Summary
Invasion of non-phagocytic cells by a number of bacterial pathogens involves the subversion of the actin cytoskeletal remodelling machinery to produce actin-rich cell surface projections designed to engulf the bacteria. The signalling that occurs to induce these actin-rich structures has considerable overlap amongst a diverse group of bacteria. The molecular organisation within these structures act in concert to internalise the invading pathogen. This dynamic process could be subdivided into three acts - actin recruitment, engulfment, and finally, actin disassembly/internalisation. This review will present the current state of knowledge of the molecular processes involved in each stage of bacterial invasion, and provide a perspective that highlights the temporal and spatial control of actin remodelling that occurs during bacterial invasion.
Introduction
Actin remodelling in eukaryotic cells is an extremely dynamic, yet well-controlled process. The recruitment of globular actin (G-actin) at the plasma membrane could be triggered by extracellular cues, usually in the form of an activating ligand binding to the appropriate plasma membrane receptor. The signal is then transduced to various signalling molecules, such as lipids, Rho GTPases, and actin-binding proteins to initiate actin nucleation, polymerisation, branching, and crosslinking of filamentous actin (F-actin). The culmination is the formation of functional structures used for motility, phagocytosis, and cell-to-cell communication. Each of these processes is associated with distinct actin-rich cell surface structures, and different signalling cascades give rise to these structural variations. For example, activation of Cdc42, a member of the Rho GTPase family results in the formation of filopodia at the cell periphery, whereas Rac activation is typically involved in lamellipodia and membrane ruffle formation (Hall, 2005). The actin nucleating components also influence the types of structures. The Arp2/3 complex plays a role in the formation of branched actin, whilst formins give rise to linear F-actin structures (Campellone et al., 2010). Interestingly, these structures are also found on cells at the early stages of infection by intracellular bacteria during adhesion and/or invasion, indicating that bacteria can subvert endogenous signalling components for their own purposes.
The invasion of host cells by a number of bacterial pathogens is an important event in their pathogenic process. This is mediated by either: (i), specialised bacterial proteins that target proteins which are known participants of cytoskeletal remodelling; or (ii), the direct engagement and clustering of host cell surface receptors that stimulate downstream effectors. Both strategies culminate in the formation of cell surface projections that promote efficient adherence, colonisation, or the eventual engulfment of the invading bacteria.
Bacterial attachment to the host cell involves primary electrostatic interactions (Hatch et al., 1981, Heckels et al., 1976), followed by binding of specific adhesins to their cognate receptors present on the surface of the host cell (Boyle et al., 2003, Humphries et al., 2001, Lambert et al., 2009, Elwell et al., 2008). Attachment of some bacteria triggers an immediate signalling cascade via conformational changes and/or clustering of receptors that promote their activation (Pizarro-Cerda et al., 2004, Patel et al., 2005, Goosney et al., 2000, Dunn et al.), with clustering defining microdomains at the plasma membrane, to which downstream signalling molecules are recruited, forming a signalling platform (Bethani et al., Himanen et al., Groves et al.). In the case of actin-related signalling, a highly localised actin remodelling occurs which results in the formation of dynamic actin-rich cell surface projections (Groves et al., Campellone et al., 2008, Campellone et al., 2004, Hayward et al., 2009, Kwiatkowska et al., 1999, Nolz et al., 2007). These projections serve to engulf the bacteria by either macropinocytosis, as found in trigger mechanisms of invasion, or by extending actin-rich cell surface structures that maintains close circumferential contacts with the bacterial surface to culminate in internalisation. Despite the differences in the mode of invasion, there are overlaps in the signalling between the two, namely the involvement of Rho GTPases, Neural Wiskott-Aldrich Syndrome Protein (N-WASP) and/or WASP family verprolin-homologous 2 protein (WAVE2), and the endogenous Arp2/3 complex. Actin nucleation kick-starts actin polymerisation. This is followed by the formation of highly dynamic actin-rich cell surface projections that enwrap and engulf the invading pathogen. Completion of bacterial invasion requires that the formed actin structures be disassembled. This may occur via the translocation of bacterial effectors to sever filamentous actin (F-actin) itself or stimulate the endogenous cellular factors that facilitate F-actin destabilisation. Thus, the process of bacterial invasion is a multistage process: (i), defining the site of attachment; (ii), initiating actin recruitment; (iii), maintaining actin-rich invasion-associated structures; and (iv), their eventual disassembly. The molecular events involved at each distinct stage are temporally and spatially coordinated to ensure the correct progression of events, thus ensuring the efficient uptake of the invading pathogen.
Initiation of actin recruitment
The degree to which the invasion structure is localised is likely reflected by the spatial constraints on the inducing event, defining a location at the membrane to which signalling components are recruited. Spatial constraints are imposed by the enrichment of a particular receptor within membrane microdomains, the multivalent engagement of the receptors by bacterial adhesins, and/or the highly localised production of signalling intermediates, such as phosphatidylinositol (PI) derivatives in the cytosolic leaflet of the host plasma membrane (Weber et al., 2009). The immediate result is the concentration of host resources for cytoskeletal remodelling at a defined site facilitating the efficient uptake of the invading bacteria. For example, the engagement of E-cadherin by InlA of Listeria effectively clusters the receptor to recruit α-catenin, β-catenin, p120, and EPLIN, and thus, assembling a signalling platform that interacts with ARHGAP10, myosin VIIa, and vezatin (Pizarro-Cerda et al., 2006). The compartmentalisation cue provided by the clusters of “activated” E-cadherin initiates the assembly of a signalling plaform that defines the area to which actin is recruited and therefore, the site of entry for Listeria. This mechanism likely cooperates with the known role of clathrin in initiating actin recruitment (Veiga et al., 2005).
An analogous strategy to the localised activation of host cell receptors during bacterial attachment is the insertion of bacterially encoded signalling proteins, like Tir and TarP of enteropathogenic/enterohaemorrhagic E. coli (EPEC/EHEC) and Chlamydia, respectively. The translocated intimin receptor (Tir) of EPEC and EHEC, when clustered at the plasma membrane triggers the recruitment of actin directly underneath the attached bacteria to form pedestal structures via the indirect activation of the actin-nucleating Arp2/3 complex (Fig. 2). Tir molecules inserted in the plasma membrane are clustered via its interaction with intimin molecules on the surface of the bacteria, essentially a domain with a critical mass of activated Tir molecules. One of the more interesting results is the enhanced activation of downstream molecules, specifically N-WASP for optimal induction of localised actin polymerisation. EPEC and EHEC recruit and cluster N-WASP using different strategies, with the former relying on the phosphorylation of Tyr474 residue by Src or Abl tyrosine kinases, and recruiting the adapter protein Nck, which in turn binds N-WASP (Gruenheid et al., 2001, Swimm et al., 2004). However, EPEC also possess the ability to establish pedestals in a Nck-independent manner (Campellone et al. 2005). EHEC, in contrast translocates a Type III secretion effector called EspF(U), which binds host proteins N-WASP and IRTKS, the latter being essential to bind Tir (Campellone et al., 2004, Garmendia et al., 2006, Vingadassalom et al., 2009).
