Summary
Mycobacterium tuberculosis is an extremely successful pathogen that demonstrates the capacity to modulate its host both at the cellular and tissue levels. At the cellular level, the bacterium enters its host macrophage and arrests phagosome maturation, thus avoiding many of the microbicidal responses associated with this phagocyte. Nonetheless, the intracellular environment places certain demands on the pathogen, which, in response, senses the environmental shifts and upregulates specific metabolic programs to allow access to nutrients, minimize the consequences of stress, and sustain infection. Despite its intracellular niche, Mycobacterium tuberculosis demonstrates a marked capacity to modulate the tissues surrounding infected cells through the release of potent, bioactive cell wall constituents. These cell wall lipids are released from the host cell by an exocytic process and induce physiological changes in neighboring phagocytes, which drives formation of a granuloma. This tissue response leads to the generation and accumulation of caseous debris and the progression of the human tuberculosis granuloma. Completion of the life cycle of tuberculosis requires damaging the host to release infectious bacteria into the airways to spread the infection. This damage reflects the pathogen’s ability to subvert the host’s innate and acquired immune responses to its own nefarious ends.
Keywords: Mycobacterium, tuberculosis, macrophage, granuloma
Introduction
Mycobacterium tuberculosis (Mtb) remains one of the most pernicious of the infectious diseases borne by mankind (1). It infects many, approximately one third of the human population, yet it causes disease in only a proportion, 3-15%, of those that are infected. The burden of disease is disproportionately distributed across the planet with 22 countries bearing 80% of the total number of cases of active disease. Not surprisingly, this distribution tracks predominantly with socio-economic status, with sub-Saharan Africa being one of the most intensely affected areas.
There are no effective vaccines against infection and the only approved vaccine, bacille Calmette Guerin (the culture-attenuated strain of M. bovis), was developed almost 100 years ago and shows slight protection against severe disease among some ethnic groups. In addition, although drug therapy is effective, it is not without serious limitations. Therapy requires treatment with multiple (four) antibiotics for nine months, which in many parts of the world is almost impossible to sustain. Such regimens are fraught with frequent cases of non-compliance, which leads to the repeated selection of multi-drug resistant strains. The effectiveness of this complex therapy depends on the existence of a health care infrastructure that is beyond the means and expertise of many of the more seriously affected regions of the world.
There are few new drugs in the development pipeline, and the vaccines under trial currently have not been shown to offer benefits beyond BCG. Therefore, we need to re-evaluate our approaches to drug and vaccine discovery. The development of new therapies or vaccines requires incorporation of a greater appreciation of the biology of the pathogen as a full partner in the process. Mtb is not a passive ‘shopping bag’ of antigens and vaccine candidates. It is an active manipulator of the immune system that utilizes this response to modulate the tissue architecture to sustain the infection and drive transmission. Nor can drug development be based on the physiology of Mtb in rich broth culture. The metabolism of the bacterium is linked intimately with the changing environments within its host. For these reasons, understanding the biochemical and physiological interplay between Mtb and it host cell, the macrophage, extends beyond the mere esoteric. An appreciation how the two cells communicate and modulate the behavior of one another is critical to the design of appropriate screens for the identification and development of new drug and vaccine candidates. Therefore, the cell biology of the intimate interaction between Mtb and the macrophage is a critical component in combating the infection.
Most cell biological studies of intracellular pathogens proceed by either immunofluorescent co-localization of known ‘marker’ proteins, or through the use of transfection of host cells with proteins tagged with reporters such as green fluorescent proteins (GFPs). These approaches have been and continue to be extremely informative (2-9). But, there are alternatives that place a direct emphasis on function and consequences, and these are the approaches explored in this article. Firstly, the presence of a protein does not demonstrate function. For example, cathepsin D is a lysosomal proteinase that is synthesized in an inactive proform (10, 11). The enzyme is not active until processed and does not attain full activity until it reaches a compartment with a pH equivalent to its pH optimum. None of these confounding factors can be judged by presence alone but can be quantified by functional, intraphagosomal hydrolase assays (12-15). Secondly, as microbiologists, our primary interest should be the consequences that intracellular environments have for the infecting microbe. Therefore by studying the responses shown by Mtb to the differing environments experienced by the pathogen within its host cell we can start to build a more detailed picture of the physiological state within the phagosome (16-18). This is an unbiased but long-term approach. It does not supplant the more traditional cell biological studies, but I believe that, ultimately, the two will complement one another to increase our appreciation of the physiology both of the phagosome and the pathogen.
Phagocytosis and the biogenesis of the phagosomal compartment
The macrophage is an extremely plastic cell that, unlike specialized phagocytes such as osteoclasts or dendritic cells, fulfills a range of activities that are modulated by the tissue and cytokine environment to which the cell is exposed. It is widely accepted that a macrophage in culture does not reflect the true properties of a tissue macrophage. Much of these data are phenomenological, linked to the changing expression of surface markers, nonetheless it is an important caveat to consider.
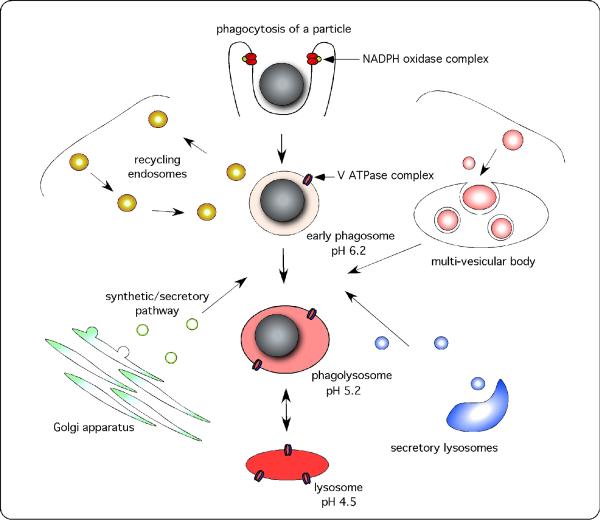
Much of the function of the macrophage is channeled through its phagosome. In the old days this was simple. Cargo was phagocytosed when appropriate receptors were ligated, and the internalized material was delivered to the lysosome. Unfortunately, this convenient model has been complicated by a growing awareness of both the diversity of intracellular organelles and the subtleties that regulate fusion between intracellular membranes (19) (Fig. 1). During phagosome maturation, it is now known that the phagocytic compartment interacts with the rapid recycling endosome, the secretory system, multi-vesicular bodies such as the major histocompatibility complex (MHC) class II (M-2C) compartment, and the endoplasmic reticulum, prior to fusion with the lysosomes themselves (20-22). During this process, the phagosome function is modified and influenced to reflect the specialized activities of all of these other organelles. Finally, this is not a defined sequence of events, this is a highly plastic system that is influenced by the immune status of the macrophage (15, 23-25), which is modulated by extrinsic and intrinsic factors of the innate and acquired immune system. The regulation of these membrane fusion events is extremely complex and has been the subject of intensive analysis of the cytosolic and membrane-associated machinery that drives and regulates the specificity of the process. However, this machinery is not directly germane to the lumen of the Mtb-containing phagosome; therefore, this article places greater emphasis on the consequences of these fusion events to the nature of the intraphagosomal environment(s) experienced by the intravacuolar bacterium.
Fig. 1. During maturation, the phagosome forms transient interactions with a wide range of intracellular organelles.
Phagosome maturation refers to the process of phagosome remodeling through a series of independent events, following its formation at the surface of the phagocyte, and culminating in the complete fusion of the phagosome with the lysosome. Following engagement of phagocytic receptors, an area of the surface is remodeled around the particle, forming the phagocytic cup. Some protein complexes, such as the NADPH oxidase complex, can be recruited and activated prior to phagosomal closure, facilitating a rapid antimicrobial response at the cell surface. The phagosome, once closed, becomes increasingly more acidic, through the accumulation of V ATPases that pump protons into the compartment, and hydrolytically competent, through the acquisition of lysosomal enzymes. This process is marked by transient fusion events with multiple intracellular organelles including the recycling endosomal machinery, the synthetic–secretory apparatus including the endoplasmic reticulum, secretory lysosomes, and multi-vesicular bodies, which may include the MIIC compartment and/or the autophagosome. Finally, the phagosome fuses with pre-existing, dense lysosomal bodies and equilibrates to a pH of 4.5-5.0.
The superoxide burst
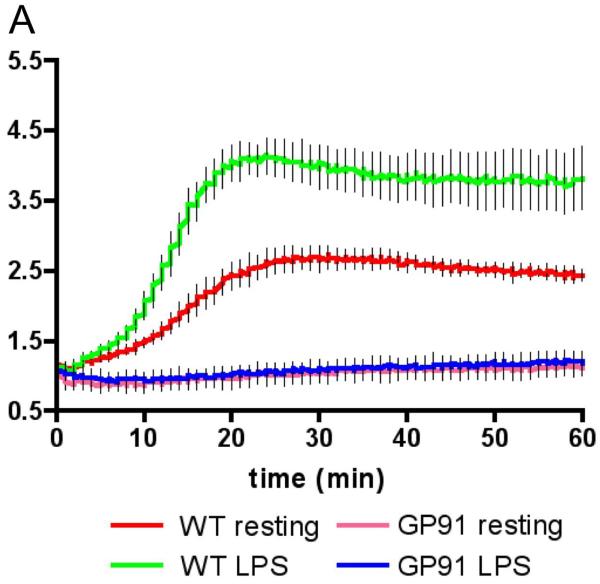
The first microbicidal activity any microbe will encounter is the superoxide burst, which is generated by the NADPH complex assembled in the phagocytic cup early in the process of phagocytosis (26-29). The complex is comprised of an integral membrane flavoprotein b588 (gp91phox and p22phox), and the cytosolic subunits p47phox, p67phox, and p40phox. Upon ligation of an appropriate receptor, such as the FcRII receptor for complexed immunoglobulin, the cytosolic subunits assemble on the flavoprotein subunit and, along with guanosine triphosphatase Rac2, form an active enzyme complex that generates O2− in the forming phagosome. The generation of the superoxide burst is transient, lasting 15-20 min in bone marrow-derived murine macrophages (Fig. 2), and while its intensity is increased upon activation of the macrophage with lipopolysaccharide (LPS) or interferon-γ (IFN-γ), its duration is unaffected (13). Superoxide and its downstream metabolites, hydrogen peroxide and hypervalent iron, are highly toxic for many microbes. However, the data on the activity of superoxide and its adducts on survival of pathogenic Mycobacterium spp. are not so impressive. M. avium infection of mice defective in gp47phox reveals no phenotype (30), and infection of NADPH oxidase-impaired macrophages with Mtb had no effect on bacterial survival (31). Mtb is known to possess several routes of avoidance of superoxide, ranging from superoxide dismutase (32-34), to the scavenging properties of its cell wall lipidoglycans (35).
