Abstract
Calcitonin gene related peptide (CGRP) and adrenomedullin are potent biologically active peptides that have been proposed to play an important role in vascular and inflammatory diseases. Their function in the central nervous system is still unclear since they have been proposed as either pro-inflammatory or neuroprotective factors. We investigated the effects of the two peptides on astrocytes and microglia, cells of the central nervous system that exert a strong modulatory activity in the neuroinflammatory processes. In particular, we studied the ability of CGRP and adrenomedullin to modulate microglia activation, i.e. its competence of producing and releasing pro-inflammatory cytokines/chemokines that are known to play a crucial role in neuroinflammation. In this work we show that the two neuropeptides exert a potent inhibitory effect on lipopolysaccharide-induced microglia activation in vitro, with strong inhibition of the release of pro-inflammatory mediators (such as NO, cytokines and chemokines). Both CGRP and adrenomedullin are known to promote cAMP elevation, this second messenger cannot fully account for the observed inhibitory effects, thereby suggesting that other signaling pathways are involved. Interestingly, the inhibitory effect of CGRP and adrenomedullin appears to be stimulus specific, since direct activation with pro-inflammatory cytokines was not affected.
Our findings clarify aspects of microglia activation, and contribute to the comprehension of the switch from reparative to detrimental function that occurs when glia is exposed to different conditions. Moreover, they draw the attention to potential targets for novel pharmacological intervention in pathologies characterized by glia activation and neuroinflammation.
Keywords: Neuroinflammation, Tumor necrosis factor α, Chemokines, cAMP, PGE2
Introduction
Calcitonin Gene-Related Peptide (CGRP) and adrenomedullin belong to the CGRP/calcitonin peptide superfamily that includes also intermedin/adrenomedullin 2 and amylin (Amara et al., 1982; Chang et al., 2004; van Rossum et al., 1997). CGRP/adrenomedullin receptors, in order to be active, require the obligatory association of the calcitonin-like receptor (CLR) with the Receptor Activity-Modifying Proteins 1–3 (RAMP1–3) (McLatchie et al., 1998). It is the association with the different members of the RAMP family that confers the specificity of these receptors to bind the various peptides of the family (Born et al., 2002; Christopoulos et al., 1999): CLR/RAMP1 complex forms the CGRP receptor (antagonized by the CGRP antagonist CGRP8–37), while CLR assembly with RAMP2 and RAMP3 gives rise to the adrenomedullin receptors 1 and 2, respectively (Poyner et al., 2002). The interaction of these receptor complexes with an additional cytoplasmic protein, the Receptor Component Protein (RCP) is required for the activation of signal transduction cascades, including cyclic adenosine monophosphate (cAMP) formation (Evans et al., 2000; Luebke et al., 1996).
CGRP/adrenomedullin receptors are widespread in endothelial, vascular smooth muscle, immune, glial and neuronal cells (Hay et al., 2004). One of the most studied and potent physiological effects elicited by CGRP/adrenomedullin receptor activation is vasodilation (Brain and Grant, 2004). In the brain, the potent and long-lasting activity of CGRP on arteries and veins (Brain and Grant, 2004) modulates local blood flow and extravasation, and is thought to be involved in headache (Geppetti et al., 2005). Also adrenomedullin, which is produced by endothelial and vascular smooth muscle cells, has been proposed to play a role in the regulation of systemic blood pressure. In particular, adrenomedullin inhibits endothelial cell contraction and junctional disassembly, thereby limiting vascular permeability and edema during inflammation (Temmesfeld-Wollbrück et al., 2007).
Among the various effects of CGRP and adrenomedullin, their ability to modulate the immune/neural-immune system is of utmost interest and the emerging picture indicates that the two peptides can exert pro- as well as anti-inflammatory actions in a cell/tissue-specific and stimulus-specific manner. For instance, experiments on animal models demonstrate anti-inflammatory effects of CGRP (Gomes et al., 2005; Kroeger et al., 2009; Tsujikawa et al., 2007) that set against the neurogenic inflammatory action widely reported in periphery and brain (Durham and Vause, 2010; Holzer, 1998). Similarly, both pro-inflammatory and anti-inflammatory roles have been proposed for adrenomedullin (see, for instance, Dackor and Caron, 2007; Ma et al., 2010; Miksa et al., 2007) although very little is known about its activity in the central nervous system. Based on these assumptions, CGRP and adrenomedullin are expected to be involved in several neuroinflammatory conditions and to play an important role in some neurodegenerative processes. For instance, the inflammatory component of multiple sclerosis is characterized by a complex interplay of cells (resident microglia, astrocytes, infiltrating macrophages and T cells), mediated by released molecules, such as tumor necrosis factor α (TNFα), interleukin-6 (IL6) and nitric oxide (NO), that can exert a detrimental role on neuronal function (see e.g. Encinas et al., 2005; Hartung et al., 1995; Martino et al., 2000). Moreover, chemokines released by vascular and perivascular resident glial cells are known to favor infiltration of lymphocytes into the parenchyma of the central nervous system, an obligatory step for the progression of the autoimmune attack (Engelhardt and Ransohoff, 2005; Szczucinski and Losy, 2007).
In this work we investigate the effects of CGRP and adrenomedullin in glial cultures, showing that CGRP and adrenomedullin exert a potent and efficient anti-inflammatory role on microglia activation by inhibiting the lipopolysaccharide (LPS)-induced release of pro-inflammatory molecules. This previously unrecognized role of CGRP and adrenomedullin on glial cells might be relevant in the neurodegenerative processes by inhibiting the inflammatory process and stimulating repair events. It follows that these findings may represent a new paradigm to devise therapeutic strategies for neuroinflammatory diseases, such as multiple sclerosis.