Fig. 2. Signalling by EHEC and Salmonella to the actin remodelling machinery.
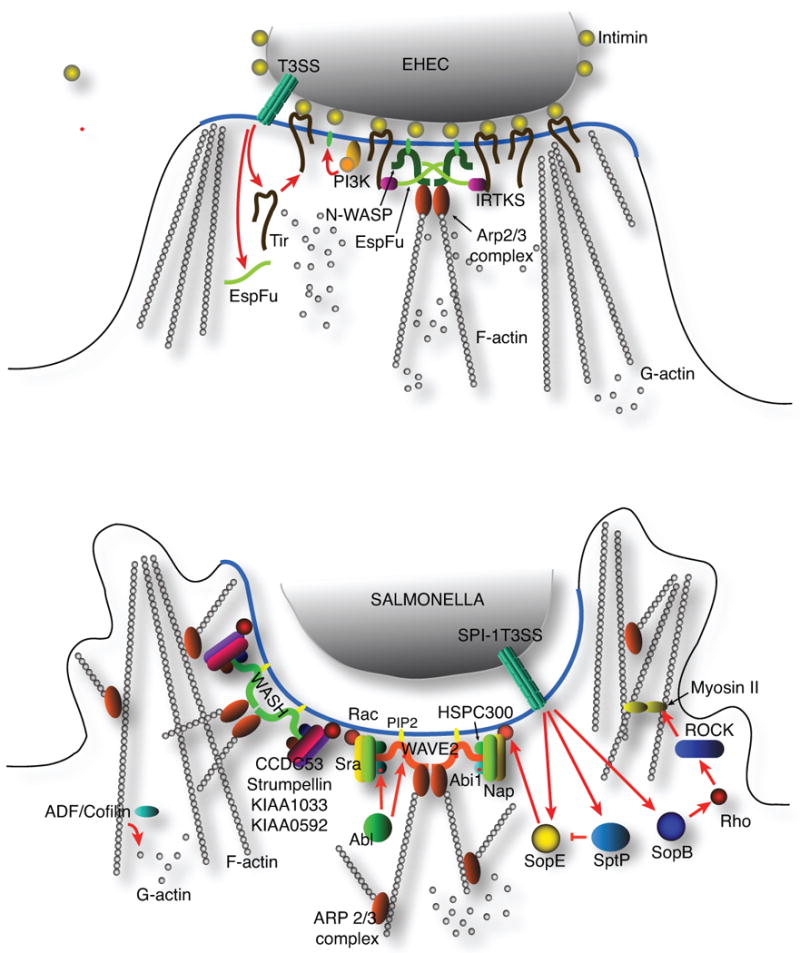
A. The formation of an actin-rich pedestal by EHEC requires the Type III translocation of Tir, which is subsequently embedded in the plasma membrane. There it recruits host signalling proteins, such as IRTKS, which in turn binds the EHEC EspF(U) effector. EspF(U) dimerise and bring N-WASP molecules in close proximity to enhance N-WASP activation.
B. Salmonella ruffle formation requires the WAVE2 signalling complex, but recent reports suggest that this is not essential to invasion. Instead, the actin remodelling required for invasion is mediated by the WASH complex. In addition, a parallel pathway consisting of Rho, Rho kinase, and Myosin II cooperates with the WASH pathway to facilitate Salmonella invasion.
The contuining elucidation of Chlamydia invasion is revealing that this pathogen may utilise a similar mechanism via the translocated invasion-associated effector TarP (Clifton et al., 2004). The configuration of TarP soon after translocation into the host cell is not known, but evidence points to a possible aggregation directly underneath the invading elementary body. For example, phosphorylated, and thus translocated TarP co-sediments with purified elementary bodies (EBs) at the early stages of Chlamydia infection (Carabeo, unpublished data) indicating that TarP molecules may be restricted in their lateral diffusion along the plasma membrane. Currently, there is no direct evidence that TarP associates with molecules on the surface of EBs, akin to the intimin-Tir interaction (Fig 1).
Fig. 1. A schematic diagram of signalling with Listeria and Chlamydia to the actin remodelling machinery.
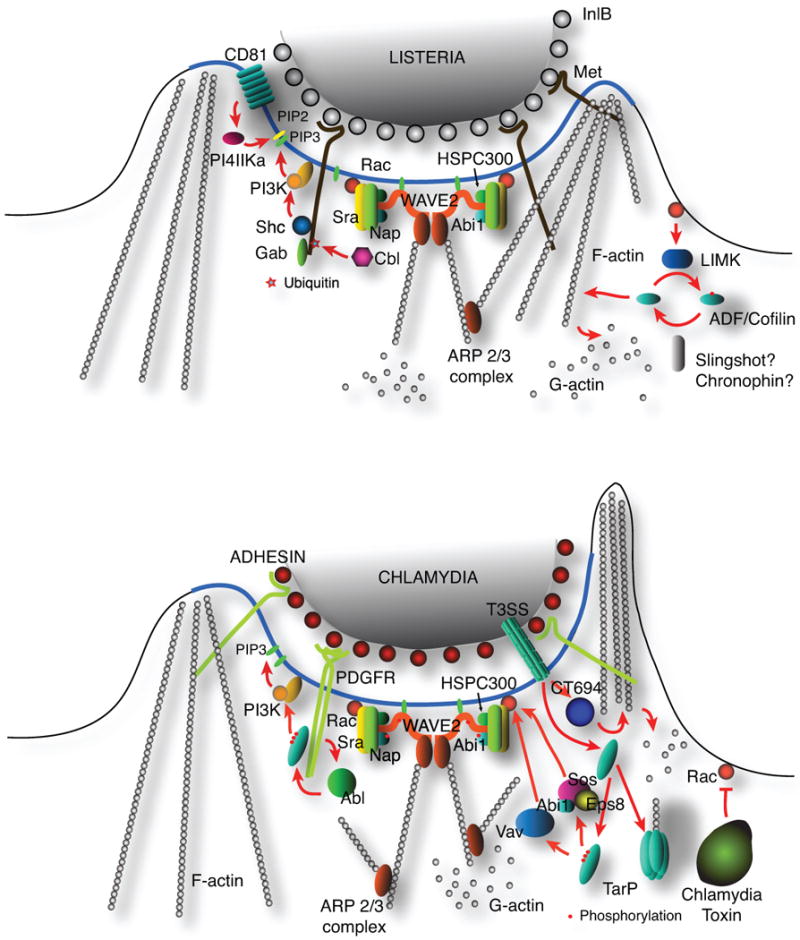
A. Listeria InlB binds to the host cell Met receptor to trigger a signal transduction that activates a variety of signalling molecules that participate in and promote the recruitment and assembly of an F-actin network during invasion. The activation of the Rac GTPase is pivotal to this process. Rac promotes a WAVE2-dependent actin polymerisation and induce LIM kinase activity to inhibit the actin depolymerising activity of cofilin. The localised synthesis of PIP3 and PIP2 ensures the enrichment of adapter/signalling molecules that recognise these phospholipids.