Fig. 2. Spectrofluorometric measurement of the superoxide burst generated by bone marrow-derived murine macrophages following internalization of IgG-coated beads.
An illustration of the assay that we have developed for measuring the superoxide burst within the phagosome. Murine macrophages were fed IgG-coated particles labeled with the oxidation sensitive fluorochrome dihydrohexafluorofluorescein, and the calibration dye Alexa-594. Both wildtype and macrophages deficient in the p91 subunit of the NADPH oxidase were examined in their resting and LPS-activated states, and the increase in fluorescence measured by spectrofluorometer. Activation of the macrophage with LPS enhanced the intensity but not the duration of the burst. Reproduced from (13).
Acidification of the phagosome and its acquisition of lysosomal hydrolases
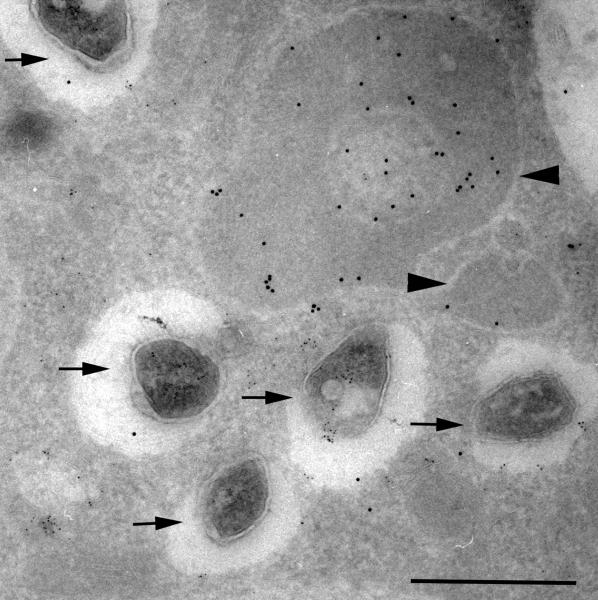
The successful function of the phagosome as a degradative organelle is linked to its acidification, which enables the activation and optimal function of a range of lysosomal hydrolases capable of degrading most biological polymers. Acidification is achieved through the accumulation of V-ATPase complexes in the phagosome membrane (36). Like the NADPH oxidase, these complexes are comprised of both integral membrane and cytosolic subunits that form an active enzyme that hydrolyses ATP and pumps protons into the phagosome. The acidification process is relatively rapid, at least around inert particles such as IgG-coated beads, where it takes 15 min to reach a pH equilibrium at pH 4.8-5.0 (14). Early studies on pathogenic Mycobacterium spp. demonstrated that this process was arrested and that the pH of the Mtb-containing phagosomes in resting macrophages remained at pH 6.2-4 (37). Comparable data have been generated by analysis of Mtb-infected macrophages isolated by broncholavage of human tuberculosis (TB) patients (38) (Fig. 3). This arrest in the acidification process is now known to be indicative of a more global block in the maturation of the Mtb-containing phagosome that limits its acquisition of a range of potentially noxious components of the lysosome.
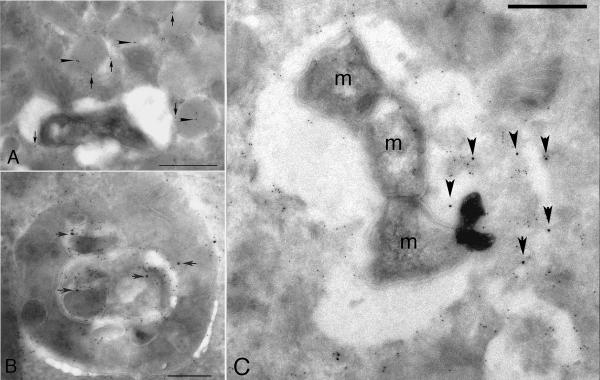
Fig. 3. Immunoelectronmicrograph from a human alveolar macrophage isolated from a TB patient in Malawi.
Following isolation the cells were incubated with the pH-indicator DAMP, which accumulates in acidic compartments, for 60 minutes prior to fixation for electron microscopy. The sample was sectioned and probed with anti-DNP (12 nm gold particles) and anti-LAMP 1 (6 nm gold particles). The acidified lysosomes label strongly (arrowhead), while the Mtb-containing vacuoles show minimal to no labeling indicating that their pH is considerably higher than that of the neighboring lysosomes. Reproduced from (38).
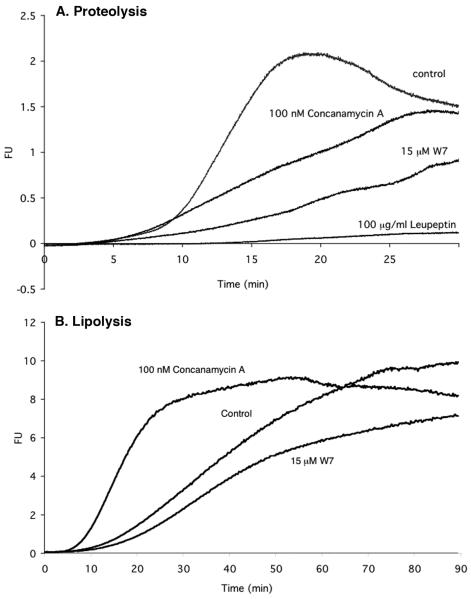
Studies on phagosomes formed around inert IgG-coated particles have provided considerable insights into the kinetics of the process of acquisition of hydrolytic activities. Most hydrolytic activities, such as proteolysis and lipolysis, can be detected within 5-10 min following particle internalization and closure of the phagosome (14). However, maximal concentration of lysosomal material does not achieve equilibrium until 90 min after initial formation of the phagosome. Pharmacological intervention that limits phagosome maturation, in general, reduces hydrolytic capacity ( Fig. 4). However, we found that the one notable exception to this observation was lipolytic activity (14). Lipolysis appears to be the product of lipases both in the early endosomal system, lipoprotein lipase (LPL), and members of the hepatic lipase family, as well as acidic lysosomal lipases (39-41). Restricting phagosome maturation by inhibition of V-ATPase activity likely renders the phagosome more accessible to the sustained activity of LPL and hepatic lipases, the significance of which is discussed later in this review.
Fig. 4. Measurement of hydrolytic activity in phagosomes using fluorogenic substrates.
(A). Cysteine proteinase substrate hydrolysis profiles for IgG-coated bead containing phagosomes. The beads were carrying the cysteine proteinase substrate Biotin-LC-Phe-Arg)2-Rhodamine 110, which increased fluorescence following the removal of the Phe-Arg quenching peptides. Manipulation of hydrolytic rates was achieved with inhibitors Concanamycin A (100 nM), W7 (15 μM) and Leupeptin (100 μg/ml), both of which reduced the rate of hydrolysis within the phagosome. (B). Triglyceride substrate hydrolysis profiles for IgG-coated bead containing phagosomes. These beads were carrying the lipase substrate 1-trinitrophenyl-amino-dodecanoyl-2-pyrenedecanoyl-3-O-hexadecyl-sn-glycerol. In contrast to proteolysis, blocking acidification of the phagosome increased its lypolytic activity. Traces were generated using the equation FU=R110/AF594 (where FU=Arbitrary Fluorescent Units, R110=Real time Rhodamine 110 fluorescence, AF594=Starting Alexa Fluor 594 fluorescence) and averaged over two experiments. Measurements were taken every second for 30 minutes. Reproduced from (14)
Modulation of phagosome-maturation by Mtb
The mechanism(s) by which Mtb prevents the maturation of its phagosome remain opaque. Not because we have yet to identify the bacterial factors involved in the process but because we have a confusing plethora of candidates.
Mycobacterial cell wall lipids such a lipoarabinomannan (LAM) (8, 42-44), the phenolic glycolipid phenolphthiocerol diester (PGL-1) (45), the isoprenoid edaxadiene (46, 47), and trehalose dimycolate (TDM) (48, 49) have all been demonstrated to modulate phagosome maturation, although the amount of supporting data varies for each candidate. LAM is thought to incorporate into the phagosomal membrane and inhibit Vps34 activity, which limits accumulation of PI3-P and the acquisition of EEA1 (9, 44, 50, 51). EEA1 is required for the loss of rab5 and its replacement with rab7 to drive fusion with late endosomal and lysosome compartments. Another cell wall lipid, TDM, is also thought to modulate phagosome maturation (48). Intriguingly, however, the ability of TDM to restrict phagosome maturation is reversed in activated macrophages, which is consistent with the studies that demonstrate that IFN-γ-activated macrophages can overcome the Mtb-mediated block in phagosome maturation (52, 53). The reversal of TDM’s inhibition is not observed in NOS2-deficient mice; however, TDM loses its capacity to arrest phagosome maturation following pre-treatment with reactive nitrogen intermediates. These data not only identify a possible mediator of phagosome arrest but also provide an explanation as to how this blockage may be overcome by activation of the host macrophage. Earlier studies had indicated that delivery to lysosomal compartments preceded death of the bacilli (52), which is consistent with the effector being a peripheral component neutralized by the host cell without affecting viability directly.
