Abstract
The expression of toxic viral proteins for the purpose of eliminating distinct populations of cells, while leaving the rest of an organism unaffected, is a valuable method for analyzing development. Using the Gal4-UAS system, we employed the M2(H37A) toxic ion channel of the influenza-A virus to selectively ablate the Drosophila eye-antennal imaginal discs, hemocytes, dorsal vessel and nervous tissue and comparatively monitored the effects of expressing the apoptosis-promoting protein Reaper in identical cell populations. In this report, we demonstrate the effectiveness of M2(H37A)-mediated ablation as a new means to selectively eliminate cells of interest during Drosophila development.
Key words: cell ablation, dorsal vessel, Drosophila, eye imaginal disc, hand, lamellocytes, M2 toxin, reaper
Introduction
Utilizing viral proteins for induction of cell-specific death is a valuable tool for the analysis of the intricacies of cell interdependency in organ formation, elucidating new functions of distinct cell populations and development of an organism as a whole.1
The wild-type M2 protein exists as a 97 amino acid protein that has a transmembrane domain comprised of four alpha helices2,3 that form the pore of a proton selective ion channel,4 which is activated to conduct transport of protons at low pH (below 6.5).5 The modified M2 channel, referred to as M2(H37A), loses channel selectivity for protons, allows for the additional transport of Na+ and K+ and reduces the pH sensitivity of the channel such that it is constitutively active.6,7 Cells that are transgenically expressing this mutant channel thus become permeable to a continuous influx of monovalent cations and are destroyed. This M2(H37A) variation of the influenza A virus M2 protein has been proven to work successfully in a vertebrate model system, Xenopus laevis, to selectively ablate both heart cells and macrophages.1 The benefits of using this specific toxic viral protein over other previously utilized transgenic toxins has been discussed in detail.1
In this study, M2(H37A) has been expressed using the Gal4-UAS system8 and cell-specific enhancers to induce selective cell death in Drosophila imaginal discs, hemocytes and heart cells. We demonstrate that use of the toxin effectively causes specific cell ablation of Drosophila tissues, and is equally effective as ablation driven by the well established pro-apoptotic gene, reaper (rpr), a regulator of programmed cell death.9 This system is thus a useful tool for studying cell lineages and the function of distinct cell populations during development. Furthermore, it may be used in conjunction with any number of different enhancers for lineage-specific cell ablation.
Results and Discussion
To introduce ablation by influenza virus M2(H37A) into the Drosophila system, UAS-M2(H37A) transgenic lines were generated. Then tissue-specific Gal4 drivers were employed for expression of the toxin in eye, blood, heart and neural tissues. Identical crosses were carried out with UAS-rpr for comparison of the M2(H37A) toxin effect.
Ablation of the eye.
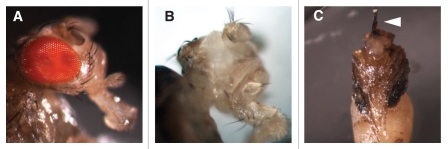
First, M2(H37A)-induced ablation was applied to eye development. The efficiency of toxin-induced cell ablation was most clearly seen in crosses with eyeless-Gal4, an eye-specific enhancer.10 Both crosses of UAS-rpr and UAS-M2(H37A)-3ME with eyeless-Gal4 resulted in a lack of eclosion of flies kept at room temperature and 25°C. When flies were removed from their pupal casings, it was found that they had arrested at different developmental stages ranging from early pupae to almost fully developed flies. As a result, some cephalic structures consisted of only the presumptive proboscis still attached to the larval mouth hook (Fig. 1C), while others were fully formed though lacking any eyes. Crosses between eyeless-Gal4 and less efficient toxin lines (ex. UAS-M2(H37A)-1LE) were also conducted to verify that the toxin was working specifically to ablate the eyes. A small number of flies from these crosses were able to eclose and they displayed a total absence of eye tissue (Fig. 1B), reduced eyes, and/or cyclopia. Premature death caused by ablation of cephalic structures, observed in M2(H37A) toxin and Rpr crosses, were due to non-specificity of the eyeless driver. This was correlated with the observed expression pattern of eyeless-Gal4>UAS-2x EGFP.
Figure 1.