Results
CGRP and adrenomedullin inhibit LPS-induced glia activation
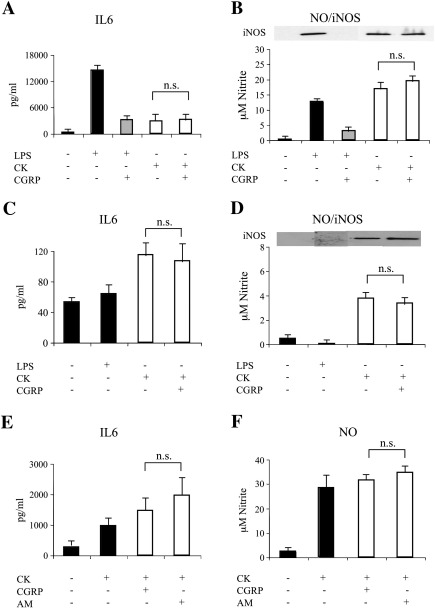
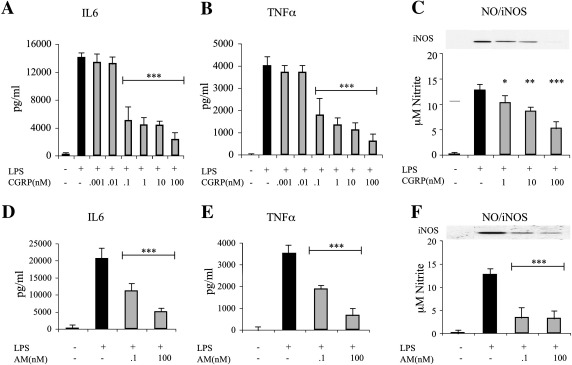
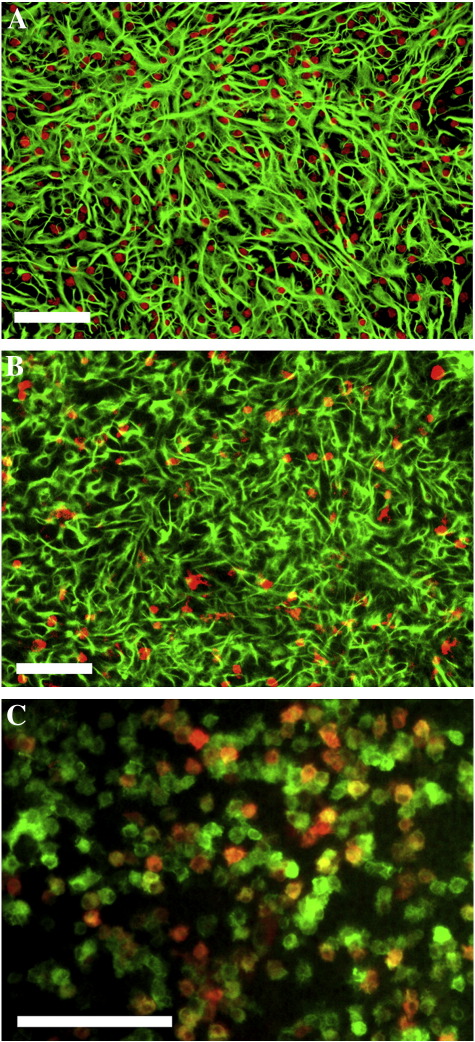
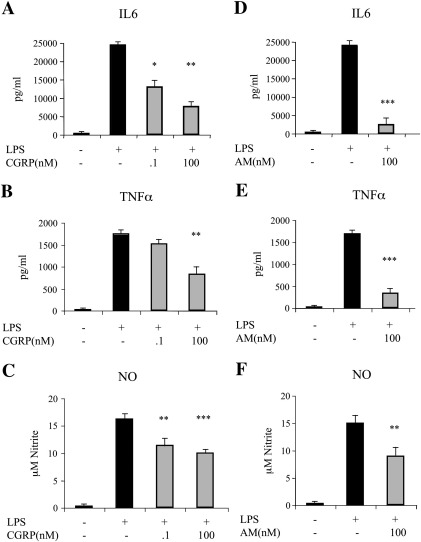
In order to evaluate the effects of CGRP and adrenomedullin on glia activation, we first established the conditions to stimulate microglia and astrocytes in culture. Cultures of either pure astrocytes or microglia seeded on a layer of pure astrocytes (co-culture ratio 1:1; see Experimental methods) were exposed to various treatments. As expected from previous data, pure astrocytes were unresponsive to 24 h exposure to 10 ng/ml LPS (Saura, 2007; see also Fig. 5). In contrast, when co-cultures were exposed to the same protocol of stimulation, a marked increase in IL6 secretion, TNFα release, NO production and iNOS induction was observed (Fig. 1). These data confirm that LPS is able to promote the expression and secretion of the major glial activation markers by directly acting on microglia that may in turn recruit astrocytes in the activation process. When LPS was applied to co-cultures in the presence of CGRP or adrenomedullin, a dose-dependent inhibition of activation was observed (Figs. 1A–F). CGRP and adrenomedullin had no effects on unstimulated cells (either microglia/astrocyte co-cultures or pure astrocytes; not shown). The immunofluorescences in Fig. 2 show the astrocyte/microglia ratio in a typical experiment (Fig. 2A) as well as the strong iNOS up-regulation induced by LPS challenge in most microglial cells, but not in astrocytes (Figs. 2B and C).
Fig. 5.
The effect of pro-inflammatory cytokines is not affected by CGRP or adrenomedullin (AM). Either microglia/astrocyte co-cultures (A and B), pure astrocytes (C and D), or highly enriched microglial cells (E and F) were stimulated by LPS (10 ng/ml) or a mix of cytokines (CK: 10 ng/ml IL1β, 30 ng/ml TNFα and 20 ng/ml INFγ) for 24 h and the effect of CGRP (100 nM) or AM (100 nM) was tested on IL6 release (A, C, and E) and NO production/iNOS expression (B, D, and F). No statistical significant decrease (n.s.) in the activation markers was observed when CGRP or AM were applied to CK stimulated cells (calculated by unpaired two-tailed Student's t-test).
Fig. 1.
CGRP and adrenomedullin (AM) inhibit LPS-induced activation in microglia/astrocyte co-cultures. Microglia/astrocyte co-cultures were treated for 24 h with LPS (10 ng/ml) and increasing concentrations of CGRP (A, B, and C) or AM (D, E, and F). At the end of the treatment the medium was tested for the release of IL6 (A and D) TNFα (B and E) and NO (C and F). Inset in (C and F) shows the corresponding western blot for iNOS performed on cell lysates. In this and the following figures, the columns in the graphs represent the average (+ SEM) of at least three independent experiments performed in triplicate. Statistical significance (*p < 0.05; **p < 0.01; ***p < 0.001) was calculated using one-way ANOVA followed by Dunnett's post hoc test (against the LPS treatment).
Fig. 2.
Immunocytochemistry of microglia/astrocyte co-cultures. Cells were fixed and double stained for the astrocytic GFAP (green in panels A and B) or the microglial IBA1 (red in panel A and green in panel C) markers at rest (panel A) or after stimulation with LPS (10 ng/ml for 24 h, panels B and C). Red staining in B and C shows the induction of iNOS in GFAP negative (B) and IBA1 positive (C) cells. Scale bar is 100 μM.
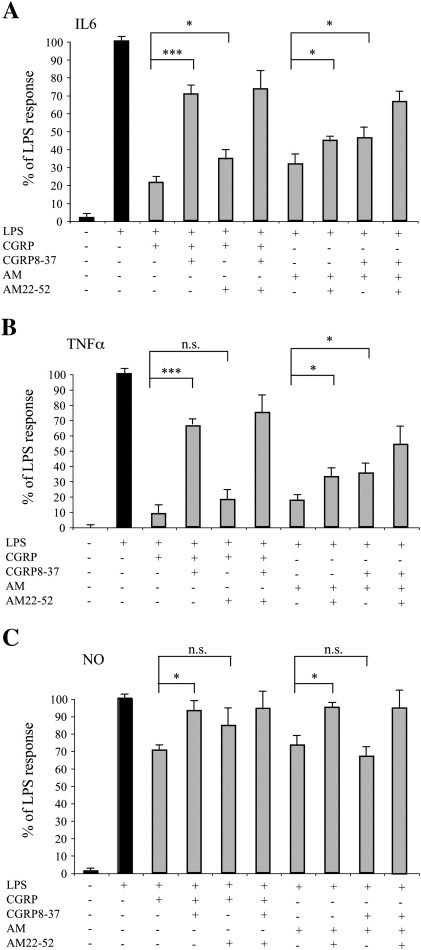
We then used CGRP8-37 and AM22-52, two antagonists of the CGRP and adrenomedullin receptors, respectively, in order to verify the pharmacological profile of the neuropeptide effects. The two antagonists reduced the inhibitory action of CGRP and adrenomedullin on LPS-treated cultures, as revealed by the effects on IL6 secretion, TNFα release and NO production (Fig. 3). This result is in line with the notion that astrocytes are potentially able to form all the receptors for CGRP and adrenomedullin (Moreno et al., 2002), but also reveals a different extent of inhibition exerted by the two antagonists. On the one hand this could reflect the ligand–receptor promiscuity of this system in which CGRP may also act on the adrenomedullin receptor 2 and adrenomedullin can also activate the CGRP receptor (Poyner et al., 2002). On the other hand, it must be considered that AM22-52 acts with different potencies on the two adrenomedullin receptors (Hay et al., 2004).