In addition to these cell wall components, two secreted proteins have also been implicated in the modulation of the Mtb phagosome. PknG, a member of the serine/threonine family of kinases of Mtb, has been proposed to access the host cell cytoplasm and modify host enzymes associated with phagsome maturation (54). However, PknG has been shown to regulate glutamine metabolism in Mtb (55), which impacts the fitness of the bacterium under certain physiological conditions. Similarly, SapM, a bacterial phosphatase capable of hydrolyzing PI3P, is proposed to access the cytoplasmic face of the phagosome to reduce the concentration of PI3P and thus restrict maturation of the phagosome (51). This activity is consistent with the delayed accumulation of PI3P on the phagosomes of pathogenic Mycobacterium spp. However, the same caveat attends the mode of action of both these proteins. How do they gain access to the host cell cytoplasm? Mtb lacks any specialized type III secretion system so delivery of these candidate effector proteins to the host cell cytoplasm remains an issue of some conjecture.
Escape into the host cell cytosol
Some early reports described Mtb in the cytoplasm of their host macrophages (56), but these observations ran against the majority of publications on Mtb-infected macrophages. More recently, compelling evidence has been generated by Van der Wel and colleagues (57) who studied human dendritic cells and macrophages infected with Mtb. Escape from the vacuole in these cells was found to be dependent on an intact ESX1 secretion system. Mtb has also been found to exit the phagosomes of Dictyostelium, which was exploited as an alternate, genetically tractable infection model (58). These data are provocative; however, the percentage of bacteria accessing the cytoplasm was extremely variable and was observed predominantly late in infection. Until now there is no indication that this represents a privileged environment that is more permissive to bacterial growth than the phagosome. More work is needed to place these data in their biological context and clarify the significance of this process to disease.
Anti-mycobacterial activities in the infected macrophage
While there are substantial data on the ability of murine macrophages to limit growth and even kill Mtb in culture, the routes of killing remain to be fully understood, even under in vitro conditions. In vivo, in murine infections there is a clear hierarchy of efficacy that has been established with the use of knockout mice, and while this does not eliminate compensatory activities, it does reveal those responses most important to control of infection (59). The most susceptible mice are those deficient in either IFN-γ or IFN-γ receptors, implying macrophage activation as a critical component in controlling infection (60). The next most susceptible mice are those lacking NOS2 or the ability to generate reactive nitrogen intermediates, again a key function of the activated macrophage (61). For several years people debated the relevance of these data to humans because human macrophages could not be stimulated either to make NO in culture or to effectively limit Mtb growth. However, compelling data from macrophages isolated from infected patients demonstrate production of NO ex vivo (62, 63). Furthermore, a genetic screen for Mtb susceptible to the effects of NO in vitro identified components of the bacterial proteosome (64-66), which is mobilized for the degradation of bacterial proteins damaged by the action of NO radicals. These mutants demonstrated marked phenotypes in wildtype but not NOS2-deficient mice. All these data are consistent with NOS2 activity playing an extremely significant role in the control of Mtb infection in vivo.
More recently, other radical-independent means of controlling Mtb within the macrophage have been documented. Gutierrez and colleagues (67, 68) observed that drugs that induced autophagy in BCG- and Mtb-infected macrophages lead to enhanced, early killing of the bacteria. Examination of the Mtb-containing vacuoles demonstrated an increased colocalization with markers of the autophagous pathway, such as LC3. Moreover, transfection of macrophages with the immune-induced GTPase LRG-47 enhanced both this colocalization and bacterial death (69). In studies initiated from a different starting point, Alonso and colleagues (70) were pursuing a soluble, lysosomal factor capable of mediating death of Mtb in broth culture. The component turned out to be proteolysed ubiquitin. These data revealed a novel pathway for killing whereby ubiquitinated proteins, translocated from the cytoplasm into the lysosome via the autophagous pathway, were processed by cathepsins to reveal cationic peptides that permiabilized and killed Mtb (Fig. 5). In more recent studies, bacterial porins were shown to be a major susceptibility factor for the action of these peptides, and expression of the major porin of M. smegmatis in Mtb rendered it exquisitely sensitive to killing by ubiquitin-derived peptides (71). This pathway of killing through the activity of cryptic peptides revealed by lysosomal hydrolases has since been verified by Ponpuak and colleagues (72), who also identified Hepcidin-derived peptides as another family of peptides capable of killing Mtb via delivery through the autophagous pathway.
Fig. 5. Immunoelectron microscopy of Mtb-infected macrophages probed with antibodies against ubiquitinated proteins.
(A). Resting, Mtb-infected macrophages show ubiquitin label (arrowheads) in dense, LAMP1-positive (arrows) lysosomes. (B). In cells in which autophagy has been induced, the ubiquitin label can be seen in multivesicular structures that are reminiscent of autophagosomes. (C). In Mtb-infected cells in which autophagy has been induced the ubiquitinated material (arrows) can be seen in the Mtb-containing phagosomes. Reproduced from (70).
Antigen presentation and the infected macrophage
The maturation of the Mtb-containing phagosome is modulated by the pathogen, but it is unclear to what extent, if any, this impacts antigen processing and presentation. The bacterium continues to secrete or release both proteins and lipids inside the cell, and these are potential antigens, which could be loaded onto the appropriate MHC class II or CD1 molecules.
In order for MHC class II-mediated antigen presentation to occur, the MHC class II antigens must undergo compartment-specific maturation processes such as glycosylation and the proteolytic removal of the invariant chain prior to binding of ‘immunogenic’ peptides (73, 74). The loading of peptide requires removal of the invariant chain-derived peptide (CLIP) from the antigen-binding groove at low pH by the chaperone HLA-DM (human) or H2-M (mice). This must take place prior to the delivery of the antigen-loaded heterodimer to the cell surface for functional presentation.
In studies on M. avium-infected murine and human macrophages, Ullrich and colleagues (75) reported detection of MHC class II molecules in early bacteria-containing phagosomes. However, these molecules are already peptide-loaded and were trafficked from the cell surface, indicating that they were not competent to sample bacterial antigens. However, upon activation of the macrophage with INF-γ, the bacteria-containing vacuoles became accessible to the class II chaperone H2-M, implying that the compartments were now capable of antigen sampling and functional presentation. But, these data refer only to antigens in the bacteria-containing phagosome, and Mtb is known to secrete an abundance of proteins that actually accumulate in the multivesicular lysosome or the M2C compartment (76-78). Several groups report effective antigen presentation of secreted antigens such as the highly immunogenic Ag85 by infected macrophages (79). Infected mice exhibit abroad spectrum of responses to both protein and lipid antigens of Mtb (80), implying that antigen presentation, by either infected macrophages or bystander antigen-presenting cells, is not adversely affected to any great extent by the infection.
Clearly the data regarding the immunocompetence of infected macrophages to present antigen are conflicting, which likely reflects key differences in the design and execution of the experiments. Mycobacterial species are extremely immunogenic particles, and while immunogenicity may be limited early, they very rapidly generate a very robust immune response in both animal models and humans (80). A robust immune response is a critical component in the completion of the bacteria life cycle. Absent this immune response, Mtb cannot achieve the late-stage tissue damage necessary to lead to necrosis and the liquification of the granuloma that facilitates host-to-host spread of infection. So while the immune response represents a threat at the level of the infected macrophage, it is, from the perspective of Mtb, a necessary evil for the success of the infection process (81).
The consequences of the intracellular environment for Mtb
As discussed previously, the arrested maturation of the Mtb-containing phagosome indicates that the bacterium resides in a relatively high pH compartment (pH 6.4), with minimal hydrolytic activity (82), with the possible exception of lipase activity. Nonetheless, this environment must represent a considerable shift from the benign environment of rich nutrient broth, which is maintained at a static temperature and pH.
In early transcriptional profiling of intracellular Mtb, Schnappinger and colleagues (17, 83) demonstrated that upon infection of the macrophage, the bacterium upregulated expression of genes relevant to resistance to oxidative and nitrosative stresses, and growth limitation, as well as an adjustment to alternative nutrient sources. Two more recent studies by Rohde and colleagues (16, 84) build on these earlier observations. Firstly, Rohde performed a temporal dissection of the transcriptional response mounted by Mtb during the first 2 h of infection and found that the patterns of upregulation or downregulation of these genes were sustained at 24 h post-infection. Many of the transcriptional signatures observed in this study coincided with the themes reported previously by Schnappinger (17, 83). However, Rohde went on to manipulate the phagosomal environment and, through negating the pH drop from pH 7.0 (extracellular) to pH 6.4 (intracellular), demonstrated that one of the dominant environmental cues that shaped the transcriptional response of intracellular Mtb was the pH shift. Over one third of the change in the transcription profile was suppressed in the absence of a pH drop to pH 6.4 (16).
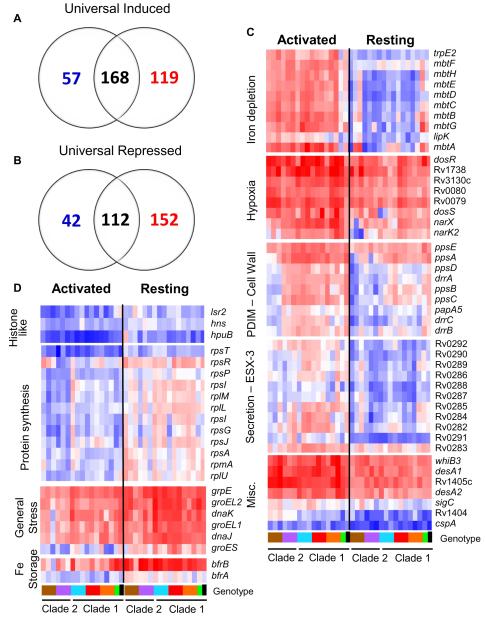
More recently, Homolka and colleagues (84) employed a different approach to identify the genes that Mtb require for intracellular success. They examined the intracellular transcriptome from a panel of 15 recent clinical isolates that represent the global genetic diversity of the Mtb complex, including the more ‘ancient’ lineages such as M. africanum. The underpinning logic to this approach was that genes up-regulated in all isolates would represent the ‘core’ transcriptome required by all Mtb isolates to sustain intracellular infection, and the divergent transcriptional profiles would indicate lineage-specific traits that could reflect increased virulence of specific strains, such as the Beijing strain. The study revealed a hitherto unexpected level of functional genetic diversity across the Mtb complex, which belayed previous beliefs that Mtb were a homogeneous and extensively clonal species. Nonetheless, a highly conserved ‘core’ transcriptome induced in resting macrophages was defined that corresponded to 280 genes, including 168 upregulated and 112 downregulated transcripts (Fig. 6). The majority of the upregulated genes were known to associate with stresses such as hypoxia, oxidative and nitrosative stress, cell wall remodeling, and fatty acid metabolism. The downregulated genes were associated with cessation of growth and reduced bacterial metabolism. Approximately 40% of the genes that constituted the ‘core’ intracellular transcriptome were genes of unknown function, which might now merit increased study. Also of interest was the comparison of the conserved transcriptome between Mtb in resting and activated macrophages. Fifty-seven genes were upregulated preferentially in resting compared to activated macrophages. Many of these genes had a clear association with growth such as ribosomal proteins and histones, and iron-storage proteins. In addition, several of the chaperones normally associated with stress were upregulated, implying that these proteins were required to sustain growth inside the macrophage rather than simply resistance to stress.