B. Actin remodelling during chlamydial invasion depends on two complementary pathways: (i), a signalling mechanism that involves host factors such as the WAVE2 complex; and (ii), a nucleating function of the Type III effector TARP. The signalling component involves the binding of PDGFR molecules at the cell surface to trigger activation of the Rac GTPase. Actin disassembly may involve the effector CT694 and the putative Chlamydia toxin.
TarP is able to induce actin remodelling directly through two distinct pathways - the actin nucleating activity of the conserved WH2-like motif at the C-terminal portion of the protein (Jewett et al., 2006, Jewett et al., 2010), or for C. trachomatis via the tyrosine-phosphorylated domain in the N-terminus (Lane et al., 2008, Jewett et al., 2008). The phosphorylated domain binds host proteins, such as PI-3 kinase, WAVE2, Rac, Sos1, Vav2 amongst others that signal to the actin cytoskeletal remodelling machinery. Thus, it appears that the mechanisms employed by EPEC/EHEC and Chlamydia to define the sites to which actin would be recruited share the same mechanistic principle as that of Listeria, via the clustering and activation of host cell receptors.
The enrichment of lipid signalling molecules also contributes to the definition of the domain in the plasma membrane to which signal transduction components are recruited. An example is the activation of Class I PI3 kinase during Chlamydia invasion to synthesise PI(3,4,5)P3 (PIP3), which is involved in the activation of the Rac-specific guanine nucleotide exchange factor (GEF), Vav2 (Lane et al., 2008)(Fig. 1). This localised activation reinforces the spatial cues provided by upstream signalling components. Another consequence of the enrichment of this lipid is the optimal activation of WAVE2 as will be discussed in more detail below. In addition to localised activation of receptors, the creation of signalling microdomains is further strengthend by the stimulation of phosphatidylinositol kinases, which synthesise phospholipid species essential for localised actin recruitment.
Enhancement of signalling to the actin remodelling machinery: a focus on N-WASP and WAVE2
Bacterial signalling via the activated host cell receptor (e.g Listeria), or through the translocation of receptor-like molecules encoded by the pathogen (e.g. Chlamydia) often converge at members of the Rho GTPase family. Bacterial activation of Rho, Rac, and Cdc42 has been described in a number of excellent reviews and will not be covered in detail here (Knodler et al. 2001; Cossart et al. 2005). What is emerging in more recent studies are the interesting biochemical bases of activation of two of the prominent downstream effectors of Rac and Cdc42, namely WAVE2 and N-WASP.
Pathogen-directed engulfment requires the extensive remodelling of the actin cytoskeleton, which manifests as intricate and unique structures that surround the invading bacteria. The formation of these structures, like the ruffles observed during Salmonella invasion is mediated by the Arp2/3 complex. Arp2/3 activation depends upon its interaction with either N-WASP or WAVE2. The activation of N-WASP by a phosphorylation through members of the Src tyrosine kinase family, and interaction with various signalling molecules (e.g. binding by acidic phospholipids, and interaction with Cdc42) relieve N-WASP from its autoinhibitory conformation revealing the C-terminal VCA region for binding by the Arp2/3 complex (Rohatgi et al. 1999, Rohatgi et al. 2001, Torres et al. 2006). The related protein WAVE2 is also maintained in an inhibited conformation, but unlike N-WASP, this configuration is in the form of a multiprotein complex composed of Sra, Nap, Hspc300, and Abi1 (Innocenti et al. 2004). Like N-WASP, optimal WAVE2 activation requires multiple events, such as phosphorylation, and binding to Rac and acidic phospholipids (Lebensohn et al. 2009). Once activated, N-WASP and WAVE2 are able to bind the Arp2/3 complex via their respective VCA (Verprolin, Central hydrophobic, Acidic) regions (Padrick et al., 2008).
Recent studies revealed that both N-WASP and WAVE2 could be further enhanced by oligomerisation that brings the VCA domains in close proximity. Indeed, a decade-old observation by Higgs & Pollard has hinted on this mode of enhanced activation. They reported that VCA dimers were approximately 2-logs more efficient in activating the Arp2/3 complex than VCA monomers (Higgs et al., 2000). Within the last year, the molecular basis of oligomerisation-dependent enhancement of WAVE2 and N-WASP activation has been elucidated biochemically, genetically, and structurally (Lebensohn et al. 2009, Padrick et al., 2008). Remarkably, this enhancement mechanism occurs in bacterial subversion of actin remodelling at the plasma membrane, highlighting a fascinating aspect of evolutionary convergence.
The role of WAVE-2 in Salmonella invasion
The full activation of WAVE2 requires four coincident events: (i), phosphorylation of WAVE2; (ii), presence of PIP2 on the membrane; (iii), synthesis of PIP3; and (iv), the membrane localisation of active Rac (Lebensohn et al., 2009). Does WAVE2 activation during bacterial invasion require all four molecular events? The presence of PIP2 at the plasma membrane contributes to the enhanced activation of WAVE2 by enriching it within a confined area on the plasma membrane, favouring homotypic interactions (Padrick et al., 2008); and this same lipid species is enriched at the plasma membrane during invasion of Salmonella. However, this lipid enrichment was never directly implicated, or even correlated with enhanced WAVE2 activation (Shi et al., 2005, Unsworth et al., 2004, Mallo et al., 2008, Patel et al., 2006). It is possible that this WAVE2 enrichment that leads to optimal activity could be preceded by the collective activation of multiple Rac molecules at the specified signalling microdomain at the plasma membrane.