Fig. 3.
CGRP8–37 and AM22–52 partially revert the effects of CGRP and adrenomedullin (AM). Microglia/astrocyte co-cultures were activated for 24 h with LPS (10 ng/ml) and various combinations of agonists (CGRP or AM, 10 nM each) and antagonists (CGRP8–37 or AM22–52, 3 μM and 9 μM, respectively) of CGRP and AM receptors. At the end of the treatment the medium was tested for the release of IL6 (A) TNFα (B) and NO (C). Statistical significance (*p < 0.05; **p < 0.01; ***p < 0.001) was calculated using one-way ANOVA followed by Bonferroni post hoc test.
To verify whether the results obtained in the co-cultures were influenced by the interplay between the two cell types, we performed experiments on highly-enriched microglia cultures (~ 95% purity). LPS was able to increase the three markers of activation (Fig. 4) although with a different pattern of release. In fact, in microglia cultures the ratio between TNFα and IL6 was reduced (about 3–4 folds) with respect to co-cultures suggesting that astrocytes can significantly contribute to the release of some cytokines. Also in this experimental cellular model CGRP and adrenomedullin exerted an inhibitory effect on the induction of LPS activation (Fig. 4).
Fig. 4.
CGRP and adrenomedullin (AM) inhibit LPS-induced activation in microglia-enriched culture. LPS (10 ng/ml) was given to preparations of primary microglia cells for 24 h in the presence or absence of CGRP (100 nM) or AM (100 nM). Secretion of interleukin-6 (A and D) tumor necrosis factor α (B and E) and NO (C and F) was then tested in the cell supernatant. Statistical significance (*p < 0.05; **p < 0.01; ***p < 0.001) was calculated using one-way ANOVA followed by Dunnett's post hoc test (against the LPS treatment).
CGRP and adrenomedullin do not inhibit glia activation promoted by cytokine administration
Since also pro-inflammatory cytokines have been reported to cause glia activation, we evaluated the effects of the two neuropeptides upon exposure of cells to a mix of cytokines (IL1β, TNFα, and INFγ). In all cell cultures (microglia/astrocytes, pure astrocytes and highly-enriched microglia) CGRP treatment did not exert inhibitory effects on NO/iNOS and IL6 levels (Fig. 5), thus suggesting that, under this condition, the activation process is sustained by different mechanisms and that CGRP modulates only specific pathways leading to cytokine and NO release from microglia. Similar results were obtained with adrenomedullin (see Figs. 5E and F for the effects on microglia).
Role of cAMP and cAMP-related stimuli in the modulation of microglia activation
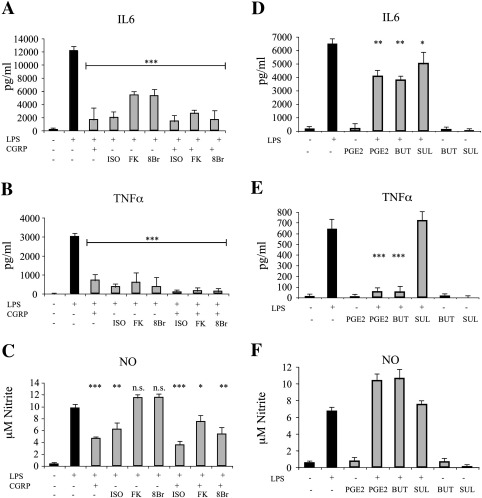
In view of the fact that CGRP and adrenomedullin are known to activate adenylyl cyclase and promote cAMP increase, we exposed cells to various treatments able to stimulate or mimic the cAMP pathway (Fig. 6). Isoproterenol, which is reported to activate β-adrenergic receptors on microglia (Tanaka et al., 2002), displayed the same effect of CGRP and adrenomedullin, while other, non receptor-mediated protocols to elevate cAMP, had a more complex behavior. In fact, treatment with either 8-Br-cAMP, an analog of cAMP, or forskolin, an activator of adenylyl cyclase, prevented the LPS-induced increase in IL6 production and TNFα release, but not NO elevation (Figs. 6A–C). Hence, receptor stimulation elicits effects that go beyond a simple cAMP increase. Indeed, another stimulus commonly used to raise cAMP in microglia, PGE2, was effective in reducing LPS-mediated IL6 and TNFα release, but was not able to produce the same inhibitory effect on NO production (Figs. 6D–F). Rather, PGE2 reinforced the LPS-mediated NO increase, most likely by stimulation of the prostaglandin E receptor 2 (EP2), as revealed by the similar effects obtained with butaprost (specific agonist for EP2), but not sulprostone (specific agonist for EP3).
Fig. 6.
Role of cAMP and cAMP-related stimuli on the modulation of microglia activation. Microglia/astrocyte co-cultures were stimulated with LPS (10 ng/ml) in the presence of various cAMP-modulating agents and the secretion of IL6 (A and D), TNFα (B and E), and NO (C and F) was measured. In addition to LPS and CGRP (100 nM), the other stimuli used were isoproterenol (ISO, 1 μM), forskolin (FK, 10 μM), 8Br-cAMP (8Br, 100 μM), PGE2 (1 μM), butaprost (BUT, 1 μM) and sulprostone (SUL, 1 μM). Statistical significance (n.s. p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001) was calculated using one-way ANOVA followed by Dunnett's post hoc test (against the LPS treatment).
In light of the possibility that CGRP and adrenomedullin mat activate signaling pathways other than cAMP (Wang et al., 2009) we exposed LPS-activated cultures to PD98059 (50 μM) and SB203580 (10 μM), two specific inhibitors of the pathways linked to activation of mitogen activated protein kinases (MAPK). These drugs showed a strong inhibitory effect on LPS-induced microglia activation, but did not influence the effects of CGRP and adrenomedullin (data not shown).