Fig. 6. The ‘universal’ intracellular transcriptome of Mtb.
The MTC universal intracellular transcriptome: identification of genes with conserved expression and regulation inside macrophage phagosome across global panel of MTC clinical isolates. Global gene expression profiles of 17 MTC strains 24h post-infection of resting and activated macrophages were determined by microarray. Normalized expression ratios for each strain were determined by comparison of RNA from intracellular bacteria versus control bacteria of the same strain treated identically except for phagocytosis by macrophages. This serves to identify relative responses to phagosomal cues rather than inherent strain-dependent differences in gene expression. (A-B) Venn diagrams showing activation-dependent (red=activated, blue=resting) and independent (black) genes with conserved expression patterns across clinical isolates. ‘Universal genes’ were selected based on trending up or down in all strains (up or down >1.2-fold in 15 of 17 strains) and significant induction or repression in >50% of strains (up or down >1.5X in 8 of 17 strains) in each macrophage type. The starting gene list for this analysis included only genes flagged as present in the majority of samples from both macrophage types. (C) Select genes with higher expression levels in activated versus resting macrophages conserved across all or most clinical isolates. Represents subset of activation-dependent genes identified by one-way ANOVA analysis of all intracellular transcription profiles (Benjamini and Hochberg False Discovery Rate p<0.01). (D) Select genes with higher expression in resting versus activated macrophages. See (C) above for analysis description. Refer to Fig. 1B for genotype color bar definition (black box denotes reference strain CDC1551). Reproduced from (84).
These studies exploit Mtb as a probe for the environment inside the phagosome of the macrophage. Interpretation is complex, yet the information return from an unbiased approach such as this continues to be extremely informative. When placed in the context of more traditional cell biological studies, we feel that the two datasets afford tremendous complementation in unraveling the physiology of this compartment.
Bacterial nutrition and the fight for food
It has long been appreciated the Mtb must realign its metabolism upon infecting its host. Bacterial mutants defective in several genes involved in lipid processing and metabolism have been shown to have defects in murine hosts, while showing no defects when grown in rich bacterial broth. Although the underlying mechanisms behind the phenotypes of these mutants may vary from simple nutrient acquisition, through the accumulation of toxic intermediates, to the need to modulate the lipid components of the bacterial cell wall, they all emphasize the critical importance the lipid metabolism has for intracellular Mtb. In previous studies, isocitrate lyase (icl1) was found to be necessary for sustained survival in macrophages and mice in the face of an intact immune response that was capable of activating the host cell (85). ICL1 mediates the flux of carbon into the glyoxylate cycle, through the diversion of isocitrate away from the Kreb’s cycle into the generation of pyruvate and possibly feeds into gluconeogenesis. The glyoxylate cycle enables the retention of carbon when an organism is growing on fatty acids as its primary carbon source. More recently, ICL1 and ICL2 have also been shown to play a role both in vitro and in vivo in the 2-methylcitrate cycle (86, 87). C3 compounds such as propionate or propionyl-Co are products of the metabolism of cholesterol, methyl-branched fatty acids and amino acids, and odd-chain-length fatty acids. The accumulation of these compounds is toxic to Mtb unless they can be degraded or incorporated into inert polymers (88) (Fig. 7). The 2-methylcitrate cycle condenses propionyl-CoA with oxaloacetate to produce pyruvate and succinate, which is an intermediate in the Kreb’s cycle. In the context of 2-methylcitrate cycle, ICL1 and ICL2 operate as methylisocitrate lyases (87). Therefore, in addition to functioning in the glyoxylate cycle, ICL1/2 also appear important for relieving the toxicity caused by accumulation of propionyl-CoA.
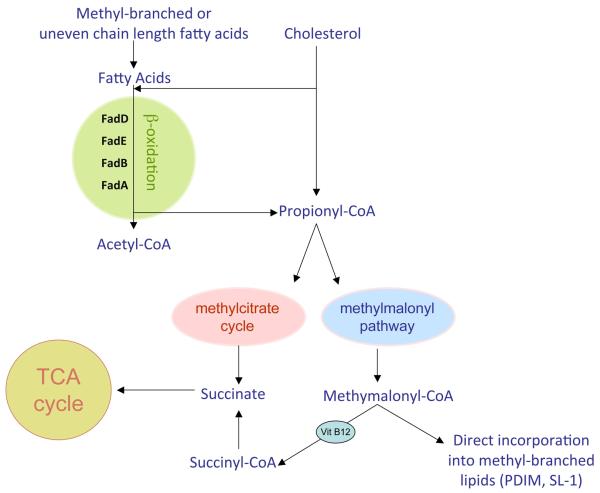
Fig. 7. Outline of the pathways relevant to C3 metabolism.
The metabolism of cholesterol, methyl-branched fatty acids and odd-chain length lipids will raise the intracellular levels of the C3 compounds propionate or propionyl-CoA, which Mtb finds highly toxic. The bacterium has developed three different strategies to detoxify propionyl-CoA. Isocitrate lyase activity has been suborned to fulfill the function of methylcitrate lyase in the last step of the methyl citrate cycle to generate the TCA cycle intermediate succinate. The methylmalonyl pathway has also been mobilized to metabolize propionyl-CoA to produce succinyl-CoA via the VitB12-dependent activity of methylmalonyl-CoA mutase. Finally, intermediates from the methylmalonyl pathway can be incorporated directly into the abundant, methyl-branched lipids of the bacterial cell wall, such as PDIM and SL-1. Reproduced from (129).
Detoxification of propionate and propionyl-CoA appears to present a significant issue for intracellular Mtb. In addition to the mobilization of the 2-methylcitrate cycle, the bacterium also subverts methylmalonyl-CoA intermediates to fulfill a comparable function. Mtb is capable of converting propionyl-CoA to methylmalonyl-CoA. The last step of the methylmalonyl pathway is the generation of succinyl-CoA from methylmalonyl-CoA, a conversion that is performed by the VitB12-dependent enzyme methylmalonyl-CoA mutase (MutAB) (88). Through the activation of the methylmalonyl pathway, Mtb is able to grow on propionate even in the presence of inhibitors of ICL activity. However, this requires supplementation of the culture medium with VitB12, because methylmalonyl-CoA mutase, which is the last enzyme of the methylmalonyl pathway, requires VitB12 as a cofactor. In addition to the generation of succinyl-CoA, intermediates from the methylcitrate pathway can be incorporated into methyl-branched fatty acids, thus detoxifying them through their incorporation into the cell wall. Mtb are known to synthesize many complex lipids containing methyl-branched fatty acid side chains that are integral to the cell wall (89). It was demonstrated recently that Mtb grown on propionate exhibit enhanced synthesis of the virulence-associated methyl-branched lipids sulfolipid (SL-1) and phthiocerol dimycocerosates (PDIM) (90). Furthermore, when Mtb was grown on propionate or odd chain length fatty acids, the mycocerosic acids in PDIM increased their length by 3-carbons. This observation suggests that C3 compounds or methylmalonyl-CoA intermediates can be incorporated directly into PDIM. Finally, PDIM purified from Mtb isolated from mouse lung tissue synthesized mycocerosic acids with similarly extended carbon chains indicating that this buildup of propionyl-CoA was not merely an in vitro artifact but was also an issue during in vivo infection.
Although we have little direct knowledge of the range of carbon sources utilized by Mtb during infection, there is genetic evidence that Mtb can access and metabolize cholesterol from the host (91). Mce4 encodes a bacterial cholesterol transporter (91, 92), and Mtb deficient in this gene demonstrate an inability to sustain a persistent infection in mice and in IFN-γ-activated macrophages. These data indicate that cholesterol is exploited by Mtb as a carbon source during certain phases of infection. The phenotype of the mce4-deficient mutant is similar to the icl1-deficient mutant (85), which also showed impaired survival in the face of a functional immune response, ie. later in the infection process.
Singh and colleagues (93) reported recently that Mtb also ameliorates redox fluctuations by selectively incorporating propionate into cell wall lipids such as polyacyltrehalose (PAT), sulfolipids (SL-1), phthiocerol dimycocerosate (PDIM), and triacylglycerol (TAG). This utilization of propionate was observed in murine macrophage infections in vitro and was controlled by the transcriptional regulator WhiB3. WhiB3 is a transcriptional regulator that contains an iron-sulfur cluster and is regulated by a thiol-disulfide redox switch. A mutant deficient in WhiB3 is resistant to propionate toxicity and exhibits increased levels of PDIM both in broth culture and within resting macrophages. Previous transcriptional profiling of Mtb in macrophages demonstrated upregulation in expression of WhiB3, as well as genes involved in the synthesis of PAT, SL-1, and TAG (16), providing further evidence that Mtb channels C3-containing compounds to its cell wall lipids as a means of detoxification, and that this pressure is felt acutely by the bacterium inside its host cell.
Inside-out signaling and control of the external environment
Mtb resides inside the vacuole that fails to fuse within lysosomes. But, this compartment is neither inert nor sequestered from the normal membrane-trafficking machinery inside the cell. Materials that are released by the bacterium, including both proteins and lipids, traffic actively through the infected cell (76-78). Immunoelectron microscopical studies revealed the accumulation of Mtb-derived components within the internal vesicles in multi-vesicular bodies. These compartments are also rich in MHC class II molecules and resemble the MIIC compartments that immunologists believe are the major site of loading of antigen into MHC class II molecules. However, these bacterial constituents fulfill other functions that extend beyond their antigenic properties.
