Abstract
The medial shell of nucleus accumbens (NAc) and its mesolimbic dopamine inputs mediate forms of fearful as well as of incentive motivation. For example, either appetitive and/or actively fearful behaviors are generated in a keyboard pattern by localized glutamate disruptions in NAc (via microinjection of the AMPA receptor antagonist DNQX) at different anatomical locations along a rostrocaudal gradient within the medial shell of rats. Rostral glutamate disruptions produce intense increases in eating, but more caudally placed disruptions produce increasingly fearful behaviors: distress vocalizations and escape attempts to human touch, and a spontaneous and directed antipredator response called defensive treading/burying. Local endogenous dopamine is required for either intense motivation to be generated by AMPA disruptions. Here we report that only endogenous local signaling at D1 dopamine receptors is needed for rostral generation of excessive eating, potentially implicating a direct output pathway contribution. In contrast, fear generation at caudal sites requires both D1 and D2 signaling simultaneously, potentially implicating an indirect output pathway contribution. Finally, when motivation valence generated by AMPA disruptions at intermediate sites was flipped by manipulating environmental ambience, from mostly appetitive in a comfortable home environment to mostly fearful in a stressful environment, the roles of local D1 and D2 signaling in dopamine/glutamate interaction at microinjection sites also switched dynamically to match the motivation valence generated at the moment. Thus, NAc D1 and D2 receptors, and their associated neuronal circuits, play different and dynamic roles in enabling desire and dread to be generated by localized NAc glutamate disruptions in medial shell.
Introduction
Intense aberrant motivation is an important feature of psychopathological disorders, ranging from intense appetitive motivation in addiction and binge eating to more fearful paranoia in schizophrenia and anxiety disorders (Barch, 2005; Kalivas and Volkow, 2005; Howes and Kapur, 2009; Woodward et al., 2011). Both appetitive and fearful motivations involve interactions between dopamine and glutamate in overlapping mesocorticolimbic circuits that converge on nucleus accumbens (NAc) (Kelley et al., 2005; Faure et al., 2008; Meredith et al., 2008; Carlezon and Thomas, 2009; Kalivas et al., 2009; Humphries and Prescott, 2010).
NAc and dopamine-related circuits are best known for roles in appetitive motivation (Schultz, 2007; Wise, 2008), but are also implicated in some forms of aversive motivation related to fear, stress, disgust, and pain (Levita et al., 2002; Salamone et al., 2005; Ventura et al., 2007; Matsumoto and Hikosaka, 2009; Zubieta and Stohler, 2009; Cabib and Puglisi-Allegra, 2011). Within medial shell of NAc, neuroanatomical coding plays an important role in determining appetitive versus fearful valence of intense motivations generated by glutamate disruptions.
Local AMPA blockade [e.g., by 6,7-dinotroquinoxaline-2,3(1H,4H)-dione (DNQX) microinjection] produces intense eating and/or fearful reactions in an anatomical keyboard pattern along a rostrocaudal gradient (Reynolds and Berridge, 2001, 2003; Faure et al., 2008; Reynolds and Berridge, 2008). At rostral sites in medial shell, purely positive/appetitive behavior, such as intense eating, is produced by local glutamate disruptions (Maldonado-Irizarry et al., 1995; Kelley and Swanson, 1997). In contrast, as locations move caudally, disruptions generate progressively more fearful behaviors, including reactive distress vocalizations and escape dashes in response to touch, and spontaneous actively fearful behaviors such as an antipredator response of defensive treading/burying, in which rodents use rapid forepaw movements to toss sand or bedding at a threatening stimulus (e.g., rattlesnake) (Coss and Owings, 1978; Treit et al., 1981; Reynolds and Berridge, 2001, 2003; Faure et al., 2008; Reynolds and Berridge, 2008). At intermediate sites in NAc shell, glutamate disruptions generate a mixture of both behaviors, and the dominant valence can be flexibly flipped between positive and negative by changing environmental ambience between familiar and stressful (Reynolds and Berridge, 2008).