Varying strengths of different toxin lines in ablating the eye. (A) Control: y1 w67c23 x eyeless-Gal4. (B) Crosses between eyeless-Gal4 and the less effective UAS-M2(H37A)-1LE toxin line resulted in a rate of eclosion of 1:10. The progeny from this cross displayed the total absence of eyes, reduced eyes, and/or cyclopia. (C) In contrast, crosses with both the highly efficient toxin lines UAS-M2(H37A)-3ME and UAS-rpr resulted in flies arresting at different stages of development within pupal casings. Progeny from UAS-M2(H37A)-3ME toxin strain x eyeless-Gal4 cross showed cephalic structure consisting of only the presumptive proboscis still attached to the larval mouth hook (arrowhead). Ablation of cephalic structures were due to the non-specificity of the eyeless driver, as verified by eyeless-Gal4>UAS-GFP screening.
Blood cell ablation.
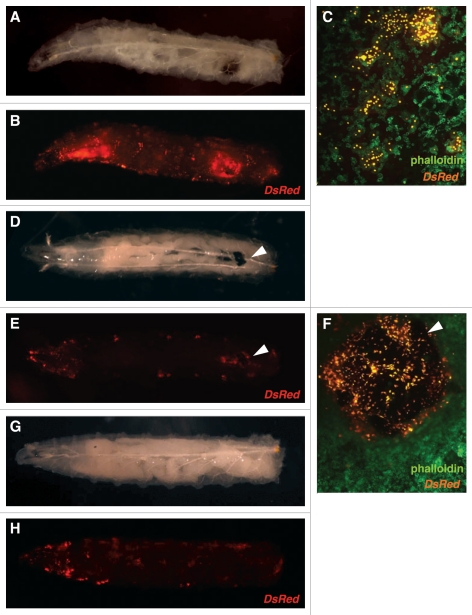
Next, blood cell-specific ablation was achieved with the M2(H37A) toxin utilizing the lamellocyte enhancer line MSNF9mo-Gal4>UAS-Red Stinger in a hopTuml background. The dominant hopTuml mutation in the Hop Janus kinase causes hyperactivation of the Hop-DStat92e pathway11 and premature differentiation of prohemocytes leading to leukemia-like defects in Drosophila.12 MSNF9mo is a characterized enhancer region that is active in lamellocytes13 and MSNF9mo-Gal4>UAS-Red Stinger larvae display strong fluorescence due to the massive amounts of lamellocytes produced in the hopTuml background. When crossed with UAS-M2(H37A)-3ME, however, almost all lamellocytes were destroyed, and those that remained were aggregated into large melanotic masses (Fig. 2D–F and arrowheads). In comparison, UAS-rpr induced by MSNF9moGal4 resulted in substantial cell death but only a few small melanotic masses, as expected with normal clearance of cells undergoing apoptosis. Blood smears of third instar larvae confirmed specificity of toxin ablation and demonstrated a significant reduction in circulating lamellocytes, with other hemocyte populations unaffected. The observation that M2(H37A) and Rpr function led to qualitatively different results post lamellocyte ablation, in terms of enhanced melanotic tumor formation being observed in M2(H37A) animals, suggested the two proteins do not act via the same apoptosis-promoting mechanism.
Figure 2.
Increase in size of melanotic masses in third instar larvae. hopTum-l MSNF9mo-Gal4>UAS-Red Stinger was crossed with y1 w67c23 (A–C), UAS-M2(H37A)-3ME (D–F) and UAS-rpr (G and H). A significant reduction in circulating lamellocytes marked with DsRed was seen in crosses with both UAS-M2(H37A)-3ME and UAS-rpr as shown in (E and H), respectively. In addition, large melanotic masses formed in more than half of all larvae from the UAS-M2(H37A)-3ME crosses (arrowheads in D–F). In contrast, resulting progeny from crosses between UAS-rpr and hopTum-l MSNF9-Gal4 UAS-Red Stinger did not display large melanotic masses (G and H). In third instar circulating hemocytes, a reduction in lamellocytes was seen in the progeny from UAS-M2(H37A)-3ME crosses (F) as compared with the control crosses with y1 w67c23 (C).
Similar results were seen with the crosses with the hemocyte enhancer hemese-Gal4, which is expressed in about 80% of circulating hemocytes, and displays strong ectopic expression in the salivary glands.14 Expression of the toxin resulted in a significant reduction in fluorescent cells, including ablation of the salivary glands in all third instar larvae and melanotic masses in the hemolymph that attached to organelles in roughly half of the total population screened. Though ablation with UAS-rpr resulted in a reduction in fluorescence comparable to that of larvae from crosses with UAS-M2(H37A)-3ME, it was found that Rpr was not always completely effective in that some third instar larvae still had aggregations of GFP-expressing hemocytes and GFP-positive blood smears though melanotic masses were rarely seen.