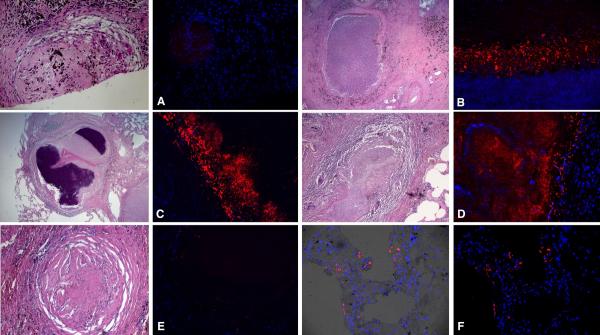
The mycobacterial lipids released by intracellular Mtb include most of the peripheral cell lipids such as trehalose monomycolate and dimycolate (TMM, TDM) phosphatidylinositol mannoside (PIMs), LAM, and PGL-1 (94). Interestingly, the vesicles into which these bacterial lipids incorporate can be exocytosed in a calcium-dependent manner by infected macrophages (78). In mixed cultures containing both infected macrophages and CellTracker dye-labeled uninfected macrophages, the Mtb cell wall lipid-containing vesicles can be shown to transfer between cells (76) (Fig. 8). More recently, van den Elzen and colleagues (95) confirmed this observation and demonstrated that cell-to-cell transfer of mycobacterial lipids could also be mediated by host apolipoprotein E. Winau and colleagues (96) reported that apoptosis of infected macrophages resulted in release of vesicles that contained Mtb-derived antigens. All of these processes enable Mtb antigens to be taken up and presented by bystander antigen-presenting cells. But, in addition to their antigenic properties, many of these cell wall lipids are known to be potent modulators of cell function, so this cell-cell transfer activity could benefit Mtb by extending its sphere of influence beyond its own host cell. Rhoades and colleagues (97) reported the development of an in vivo model to probe the bioactivity of these released cell wall components. In brief, large, 90 μm diameter polystyrene beads were coated with Mtb lipids, either individually or in combination, mixed with Matrigel and were inoculated either intraperitoneally or subcutaneously into recipient mice. The resultant experimental granuloma was isolated and analyzed for cell content, cytokine production, and histology. Head-to-head comparison of bacterial lipids demonstrated that the mycolates TDM and TMM were the most potent, biologically active of the released lipids (98). The response was abrogated in MyD88-deficient mice, indicating that the process was dependent on innate immune recognition. The granuloma induced by these lipids shared many histological features with human TB granulomas including neovascularization, fibrosis, the generation of multinucleate giant cells, epithelioid macrophages and foamy macrophages (Fig. 9). This potent biological activity could explain why the tissue response to infection in humans is so extreme, even at low apparent bacterial burden.
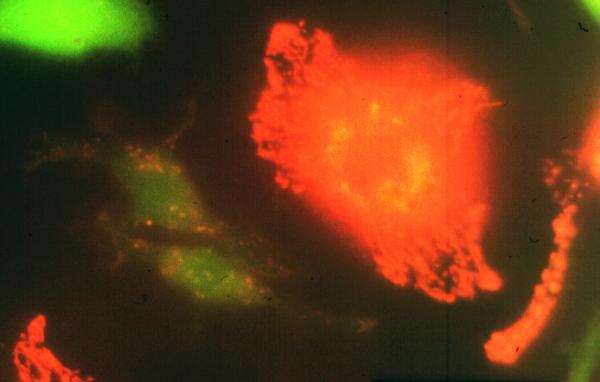
Fig. 8. Mtb-derived cell wall lipids transfer from infected to uninfected macrophages.
Transfer of mycobacterial material from BCG-infected macrophages to uninfected bystanders following overnight incubation. BMMØ infected with Texas Red hydrazide-labeled BCG were incubated with an equal number of uninfected macrophages labeled with chloromethylfluorescein diacetate. Uninfected bystander cells (green fluorescence) acquired Texas Red-labeled mycobacterial material from BCG-infected macrophages. Reproduced from (76).
Fig. 9. Cells associated with BCG lipid-coated beads.
Matrices containing BMMØ and BCG lipid-coated beads were injected i.p. and recovered at 14 h (A), 4 days (B) and 12 days (C - F). (A): Neutrophils (blue arrows) were present in spaces that had been occupied by beads after 14 hr. Scale bar, 35 mm. (B): A diverse infiltrate of macrophages (black arrows), neutrophils (blue arrow), eosinophils (orange arrow) and lymphocytes (small cells with little cytoplasm) accumulated at beads at 4 days. Scale bar, 25 mm. (C): A dense cellular infiltrate composed of mononuclear and polymorphonuclear leukocytes and lymphocytes, including plasma cells (black arrow) formed at 12 days. Infiltrates were especially florid in spaces between lipid-coated beads. Scale bar, 35 mm. (D): Epithelioid macrophages and occasional neutrophils (blue arrow) typically adhered to beads. Scale bar represents 50 mm. (E): Multi-nucleated giant cell in association with a bead at 12 days. Scale bar, 35 mm. (F): Fibrotic material (dark blue-staining material) was deposited in the dense aggregates of leukocytes at 12 days. The uniformly staining blue material in the lower left corner of the panel is the collagen in the Matrigel. Scale bar, 35 mm. Representative H & E-stained sections (A – E) and a trichrome-stained section (F) are shown. Reproduced from (97).
Consistent with the lack of response to TDM observed in MyD88-deficient mice, Bowdish and colleagues (99) demonstrated that TDM is recognized by the scavenger receptor MARCO, which forms a signaling complex with the Toll-like receptor 2 (TLR2). More recently Mincle has been reported as another cellular receptor for TDM (100, 101), but it is unclear if Mincle also generates a functional signaling complex that activates the innate immune response in a manner comparable to MARCO.
Modulation of the granuloma from within the host cell
The active release of cell wall components by Mtb is an extremely expensive behavior with respect to both carbon and energy utilization; therefore, one has to imagine that this activity has been retained through evolution because it fulfills a purpose important to the success of the infection. To better understand the tissue reaction in active infections, we performed microarray analysis on caseating granulomas isolated by laser-capture microdissection from tissue excised from TB patients (102). The arrays were grounded on the transcriptional profile from neighboring, uninvolved lung tissue. Among many of the themes of interest, we focused initially on the marked re-alignment of host lipid metabolism. Several genes that played key roles in the synthesis, sequestration and processing of lipids, most notably those lipids derived from low-density lipoprotein (LDL) showed marked upregulation in expression in the caseous lesions. We selected 3 key players in these processes for further characterization. The selected genes encode adipophilin (ADFP), acyl Co-A synthase long chain family member 1 (ACSL1), and prosaposin (PSAP), the precursor of SapC. These three proteins play different but central roles in the processing and sequestration of lipids within mammalian cells. ADFP is required for lipid droplet synthesis and is strongly associated with the periphery of these cytoplasmic structures following their formation (103). The increased expression of ADFP enhances sequestration of cholesterol ester (CE), increases synthesis of long-chain fatty acid (LCFA) and triacylglycerol (TAG), and reduces the breakdown of lipid through the β-oxidation pathway (104-106). ACSL1 has also been shown to increase lipid droplet formation through the de novo synthesis of long chain fatty acids (LCFAs) that can be incorporated into TAG (107, 108). Lastly, SapC is essential for the metabolism of sphingolipids, which is required to maintain the balance of lipid species in cellular membranes and is highly upregulated in cells experiencing stress through lipid overload (109, 110).
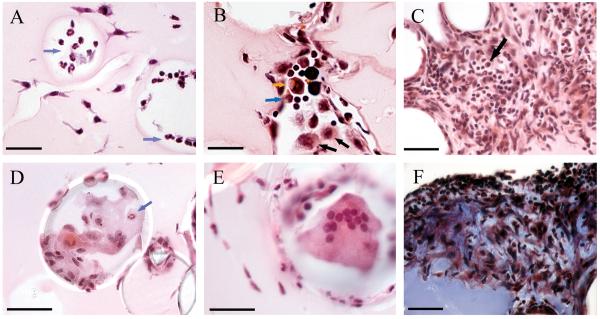
Immunohistological examination of an extensive panel of human TB granulomas in differing stages of development revealed that ADFP and ACSL1 expression correlated tightly with the appearance of caseum in the granuloma center (111) (Fig. 10). These proteins were expressed highly in macrophages subtending the caseous region. In contrast, while SapC was also expressed highly in the same cellular region, it was also abundant in early, or nascent, granulomas prior to the appearance of caseum suggesting that this represented a physiological shift that occurred upstream of caseum formation/accumulation.
Fig. 10. ADFP expression in human TB granulomas.
Immunofluorescence signals were obtained for each granuloma, and a representative image (right) and the corresponding region from an H&E stained slide (left, boxed) are shown. Nuclei are seen in blue and antigens in red. (A). Nascent granulomas exhibit weak ADFP expression. (B). Caseous granulomas stain strongly for ADFP. (C). Fibrocaseous granulomas also label strongly for ADFP expression. (D). The caseous center also has intense ADFP expression, together with nuclear debris. E. Resolved granulomas exhibit low levels of ADFP labeling. F. Control normal lung parenchyma shows ADFP expression in pneumocytes and alveolar macrophages (the left image is a merged image with bright field). Scale bar is 50 mm. Reproduced from (102).
So where does the caseum come from, and what are its major components? Prior to the work of Kim and colleagues, no-one had performed analysis of caseous material. The lipids from isolated caseum were fractionated by thin-layer chromatography, alongside known lipid standards, and their identity was confirmed by mass spectrometry. The major lipid species present in the caseum were cholesterol, CE, TAG, and lactosylceramide (Lac-Cer). All of these lipids are known constituents of LDL, although Lac-Cer is usually a minor constituent of the LDL particle. However, cell synthesis of Lac-Cer is increased markedly in response to sustained stress (112). Mtb has been known for a while to be an active inducer of lipid droplet formation in macrophages, and the primary source of the lipids in the droplets is LDL in the serum. The cholesterol in LDL occurs predominantly in a non-esterified form, and it is only during the passage through the macrophage cytosol and incorporation into droplets formed within the leaflet of the ER membrane that the cholesterol is esterified. Therefore the over-representation of these lipids, and most notably cholesterol in the form of CE, suggests that the most likely source of the lipids in the caseum are the foamy macrophages that lie within the fibrous cuff of the granuloma. We propose that the foamy macrophages become necrotic, die, and release the lipid-rich debris into the granuloma center to become caseum (111, 113).