Inhibition of chemokine expression by CGRP and adrenomedullin
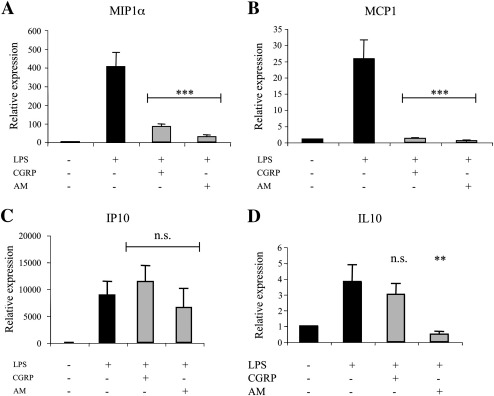
Having established that CGRP and adrenomedullin reduce microglial activation, we tested whether these same treatments were also able to affect chemokine production in LPS-stimulated co-cultures. Indeed, the transcription of some chemokines known to play a major role in neuroinflammation (e.g. CCL2/MCP1, CCL3/MIP1α, CXCL10/IP10 and IL10) was promoted by LPS (Fig. 7). Of note, CGRP and adrenomedullin differentially affected these increases: CCL2/MCP1 and CCL3/MIP1α induction was strongly repressed by both neuropeptides, while CXCL10/IP10 increase was not significantly inhibited. On the other hand, the expression of IL10, which is potentiated in alternative pathways of microglia activation, was not increased by the two neuropeptides and even lowered by adrenomedullin (Fig. 7D). CGRP and adrenomedullin were also ineffective in inducing genes related to the appearance of anti-inflammatory and neurotrophic phenotypes of microglia (Colton, 2009), such as Fc epsilon RII (CD23), transforming growth factor β, and nerve growth factor (data not shown).
Fig. 7.
CGRP and adrenomedullin (AM) revert induction of chemokines by LPS. Relative variations in the expression of MIP1α (A), MCP1 (B), IP10/CXCL10 (C) and IL10 (D) measured by RT-qPCR in microglia/astrocyte co-cultures. Statistical significance (n.s. p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001) was calculated using one-way ANOVA followed by Dunnett's post hoc test (against the LPS treatment).
Discussion
In this work we demonstrate that CGRP and adrenomedullin can inhibit microglia activation starting from the low nanomolar range. This evidence was obtained by challenging primary cultures of microglia with LPS and assessing the inhibitory effect exerted by the two neuropeptides on the release of molecules reported to have detrimental roles in inflammatory processes, such as cytokines (TNFα, IL6), NO, and chemokines (MIP1α, MCP1) (Kerschensteiner et al., 2009; Lehnardt, 2010). These results extend previous reports of an anti-inflammatory action of CGRP and adrenomedullin in macrophagic cell lines and primary macrophages (Feng et al., 1997; Liu et al., 2000; Ma et al., 2010; Miksa et al., 2007; Wong et al., 2005; Wu et al., 2003), i.e. cells belonging to the same lineage as microglia. The effects we report were clearly stimulus dependent, as well as cell specific. We employed the well-known paradigm of microglia activation by LPS to induce the release of pro-inflammatory molecules able to activate astrocytes with consequent additional release of cytokines. Indeed, our results provide evidence of an increased release of TNFα when microglial cells are co-cultured with astrocytes. With this model, we demonstrate that CGRP and adrenomedullin efficiently suppress the LPS-mediated effects. Accordingly, our data point to microglia, not to astrocytes, as the primary target of this cascade of events, in line with a recent cautionary note on the real effects of LPS on pure astrocytes (Saura, 2007). It is worth mentioning that, according to the current view on the neuroinflammatory process during neurodegeneration, microglia is considered the causal player, with astrocytes exerting either a protective feedback role, by limiting microglial activation (Harris et al., 2002), or a deleterious feed-forward effect, by favoring the development of chronic inflammation (Farina et al., 2007). Notwithstanding the extent of interplay between microglia and astrocytes, which is far from being elucidated, it is clear that CGRP and adrenomedullin can play an important role on the effects of microglia activation. According to our results, the two neuropeptides reverse the LPS effects rather than redirect microglia to different pathways of activation, such as the anti-inflammatory “alternative activation” or the immunosuppressant “acquired deactivation” phenotypes proposed in the literature (Colton, 2009). In addition, it should be pointed out that CGRP and adrenomedullin inhibition on microglia activation is exerted on specific pathways. In fact the inhibitory effect was not observed when cells, either astrocytes or microglia, were challenged with a mix of pro-inflammatory cytokines.
Since both neuropeptides activate CLR complexes, it can be inferred that a common signal transduction pathway may sustain their effects. Indeed, cAMP is produced upon stimulation of CGRP or adrenomedullin receptors and is thus expected to be involved in the inhibitory activity observed on LPS action. However, elevation of cAMP levels by common pharmacological tools mimicked the effect only partially, thereby suggesting, as already reported (Walker et al., 2010), that receptor activation stimulates other signaling pathways as well. Interestingly, stimulation of different receptors coupled to adenylyl cyclase showed similar, as well as specific effects. For instance, activation of β-adrenergic receptors mimicked the action of the two neuropeptides, while activation of EP2 receptors had an opposite effect on NO production. Altogether, the heterogeneous responses we have observed are in line with the possibility that receptors coupled to adenylyl cyclase activation can stimulate, along with cAMP production, also other signaling pathways, thereby driving glia to specific activated phenotypes.
Interestingly, the pro-inflammatory reaction caused by LPS implies the initial activation of the Toll-like receptors, a mechanism proposed to be involved in the development of experimental autoimmune encephalomyelitis (EAE), i.e. the main model for multiple sclerosis in mice (Farez et al., 2009; Marta, 2009). Since it is possible that CGRP and adrenomedullin modulate this pathway, a better definition of their mechanism of action might have a potential impact on this neuroinflammatory pathology. Looking at the possible consequences of microglia activation in the neuroinflammatory processes, we focused on chemokine release. This is a crucial step in the recruitment of leukocytes from the periphery, with ensuing propagation of the inflammatory attack to the parenchyma (via Th1/Th17 response; Gutcher and Becher, 2007) and further amplification of glia activation in a dangerous feed-forward loop. Noticeably, CGRP and adrenomedullin strongly inhibited the upregulation of MIP1α and MCP1, two chemokines responsible for the recruitment of T lymphocytes into the parenchyma of the central nervous system.
Among the CGRP-induced effects on the central nervous system, its role in the development of neuropathic pain and migraine has attracted most of the attention (Recober and Russo, 2009). Our results bring awareness to the role of CGRP and adrenomedullin as anti-inflammatory agents and, at the same time, open new perspectives in the pharmacological treatment of pathologies characterized by microglia activation and neuroinflammation. In this respect, multiple sclerosis is a paradigm since all demyelinating lesions occur on an inflammatory background that sees the involvement of several cells, including activated microglia and locally recruited lymphocytes and macrophages (Lassmann et al., 2001). The mechanisms leading to this pathological pattern are complex and poorly understood. It has been hypothesized that unknown inflammatory events may cause a local release of inflammatory cytokines (TNFα, IL1β, and IL6) capable of triggering the production of secondary inflammatory mediators, such as chemokines, colony stimulating factors and lipid-derived molecules (Martino and Hartung, 1999). Cytokines and chemokines would, in turn, allow the recruitment of leukocytes across the blood–brain barrier, including autoreactive T cells able to interact with resident antigen-presenting cells (Engelhardt and Ransohoff, 2005; Flügel et al., 2007), such as microglia and astrocytes. This local interplay is considered a crucial event leading to perpetuation and amplification of the inflammatory reaction since the initial damage introduced by T cells is a stimulus for microglia activation and further recruitment of macrophages. Within this pathological framework, activated microglia and infiltrating macrophages are expected to produce a large number of harmful soluble factors, such as nitric oxide (Redford et al., 1997), inflammatory cytokines/chemokines (Muzio et al., 2007), excitotoxins (Smith et al., 2000), matrix metalloproteinases (Leppert et al., 2001; Lindberg et al., 2004) and other proteases (Anthony et al., 1998). However, a view in which microglia might exert a reparative and anti-inflammatory action, with mechanisms still to be clarified, has recently emerged (Muzio et al., 2007). Based on these premises, the neural-immune activity of CGRP and adrenomedullin might exert either a control on the initial immunological attack to the central nervous system parenchyma, or on subsequent mechanisms of the disease via modulation of glia activation.