Ablation of the heart.
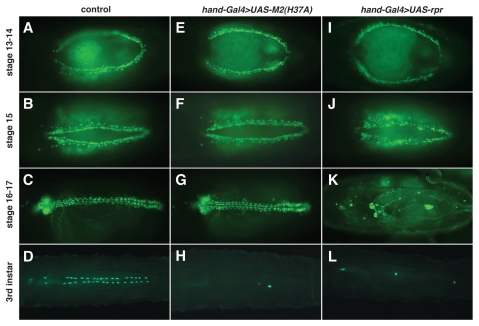
The M2(H37A) toxin was also used to ablate heart cells. To induce the M2(H37A) transgene in the dorsal vessel, crosses were made with hand-GFP; hand-Gal4, which displays expression in cardioblasts and pericardial cells of the embryonic, larval and adult dorsal vessel. Similar to what was observed in eye tissue, M2(H37A)-induced ablation (Fig. 3E–H) did not commence until mid to late second instar larval stage, whereas Rpr-induced cell death began at about embryonic stage 15 (Fig. 3J), leaving the lymph glands freely floating by the beginning of first larval instar stage. Corresponding to the findings in larvae, M2(H37A)-expressing adult flies displayed zero to one pericardial cell associated with the dorsal vessel (Fig. 4C). In contrast, cardioblasts seemed to be intact for crosses done with UAS-M2(H37A)-3ME, indicating that the efficiency of ablation is dependent on cardiac cell type. Analysis of the phase of lethality indicated no significant death, as compared to the control cross (y1 w67c23 crossed with hand-GFP; hand-Gal4) during embryogenesis, larval or pupal stages at 29°C for progeny expressing the toxin (Suppl. Fig. 1).
Figure 3.
Developmental profile of dorsal vessel formation following crosses with UAS-(H37A)-3ME and UAS-rpr at 29°C. hand-GFP; hand-Gal4 was crossed with y1w67c23 (A–D), UAS-M2(H37A)-3ME (E–H) and UAS-rpr (I–L). Rpr-mediated ablation of cardioblasts begins at embryonic stage 15, whereas the M2(H37A) toxin is only activated at the late second larval instar stage. Embryonic stage was calculated from the middle of a 2 hr egg lay. Embryonic stage 13–14 (A, E and I), 15 (B, F and J), 16–17 (C, G and K) and third instar larvae (D, H and L). Green indicates hand-GFP-expressing cardioblasts, pericardial cells or lymph gland cells.
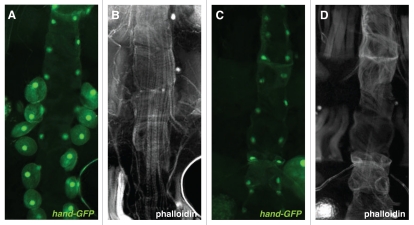
Figure 4.
Visualization of the adult heart. (A and B) Control cross: hand-GFP; hand-Gal4 with y1w67c23. (C and D) hand-GFP; hand-Gal4 crossed with UAS-M2(H37A)-3ME. Dorsal vessel myofibrils of dissected adult females (≤2 days old) were visualized with Alexa594-conjugated phalloidin, with cardioblasts and pericardial cells marked by hand-GFP. All pericardial cells except for one, in one third of population screened, were ablated by crosses with the M2(H37A) toxin. As a result of the ablation crosses, all dorsal vessels were characterized by abnormally organized myofibrils but maintained cardioblast GFP expression.
Adult progeny from crosses with UAS-rpr, which produced almost complete ablation of the dorsal vessel, resulted in significantly reduced life spans, with a precipitous increase in death on average at about five days post-eclosion (Suppl. Fig. 1). In contrast, adult progeny from crosses with UAS-M2(H37A)-3ME, which specifically ablated the excretory cells (pericardial nephrocytes) that flank the dorsal vessel (Fig. 4C), did not have as severe of a reduction in life span, but did die prematurely relative to control animals (y1 w67c23 crossed with hand-GFP; hand-Gal4) (Suppl. Fig. 1).
Ablation of nervous tissue.