M. leprae has also been shown to realign the lipid metabolism of its host and induce formation of foamy macrophages (114, 115); however, there is no formation of caseum. Similarly, the mouse is frequently called into question as a model for TB disease, because it fails to generate caseum and a degenerative granuloma that typifies active human tuberculosis. However, we would argue that what differs in these cases may not be the lipid metabolism but the formation of a fibrous capsule that retains the caseous debris. More recently, Hunter and colleagues (116) have demonstrated that secondary granulomas in a reactivation model for TB in mice do form caseous granulomas enclosed within a fibrotic capsule. This is consistent with our model and offers the attractive possibility that this late-stage progression of the TB granuloma could be induced in the murine model, with all the attendant genetic tools.
Another metabolic program that is upregulated in the caseous granuloma, which is likely linked to lipid excess, is the endoplasmic reticulum (ER) stress pathway (117). The ER stress pathway, or unfolded protein response (UPR), is an adaptive survival pathway that is induced when cells experience stress that leads to accumulation of unfolded proteins in the ER (118, 119). However, if the stress is prolonged or extreme in nature, the pathway leads to cell death via apoptosis (120, 121). ER stress-induced apoptosis is dependent on the upregulated expression of C/EBP homologous protein (CHOP) (122, 123). Microarray analysis of human TB granulomas demonstrate marked upregulation of CHOP, as well as ER proteins associated with stress such as calreticulin (CALR), calnexin (CANX), heat shock protein, and the transcription factors XBP1, ATF3, and ATF4 (117). Immunohistological analysis of human TB granulomas with anti-CHOP antibodies demonstrated strong labeling in the macrophages subtending the caseum. This region also labeled intensely with TUNEL stain for apopototic cells. Comparable labeling was seen in Mtb-infected mice. The UPR stress pathway has been observed in atherosclerotic lesions (124), which show a similar accumulation of foamy macrophages. It is therefore possible that the TB-induced lipid sequestration also drives the cell death that releases these lipids into the caseous center of the granuloma.
Foamy macrophage generation is a pathogen-mediated phenomenon induced by cell wall lipids, including TDM
To complete the argument, it was necessary to revisit the earlier studies of Peyron and D’Avila (125, 126), who reported induction of foamy macrophages by Mtb and BCG in culture to demonstrate that this phenomenon could be induced by the cell wall lipids released by intracellular Mtb. Isolated TDM was coated onto polystyrene beads and inoculated into mice in the artificial granuloma model detailed earlier (97, 111). Histological examination of the induced granuloma demonstrated abundant lipid droplets and markedly enhanced expression of ADFP, neither of which was observed in the granulomas induced by beads coated in phosphatidylglycerol. These data provide strong evidence that development of the caseous center of the granuloma is a pathogen-mediated process (Fig. 12).
Caseum formation and accumulation could benefit Mtb in two ways (81, 113). Firstly, the successful completion of the pathogen’s life cycle requires the liquification of the granuloma and the release of infectious bacilli into the airways. The induction of foam cell formation and accumulation of the caseum likely contributes to the increased tissue pathology. This pathogen-induced response turns the host’s innate immune response against itself via a process that is exacerbated by the presence of a robust acquired immune response, which will amplify the inflammatory process. Secondly, histological studies of human TB report abundant extracellular bacteria in active, cavitated lesions. These bacteria may be supported by the lipid-rich milieu of the caseum. Mtb metabolizes cholesterol and TAG, and bacilli isolated from the sputum of human TB patients show marked accumulation of intracellular TAG (127, 128), so these lipids may provide an accessible energy source for Mtb during the infection cycle.
Concluding remarks: from the cellular to the organismal level
This article was supposed to focus on the cell biology of Mtb infection; however, it is difficult to discuss the cellular aspects of a pathogen if you do not delve into the relevance of these findings at the level of disease. Mtb manipulates its host cell to enable its survival within that cell, but it does much more. We propose that it has hijacked the normal housekeeping properties of that cell to traffic extremely potent, Mtb-derived biological mediators that act, much-like evangelists, to spread the word to neighboring tissues. The cell-based infection sustains a tissue response that becomes the granuloma. Each granuloma behaves like an individual entity, and it is the interplay between the granuloma and the host’s immune response that determines whether each individual granuloma will resolve or progress to active disease.
The field of tuberculosis research has progressed enormously over the past few years. We now have the capacity to pose questions locally and analyze answers globally. Our ability to determine the establish links between cellular events and systemic disease is expanding constantly, making this a fascinating time to work in the field.
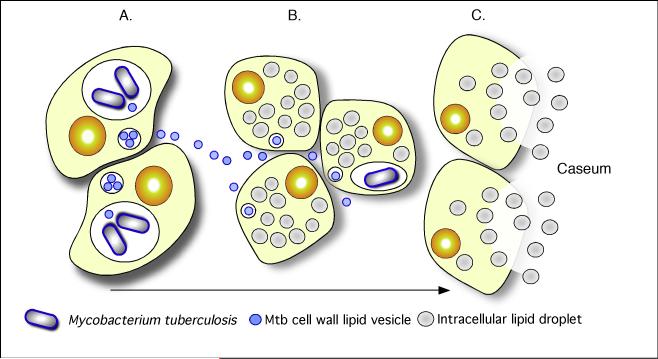
Fig. 11. A model illustrating the linkages between Mtb-infection, foam cell formation and accumulation of caseum in the human TB granuloma.
(A). Intracellular Mtb bacilli synthesize and release cell wall components inside their host cells. We have demonstrated previously that these lipids accumulate in the internal vesicles in multi-vesicular bodies, which are exocytosed from the cell in vesicular form (B). Because of the release of these vesicles, both infected and uninfected macrophages are exposed to cell wall mycolates and induced to form foamy macrophages, as illustrated in Fig. 7. The foamy macrophages have been shown to support the maintenance and growth of persistent bacteria (C). We now propose that these cells die via an inflammatory, necrotic process and release their lipid droplets into the extracellular milieu within the granuloma. As a result of the fibrotic capsule, the human granuloma is an enclosed, isolated structure with minimal vasculature. The enclosed nature of the human granuloma leads to accumulation of necrotic debris as caseum. In this model, this process is an integral part of the pathology that leads to active disease and transmission. Reproduced from (102).
Acknowledgements
This work was supported by USPHS grants AI067027, AI057086, HL055936, AI 080651 and HL100928 to DGR and by funds from the Bill and Melinda Gates Foundation.
References
- 1.Russell DG, Barry CE, 3rd, Flynn JL. Tuberculosis: what we don’t know can, and does, hurt us. Science. 2010;328:852–856. doi: 10.1126/science.1184784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardoso CM, Jordao L, Vieira OV. Rab10 regulates phagosome maturation and its overexpression rescues Mycobacterium-containing phagosomes maturation. Traffic. 2010;11:221–235. doi: 10.1111/j.1600-0854.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 3.Philips JA, Porto MC, Wang H, Rubin EJ, Perrimon N. ESCRT factors restrict mycobacterial growth. Proc Natl Acad Sci USA. 2008;105:3070–3075. doi: 10.1073/pnas.0707206105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts EA, Chua J, Kyei GB, Deretic V. Higher order Rab programming in phagolysosome biogenesis. J Cell Biol. 2006;174:923–929. doi: 10.1083/jcb.200603026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts EA, Deretic V. The Mycobacterium tuberculosis phagosome. Methods Mol Biol. 2008;445:439–449. doi: 10.1007/978-1-59745-157-4_28. [DOI] [PubMed] [Google Scholar]
- 6.Seto S, Matsumoto S, Ohta I, Tsujimura K, Koide Y. Dissection of Rab7 localization on Mycobacterium tuberculosis phagosome. Biochem Biophys Res Commun. 2009;387:272–277. doi: 10.1016/j.bbrc.2009.06.152. [DOI] [PubMed] [Google Scholar]
- 7.Seto S, Matsumoto S, Tsujimura K, Koide Y. Differential recruitment of CD63 and Rab7-interacting-lysosomal-protein to phagosomes containing Mycobacterium tuberculosis in macrophages. Microbiol Immunol. 2010;54:170–174. doi: 10.1111/j.1348-0421.2010.00199.x. [DOI] [PubMed] [Google Scholar]
- 8.Sweet L, Singh PP, Azad AK, Rajaram MV, Schlesinger LS, Schorey JS. Mannose receptor-dependent delay in phagosome maturation by Mycobacterium avium glycopeptidolipids. Infect Immun. 2010;78:518–526. doi: 10.1128/IAI.00257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergne I, Chua J, Singh SB, Deretic V. Cell biology of mycobacterium tuberculosis phagosome. Annu Rev Cell Dev Biol. 2004;20:367–394. doi: 10.1146/annurev.cellbio.20.010403.114015. [DOI] [PubMed] [Google Scholar]
- 10.Sturgill-Koszycki S, Schaible UE, Russell DG. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 1996;15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- 11.Ullrich HJ, Beatty WL, Russell DG. Direct delivery of procathepsin D to phagosomes: implications for phagosome biogenesis and parasitism by Mycobacterium. Eur J Cell Biol. 1999;78:739–748. doi: 10.1016/S0171-9335(99)80042-9. [DOI] [PubMed] [Google Scholar]