Interestingly, by working on the EAE mouse model of relapsing–remitting multiple sclerosis we have found that, in the lesion areas with infiltrating lymphocytes, glial cells display changes in CGRP, adrenomedullin and RCP levels (Morara et al., in preparation). Altogether, these findings are in line with the expectation of a possible role played by these neuropeptides in multiple sclerosis. The recent development of non-peptidic antagonists for the CGRP receptor, already in clinical use (Doods et al., 2000), paves the way also to the discovery of non-peptidic agonists for the same receptor family (Katayama et al., 2001), which might be employed in the treatment of neuroinflammatory diseases. Finally, our results call attention to the use of CGRP inhibitors, widely employed for the therapy of migraine (Durham and Vause, 2010). In fact, in light of a possible physiological role of these neuropeptides in keeping neuroinflammation at bay, treatments with inhibitors might have previously unpredicted harmful effects.
In conclusion, we provide evidence for a role of CGRP and adrenomedullin as modulators of microglia activation. A better comprehension of the molecular basis of their action is expected to advance our knowledge of the neuroinflammation mechanisms as well as to devise new therapeutic paradigms (Schreiner et al., 2009).
Experimental methods
Materials
Cell culture media and reagents were from Lonza. Other chemicals, if not otherwise stated, were from Sigma-Aldrich. Culture flasks and multiwell plates were from Nalge Nunc.
Cell culture
The animal use procedures, performed according to the EC Directive 86/609/EEC, were approved by the Institutional Animal Care and Use Committee of the San Raffaele Scientific Institute. Animals (about 100 pups were used across this study) were sacrificed after gentle carbonarcosis (by slowly rising CO2 inside the cage) to minimize pain and discomfort. Primary cultures of cortical astrocytes were obtained from 1 to 2 day-old Sprague–Dawley rats (Charles River) according to McCarthy and De Vellis (1980). Cortices were freshly dissected, cut into small sections and washed in Hank's Balanced Salt Solution supplemented with Hepes/Na pH 7.4 (10 mM), MgSO4 (12 mM), 50 U/ml Penicillin and 50 μg/ml Streptomycin. Then, they were dissociated with 2.5 mg/ml trypsin type IX in presence of 1 mg/ml deoxyribonuclease (DNase, Calbiochem) for 10 min at 37 °C in two subsequent steps and the supernatants obtained were diluted 1:1 in medium containing 10% horse serum (PAA Laboratories). The cell suspension was spun (100 g for 10 min) and cells (about 30 × 106 cells per pup) were put in culture in Minimum Essential Medium Eagle (EMEM) supplemented with 10% horse serum, 33 mM glucose, 2 mM Glutamax (Gibco), 50 U/ml penicillin, 50 μg/ml streptomycin. Cells were maintained in 75 cm2 flasks (1 per pup) at 37 °C in a humidified 5% CO2 incubator. Pure cultures (> 99.5%) of type-1 astrocytes were obtained by shaking flasks at 200 rpm for 24 h at 37 °C at days 2 and 6 after plating to remove microglial cells and oligodendrocyte progenitors (also known as O2A). Shaking medium (10 ml/flask) was Minimum Essential Medium with Hank's salts, supplemented with 10% horse serum, 33 mM glucose, 2 mM Glutamax and 10 mM Hepes/Na pH 7.4. For biochemical and activation experiments cells were detached with buffered trypsin (0.25%)/ethylenediaminetetraacetic acid (EDTA, 1 mM) and re-plated with fresh medium on plastic multiwells (24 well plates; 100,000 cells per well) coated with poly-l-lysine (100 μg/ml, 5 min on the surface and then washed with H2O). Cells were used within 3 days after re-plating. Purity of astrocytic cells was assessed by morphological examination (immunofluorescence for glial fibrillary acidic protein, GFAP, and ionized calcium binding adaptor molecule 1, IBA1, markers for astrocytes and microglia, respectively) and the absence of response to LPS (in terms of upregulation of inducible nitric oxide synthase, iNOS, and secretion of IL6; see also Fig. 5).
Microglia cells were obtained from astrocytic flasks by gentle manual shaking three days after dissection. Detached cells (about 80–90% microglia with a 20–10% astrocytic contamination) were plated on the top of pure astrocyte monolayers, making possible the preparation of co-cultures with known relative percentage of cells, or, alternatively, detached cells were plated in multiwells (150,000 cells per well in 24 well plates) coated with poly-l-Lysine (100 μg/ml) to obtain, within 2–3 days, astrocyte/microglia co-cultures with approximately a 1:1 ratio. Highly enriched (> 95%) microglial cultures were obtained by adding granulocyte-macrophage colony-stimulating factor (GM-CSF, 25 ng/ml; R&D Systems) to culture medium just after re-plating detached cells onto uncoated plastic multiwells. The resting state of unstimulated microglia was confirmed by the almost undetectable levels of IL6 secretion and iNOS expression (see Results).
Cell treatments
Recombinant rat interleukin-1β (IL1β), TNFα and interferon γ (INFγ) were from R&D Systems; LPS, isoproterenol, prostaglandin E2 (PGE2), sulprostone and butaprost were from Sigma; rat α-CGRP, CGRP8-37 (inhibitor of the CGRP receptor) and AM22-52 (inhibitor of the adrenomedullin receptors) were from Polypeptide group; adrenomedullin was from Bachem; forskolin was from Calbiochem and 8-bromoadenosine 3′,5′-cyclic monophosphate (8Br-cAMP) was from Biaffin. Stock solutions of LPS, TNFα, IL1β, INFγ, CGRP, adrenomedullin, CGRP8-37, AM22-52, isoproterenol and 8Br-cAMP were prepared in EMEM and stimuli administered with a 1:1000 dilution (with the exception of LPS that was 1:100). Forskolin, sulprostone, PGE2 and butaprost were dissolved in dimethyl sulfoxide and administered with a 1:1000 dilution, i.e. a condition in which solvent alone was found to be ineffective on both basal activation of glia and LPS effects. Stimuli were administered directly to the culture medium as follows. Rat cortical co-cultures astrocytes/microglia were stimulated with IL1β (10 ng/ml), TNFα (30 ng/ml), INFγ (20 ng/ml), CGRP (100 nM), adrenomedullin (100 nM), and LPS (10 ng/ml; Sigma-Aldrich cod. L2654), for 24 h at 37 °C. Activated phenotype was tested by measuring the release of IL6, TNFα, and NO, and the upregulation of iNOS. Isoproterenol (1 μM), 8Br-cAMP (100 μM), forskolin (10 μM), CGRP8–37 (3 μM) and AM22–52 (9 μM) were administered 30 min before pro-inflammatory stimuli administration. Cell viability at the end of the treatments was assessed biochemically by standard 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and by fluorescence microscopy using sytox blue (Invitrogen) as dead-cell indicator. Changes in vitality, as well as total cellular protein content, upon treatments and among samples were less than 5%.