The toxin line was also tested with the embryonic nervous system driver elav-Gal4, which is expressed in glial cells and mitotically active neuronal progenitor cells in the brain and presumptive ventral ganglion beginning at embryonic stage 12,15,16 and continues to be expressed in the central nervous system of third instar larva. Similar to what was observed in the heart, no ablation of elav-expressing nervous system cells occurred during embryonic stages. Ablation of cells only began to be apparent during the middle of the second instar larval stage (data not shown). Notably, UAS-rpr did not work as efficiently in ablating cells expressing elav-Gal4. Stage 17 embryos showed a reduction in fluorescence intensity and an absence of the presumptive salivary glands. However, the ventral ganglion remained intact through the end of the larval first instar stage (data not shown).
In summary, it has been determined that M2(H37A) toxin-mediated cell destruction is as efficient as rpr in selectively ablating hemocytes and imaginal discs. However unlike rpr, the toxin displays a characteristic of being more efficient in ablating certain cell types (ex. pericardial cells vs. cardioblasts) and showing a delay in cell ablation until post-embryonic development. Utilization of this new method of cell ablation in Drosophila may be highly beneficial to studies which require a method of cell ablation that is not completely dependent on apoptosis and allows for selective elimination of imaginal or polytene cells during larval stages of development.
Materials and Methods
Drosophila strains.
Fly stocks were kept on standard cornmeal-based medium. The following lines were used: hand-GFP; hand-Gal4, a gift from Zhe Han (University of Michigan) and hopTuml, a gift from Shubha Govind (City College of New York). The following fly stocks were obtained from the Bloomington Drosophila Sock Center: eyeless-Gal4 (FBst0005534), rpr-Gal4 (FBst0005824), elavC155-Gal4 UAS-eGFP (FBst0006925), UAS-2xEGFP (FBst0006874), UAS-Red Stinger (FBst0008545) and hemese-Gal4 UAS-GFP (FBst0008700). y1 w67c23 (FBst0006599) was used as a wild-type strain. While these Gal4-inducing strains are well characterized as to their cellular sites of expression, the initial characterization of other Gal4 strains by the G-TRACE cell lineage analysis technique may prove worthwhile prior to their use in directing UAS-M(H37A) for the purpose of selective cell ablation.17
DNA construction and generation of transgenic flies.
Directed expression of the toxin gene was accomplished using the Gal4-UAS system.8 pUAST-M2(H37A) was derived by inserting the M2(H37A) toxin-coding sequence from pCS2-M2(H37A)1 into the BgLII and XbaI sites of the pUAST vector.8 For the generation of the lamellocyte specific Gal4 driver, the misshapen 140 bp region was cut with Bgl II and BamHI from pHStinger-MSNF9mo13 and subcloned into a pPTGAL vector.18 Injections were performed as described previously.19 At least four independent transgenic lines were generated and analyzed for pUAST-M2(H37A) construct function. All four demonstrated the ability to ablate cells, but with different efficacies presumably due to positional effects of transgene chromosome site insertions. The UAS-M2(H37A)-1LE strain was shown to be less efficient in killing cells while the UAS-M2(H37A)-3ME strain proved highly efficient in this process. We also recombined an MSNF9mo-Gal4 line with UAS-Red Stinger and hopTum-l on the same X chromosome. Due to the temperature-dependent nature of Gal4 induction, experiments were carried out at room temperature, 25°C and 29°C to monitor the effect of induction at different levels.
GFP assessment of embryos and larva at specific stages.
UAS-M2(H37A)-3ME males were crossed to females of the indicated transgenic strains, with females allowed to lay eggs for 2 hr on grape agar plates with yeast paste before removal of the parents. Embryos and larva were kept on the plates and grown at indicated rearing temperatures (ambient, 25°C, 29°C). Embryos were dechorionated with 50% bleach for 3 min and washed with DI water prior to imaging. Embryos and first instar larva were imaged using a Zeiss Axioplan2 microscope (Thornwood, NY, USA) and subsequent larval stages were imaged using a Zeiss Lumar.V12 Stereoscope. Larval ages were calculated from the midpoint of the egg lay period.
Analysis of the adult dorsal vessel.
Dissection and analysis of the adult fly heart was achieved using a modified protocol.20 A live adult female (≤2 days post-eclosion) was mounted at the wings and dorsal side of the thorax, with its ventral side up, onto a glass slide, with melted paraffin. The legs were removed and abdomen was then dissected under 50 µl 1x PBS. After the ventral epithelium, Crop, Malpighian tubules, gut, rectal ampulla, ovaries and fat bodies were removed, the abdomen was fixed with 50 µl 3.7% formaldehyde in PBS for 20 min and then incubated with Alex594 conjugated phalloidin (1:40 in PBS, Molecular Probes Invitrogen) for 72 hr, in the dark, at 18°C for actin staining of cardiac myofibrils.