- 12.Russell DG, Vanderven BC, Glennie S, Mwandumba H, Heyderman RS. The macrophage marches on its phagosome: dynamic assays of phagosome function. Nat Rev. 2009;9:594–600. doi: 10.1038/nri2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.VanderVen BC, Yates RM, Russell DG. Intraphagosomal measurement of the magnitude and duration of the oxidative burst. Traffic. 2009;10:372–378. doi: 10.1111/j.1600-0854.2009.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yates RM, Hermetter A, Russell DG. The kinetics of phagosome maturation as a function of phagosome/lysosome fusion and acquisition of hydrolytic activity. Traffic. 2005;6:413–420. doi: 10.1111/j.1600-0854.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 15.Yates RM, Hermetter A, Taylor GA, Russell DG. Macrophage activation downregulates the degradative capacity of the phagosome. Traffic. 2007;8:241–250. doi: 10.1111/j.1600-0854.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 16.Rohde KH, Abramovitch RB, Russell DG. Mycobacterium tuberculosis invasion of macrophages: linking bacterial gene expression to environmental cues. Cell Host Microbe. 2007;2:352–364. doi: 10.1016/j.chom.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Schnappinger D, et al. Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J Exp Med. 2003;198:693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waddell SJ, Butcher PD. Microarray analysis of whole genome expression of intracellular Mycobacterium tuberculosis. Curr Mol Med. 2007;7:287–296. doi: 10.2174/156652407780598548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: aging gracefully. Biochem J. 2002;366:689–704. doi: 10.1042/BJ20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desjardins M. The good fat: a link between lipid bodies and antigen cross-presentation. Immunity. 2009;31:176–178. doi: 10.1016/j.immuni.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Desjardins M, Houde M, Gagnon E. Phagocytosis: the convoluted way from nutrition to adaptive immunity. Immunol Rev. 2005;207:158–165. doi: 10.1111/j.0105-2896.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 22.Stuart LM, et al. A systems biology analysis of the Drosophila phagosome. Nature. 2007;445:95–101. doi: 10.1038/nature05380. [DOI] [PubMed] [Google Scholar]
- 23.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 24.Trost M, English L, Lemieux S, Courcelles M, Desjardins M, Thibault P. The phagosomal proteome in interferon-gamma-activated macrophages. Immunity. 2009;30:143–154. doi: 10.1016/j.immuni.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Yates RM, Russell DG. Phagosome maturation proceeds independently of stimulation of toll-like receptors 2 and 4. Immunity. 2005;23:409–417. doi: 10.1016/j.immuni.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 26.El-Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med. 2009;41:217–225. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson JM. Reactive oxygen species in phagocytic leukocytes. Histochem Cell Biol. 2008;130:281–297. doi: 10.1007/s00418-008-0461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 29.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 30.Gomes MS, Appelberg R. NRAMP1- or cytokine-induced bacteriostasis of Mycobacterium avium by mouse macrophages is independent of the respiratory burst. Microbiol. 2002;148:3155–3160. doi: 10.1099/00221287-148-10-3155. [DOI] [PubMed] [Google Scholar]
- 31.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spagnolo L, et al. Unique features of the sodC-encoded superoxide dismutase from Mycobacterium tuberculosis, a fully functional copper-containing enzyme lacking zinc in the active site. J Biol Chem. 2004;279:33447–33455. doi: 10.1074/jbc.M404699200. [DOI] [PubMed] [Google Scholar]
- 33.Dussurget O, Stewart G, Neyrolles O, Pescher P, Young D, Marchal G. Role of Mycobacterium tuberculosis copper-zinc superoxide dismutase. Infect Immun. 2001;69:529–533. doi: 10.1128/IAI.69.1.529-533.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piddington DL, Fang FC, Laessig T, Cooper AM, Orme IM, Buchmeier NA. Cu,Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect Immun. 2001;69:4980–4987. doi: 10.1128/IAI.69.8.4980-4987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan J, et al. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc Natl Acad Sci USA. 1989;86:2453–2457. doi: 10.1073/pnas.86.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hackam DJ, et al. Regulation of phagosomal acidification. Differential targeting of Na+/H+ exchangers, Na+/K+-ATPases, and vacuolar-type H+-atpases. J Biol Chem. 1997;272:29810–29820. doi: 10.1074/jbc.272.47.29810. [DOI] [PubMed] [Google Scholar]
- 37.Sturgill-Koszycki S, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 38.Mwandumba HC, et al. Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J Immunol. 2004;172:4592–4598. doi: 10.4049/jimmunol.172.7.4592. [DOI] [PubMed] [Google Scholar]
- 39.Heeren J, Grewal T, Jackle S, Beisiegel U. Recycling of apolipoprotein E and lipoprotein lipase through endosomal compartments in vivo. J Biol Chem. 2001;276:42333–42338. doi: 10.1074/jbc.M107461200. [DOI] [PubMed] [Google Scholar]
- 40.Sugii S, Reid PC, Ohgami N, Du H, Chang TY. Distinct endosomal compartments in early trafficking of low density lipoprotein-derived cholesterol. J Biol Chem. 2003;278:27180–27189. doi: 10.1074/jbc.M300542200. [DOI] [PubMed] [Google Scholar]
- 41.Verges M, Bensadoun A, Herz J, Belcher JD, Havel RJ. Endocytosis of hepatic lipase and lipoprotein lipase into rat liver hepatocytes in vivo is mediated by the low density lipoprotein receptor-related protein. J Biol Chem. 2004;279:9030–9036. doi: 10.1074/jbc.M312908200. [DOI] [PubMed] [Google Scholar]
- 42.Fratti RA, Chua J, Vergne I, Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc Natl Acad Sci USA. 2003;100:5437–5442. doi: 10.1073/pnas.0737613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayakawa E, Tokumasu F, Nardone GA, Jin AJ, Hackley VA, Dvorak JA. A Mycobacterium tuberculosis-derived lipid inhibits membrane fusion by modulating lipid membrane domains. Biophys J. 2007;93:4018–4030. doi: 10.1529/biophysj.107.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vergne I, Chua J, Deretic V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J Exp Med. 2003;198:653–659. doi: 10.1084/jem.20030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson N, Wolke M, Ernestus K, Plum G. A mycobacterial gene involved in synthesis of an outer cell envelope lipid is a key factor in prevention of phagosome maturation. Infect Immun. 2007;75:581–591. doi: 10.1128/IAI.00997-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mann FM, Xu M, Chen X, Fulton DB, Russell DG, Peters RJ. Edaxadiene: a new bioactive diterpene from Mycobacterium tuberculosis. J Am CHem Soc. 2009;131:17526–17527. doi: 10.1021/ja9019287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mann FM, Prisic S, Hu H, Xu M, Coates RM, Peters RJ. Characterization and inhibition of a class II diterpene cyclase from Mycobacterium tuberculosis: implications for tuberculosis. J Biol Chem. 2009;284:23574–23579. doi: 10.1074/jbc.M109.023788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Axelrod S, et al. Delay of phagosome maturation by a mycobacterial lipid is reversed by nitric oxide. Cell Microbiol. 2008;10:1530–1545. doi: 10.1111/j.1462-5822.2008.01147.x. [DOI] [PubMed] [Google Scholar]
- 49.Indrigo J, Hunter RL, Jr., Actor JK. Cord factor trehalose 6,6′-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiol. 2003;149:2049–2059. doi: 10.1099/mic.0.26226-0. [DOI] [PubMed] [Google Scholar]
- 50.Vergne I, Chua J, Deretic V. Mycobacterium tuberculosis phagosome maturation arrest: selective targeting of PI3P-dependent membrane trafficking. Traffic. 2003;4:600–606. doi: 10.1034/j.1600-0854.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 51.Vergne I, Chua J, Lee HH, Lucas M, Belisle J, Deretic V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2005;102:4033–4038. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol. 1998;160:1290–1296. [PubMed] [Google Scholar]
- 53.Via LE, Fratti RA, McFalone M, Pagan-Ramos E, Deretic D, Deretic V. Effects of cytokines on mycobacterial phagosome maturation. J Cell Sci. 1998;111:897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]
- 54.Walburger A, et al. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science. 2004;304:1800–1804. doi: 10.1126/science.1099384. [DOI] [PubMed] [Google Scholar]
- 55.Cowley S, et al. The Mycobacterium tuberculosis protein serine/threonine kinase PknG is linked to cellular glutamate/glutamine levels and is important for growth in vivo. Mol Microbiol. 2004;52:1691–1702. doi: 10.1111/j.1365-2958.2004.04085.x. [DOI] [PubMed] [Google Scholar]
- 56.McDonough KA, Kress Y, Bloom BR. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun. 1993;61:2763–2773. doi: 10.1128/iai.61.7.2763-2773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Wel N, et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 58.Hagedorn M, Rohde KH, Russell DG, Soldati T. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science. 2009;323:1729–1733. doi: 10.1126/science.1169381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 60.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaufmann SH, Cole ST, Mizrahi V, Rubin E, Nathan C. Mycobacterium tuberculosis and the host response. J Exp Med. 2005;201:1693–1697. doi: 10.1084/jem.20050842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearce MJ, Arora P, Festa RA, Butler-Wu SM, Gokhale RS, Darwin KH. Identification of substrates of the Mycobacterium tuberculosis proteasome. EMBO J. 2006;25:5423–5432. doi: 10.1038/sj.emboj.7601405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Darwin KH, Lin G, Chen Z, Li H, Nathan CF. Characterization of a Mycobacterium tuberculosis proteasomal ATPase homologue. Mol Microbiol. 2005;55:561–571. doi: 10.1111/j.1365-2958.2004.04403.x. [DOI] [PubMed] [Google Scholar]
- 66.Darwin KH, Ehrt S, Gutierrez-Ramos JC, Weich N, Nathan CF. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science. 2003;302:1963–1966. doi: 10.1126/science.1091176. [DOI] [PubMed] [Google Scholar]
- 67.Deretic V, et al. Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism. Cell Microbiol. 2006;8:719–727. doi: 10.1111/j.1462-5822.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- 68.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 69.Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313:1438–1441. doi: 10.1126/science.1129577. [DOI] [PubMed] [Google Scholar]
- 70.Alonso S, Pethe K, Russell DG, Purdy GE. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc Natl Acad Sci USA. 2007;104:6031–6036. doi: 10.1073/pnas.0700036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Purdy GE, Niederweis M, Russell DG. Decreased outer membrane permeability protects mycobacteria from killing by ubiquitin-derived peptides. Mol Microbiol. 2009;73:844–857. doi: 10.1111/j.1365-2958.2009.06801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ponpuak M, et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity. 2010;32:329–341. doi: 10.1016/j.immuni.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Busch R, Doebele RC, Patil NS, Pashine A, Mellins ED. Accessory molecules for MHC class II peptide loading. Curr Opin Immunol. 2000;12:99–106. doi: 10.1016/s0952-7915(99)00057-6. [DOI] [PubMed] [Google Scholar]
- 74.Jensen PE, Weber DA, Thayer WP, Chen X, Dao CT. HLA-DM and the MHC class II antigen presentation pathway. Immunol Res. 1999;20:195–205. doi: 10.1007/BF02790403. [DOI] [PubMed] [Google Scholar]