Western blotting
Cells were washed 2 times with phosphate buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM sodium phosphate dibasic, 2 mM potassium phosphate monobasic, pH 7.4) and lysed for 15 min at 4 °C with 300 μl/well of lysis buffer (phosphate buffered saline supplemented with 2% Nonidet P-40, 0.2% sodium dodecyl sulfate, 10 mM EDTA/Na, and a cocktail of protease inhibitors, chimostatin, leupeptin, antipain, pepstatin, 10 μg/ml each). Lysates were centrifuged for 15 min at 15,000 g at 4 °C, the supernatants were collected and their total protein content analyzed by the MicroBCA reagent (Pierce). About 25 μg of proteins were separated by standard sodium dodecyl sulfate polyacrylamide gel electrophoresis (1.5 mm gel thickness) and transferred onto nitrocellulose membrane (0.45 um pore size, Protran BA85, Whatman). The membrane was stained with Ponceau S (0.2% in 3% trichloroacetic acid) for protein visualization and de-stained with bi-distilled water. After overnight blocking with Tris buffered saline (TBS: 50 mM Tris, 150 mM NaCl, pH 7.6) containing 0.2% Tween-20 and 5% skimmed milk, membranes were incubated 2 h (room temperature, shaking) with 0.5 μg/ml of the primary antibody (mouse monoclonal anti iNOS antibody from BD Biosciences) and then, after an extensive washing with TBS containing 0.2% Tween-20, with horseradish peroxidase-conjugated anti-mouse secondary antibody (Bio-Rad). Protein signals were revealed on autoradiographic films by Super Signal West Pico (or Femto) Chemiluminescent Substrate (Pierce). ImageJ software (Collins, 2007) was used to perform the densitometric analysis of Ponceau S stained membranes in order to evaluate correct loading.
Interleukin-6, tumor necrosis factor α and NO determinations
IL6 and TNFα were measured by sandwich enzyme-linked immunosorbent assay in 50 μl of supernatants collected from co-cultures, according to manufacturer's instructions (Biotrak ELISA Systems, Amersham Bioscience). Limits of detection for IL6 and TNFα were 10 and 5 pg/ml, while limits of quantification were 40 and 15 pg/ml, respectively. NO production was determined by measuring the accumulation of nitrite in the culture medium. Nitrite was assayed colorimetrically by a diazotization reaction using the Griess reagent, composed by a 1:1 mixture of 1% sulfanilamide in 5% ortophosphoric acid and 0.1% naphtylenethylenediamine dihydrochloride in H2O. 100 μl of culture medium were mixed to 100 μl of Griess reagent in a 96-multiwell plate and the O.D. at 550 nm was measured within 10 min. The nitrite concentration in the samples was interpolated from a NaNO2 standard curve ranging from 0 to 100 μΜ. The limits of detection and quantification were 0.25 and 0.7 μM, respectively.
Immunofluorescence
Rat cortical co-cultures, plated at subconfluent density on poly-lysine coated multiwell plates, were washed with PBS and fixed for 15 min with 3.7% paraformaldehyde in PBS at room temperature. After two washes with PBS, paraformaldehyde was blocked by two 5 min incubations with 50 mM NH4Cl. Cells were then permeabilized with 0.1% Triton X-100 in PBS for 5 min and incubated for 20 min with blocking solution (0.2% gelatine in PBS). The primary antibodies were diluted in blocking buffer and incubated for 30 min at room temperature. The following antibodies were used: polyclonal (Dako, 1:250 dilution) and monoclonal (Sigma, 1:200 dilution) anti GFAP antibody as astrocytic marker; polyclonal anti IBA1 (Wako Pure Chemical Industries, 1:500 dilution) as microglial marker; monoclonal anti iNOS (BD Biosciences, 1:100 dilution) as microglia activation marker. After three washes of 10 min with blocking solution, the secondary antibodies, either fluorescein isothiocyanate (FITC)- or rhodamine-conjugated goat anti mouse and anti rabbit immunoglobulin G (Invitrogen, both diluted 1:150 in blocking buffer), were incubated for 30 min at room temperature, in the dark. Multiwells were washed with blocking solution (three washes of 10 min), left in PBS, and observed by epifluorescence microscopy.
RNA extraction and RT-qPCR
RNA was extracted from treated or untreated cells plated on 3.5 cm Petri dishes with TRIzol (Invitrogen) and phenol/chlorophorm/isoamyl alcohol (PCI, 25:24:1 v/v), following manufacturer instruction. Briefly, cells were lysed in 1 ml of TRIzol, to which were added 200 μl of PCI. After centrifugation (12,000 g, 15 min), the upper aqueous phase was transferred in a new tube and RNA was precipitated through addition of an equivalent amount of isopropanol. Samples were centrifuged (12,000 g, 10 min) and washed with 70% ethanol. RNA pellets were air-dried for 5 min, resuspended in 20 μl of RNase-free water and stored at − 80 °C.
Reverse transcription (RT) was carried out with random hexamers as primers, using Superscript III Retrotranscription Kit (Invitrogen) following manufacturer instruction. RT was carried on for 50 min at 50 °C then stopped incubating samples at 85 °C for 5 min. Single strand cDNA was obtained digesting complementary RNA strand with provided RNase H for 20 min at 37 °C.
Quantitative polymerase chain reaction (PCR) was performed on a LightCycler 480 machine (Roche Diagnostics), with proprietary SybrGreen mix (LightCycler 480 Master Mix, Roche), following manufacturer instruction. Both forward and reverse primers were used at a 0.5 μM concentration. RT-derived cDNA was typically diluted 1:16 before use. PCR program was performed with 10 min of denaturation step at 95 °C and 35 to 45 cycles of amplification. Each cycle consisted of a denaturation step (95 °C, 10 s), an annealing step (60 °C, 25 s) and an elongation step (72 °C, 15 s). After amplification, a melting step was performed (95 °C for 30 s, 60 °C for 1 min). Determination of Crossing points and Melting peaks was performed with LightCycler 480 Software (version 1.5.0.39, Roche). Primers used (forward and reverse) were: gatccacattcggaggctaa and acgtgaaggttcaaggatgc for the gene encoding chemokine (C–C motif) ligand 2 (CCL2, also known as monocyte chemotactic protein-1 or MCP1); ccaccgctgcccttgctgtt and cacccggctgggagcaaagg for the gene encoding chemokine (C–C motif) ligand 3 (CCL3, also known as macrophage inflammatory protein-1α or MIP1α); acgagagccacaacgcagcc and tcaccccggatggaatggcct for the gene encoding interleukin 10 (IL10); gagcccagccacatcccgag and gtgcagcgcaccgttcttgc for the gene encoding C–X–C motif chemokine 10 (CXCL10, also known as interferon gamma-induced protein 10 kDa or IP10); gtatgaacagcgatgatgcact and gaagaccagagcagattttcaatag for the gene encoding IL6 (used as positive control for activation); gaagaagaaattagagaagcgttcc and gtagtttacctgaccatccccat for CALM2 (i.e. the gene coding for calmodulin 2, used as internal reference for normalization).