Acknowledgements
We are grateful to T. Mohun and S. Smith for providing the M2(H37A) cDNA clone, Z. Han for sending the hand driver/reporter stock and the Bloomington Stock Center for providing the eyeless-Gal4 and UAS-reaper fly lines. This research was supported by grants from the National Institutes of Health to Robert A. Schulz (HL059151, HL071540) and facilitated through the use of the Notre Dame Integrated Imaging Facility.
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/13114
Supplementary Material
References
- 1.Smith SJ, Kotecha S, Towers N, Mohun TJ. Targeted cell-ablation in Xenopus embryos using the conditional, toxic viral protein M2(H37A) Dev Dyn. 2007;236:2159–2171. doi: 10.1002/dvdy.21233. [DOI] [PubMed] [Google Scholar]
- 2.Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40:627–633. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- 3.Sugrue RJ, Hay AJ. Structural characteristics of the M2 protein of influenza A viruses: Evidence that it forms a tetrameric channel. Virology. 1991;180:617–624. doi: 10.1016/0042-6822(91)90075-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 5.Lamb R, Pinto L. The M2 proton channels of influenza A and B viruses. J Biol Chem. 2005;281:8997–9000. doi: 10.1074/jbc.R500020200. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi CS, Shuck K, Lear JD, Dieckmann GR, DeGrado WF, Lamb RA, Pinto LH. Cu(II) inhibition of the proton translocation machinery of the influenza A virus M2 protein. J Biol Chem. 1999;274:5474–5482. doi: 10.1074/jbc.274.9.5474. [DOI] [PubMed] [Google Scholar]
- 7.Smith CA, Graham CM, Mathers K, Skinner A, Hay AJ, Schroeder C, Thomas DB. Conditional ablation of T-cell development by a novel viral ion channel transgene. Immunology. 2002;105:306–313. doi: 10.1046/j.0019-2805.2002.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 9.White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- 10.Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the small eye gene in mice and aniridia in humans. Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- 11.Luo H, Rose P, Barber D, Hanratty WP, Lee S, Roberts TM, et al. Mutation in the jak kinase JH2 domain hyperactivates Drosophila and mammalian jak-stat pathways. Mol Cell Biol. 1997;17:1562–1571. doi: 10.1128/mcb.17.3.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanratty WP, Ryerse JS. A genetic melanotic neoplasm of Drosophila melanogaster. Dev Biol. 1981;83:238–249. doi: 10.1016/0012-1606(81)90470-x. [DOI] [PubMed] [Google Scholar]
- 13.Tokusumi T, Sorrentino RP, Russell M, Ferrarese R, Govind S, Schulz RA. Characterization of a lamellocyte transcriptional enhancer located within the misshapen gene of Drosophila melanogaster. PLoS ONE. 2009;4:e6429. doi: 10.1371/journal.pone.0006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, Ando I, Hultmark D. A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci USA. 2004;101:14192–14197. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin DM, Goodman CS. Ectopic and increased expression of fasciclin II alters motoneuron growth cone guidance. Neuron. 1994;13:507–523. doi: 10.1016/0896-6273(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 16.Berger C, Renner S, Luer K, Technau GM. The commonly used marker ELAV is transiently expressed in neuroblasts and glial cells in the Drosophila embryonic CNS. Dev Dyn. 2007;236:3562–3568. doi: 10.1002/dvdy.21372. [DOI] [PubMed] [Google Scholar]
- 17.Evans CJ, Olson JM, Ngo KT, Kim E, Lee NE, Kuoy E, et al. G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Methods. 2009;6:603–605. doi: 10.1038/nmeth.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma Y, Cheung U, Larsen EW, Eberl DF. PPTGAL, a convenient Gal4 P-element vector for testing expression of enhancer fragments in Drosophila. Genesis. 2002;34:115–118. doi: 10.1002/gene.10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gajewski K, Fossett N, Molkentin JD, Schulz RA. The zinc finger proteins pannier and GATA4 function as cardiogenic factors in Drosophila. Development. 1999;126:5679–5688. doi: 10.1242/dev.126.24.5679. [DOI] [PubMed] [Google Scholar]
- 20.Lo PC, Zaffran S, Sénatore S, Frasch M. The Drosophila hand gene is required for remodeling of the developing adult heart and midgut during metamorphosis. Dev Biol. 2007;311:287–296. doi: 10.1016/j.ydbio.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.