We reported previously that endogenous dopamine activity was required locally for glutamate disruptions in NAc shell to generate feeding or fear (Faure et al., 2008). What remains unknown are the relative roles of D1-like versus D2-like dopamine receptors and their associated direct versus indirect output circuits in DNQX-generated motivations. Here we addressed these roles, and found that only D1 receptor stimulation, potentially involving the direct pathway to ventral tegmentum (VTA), was needed for glutamatergic disruptions to generate appetitive eating at rostral sites. In contrast, endogenous activity at both D1 and D2 receptors, potentially recruiting a stronger role of the indirect pathway to ventral pallidum (VP) and lateral hypothalamus (LH), was needed for DNQX to generate fearful behavior at caudal sites. Furthermore, we found that motivational valence trumped rostrocaudal location at flexible intermediate sites, which switched reversibly between an appetitive mode that required only D1 neurotransmission and a fearful mode that required simultaneous D1 and D2 neurotransmission.
Materials and Methods
Subjects
Male Sprague Dawley rats (total, n = 87; feeding and fear test groups, n = 51; Fos plume groups, n = 36), weighing 300–400 g at surgery, were housed at ∼21°C on a reverse 12 h light/dark cycle. All rats had ad libitum access to both food and water. All of the following experimental procedures were approved by the University Committee on the Use and Care of Animals at the University of Michigan.
Cranial cannulation surgery
Rats were anesthetized with intraperitoneal injections of ketamine hydrochloride (80 mg/kg) and xylazine (5 mg/kg) and treated with atropine (0.05 mg/kg) to prevent respiratory distress, and then placed in a stereotaxic apparatus (David Kopf Instruments). The incisor bar was set at 5.0 mm above intra-aural zero, angling cannula trajectory so as to avoid penetrating the lateral ventricles. Under surgical anesthesia, rats (n = 87) received bilateral implantation of permanent cranial cannulae (14 mm, 23-gauge, stainless steel) aimed at staggered points throughout the rostrocaudal extent of medial shell of NAc. Cannulae were bilaterally inserted at coordinates between +2.4 to +3.1 mm anteroposterior (AP), ±0.9 to 1.0 mm mediolateral (ML), and −5.6 to 5.7 mm dorsoventral (DV) from bregma. Cannulae were anchored to the skull using surgical screws and dental acrylic. Stainless-steel obturators (28 gauge) were inserted in cannulae to avoid occlusion. After surgery, each rat received subcutaneous injection of chloramphenicol sodium succinate (60 mg/kg) to prevent infection and carprofen (5 mg/kg) for pain relief. Rats received carprofen again 24 h later and were allowed to recover for at least 7 d before testing began.
Drugs and intracerebral microinjections
Localized glutamate disruptions in medial shell were induced before behavioral tests by bilateral microinjections of DNQX, an AMPA/kainate receptor glutamate antagonist (Sigma) at a dose of 450 ng/0.5 μl per side. Either DNQX or vehicle (0.5 μl per side) was microinjected alone, or in combination with (1) the selective D1 antagonist R(+)-7-chloro-8-hydroxy-3-methyl1-phenyl-2,3,4,5,-tetrahydro-1H-3-benzazepine (SCH23390; Sigma) at a dose of 3 μg/0.5 μl per side, (2) the selective D2 antagonist raclopride (3,5-dichloro-N-{[(2S)-1-ethylpyrrolidin-2-yl]methyl}-2-hydroxy-6-methoxybenzamide) at a dose of 5 μg/ 0.5 μl per side, or (3) both SCH23390 and raclopride. Drug doses were chosen based on studies by Faure et al. (2008) and Reynolds and Berridge (2003). All drugs were dissolved in a vehicle of 50% DMSO mixed with 50% 0.15 m saline and microinjected at a volume of 0.5 μl per side. The pH was normalized to 7.0 to 7.4 using HCl for both drug and vehicle microinjections. On test days, solutions were brought to room temperature (∼21°C), inspected to confirm the absence of precipitation, and bilaterally infused at a speed of 0.3 μl/min by syringe pump via PE-20 polyethylene tubing through stainless-steel injectors (16 mm, 29 gauge) extending 2 mm beyond the guide cannulae to reach NAc targets. Injectors were left in place for 1 min following microinjection to allow drug diffusion, after which obturators were replaced, and rats were immediately placed in the testing chamber.
Glutamate/dopamine interaction group.