A recent study revealed an added complexity in Salmonella infection (Hanisch et al., 2010) by revealing that WAVE2-dependent membrane ruffle formation could be separated from actual invasion, bringing into question the role of WAVE2-mediated actin recruitment in Salmonella invasion. Cells depleted of WAVE2 or Nap1 lacked the signature membrane ruffles, but still supported invasion via a zipper-like mechanism. It appears that a novel Arp2/3 complex regulator, called WASH function in Salmonella invasion in the absence of WAVE2 (Hanisch et al., 2010). It was recently reported that WASH, like WAVE2 is part of a multiprotein complex consisting of KIAA1033, KIAA0592, Strumpellin, and ccdc53 (Jia et al., 2010); and from in vitro actin polymerisation studies, WASH induces both bundling and branching of F-actin (Liu et al., 2009). Thus, Salmonella invasion remains actin-dependent, but WAVE2 is dispensable (Fig. 2). Because WASH could be found in a multiprotein complex, similar to WAVE2, it would not be surprising if WASH also exhibited a clustering-dependent optimal activation.
A recent report also indicated that the Arp2/3-dependent actin nucleation during Salmonella invasion functions in parallel with a RhoA/Rho kinase-dependent activation of myosin II, and this appears to require the Type III effector SopB (Hanisch et al., 2011). Thus, Salmonella possesses multiple means to subvert the host actin cytoskeleton – the SopE/SopE2-requiring pathway (WASH/WAVE, Arp2/3 complex), and the SopB-dependent pathway (RhoA, Rho kinase, myosin II) (Fig. 2). The presence of redundant invasion pathways would clearly benefit Salmonella by avoiding the full assault of the host immune system, and possibly aid in its systemic dissemination; and further exploration of the biological and/or pathological consequences of each invasion pathway may be needed to fully appreciate the full pathogenic capability of this bacterium.
Regulation of N-WASP in pedestal formation by allostery and oligomerisation
N-WASP serves a similar role as WAVE2 in its ability to activate the Arp2/3 complex and to stimulate actin remodelling. The activation of N-WASP requires a dramatic conformational unfolding to allow for its interaction with the Arp2/3 complex. The binding of active Cdc42, Nck adaptor, and/or PIP2 (or PIP3) results in allosteric activation (Derivery et al., 2010). This now appears to be just one level of activation. Recent biochemical studies also demonstrate that promoting the formation of higher order N-WASP oligomers enhances N-WASP activation (Padrick et al., 2008), thus representing another level of modulation of N-WASP activity. Oligomerisation can occur by the binding of N-WASP to the clustered activated upstream components in a configuration resembling a signalling microdomain. This is best exemplified in the formation of actin-rich pedestals during EPEC/EHEC attachment to cells (Fig. 2). In addition to the recruitment by clustered Tir molecules, interacting partners with divalent binding sites can bring together multiple N-WASP molecules. A bacterial effector fulfils this role for EHEC. EspF(U)/TccP can bind multiple N-WASP molecules via 2-7 copies of a repeated hydrophobic domain composed of 47 amino acid residues. EspF(U)/TccP also contain repeats of PxxP motifs, which is recognised by the SH3 domains of IRTKS, thus forming linked quaternary complexes consisting of Tir, IRTKS, EspF(U), and N-WASP. This effectively clusters multiple N-WASP proteins; and when accompanied by the clustering of Tir molecules via their interaction with the immobile intimin molecules on the surface of the extracellular bacteria creates a platform array of optimally activated N-WASP (Vingadassalom et al., 2009, Campellone et al., 2008, Sallee et al., 2008, Weiss et al., 2009).
The Arp2/3 complex – Subversion by Chlamydia
Actin polymerisation is necessary to form invasion-related structures, and the primary players, depending on the bacteria are Arp2/3, formin, formin-related proteins, and bacterial factors with inherent actin-nucleating activity (Campellone et al., 2010). Nucleators are essential to overcome the thermodynamic “hump” that is inherent in actin polymerisation. This thermodynamic barrier maintains the cellular balance of G-actin and F-actin, minimising undesired spontaneous actin polymerisation. Therefore, invasive bacteria have acquired strategies to overcome this thermodynamic barrier.
In Chlamydia, the Arp2/3 subversion involves the TarP-dependent actin nucleation reported by Jewett et al. (Jewett et al., 2006, Jewett et al., 2010). TarP actin nucleation requires the actin-binding domains (ABDs) within the C-terminal region of the protein, where actin monomers bind to tandemly arranged ABDs, and thus, are brought in proximity to favour formation of the initial nucleating actin trimer. In species that harbour only one copy of the ABD, an oligomerisation-dependent mechanism via the proline-rich domain (PRD) of TarP was proposed and is supported by detailed biochemical studies (Jewett et al., 2006, Jewett et al., 2010). The tandem arrangement of multiple WH2 domains resembles those that are found in Spire-like actin nucleators – a family of proteins that nucleates the formation of unbranched actin structures (Baum et al., 2005, Quinlan et al., 2005, Renault et al., 2008). The exact role of TarP actin nucleation is not fully understood. However, the introduction of anti-TarP ABD antibody in the cytosol of cell prior to infection partially blocked invasion (Jewett et al., 2010). Conversely, interference with host cell signalling led to the inhibition of chlamydial invasion, despite the presence of actin nucleating activity in TarP, (Carabeo et al., 2007, Carabeo et al., 2004, Elwell et al., 2008, Coombes et al., 2002, Subtil et al., 2004). These observations indicate that the invasion process has additional requirements beyond the nucleating activity of TarP. If indeed the case, it raises the question of how the nucleation pathways relate to each other. There are some indications that at some level, the two pathways are able to function independently. Both microvillar and pedestal structures on the cell surface of infected cells (Carabeo et al., 2002) are routinely observed in infected cells, and these cell surface projections may be related to the predicted unbranched and branched structures resulting from actin nucleation by TarP and Arp2/3, respectively. Whether both structures are required for invasion is not known.