Statistical analysis
Statistical analysis was performed with Prism software version 5.0 (GraphPad Software Inc., La Jolla, CA, USA). Columns in the graphs represent the mean (with SD or SEM) of at least three independent experiments performed in triplicate. Statistical significance was evaluated (with 95% confidence intervals) by unpaired two-tailed Student's t-test, for statistical analysis of two groups, or one-way ANOVA followed by Dunnett's (against the LPS treatment for multiple groups) or Bonferroni (for all pairwise comparisons) post hoc tests. A value of p < 0.05 was considered to be statistically significant.
Acknowledgments
We thank all the people of the Cellular Neurophysiology Unit at San Raffaele for support and Roberto Furlan for helpful discussions. The work was carried out within the framework of the Italian Ministry of Research Center of Excellence in Physiopathology of Cell Differentiation. Financial support was from the Italian Ministry of Research (PRIN, Progetti di Ricerca di Interesse Nazionale, project 2006054051 to FG) and the Italian Telethon Foundation (GGP05141 and GGP10099 grants to FG). The authors declare that there are no actual or perceived conflicts of interest.
Contributor Information
Fabio Grohovaz, Email: grohovaz.fabio@hsr.it.
Daniele Zacchetti, Email: zacchetti.daniele@hsr.it.
References
- Amara S.G., Jonas V., Rosenfeld M.G., Ong E.S., Evans R.M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Anthony D.C., Miller K.M., Fearn S., Townsend M.J., Opdenakker G., Wells G.M., Clements J.M., Chandler S., Gearing A.J., Perry V.H. Matrix metalloproteinase expression in an experimentally-induced DTH model of multiple sclerosis in the rat CNS. J. Neuroimmunol. 1998;87:62–72. doi: 10.1016/s0165-5728(98)00046-0. [DOI] [PubMed] [Google Scholar]
- Born W., Fischer J.A., Muff R. Receptors for calcitonin gene-related peptide, adrenomedullin, and amylin: the contributions of novel receptor-activity-modifying proteins. Receptors Channels. 2002;8:201–209. [PubMed] [Google Scholar]
- Brain S.D., Grant A.D. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Chang C.L., Roh J., Hsu S.Y. Intermedin, a novel calcitonin family peptide that exists in teleosts as well as in mammals: a comparison with other calcitonin/intermedin family peptides in vertebrates. Peptides. 2004;25:1633–1642. doi: 10.1016/j.peptides.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Christopoulos G., Perry K.J., Morfis M., Tilakaratne N., Gao Y., Fraser N.J., Main M.J., Foord S.M., Sexton P.M. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- Collins T.J. ImageJ for microscopy. Biotechniques. 2007;43:25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]
- Colton C.A. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackor R., Caron K. Mice heterozygous for adrenomedullin exhibit a more extreme inflammatory response to endotoxin-induced septic shock. Peptides. 2007;28:2164–2170. doi: 10.1016/j.peptides.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doods H., Hallermayer G., Wu D., Entzeroth M., Rudolf K., Engel W., Eberlein W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br. J. Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham P.L., Vause C.V. Calcitonin gene-related peptide (CGRP) receptor antagonists in the treatment of migraine. CNS Drugs. 2010;24:539–548. doi: 10.2165/11534920-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas J.M., Manganas L., Enikolopov G. Nitric oxide and multiple sclerosis. Curr. Neurol. Neurosci. Rep. 2005;5:232–238. doi: 10.1007/s11910-005-0051-y. [DOI] [PubMed] [Google Scholar]
- Engelhardt B., Ransohoff R.M. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Evans B.N., Rosenblatt M.I., Mnayer L.O., Oliver K.R., Dickerson I.M. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J. Biol. Chem. 2000;275:31438–31443. doi: 10.1074/jbc.M005604200. [DOI] [PubMed] [Google Scholar]
- Farez M.F., Quintana F.J., Gandhi R., Izquierdo G., Lucas M., Weiner H.L. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat. Immunol. 2009;10:958–964. doi: 10.1038/ni.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C., Aloisi F., Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Feng Y., Tang Y., Guo J., Wang X. Inhibition of LPS-induced TNF-alpha production by calcitonin gene-related peptide (CGRP) in cultured mouse peritoneal macrophages. Life Sci. 1997;61:PL 281–287. doi: 10.1016/s0024-3205(97)00866-7. [DOI] [PubMed] [Google Scholar]
- Flügel A., Odoardi F., Nosov M., Kawakami N. Autoaggressive effector T cells in the course of experimental autoimmune encephalomyelitis visualized in the light of two-photon microscopy. J. Neuroimmunol. 2007;191:86–97. doi: 10.1016/j.jneuroim.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Geppetti P., Capone J.G., Trevisani M., Nicoletti P., Zagli G., Tola M.R. CGRP and migraine: neurogenic inflammation revisited. J. Headache Pain. 2005;6:61–70. doi: 10.1007/s10194-005-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes R.N., Castro-Faria-Neto H.C., Bozza P.T., Soares M.B., Shoemaker C.B., David J.R., Bozza M.T. Calcitonin gene-related peptide inhibits local acute inflammation and protects mice against lethal endotoxemia. Shock. 2005;24:590–594. doi: 10.1097/01.shk.0000183395.29014.7c. [DOI] [PubMed] [Google Scholar]
- Gutcher I., Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J. Clin. Invest. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S.G., Padilla J., Koumas L., Ray D., Phipps R.P. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- Hartung H.P., Reiners K., Archelos J.J., Michels M., Seeldrayers P., Heidenreich F., Pflughaupt K.W., Toyka K.V. Circulating adhesion molecules and tumor necrosis factor receptor in multiple sclerosis: correlation with magnetic resonance imaging. Ann. Neurol. 1995;38:186–193. doi: 10.1002/ana.410380210. [DOI] [PubMed] [Google Scholar]
- Hay D.L., Conner A.C., Howitt S.G., Takhshid M.A., Simms J., Mahmoud K., Poyner D.R. The pharmacology of CGRP-responsive receptors in cultured and transfected cells. Peptides. 2004;25:2019–2026. doi: 10.1016/j.peptides.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- Katayama T., Furuya M., Yamaichi K., Konishi K., Sugiura N., Murafuji H., Magota K., Saito M., Tanaka S., Oikawa S. Discovery of a non-peptide small molecule that selectively mimics the biological actions of calcitonin. Biochim. Biophys. Acta. 2001;1526:183–190. doi: 10.1016/s0304-4165(01)00125-8. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M., Meinl E., Hohlfeld R. Neuro-immune crosstalk in CNS diseases. Neuroscience. 2009;158:1122–1132. doi: 10.1016/j.neuroscience.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Kroeger I., Erhardt A., Abt D., Fischer M., Biburger M., Rau T., Neuhuber W.L., Tiegs G. The neuropeptide calcitonin gene-related peptide (CGRP) prevents inflammatory liver injury in mice. J. Hepatol. 2009;51:342–353. doi: 10.1016/j.jhep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Lassmann H., Brück W., Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol. Med. 2001;7:115–121. doi: 10.1016/s1471-4914(00)01909-2. [DOI] [PubMed] [Google Scholar]