Each rat tested for motivated behavior (n = 23) received the following five drug microinjections on different days, spaced 48 h apart, in counterbalanced order: (1) vehicle alone, (2) DNQX alone (to elicit motivated behavior), (3) a mixture of DNQX plus SCH23390 (D1 blockade), (4) DNQX plus raclopride (D2 blockade), and (5) DNQX plus both SCH23390 and raclopride (combined dopamine blockade) (Faure et al., 2008).
Independent dopamine blockade group.
A separate group of rats (n = 18) was tested for motivated behavior after receiving microinjections of dopamine antagonists alone (without DNQX), DNQX alone, or vehicle to ensure that dopamine antagonists in NAc shell did not prevent DNQX from generating motivations by simply eliminating motoric capacity or normal motivated behavior. Use of different groups ensured that the number of microinjections any rat received was limited to five or six. This dopamine antagonist group received the following five drug conditions: (1) vehicle, (2) SCH23390 alone, (3) raclopride alone, (4) SCH23390 plus raclopride, and (5) DNQX alone (as a positive contrast to confirm that motivated behaviors could be generated at high intensities in these rats). All drug conditions were administered in counterbalanced order within each group, and tests were spaced at least 48 h apart.
Environmental shift group.
A separate environment-shift group (n = 10) was used to assess whether changing the environmental ambience flexibly altered the mode of dopamine/glutamate interactions at a particular site within the intermediate two-thirds of medial shell that is capable of generating both appetitive and fearful motivations (Reynolds and Berridge, 2008). Rats in this group had microinjection cannulae aimed at intermediate rostral–caudal sites. Each rat was tested on different days in two environments: comfortable and familiar “home” versus overstimulating and “stressful” (described below) in counterbalanced order. Rats were tested in each environment three times, also in counterbalanced order, after microinjections of either (1) vehicle, (2) DNQX, or (3) DNQX plus raclopride. Thus, each rat received six test conditions, all separated at least 48 h apart in balanced order.
Behavioral tests of spontaneous motivated behaviors
Following 3 d of handling, all rats tested for motivated behavior (n = 51) were habituated to the testing procedure and apparatus on 4 d for 1 h each. On the fourth day of habituation, rats received mock microinjections of vehicle before entering the test chamber, to habituate them to the microinjection procedure. On each test day, rats received one of the drug conditions described previously and were placed immediately in the transparent testing chamber (23 × 20 × 45 cm), which contained preweighed food (∼20 g rat chow) and ad libitum water, to allow the expression of appetitive behavior. The chamber also contained granular cob bedding spread on the floor ∼3 cm deep to allow the expression of defensive treading behavior. Behavior in the chamber was video recorded for 60 min, to be scored later offline for analysis. At the end of each session, rats were removed by the experimenter's gloved hand using a standardized slow-approach hand motion to quantify any fearful distress calls, escape attempts, or defensive bites elicited by human touch. Following a ∼5 s approach toward the testing cage, the experimenter slowly reached toward the rat, taking ∼2 s. Upon contact, the experimenter lightly brushed the side of the rat with gloved fingertips, taking ∼1 s, before lifting the rat from the chamber in a gentle movement that lasted ∼2 s. The observer recorded any attempts by the rat to escape when touched, as well as bites and audible distress vocalizations.
All behavioral tests for the glutamate/dopamine interaction and independent dopamine blockade groups (n = 41) were conducted in a “standard” lab environment (Reynolds and Berridge, 2008) following a brief transport from the home room. The standard environment was intended to be similar to most behavioral neuroscience laboratories in lighting, sounds, and odors, and to be of relatively neutral ambience (in between positive home and negative stress of the next experiment). This standard environment consisted of a conventional laboratory testing room (daylight illumination conditions of white fluorescent light intensity, 550–650 lux; ambient noise sound intensity, 65–70 dB) as described previously (Reynolds and Berridge, 2008).
Rats in the environmental shift group were tested in two environments of opposite extreme valence: (1) the home environment, which consisted of normal dim red lighting (5–10 lux) and quiet levels of ambient noise (65–70 dB, primarily rat noise and static noise from ventilation systems), as well as familiar odors and sights of the rat's own home room, versus (2) the stressful, high-intensity sensory-stimulation environment, which was conducted in the standard laboratory except that additional incandescent lamps were directed at the test chamber (1000–1300 lux within the cage), and loud, unpredictable sound was presented continuously throughout the test [raucous rock music from the continuous full-album soundtrack of Raw Power by Iggy and The Stooges (1973; Iggy Pop reissue 1997); 80–86 dB]. In preference tests, rats have been shown to prefer the home environment over the standard environment, and to prefer the standard lab environment over the stressful one (Reynolds and Berridge, 2008).