Actin disassembly after invasion
Until this point, I have discussed how signalling at the plasma membrane is established by attached bacteria, and how the initial arrangements of signalling molecules ensure the optimal activation of downstream elements via oligomerisation/clustering. Equally important to the induction of actin recruitment and polymerisation is the disassembly of the F-actin structures. Actin disassembly is a necessary step in bacterial invasion. Without disassembly, the assembled F-actin network directly underneath the bacteria would pose a formidable physical barrier that would prevent the full internalisation of the bacteria by the host cell. Conceptually, a switch that shifts the balance of actin dynamics from polymerisation to disassembly would have to occur to complete invasion. This switch could be bacterial effectors that directly or indirectly act on the F-actin network. The bacteria could stimulate the disassembly machinery of the host cell (e.g. actin depolymerisation factor (ADF)/cofilin), shut down the pro-assembly signalling (e.g. inhibition of Rho GTPases), and/or inhibit cellular actin stabilising functions (e.g. α-actinin).
For Chlamydia, it is clear that actin disassembly is crucial for invasion, as highlighted by the inhibition of invasion upon treatment with the actin stabilising drug, jasplakinolide (Carabeo, unpublished observation). The turnover of actin localised at the chlamydial entry site is relatively rapid (Carabeo et al., 2002), and the molecular mechanism mediating this process is unknown. There is a recently identified EB-associated effector, CT694, which binds the host protein AHNAK and may function as an actin-severing machinery (Hower et al., 2009), although the exact biochemical function of CT694 remains to be elucidated. In some species of Chlamydia, the putative toxin with proposed may play a role in promoting the relatively rapid disassembly of F-actin at the sites of invasion by inactivating the Rac and Cdc42 (Belland et al., 2001, Carlson et al., 2004, Thalmann et al.) (Fig. 1).
A number of pathogens rely on the endogenous actin depolymerising proteins. For example, the actin depolymerising factor (ADF)/cofilin has been implicated in Salmonella invasion (Dai et al., 2004). Disassembly of F-actin in eukaryotic cells is achieved through the action of ADF/cofilin, which enhances F-actin depolymerisation and severs actin filaments. Together with the cessation of signalling from Rac and Cdc42, which are inactivated by the GTPase-activating protein (GAP)-like effector SptP (Fu et al., 1999) result in the timely disassembly, uptake of bacteria, and the return to normal cell surface morphology.
In cells, there is an interplay between LIM kinase and actin depolymerising factor (ADF)/cofilin. The disassembly function of ADF/cofilin is counteracted by LIM kinase by phosphorylating, and thus inactivating cofilin (Bernstein et al., 2010, Huang et al., 2006, Van Troys et al., 2008). During Listeria invasion, LIMK is activated via the Rac pathway to phosphorylate cofilin and shift the balance of actin dynamics to that of polymerisation (Bierne et al., 2001). Eventually, dephosphorylation of cofilin occurs via an unknown phosphatase, leading to its activation and the destabilisation of F-actin at the site of entry (Fig. 1). Two cofilin phosphatases, Slingshot and Chronophin have been identified (Huang et al., 2006), but their specific involvement in bacterial invasion have not been investigated. The uptake of Bartonella bacterial clusters, which involves the constant remodelling of a structure called an invasome, requires cofilin (Truttmann et al., 2010), although how cofilin is regulated in this process has not been reported.
Invasion-independent role of actin recruitment
It is interesting that intracellular pathogens usually possess multiple strategies to induce highly localised actin recruitment. In the case of Salmonella, WAVE2-mediated actin recruitment was not necessary for invasion. What is its true function? One possible actin-related, but invasion-independent role for WAVE2 is in the enhancement translocation of effectors important for post-invasion processes. A similar role for actin recruitment has been postulated for EPEC, Shigella, and Yersinia (Vingadassalom et al., 2010, Mounier et al., 2009, Mejia et al., 2008). The exact mechanism of this actin-dependent enhancement of effector translocation is not known, but may involve the stabilisation of the Type III apparatus connection from the host side. This invasion-independent role of actin recruitment may also apply to Chlamydia. The actin nucleating function of TarP may be important for the further stabilisation of the Type III apparatus for enhanced translocation of effectors. This represents a clever integration of actin recruitment and translocation, with the assembled F-actin network possibly “recycled” or “refitted” for a different purpose. It would be of interest to determine if actin recruitment for this purpose has structural features that are distinct from that involved in invasion or adhesion in the case of EPEC/EHEC. Answers to this question may provide initial insights into the invasion-independent role of actin recruitment.
Concluding remarks
The act of bacterial adhesion to the surface of the host cell is so much more than just a simple attachment. It initiates in an orderly manner a series of molecular events within a defined area on the plasma membrane to facilitate optimal actin remodelling that is crucial to bacterial invasion. The invasion process clearly requires the recruitment of actin and in some cases, the formation of dramatic actin-rich cell surface projections. However, it is starting to emerge that the process of disassembling the F-actin network is also pivotal to the successful completion of invasion. The majority of the scientific literature on actin-mediated bacterial invasion are focussed on actin recruitment and remodelling dynamics, and it is becoming apparent and increasingly appreciated that multiple layers of control, such as allostery and oligomerisation determine the efficiency and robustness of the signalling cascades. Also, the process of disassembly will likely be crucial for the optimal invasion of host cells, and therefore, is a fertile ground for further research. We are also beginning to gain a greater appreciation for alternative roles of actin recruitment in bacterial pathogenesis. Given the wealth of mechanistic information on actin recruitment during bacterial infection in vitro, their roles in vivo are now being explored in greater details (Crepin et al., 2010). Whilst we are far from obtaining a detailed molecular picture of bacterial invasion, we are making rapid progress in identifying an array of tricks bacteria possess to subvert actin dynamics.
Acknowledgments
I would like to acknowledge Dr. David Banbury (Imperial College) and the anonymous reviewers for their helpful suggestions, and the Medical Research Council (UK) and the National Institutes of Health (USA) for research support. I sincerely apologise to colleagues whose work could not be cited due to space limitations.
