ullrich R, Dechend R, Park JK, Qadri F, Wellner M, et al. Vascular endothelial cell-specific NF-kappaB suppression attenuates hypertension-induced renal damage. Circ Res. 2007;101:268–276. doi: 10.1161/CIRCRESAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- Hong HJ, Loh SH, Yen MH. Suppression of the development of hypertension by the inhibitor of inducible nitric oxide synthase. Br J Pharmacol. 2000;131:631–637. doi: 10.1038/sj.bjp.0703603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD. Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension. 2008;52:195–200. doi: 10.1161/HYPERTENSIONAHA.107.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res. 2010;85:413–423. doi: 10.1093/cvr/cvp268. [DOI] [PubMed] [Google Scholar]
- Lemarie CA, Tharaux PL, Esposito B, Tedgui A, Lehoux S. Transforming growth factor-alpha mediates nuclear factor kappaB activation in strained arteries. Circ Res. 2006;99:434–441. doi: 10.1161/01.RES.0000237388.89261.47. [DOI] [PubMed] [Google Scholar]
- Lemarie CA, Tharaux PL, Lehoux S. Extracellular matrix alterations in hypertensive vascular remodeling. J Mol Cell Cardiol. 2010;48:433–439. doi: 10.1016/j.yjmcc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Martinez ML, Castro MM, Rizzi E, Fernandes K, Demacq C, Bendhack LM, et al. Lercanidipine reduces matrix metalloproteinase-2 activity and reverses vascular dysfunction in renovascular hypertensive rats. Eur J Pharmacol. 2008;591:224–230. doi: 10.1016/j.ejphar.2008.06.096. [DOI] [PubMed] [Google Scholar]
- Martinez-Maldonado M. Pathophysiology of renovascular hypertension. Hypertension. 1991;17:707–719. doi: 10.1161/01.hyp.17.5.707. [DOI] [PubMed] [Google Scholar]
- Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, et al. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000;35(1 Pt 2):193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008;29:290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby AC. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 2006;69:614–624. doi: 10.1016/j.cardiores.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Pacher P, Schulz R, Liaudet L, Szabo C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci. 2005;26:302–310. doi: 10.1016/j.tips.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Iturbe B, Ferrebuz A, Vanegas V, Quiroz Y, Mezzano S, Vaziri ND. Early and sustained inhibition of nuclear factor-kappaB prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2005;315:51–57. doi: 10.1124/jpet.105.088062. [DOI] [PubMed] [Google Scholar]
- Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart : role of oxidative stress. Am J Pathol. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Heiden K, Cuhlmann S, Luong A, Zakkar M, Evans PC. Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond) 2010;118:593–605. doi: 10.1042/CS20090557. [DOI] [PubMed] [Google Scholar]
- Watts SW, Rondelli C, Thakali K, Li X, Uhal B, Pervaiz MH, et al. Morphological and biochemical characterization of remodeling in aorta and vena cava of DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H2438–H2448. doi: 10.1152/ajpheart.00900.2006. [DOI] [PubMed] [Google Scholar]
- Zahradka P, Werner JP, Buhay S, Litchie B, Helwer G, Thomas S. NF-kappaB activation is essential for angiotensin II-dependent proliferation and migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2002;34:1609–1621. doi: 10.1006/jmcc.2002.2111. [DOI] [PubMed] [Google Scholar]
- Zhang L, Cheng J, Ma Y, Thomas W, Zhang J, Du J. Dual pathways for nuclear factor kappaB activation by angiotensin II in vascular smooth muscle: phosphorylation of p65 by IkappaB kinase and ribosomal kinase. Circ Res. 2005;97:975–982. doi: 10.1161/01.RES.0000190589.52286.41. [DOI] [PubMed] [Google Scholar]
- Zhou MS, Schulman IH, Raij L. Vascular inflammation, insulin resistance, and endothelial dysfunction in salt-sensitive hypertension: role of nuclear factor kappa B activation. J Hypertens. 2010;28:527–535. doi: 10.1097/HJH.0b013e3283340da8. [DOI] [PubMed] [Google Scholar]