Behavioral coding
The incidence of elicited fearful distress vocalizations, escape dashes, and bite attempts directed at the experimenter's hand were scored when the rat was gently picked up at the end of the test session (Reynolds and Berridge, 2003), after which total grams of chow pellets consumed were recorded. Behaviors emitted spontaneously and videotaped during the 1 h test were subsequently scored by experimenters blind to treatment for the total cumulative duration (seconds) for each of the following: eating behavior (involving both appetitive approach and voluntary initiation of ingestion plus consummatory chewing and swallowing of food), drinking behaviors (licking from water spout), and fearful defensive treading/burying behavior (defined as active spraying or pushing of bedding with rapid alternating thrusts of the forepaws, spatially directed generally toward the brightly lit front or corners of the cage). Additionally, the number of bouts of appetitive behaviors such as food carrying and food sniffs, as well as less valenced behaviors such as rearing, cage crosses, and grooming behavior were also recorded.
Histology
Following behavioral testing, rats were deeply anesthetized with an overdose of sodium pentobarbital. Rats in which Fos plumes were measured were perfused and brains treated as described previously (Reynolds and Berridge, 2008). These included rats behaviorally tested in the environmental shift group (n = 10; which therefore received a seventh final drug or vehicle microinjection and behavioral test 90 min before perfusion) and a separate dedicated Fos group (n = 36; which were histologically assessed after just a single drug or vehicle microinjection into locations staggered throughout medial shell, administered under conditions identical to the first day of testing for behavioral rats). The purpose of the dedicated Fos group was to assess maximal local impact radius and avoid the danger of underestimating plume size due to progressive necrosis/gliosis over a series of microinjections that might shrink a final plume. If shrinkage occurred in the behaviorally tested group, that in turn could give rise to overly precise estimates of localization of function in brain maps. This potential distortion of impact estimates by plume shrinkage was prevented in the dedicated group that received only one microinjection.
All rats used for Fos analysis were anesthetized and transcardially perfused 90 min after their final or sole bilateral microinjection of vehicle (n = 10), DNQX alone (n = 13), DNQX plus SCH23390 (n = 6), DNQX plus raclopride (n = 10), DNQX plus raclopride and SCH23390 (n = 3), or no solution (normal, n = 3). Brain slices were processed for Fos-like immunoreactivity using normal donkey serum, goat anti-c-fos (Santa Cruz Biotechnology), and donkey anti-goat Alexa Fluor 488 (Invitrogen) (Faure et al., 2008; Reynolds and Berridge, 2008). Sections were mounted, air-dried, and coverslipped with ProLong Gold antifade reagent (Invitrogen). Zones where the expression of fluorescent Fos was elevated in neurons surrounding microinjection sites (“Fos plumes”) were assessed via microscope as described previously (Reynolds and Berridge, 2008).
Other brains were removed and fixed in 10% paraformaldehyde for 1–2 d and in 25% sucrose solution (0.1 m NaPB) for 3 d. For assessment of microinjection site locations in behaviorally tested rats, brains were sliced at 60 μm on a freezing microtome, mounted, air-dried, and stained with cresyl violet for verification of microinjection sites. Bilateral microinjection sites for each rat were placed on coronal slices from a rat brain atlas (Paxinos and Watson, 2007), which were used to extrapolate the position of each site on one sagittal slice. Mapping in the sagittal view allows for the presentation on the same map of the entire rostrocaudal and dorsoventral extents of NAc medial shell. Functional effects on appetitive and fearful behaviors were mapped using color coding to express the intensity of changes in motivated behaviors for individual behaviorally tested rats. Symbols were sized to match the maximal diameter of Fos plumes measured as described below. Sites were classified as rostral shell if their NAc placements were located +1.4 to +2.6 mm ahead of bregma, and as caudal shell if their placements were located +0.4 to +1.4 mm ahead of bregma.