References
- Baum B, Kunda P. Actin nucleation: spire - actin nucleator in a class of its own. Curr Biol. 2005;15:R305–308. doi: 10.1016/j.cub.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Belland RJ, Scidmore MA, Crane DD, Hogan DM, Whitmire W, McClarty G, Caldwell HD. Chlamydia trachomatis cytotoxicity associated with complete and partial cytotoxin genes. Proc Natl Acad Sci U S A. 2001;98:13984–13989. doi: 10.1073/pnas.241377698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethani I, Skanland SS, Dikic I, Acker-Palmer A. Spatial organization of transmembrane receptor signalling. EMBO J. 2010;29:2677–2688. doi: 10.1038/emboj.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierne H, Gouin E, Roux P, Caroni P, Yin HL, Cossart P. A role for cofilin and LIM kinase in Listeria-induced phagocytosis. J Cell Biol. 2001;155:101–112. doi: 10.1083/jcb.200104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierne H, Miki H, Innocenti M, Scita G, Gertler FB, Takenawa T, Cossart P. WASP-related proteins, Abi1 and Ena/VASP are required for Listeria invasion induced by the Met receptor. J Cell Sci. 2005;118:1537–1547. doi: 10.1242/jcs.02285. [DOI] [PubMed] [Google Scholar]
- Boyle EC, Finlay BB. Bacterial pathogenesis: exploiting cellular adherence. Curr Opin Cell Biol. 2003;15:633–639. doi: 10.1016/s0955-0674(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Campellone KG, Cheng HC, Robbins D, Siripala AD, McGhie EJ, Hayward RD, et al. Repetitive N-WASP-binding elements of the enterohemorrhagic Escherichia coli effector EspF(U) synergistically activate actin assembly. PLoS Pathog. 2008;4:e1000191. doi: 10.1371/journal.ppat.1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone KG, Leong JM. Nck-independent actin assembly is mediated by two phosphorylated tyrosines within enterohathogenic Escherichia coli Tir. Mol Microbiol. 2005;56:416–432. doi: 10.1111/j.1365-2958.2005.04558.x. [DOI] [PubMed] [Google Scholar]
- Campellone KG, Rankin S, Pawson T, Kirschner MW, Tipper DJ, Leong JM. Clustering of Nck by a 12-residue Tir phosphopeptide is sufficient to trigger localized actin assembly. J Cell Biol. 2004;164:407–416. doi: 10.1083/jcb.200306032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone KG, Robbins D, Leong JM. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev Cell. 2004;7:217–228. doi: 10.1016/j.devcel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–251. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabeo RA, Dooley CA, Grieshaber SS, Hackstadt T. Rac interacts with Abi-1 and WAVE2 to promote an Arp2/3-dependent actin recruitment during chlamydial invasion. Cell Microbiol. 2007;9:2278–2288. doi: 10.1111/j.1462-5822.2007.00958.x. [DOI] [PubMed] [Google Scholar]
- Carabeo RA, Grieshaber SS, Fischer E, Hackstadt T. Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells. Infect Immun. 2002;70:3793–3803. doi: 10.1128/IAI.70.7.3793-3803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabeo RA, Grieshaber SS, Hasenkrug A, Dooley C, Hackstadt T. Requirement for the Rac GTPase in Chlamydia trachomatis invasion of non-phagocytic cells. Traffic. 2004;5:418–425. doi: 10.1111/j.1398-9219.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- Carlson JH, Hughes S, Hogan D, Cieplak G, Sturdevant DE, McClarty G, et al. Polymorphisms in the Chlamydia trachomatis cytotoxin locus associated with ocular and genital isolates. Infect Immun. 2004;72:7063–7072. doi: 10.1128/IAI.72.12.7063-7072.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton DR, Fields KA, Grieshaber SS, Dooley CA, Fischer ER, Mead DJ, et al. A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin. Proc Natl Acad Sci U S A. 2004;101:10166–10171. doi: 10.1073/pnas.0402829101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes BK, Mahony JB. Identification of MEK- and phosphoinositide 3-kinase-dependent signalling as essential events during Chlamydia pneumoniae invasion of HEp2 cells. Cell Microbiol. 2002;4:447–460. doi: 10.1046/j.1462-5822.2002.00203.x. [DOI] [PubMed] [Google Scholar]
- Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- Crepin VF, Girard F, Schüller S, Phillips AD, Mousnier A, Frankel G. Dissecting the role of the Tir:Nck and Tir:IRTKS/IRSp53 signalling pathways in vivo. Mol Microbiol. 2010;75:308–323. doi: 10.1111/j.1365-2958.2009.06938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Sarmiere PD, Wiggan O, Bamburg JR, Zhou D. Efficient Salmonella entry requires activity cycles of host ADF and cofilin. Cell Microbiol. 2004;6:459–471. doi: 10.1111/j.1462-5822.2004.00375.x. [DOI] [PubMed] [Google Scholar]
- Derivery E, Gautreau A. Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. Bioessays. 2010;32:119–131. doi: 10.1002/bies.200900123. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Valdivia RH. Uncivil engineers: Chlamydia, Salmonella and Shigella alter cytoskeleton architecture to invade epithelial cells. Future Microbiol. 2010;5:1219–1232. doi: 10.2217/fmb.10.77. [DOI] [PubMed] [Google Scholar]
- Elwell CA, Ceesay A, Kim JH, Kalman D, Engel JN. RNA interference screen identifies Abl kinase and PDGFR signaling in Chlamydia trachomatis entry. PLoS Pathog. 2008;4:e1000021. doi: 10.1371/journal.ppat.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis CL, Ryan TA, Jones BD, Smith SJ, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- Fu Y, Galan JE. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401:293–297. doi: 10.1038/45829. [DOI] [PubMed] [Google Scholar]
- Garmendia J, Carlier MF, Egile C, Didry D, Frankel G. Characterization of TccP-mediated N-WASP activationi during enterohaemorrhagic Escherichia coli infection. Cell Microbiol. 2006;8:1444–1455. doi: 10.1111/j.1462-5822.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- Ginocchio CC, Olmsted SB, Wells CL, Galan JE. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994;76:717–724. doi: 10.1016/0092-8674(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Goosney DL, Gruenheid S, Finlay BB. Gut feelings: enteropathogenic E. coli (EPEC) interactions with the host. Annu Rev Cell Dev Biol. 2000;16:173–189. doi: 10.1146/annurev.cellbio.16.1.173. [DOI] [PubMed] [Google Scholar]