- 75.Ullrich HJ, Beatty WL, Russell DG. Interaction of Mycobacterium avium-containing phagosomes with the antigen presentation pathway. J Immunol. 2000;165:6073–6080. doi: 10.4049/jimmunol.165.11.6073. [DOI] [PubMed] [Google Scholar]
- 76.Beatty WL, Rhoades ER, Ullrich HJ, Chatterjee D, Heuser JE, Russell DG. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1:235–247. doi: 10.1034/j.1600-0854.2000.010306.x. [DOI] [PubMed] [Google Scholar]
- 77.Beatty WL, Russell DG. Identification of mycobacterial surface proteins released into subcellular compartments of infected macrophages. Infect Immun. 2000;68:6997–7002. doi: 10.1128/iai.68.12.6997-7002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beatty WL, Ullrich HJ, Russell DG. Mycobacterial surface moieties are released from infected macrophages by a constitutive exocytic event. Eur J Cell Biol. 2001;80:31–40. doi: 10.1078/0171-9335-00131. [DOI] [PubMed] [Google Scholar]
- 79.Singh CR, et al. Processing and presentation of a mycobacterial antigen 85B epitope by murine macrophages is dependent on the phagosomal acquisition of vacuolar proton ATPase and in situ activation of cathepsin D. J Immunol. 2006;177:3250–3259. doi: 10.4049/jimmunol.177.5.3250. [DOI] [PubMed] [Google Scholar]
- 80.Kaufmann SH, Schaible UE. Antigen presentation and recognition in bacterial infections. Curr Opin Immunol. 2005;17:79–87. doi: 10.1016/j.coi.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 81.Russell DG. Who puts the tubercle in tuberculosis? Nature reviews. 2007;5:39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 82.Rohde K, Yates RM, Purdy GE, Russell DG. Mycobacterium tuberculosis and the environment within the phagosome. Immunol Rev. 2007;219:37–54. doi: 10.1111/j.1600-065X.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 83.Schnappinger D, Schoolnik GK, Ehrt S. Expression profiling of host pathogen interactions: how Mycobacterium tuberculosis and the macrophage adapt to one another. Microbes Infect. 2006;8:1132–1140. doi: 10.1016/j.micinf.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 84.Homolka S, Niemann S, Russell DG, Rohde KH. Functional genetic diversity among Mycobacterium tuberculosis complex clinical isolates: delineation of conserved core and lineage-specific transcriptomes during intracellular survival. PLoS Pathogens. 2010;6:e1000988. doi: 10.1371/journal.ppat.1000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McKinney JD, et al. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406:735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- 86.Munoz-Elias EJ, McKinney JD. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat Med. 2005;11:638–644. doi: 10.1038/nm1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Munoz-Elias EJ, Upton AM, Cherian J, McKinney JD. Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol Microbiol. 2006;60:1109–1122. doi: 10.1111/j.1365-2958.2006.05155.x. [DOI] [PubMed] [Google Scholar]
- 88.Savvi S, Warner DF, Kana BD, McKinney JD, Mizrahi V, Dawes SS. Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: implications for propionate metabolism during growth on fatty acids. J Bacteriol. 2008;190:3886–3895. doi: 10.1128/JB.01767-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jackson M, Stadthagen G, Gicquel B. Long-chain multiple methyl-branched fatty acid-containing lipids of Mycobacterium tuberculosis: biosynthesis, transport, regulation and biological activities. Tuberculosis. 2007;87:78–86. doi: 10.1016/j.tube.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 90.Jain M, et al. Lipidomics reveals control of Mycobacterium tuberculosis virulence lipids via metabolic coupling. Proc Natl Acad Sci USA. 2007;104:5133–5138. doi: 10.1073/pnas.0610634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci USA. 2008;105:4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mohn WW, et al. The actinobacterial mce4 locus encodes a steroid transporter. J Biol Chem. 2008;283:35368–35374. doi: 10.1074/jbc.M805496200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh A, et al. Mycobacterium tuberculosis WhiB3 maintains redox homeostasis by regulating virulence lipid anabolism to modulate macrophage response. PLoS Pathogens. 2009;5:e1000545. doi: 10.1371/journal.ppat.1000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rhoades E, Hsu F, Torrelles JB, Turk J, Chatterjee D, Russell DG. Identification and macrophage-activating activity of glycolipids released from intracellular Mycobacterium bovis BCG. Mol Microbiol. 2003;48:875–888. doi: 10.1046/j.1365-2958.2003.03473.x. [DOI] [PubMed] [Google Scholar]
- 95.van den Elzen P, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437:906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- 96.Winau F, et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 97.Rhoades ER, Geisel RE, Butcher BA, McDonough S, Russell DG. Cell wall lipids from Mycobacterium bovis BCG are inflammatory when inoculated within a gel matrix: characterization of a new model of the granulomatous response to mycobacterial components. Tuberculosis. 2005;85:159–176. doi: 10.1016/j.tube.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 98.Geisel RE, Sakamoto K, Russell DG, Rhoades ER. In vivo activity of released cell wall lipids of Mycobacterium bovis bacillus Calmette-Guerin is due principally to trehalose mycolates. J Immunol. 2005;174:5007–5015. doi: 10.4049/jimmunol.174.8.5007. [DOI] [PubMed] [Google Scholar]
- 99.Bowdish DM, et al. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathogens. 2009;5:e1000474. doi: 10.1371/journal.ppat.1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ishikawa E, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matsunaga I, Moody DB. Mincle is a long sought receptor for mycobacterial cord factor. J Exp Med. 2009;206:2865–2868. doi: 10.1084/jem.20092533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim MJ, et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med. 2010;2:258–274. doi: 10.1002/emmm.201000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Robenek H, et al. Lipid droplets gain PAT family proteins by interaction with specialized plasma membrane domains. J Biol Chem. 2005;280:26330–26338. doi: 10.1074/jbc.M413312200. [DOI] [PubMed] [Google Scholar]
- 104.Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chang BH, Chan L. Regulation of Triglyceride Metabolism. III. Emerging role of lipid droplet protein ADFP in health and disease. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1465–1468. doi: 10.1152/ajpgi.00566.2006. [DOI] [PubMed] [Google Scholar]
- 106.Larigauderie G, et al. Adipophilin enhances lipid accumulation and prevents lipid efflux from THP-1 macrophages: potential role in atherogenesis. Arterioscler Thromb Vasc Biol. 2004;24:504–510. doi: 10.1161/01.ATV.0000115638.27381.97. [DOI] [PubMed] [Google Scholar]
- 107.Parkes HA, et al. Overexpression of acyl-CoA synthetase-1 increases lipid deposition in hepatic (HepG2) cells and rodent liver in vivo. Am J Physiol Endocrinol Metab. 2006;291:E737–744. doi: 10.1152/ajpendo.00112.2006. [DOI] [PubMed] [Google Scholar]
- 108.Soupene E, Kuypers FA. Mammalian long-chain acyl-CoA synthetases. Exp Biol Med. 2008;233:507–521. doi: 10.3181/0710-MR-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harzer K, Hiraiwa M, Paton BC. Saposins (sap) A and C activate the degradation of galactosylsphingosine. FEBS Lett. 2001;508:107–110. doi: 10.1016/s0014-5793(01)03044-7. [DOI] [PubMed] [Google Scholar]
- 110.Kolter T, Sandhoff K. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu Rev Cell Dev Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- 111.Kim MJ, et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med. 2010 doi: 10.1002/emmm.201000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chatterjee S, Pandey A. The Yin and Yang of lactosylceramide metabolism: implications in cell function. Biochim Biophys Acta. 2008;1780:370–382. doi: 10.1016/j.bbagen.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 113.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cruz D, et al. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest. 2008;118:2917–2928. doi: 10.1172/JCI34189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mattos KA, et al. Lipid droplet formation in leprosy: Toll-like receptor-regulated organelles involved in eicosanoid formation and Mycobacterium leprae pathogenesis. J Leukoc Biol. 2010;87:371–384. doi: 10.1189/jlb.0609433. [DOI] [PubMed] [Google Scholar]
- 116.Hunter RL, Jagannath C, Actor JK. Pathology of postprimary tuberculosis in humans and mice: contradiction of long-held beliefs. Tuberculosis. 2007;87:267–278. doi: 10.1016/j.tube.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 117.Seimon TA, et al. Induction of ER stress in macrophages of tuberculosis granulomas. PloS one. 2010 doi: 10.1371/journal.pone.0012772. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 119.Medigeshi GR, et al. West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis. J Virol. 2007;81:10849–10860. doi: 10.1128/JVI.01151-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxidants Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 121.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Mol Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Devries-Seimon T, et al. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe-/- and Ldlr-/- mice lacking CHOP. Cell Metabolism. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.D’Avila H, Melo RC, Parreira GG, Werneck-Barroso E, Castro-Faria-Neto HC, Bozza PT. Mycobacterium bovis bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J Immunol. 2006;176:3087–3097. doi: 10.4049/jimmunol.176.5.3087. [DOI] [PubMed] [Google Scholar]
- 126.Peyron P, et al. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathogens. 2008;4:e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garton NJ, Christensen H, Minnikin DE, Adegbola RA, Barer MR. Intracellular lipophilic inclusions of mycobacteria in vitro and in sputum. Microbiol. 2002;148:2951–2958. doi: 10.1099/00221287-148-10-2951. [DOI] [PubMed] [Google Scholar]
- 128.Garton NJ, et al. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 2008;5:e75. doi: 10.1371/journal.pmed.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Russell DG, et al. Mycobacterium tuberculosis wears what it eats. Cell Host Microbe. 2010;8:68–76. doi: 10.1016/j.chom.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]