Statistical analysis
The effects of DNQX on parametric behaviors were assessed using a three-factor mixed within- and between-subjects ANOVA [drug by group (glutamate/dopamine interaction vs independent dopamine blockade) by anatomical level (rostral vs caudal)] to verify elicitation of eating and defensive behavior along a rostrocaudal gradient. The effects of antagonism at D1- and D2-like receptors on DNQX-induced behavior were assessed using an additional two-factor mixed within- and between-subjects ANOVA to compare with behavior on DNQX alone (D1 antagonism by D2 antagonism). The effects of environmental modulation were assessed using a two-factor within-subject ANOVA (environment by drug). When significant effects were found, rats were split by anatomical location, and additional analysis was done using a one-way ANOVA and pairwise comparisons using Sidak corrections for multiple comparisons. For nominal data, differences between drug conditions were assessed using McNemar's repeated-measures test.
Results
Local AMPA receptor blockade in medial shell elicits eating and defensive treading behavior in a rostrocaudal gradient
Localized glutamate disruptions in medial shell induced by microinjections of DNQX, an AMPA/kainate receptor glutamate antagonist, stimulated intense appetitive and/or fearful behaviors depending on placement along a rostrocaudal gradient as expected (Fig. 1a). At rostral sites in medial shell, NAc glutamate disruptions generated robust elevations nearly five times over vehicle levels in amounts of eating behavior and food consumed during the 1 h test (cumulative duration of eating, drug by site interaction, F(1,32) = 10.0, p = 0.003; food intake measured in grams consumed, drug by site interaction, F(1,32) = 14.5, p = 0.001; Figures 2a,b, 3a). Conversely, at caudal sites in medial shell, DNQX microinjections did not elevate food intake (and in some caudal rats actually suppressed eating and food intake below control vehicle levels; Fig. 2a,b), but instead generated profound elevations in the incidence of fearful distress vocalizations (Figs. 2d, 3c; 73% of rats after DNQX microinjection vs 0% after vehicle; McNemar's test, p = 0.001) and of fearful escape attempts to human touch (Figs. 2e, 3c; 40% of rats after DNQX vs 0% after vehicle, McNemar's test, p = 0.031). Likewise, caudal DNQX microinjections generated nearly 10-fold increases in the spontaneous emission of defensive treading/burying behavior over vehicle control levels (Figs. 1a, 2c, 3b; drug by site interaction in cumulative duration of treading, F(1,32) = 6.9, p = 0.013). Defensive treading typically was not diffuse or random, but rather was directionally focused on a particular target, usually toward the transparent front of the cage (beyond which objects and people in the room could be seen) and toward light-reflecting front corners of the transparent plastic chamber.
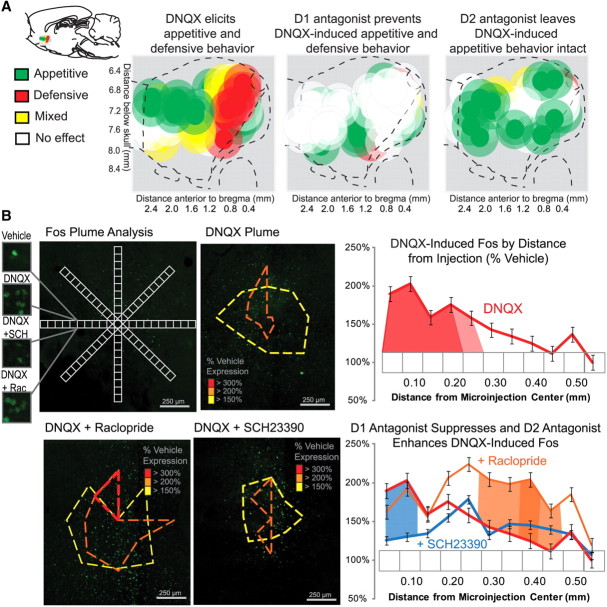
Figure 1.