- Groves JT, Kuriyan J. Molecular mechanisms in signal transduction at the membrane. Nat Struct Mol Biol. 2010;17:659–665. doi: 10.1038/nsmb.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenheid S, DeVinney R, Bladt F, Goosney D, Gelkop S, Gish GD, Pawson T, Finlay BB. Enterophathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat Cell Biol. 2001;3:856–859. doi: 10.1038/ncb0901-856. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Hanisch J, Ehinger J, Ladwein M, Rohde M, Derivery E, Bosse T, et al. Molecular dissection of Salmonella-induced membrane ruffling versus invasion. Cell Microbiol. 2010;12:84–98. doi: 10.1111/j.1462-5822.2009.01380.x. [DOI] [PubMed] [Google Scholar]
- Hanisch J, Kolm R, Wozniczka M, Bumann D, Rottner K, Stradal TE. Activation of a RhoA/Myosin II-Dependent but Arp2/3 Complex-Independent Pathway Facilitates Salmonella Invasion. Cell Host Microbe. 2011;9:273–285. doi: 10.1016/j.chom.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Hardt WD, Chen LM, Schuebel KE, Bustelo XR, Galan JE. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 1998;93:815–826. doi: 10.1016/s0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- Hatch TP, Vance DW, Jr, Al-Hossainy E. Attachment of Chlamydia psittaci to formaldehyde-fixed and unfixed L cells. J Gen Microbiol. 1981;125:273–283. doi: 10.1099/00221287-125-2-273. [DOI] [PubMed] [Google Scholar]
- Hayward RD, Hume PJ, Humphreys D, Phillips N, Smith K, Koronakis V. Clustering transfers the translocated Escherichia coli receptor into lipid rafts to stimulate reversible activation of c-Fyn. Cell Microbiol. 2009;11:433–441. doi: 10.1111/j.1462-5822.2008.01265.x. [DOI] [PubMed] [Google Scholar]
- Heckels JE, Blackett B, Everson JS, Ward ME. The influence of surface charge on the attachment of Neisseria gonorrhoeae to human cells. J Gen Microbiol. 1976;96:359–364. doi: 10.1099/00221287-96-2-359. [DOI] [PubMed] [Google Scholar]
- Higgs HN, Pollard TD. Activation by Cdc42 and PIP(2) of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J Cell Biol. 2000;150:1311–1320. doi: 10.1083/jcb.150.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, et al. Architecture of Eph receptor clusters. Proc Natl Acad Sci U S A. 2010;107:10860–10865. doi: 10.1073/pnas.1004148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hower S, Wolf K, Fields KA. Evidence that CT694 is a novel Chlamydia trachomatis T3S substrate capable of functioning during invasion or early cycle development. Mol Microbiol. 2009;72:1423–1437. doi: 10.1111/j.1365-2958.2009.06732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TY, DerMardirossian C, Bokoch GM. Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol. 2006;18:26–31. doi: 10.1016/j.ceb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Humphries AD, Townsend SM, Kingsley RA, Nicholson TL, Tsolis RM, Baumler AJ. Role of fimbriae as antigens and intestinal colonization factors of Salmonella serovars. FEMS Microbiol Lett. 2001;201:121–125. doi: 10.1111/j.1574-6968.2001.tb10744.x. [DOI] [PubMed] [Google Scholar]
- Innocenti M, Zucconi A, Disanza A, Frittoli E, Areces LB, Steffen A, Stradal TE, Di Fiore PP, Carlier MF, Scita G. Abi1 is essential for the formation and activation of a WAVE2 signalling complex. Nat Cell Biol. 2004;6:319–27. doi: 10.1038/ncb1105. [DOI] [PubMed] [Google Scholar]
- Jewett TJ, Dooley CA, Mead DJ, Hackstadt T. Chlamydia trachomatis tarp is phosphorylated by src family tyrosine kinases. Biochem Biophys Res Commun. 2008;371:339–344. doi: 10.1016/j.bbrc.2008.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett TJ, Fischer ER, Mead DJ, Hackstadt T. Chlamydial TARP is a bacterial nucleator of actin. Proc Natl Acad Sci U S A. 2006;103:15599–15604. doi: 10.1073/pnas.0603044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett TJ, Miller NJ, Dooley CA, Hackstadt T. The conserved Tarp actin binding domain is important for chlamydial invasion. PLoS Pathog. 2010;6:e1000997. doi: 10.1371/journal.ppat.1000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D, Gomez TS, Metlagel Z, Umetani J, Otwinowski Z, Rosen MK, Billadeau DD. WASH and WAVE actin regulators of the Wiskott-Aldrich syndrome protein (WASP) family are controlled by analogous structurally related complexes. Proc Natl Acad Sci U S A. 2010;107:10442–10447. doi: 10.1073/pnas.0913293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleba B, Clark TR, Lutter EI, Ellison DW, Hackstadt T. Disruption of the Rickettsia rickettsii Sca2 autotransporter inhibits actin-based motility. Infect Immun. 2010;78:2240–2247. doi: 10.1128/IAI.00100-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler LA, Celli J, Finlay BB. Pathogenic trickery: deception of host cell processes. Nat Rev Mol Cell Biol. 2001;2:578–588. doi: 10.1038/35085062. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska K, Sobota A. Signaling pathways in phagocytosis. Bioessays. 1999;21:422–431. doi: 10.1002/(SICI)1521-1878(199905)21:5<422::AID-BIES9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lambert MA, Smith SG. The PagN protein mediates invasion via interaction with proteoglycan. FEMS Microbiol Lett. 2009;297:209–216. doi: 10.1111/j.1574-6968.2009.01666.x. [DOI] [PubMed] [Google Scholar]
- Lane BJ, Mutchler C, Al Khodor S, Grieshaber SS, Carabeo RA. Chlamydial entry involves TARP binding of guanine nucleotide exchange factors. PLoS Pathog. 2008;4:e1000014. doi: 10.1371/journal.ppat.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebensohn AM, Kirschner MW. Activation of the WAVE complex by coincident signals controls actin assembly. Mol Cell. 2009;36:512–524. doi: 10.1016/j.molcel.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M, Ohayon H, Braun L, Mengaud J, Cossart P. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect Immun. 1997;65:5309–5319. doi: 10.1128/iai.65.12.5309-5319.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Abreu-Blanco MT, Barry KC, Linardopoulou EV, Osborn GE, Parkhurst SM. Wash functions downstream of Rho and links linear and branched actin nucleation factors. Development. 2009;136:2849–2860. doi: 10.1242/dev.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly KT, Casanova JE. Abelson tyrosine kinase facilitates Salmonella enterica serovar Typhimurium entry into epithelial cells. Infect Immun. 2009;77:60–69. doi: 10.1128/IAI.00639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo GV, Espina M, Smith AC, Terebiznik MR, Aleman A, Finlay BB, et al. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J Cell Biol. 2008;182:741–752. doi: 10.1083/jcb.200804131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia E, Bliska JB, Viboud GI. Yersinia controls type III effector delivery into host cells by modulating Rho activity. PLoS Pathog. 2008;4:e3. doi: 10.1371/journal.ppat.0040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J, Popoff MR, Enninga J, Frame MC, Sansonetti PJ, Van Nhieu GT. The IpaC carboxyterminal effector domain mediates Src-dependent actin polymerization during Shigella invasion of epithelial cells. PLoS Pathog. 2009;5:e1000271. doi: 10.1371/journal.ppat.1000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolz JC, Medeiros RB, Mitchell JS, Zhu P, Freedman BD, Shimizu Y, Billadeau DD. WAVE2 regulates high-affinity integrin binding by recruiting vinculin and talin to the immunological synapse. Mol Cell Biol. 2007;27:5986–6000. doi: 10.1128/MCB.00136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrick SB, Cheng HC, Ismail AM, Panchal SC, Doolittle LK, Kim S, et al. Hierarchical regulation of WASP/WAVE proteins. Mol Cell. 2008;32:426–438. doi: 10.1016/j.molcel.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JC, Galan JE. Manipulation of the host actin cytoskeleton by Salmonella--all in the name of entry. Curr Opin Microbiol. 2005;8:10–15. doi: 10.1016/j.mib.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Patel JC, Galan JE. Differential activation and function of Rho GTPases during Salmonella-host cell interactions. J Cell Biol. 2006;175:453–463. doi: 10.1083/jcb.200605144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistor S, Chakraborty T, Niebuhr K, Domann E, Wehland J. The ActA protein of Listeria monocytogenes acts as a nucleator inducing reorganization of the actin cytoskeleton. EMBO J. 1994;13:758–763. doi: 10.1002/j.1460-2075.1994.tb06318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Cerdá J, Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124:715–27. doi: 10.1016/j.cell.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Pizarro-Cerda J, Sousa S, Cossart P. Exploitation of host cell cytoskeleton and signalling during Listeria monocytogenes entry into mammalian cells. C R Biol. 2004;327:115–123. doi: 10.1016/j.crvi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Quinlan ME, Heuser JE, Kerkhoff E, Mullins RD. Drosophila Spire is an actin nucleation factor. Nature. 2005;433:382–388. doi: 10.1038/nature03241. [DOI] [PubMed] [Google Scholar]
- Renault L, Bugyi B, Carlier MF. Spire and Cordon-bleu: multifunctional regulators of actin dynamics. Trends Cell Biol. 2008;18:494–504. doi: 10.1016/j.tcb.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Nollau P, Ho HY, Kirschner MW, Mayer BJ. Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J Biol Chem. 2001;276:26448–26452. doi: 10.1074/jbc.M103856200. [DOI] [PubMed] [Google Scholar]
- Sallee NA, Rivera GM, Dueber JE, Vasilescu D, Mullins RD, Mayer BJ, Lim WA. The pathogen protein EspF(U) hijacks actin polymerization using mimicry and multivalency. Nature. 2008;454:1005–1008. doi: 10.1038/nature07170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Scita G, Casanova JE. WAVE2 signaling mediates invasion of polarized epithelial cells by Salmonella typhimurium. J Biol Chem. 2005;280:29849–29855. doi: 10.1074/jbc.M500617200. [DOI] [PubMed] [Google Scholar]
- Subtil A, Wyplosz B, Balana ME, Dautry-Varsat A. Analysis of Chlamydia caviae entry sites and involvement of Cdc42 and Rac activity. J Cell Sci. 2004;117:3923–3933. doi: 10.1242/jcs.01247. [DOI] [PubMed] [Google Scholar]
- Swimm A, Bommarius B, Reeves P, Sherman M, Kalman D. Complex kinase requirements for EPEC pedestal formation. Nat Cell Biol. 2004;6:795. doi: 10.1038/ncb0904-795a. [DOI] [PubMed] [Google Scholar]
- Thalmann J, Janik K, May M, Sommer K, Ebeling J, Hofmann F, et al. Actin re-organization induced by Chlamydia trachomatis serovar D--evidence for a critical role of the effector protein CT166 targeting Rac. PLoS One. 2010;5:e9887. doi: 10.1371/journal.pone.0009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres E, Rosen MK. Protein-tyrosine kinase and GTPase signals cooperate to phosphorylate and activate Wiskott-Aldrich syndrome protein (WASP)/neuronal WASP. J Biol Chem. 2006;281:3513–20. doi: 10.1074/jbc.M509416200. [DOI] [PubMed] [Google Scholar]
- Truttmann MC, Rhomberg TA, Dehio C. Combined action of the type IV secretion effector proteins BepC and BepF promotes invasome formation of Bartonella henselae on endothelial and epithelial cells. Cell Microbiol. 2010;13:284–299. doi: 10.1111/j.1462-5822.2010.01535.x. [DOI] [PubMed] [Google Scholar]
- Unsworth KE, Way M, McNiven M, Machesky L, Holden DW. Analysis of the mechanisms of Salmonella-induced actin assembly during invasion of host cells and intracellular replication. Cell Microbiol. 2004;6:1041–1055. doi: 10.1111/j.1462-5822.2004.00417.x. [DOI] [PubMed] [Google Scholar]
- Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C. Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol. 2008;87:649–667. doi: 10.1016/j.ejcb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol. 2005;7:894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- Vingadassalom D, Campellone KG, Brady MJ, Skehan B, Battle SE, Robbins D, et al. Enterohemorrhagic E. coli requires N-WASP for efficient type III translocation but not for EspFU-mediated actin pedestal formation. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingadassalom D, Kazlauskas A, Skehan B, Cheng HC, Magoun L, Robbins D, Rosen MK, Saksella K, Leong JM. Insulin receptor tyrosine kinase substrate links the E. coli O157:H7 actin assembly effectors Tir and EspFU during pedestal formation. Proc Natl Acad Sci USA. 2009;106:6754–6759. doi: 10.1073/pnas.0809131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber SS, Ragaz C, Hilbi H. Pathogen trafficking pathways and host phosphoinositide metabolism. Mol Microbiol. 2009;71:1341–1352. doi: 10.1111/j.1365-2958.2009.06608.x. [DOI] [PubMed] [Google Scholar]
- Weiss SM, Ladwein M, Schmidt D, Ehinger J, Lommel S, Stading K, et al. IRSp53 links the enterohemorrhagic E. coli effectors Tir and EspFU for actin pedestal formation. Cell Host Microbe. 2009;5:244–258. doi: 10.1016/j.chom.2009.02.003. [DOI] [PubMed] [Google Scholar]