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- Leppert D., Lindberg R.L.P., Kappos L., Leib S.L. Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res. Rev. 2001;36:249–257. doi: 10.1016/s0165-0173(01)00101-1. [DOI] [PubMed] [Google Scholar]
- Lindberg R.L., De Groot C.J., Certa U., Ravid R., Hoffmann F., Kappos L., Leppert D. Multiple sclerosis as a generalized CNS disease—comparative microarray analysis of normal appearing white matter and lesions in secondary progressive MS. J. Neuroimmunol. 2004;152:154–167. doi: 10.1016/j.jneuroim.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Liu J., Chen M., Wang X. Calcitonin gene-related peptide inhibits lipopolysaccharide-induced interleukin-12 release from mouse peritoneal macrophages, mediated by the cAMP pathway. Immunology. 2000;101:61–67. doi: 10.1046/j.1365-2567.2000.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke A.E., Dahl G.P., Roos B.A., Dickerson I.M. Identification of a protein that confers calcitonin gene-related peptide responsiveness to oocytes by using a cystic fibrosis transmembrane conductance regulator assay. Proc. Natl. Acad. Sci. U. S. A. 1996;93:3455–3460. doi: 10.1073/pnas.93.8.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Dumont Y., Vercauteren F., Quirion R. Lipopolysaccharide induces calcitonin gene-related peptide in the RAW264.7 macrophage cell line. Immunology. 2010;130:399–409. doi: 10.1111/j.1365-2567.2009.03239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marta M. Toll-like receptors in multiple sclerosis mouse experimental models. Ann. N. Y. Acad. Sci. 2009;1173:458–462. doi: 10.1111/j.1749-6632.2009.04849.x. [DOI] [PubMed] [Google Scholar]
- Martino G., Hartung H.P. Immunopathogenesis of multiple sclerosis: the role of T cells. Curr. Opin. Neurol. 1999;12:309–321. doi: 10.1097/00019052-199906000-00010. [DOI] [PubMed] [Google Scholar]
- Martino G., Furlan R., Brambilla E., Bergami A., Ruffini F., Gironi M., Poliani P.L., Grimaldi L.M.E., Comi G. Cytokines and immunity in multiple sclerosis: the dual signal hypothesis. J. Neuroimmunol. 2000;109:3–9. doi: 10.1016/s0165-5728(00)00295-2. [DOI] [PubMed] [Google Scholar]
- McCarthy K.D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie L.M., Fraser N.J., Main M.J., Wise A., Brown J., Thompson N., Solari R., Lee M.G., Foord S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Miksa M., Wu R., Cui X., Dong W., Das P., Simms H.H., Ravikumar T.S., Wang P. Vasoactive hormone adrenomedullin and its binding protein: anti-inflammatory effects by up-regulating peroxisome proliferator-activated receptor-gamma. J. Immunol. 2007;179:6263–6272. doi: 10.4049/jimmunol.179.9.6263. [DOI] [PubMed] [Google Scholar]
- Moreno M.J., Terrón J.A., Stanimirovic D.B., Doods H., Hamel E. Characterization of calcitonin gene-related peptide (CGRP) receptors and their receptor-activity-modifying proteins (RAMPs) in human brain microvascular and astroglial cells in culture. Neuropharmacology. 2002;42:270–280. doi: 10.1016/s0028-3908(01)00176-9. [DOI] [PubMed] [Google Scholar]
- Muzio L., Martino G., Furlan R. Multifaceted aspects of inflammation in multiple sclerosis: the role of microglia. J. Neuroimmunol. 2007;191:39–44. doi: 10.1016/j.jneuroim.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Poyner D.M., Sexton P.M., Marshall I., Smith D.M., Quirion R., Born W., Muff R., Fischer J.A., Foord S.M. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Recober A., Russo A.F. Calcitonin gene-related peptide: an update on the biology. Curr. Opin. Neurol. 2009;22:241–246. doi: 10.1097/wco.0b013e32832b2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redford C.J., Kapoor P., Smith K.J. Nitric oxide donors reversibly block axonal conduction: demyelinated axons are especially susceptible. Brain. 1997;120:2149–2157. doi: 10.1093/brain/120.12.2149. [DOI] [PubMed] [Google Scholar]
- Saura J. Microglial cells in astroglial cultures: a cautionary note. J. Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner B., Heppner F.L., Becher B. Modeling multiple sclerosis in laboratory animals. Semin. Immunopathol. 2009;31:479–495. doi: 10.1007/s00281-009-0181-4. [DOI] [PubMed] [Google Scholar]
- Smith S.R., Terminelli C., Denhardt G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J. Pharmacol. Exp. Ther. 2000;293:136–150. [PubMed] [Google Scholar]
- Szczucinski A., Losy J. Chemokines and chemokine receptors in multiple sclerosis. Potential targets for new therapies. Acta Neurol. Scand. 2007;115:137–146. doi: 10.1111/j.1600-0404.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K.F., Kashima H., Suzuki H., Ono K., Sawada M. Existence of functional beta1- and beta2-adrenergic receptors on microglia. J. Neurosci. Res. 2002;70:232–237. doi: 10.1002/jnr.10399. [DOI] [PubMed] [Google Scholar]
- Temmesfeld-Wollbrück B., Hocke A.C., Suttorp N., Hippenstiel S. Adrenomedullin and endothelial barrier function. Thromb. Haemost. 2007;98:944–951. doi: 10.1160/th07-02-0128. [DOI] [PubMed] [Google Scholar]
- Tsujikawa K., Yayama K., Hayashi T., Matsushita H., Yamaguchi T., Shigeno T., Ogitani Y., Hirayama M., Kato T., Fukada S., Takatori S., Kawasaki H., Okamoto H., Ikawa M., Okabe M., Yamamoto H. Hypertension and dysregulated proinflammatory cytokine production in receptor activity-modifying protein 1-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16702–16707. doi: 10.1073/pnas.0705974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum D., Hanisch U.K., Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci. Biobehav. Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- Walker C.S., Conner A.C., Poyner D.R., Hay D.L. Regulation of signal transduction by calcitonin gene-related peptide receptors. Trends Pharmacol. Sci. 2010;31:476–483. doi: 10.1016/j.tips.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Wang Z., Ma W., Chabot J.G., Quirion R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J. 2009;23:2576–2586. doi: 10.1096/fj.08-128348. [DOI] [PubMed] [Google Scholar]
- Wong L.Y., Cheung B.M., Li Y.Y., Tang F. Adrenomedullin is both proinflammatory and antiinflammatory: its effects on gene expression and secretion of cytokines and macrophage migration inhibitory factor in NR8383 macrophage cell line. Endocrinology. 2005;146:1321–1327. doi: 10.1210/en.2004-1080. [DOI] [PubMed] [Google Scholar]
- Wu R., Zhou M., Wang P. Adrenomedullin and adrenomedullin binding protein-1 downregulate TNF-alpha in macrophage cell line and rat Kupffer cells. Regul. Pept. 2003;112:19–26. doi: 10.1016/s0167-0115(03)00018-1. [DOI] [PubMed] [Google Scholar]