Summary maps of behavior and Fos plume analysis. A, Summary maps show in sagittal section the motivated behaviors generated by microinjections in NAc medial shell of DNQX alone (left), DNQX plus SCH23390 (D1 antagonist, middle), or DNQX plus raclopride (D2 antagonist, right) in the standard laboratory environment. Each subject (n = 23) was designated as producing primarily appetitive (green symbols), fearful (red symbols), or mixed appetitive and fearful (yellow symbols) motivated behavior following DNQX microinjection. Purely appetitive eating behavior and food intake (criteria for including a site was a >200% increase in eating) was primarily stimulated in rostral shell by DNQX. Fearful distress calls, escape attempts, and spontaneous emission of defensive treading/burying behavior directed toward specific targets (criteria for including a site was a >500% increase over vehicle levels) were primarily stimulated in caudal shell by DNQX. Intermediate sites in medial shell often met both appetitive and defensive criteria and were designated as generating “mixed valence.” Combining the D1 antagonist in the DNQX microinjection prevented the elicitation of all appetitive (rostral) and fearful (caudal) behaviors. In contrast, combining the D2 antagonist in the DNQX microinjection only prevented the elicitation of defensive behavior, but left appetitive behavior intact. B, Maximal Fos plumes were analyzed for each drug microinjection condition. Fos-labeled cells were counted individually within successive blocks (50 × 50 μm), along eight radial arms emanating from the center of the site, with 10× magnification. Colors indicate levels of Fos expression of 3× (red), 2× (orange), and 1.5× (yellow) vehicle-level Fos expression. Line graphs show that DNQX (red) produced elevated Fos expression starting at the center of the microinjection to zones ∼0.3 mm away. DNQX-induced increases in Fos expression were suppressed by adding D1 antagonist (SCH23390; blue) but were enhanced by adding D2 antagonist (raclopride; orange) to the DNQX microinjection. Error bars indicate SEM.
Figure 2.
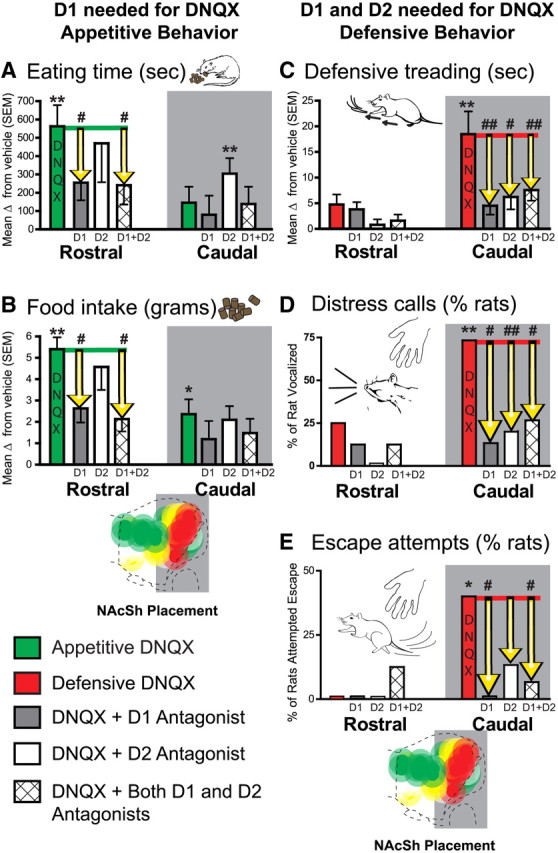
Motivated behavior summary graphs. A–E, Generation of increases in eating behavior (A), food intake (B), spontaneous defensive treading/burying behavior (C), incidence of distress vocalizations in response to human touch after the test (D), and of escape attempts in response to human touch (E). Effects are shown for microinjections of DNQX alone, DNQX plus SCH23390 (D1 antagonist), DNQX plus raclopride (D2 antagonist), and DNQX plus both D1 and D2 antagonists in rostral (n = 9) and caudal (n = 14) regions of medial NAc shell (relative to vehicle microinjections in the same rats). Data are presented as change from vehicle. Errors bars indicate SEM. *p < 0.05; **p < 0.01 (change from vehicle); #p < 0.05; ##p < 0.01 [change from DNQX; pairwise comparisons using Sidak corrections for multiple comparisons (eating, food intake, and defensive treading) or McNemar's test (distress vocalizations and escape attempts)].
Figure 3.

Effects of D1 and D2 antagonism on DNQX-induced eating and defensive fearful behaviors. Fos plume maps (n = 23) in sagittal plane of the generation in medial shell of eating (A), defensive treading (B), and fearful vocalizations and escape attempts (C) by DNQX (left), DNQX plus SCH23390 (D1 antagonist, middle), and DNQX plus raclopride (D2 antagonist, right). The D1 antagonist prevented DNQX from generating either eating or fear, whereas the D2 antagonist left DNQX-induced eating intact but prevented DNQX-induced generation of fear. Histogram bars below the maps show behavior as a percentage of vehicle (eating, A; treading, B) or percentage of subjects (vocalizations and escape attempts, C) for each behavior at rostrocaudal level as marked along the medial shell (error bars indicate SEM).
D1 dopamine receptor transmission alone needed for DNQX to generate appetitive behaviors at rostral sites
A novel finding here was that endogenous local dopamine stimulation was needed only at D1-like (D1, D5) receptors around the microinjection site in rostral shell for the generation of intense appetitive behavior by DNQX microinjections. Rostral D2-like receptors (D2, D3, D4) appeared essentially irrelevant to glutamate-related amplification of eating behavior and food intake (Figs. 1–3); that is, when the dopamine D1 antagonist SCH23390 was added to the rostral DNQX microinjection, the D1 blockade abolished the ability of DNQX to increase time spent eating or food intake, leaving eating behavior and intake at control levels seen after vehicle microinjections (Figs. 2a,b, 3a, eating, SCH23390, F(1,7) = 13.3, p = 0.008; gram intake, SCH23390, F(1,7) = 11.1, p = 0.010).
In contrast, combining the D2-like antagonist raclopride with DNQX microinjection for rostral sites failed to prevent or even impair the DNQX-enhancement of eating (cumulative duration; Figs. 2a,b, 3a; raclopride, F(1,8) < 1, p = 0.743) or food intake (grams consumed; Fig. 2b; raclopride, F(1,8) < 1, p = 0.517). Quite the opposite, at least at caudal shell sites, adding the D2 antagonist allowed caudal DNQX to further increase time spent eating to even higher levels that were 245% above vehicle, or 156% above eating levels produced by DNQX alone (Figs. 2a, 3a; DNQX stimulation of eating at caudal sites was usually low due to the rostrocaudal gradient, average of 566 ± 101 s on DNQX plus raclopride vs 362 s on DNQX alone and 230 s on vehicle; raclopride by DNQX, F(1,10) = 6.0, p = 0.035). A slight caveat to this additional enhancement is that adding the D2 antagonist did not actually boost the physical amount of food consumed for this group, even though it nearly doubled the proportion of time during the trial in which rats ate (Fig. 2b; raclopride, F(1,11) < 1, p = 0.930); however, we note that raclopride did boost stimulation of food consumption as well as of eating behavior for caudal DNQX microinjections in a separate experiment tested below (in tests conducted in a more stressful environment).
As expected, combining both the D1 antagonist and the D2 antagonist together with DNQX completely prevented DNQX from enhancing eating (similar to D1 antagonist above), and kept levels of intake equivalent to vehicle baseline levels (Fig. 2a,b; gram intake vs vehicle, F(1,7) < 1, p = 0.973; eating, F(1,7) = 1.1, p = 0.322). However, the D1–D2 mixture of antagonists was no more effective than adding just the D1 antagonist alone to DNQX, which also completely prevented appetitive increases (Fig. 2a; eating, SCH23390 plus raclopride vs SCH23390 alone, F < 1, p = 1.000). In short, we conclude that only local endogenous D1 receptor neurotransmission is needed to enable glutamate disruptions in rostral sites of medial shell to stimulate appetitive behavior and food intake. In contrast, local D2 receptor neurotransmission is essentially irrelevant to rostral eating stimulation, being neither necessary nor even contributing additively in any detectable way (and possibly even inhibiting the stimulation of eating at caudal sites, perhaps via generation of fearful reactions as described below that could compete with or suppress appetitive eating).
Ruling out general suppression of appetitive/fearful behavior by dopamine antagonists
Finally, the prevention of DNQX-induced increases in food intake or eating by D1 receptor blockade appeared to reflect a specific interaction of dopamine receptors with glutamate disruptions rather than a general independent suppression of eating motivation or capacity induced by dopamine blockade. Neither microinjections of the D1 antagonist by itself (without DNQX) nor of the D2 antagonist by itself (without DNQX) suppressed baseline levels of eating below control vehicle levels of about 1 g of chow per session (eating, SCH23390, F(1,14) = 1.9, p = 0.194, 149 ± 52 s on SCH23390 vs 166 ± 54 s on vehicle; raclopride, F(1,14) < 1, p = 0.389, 227 ± 56 s; grams intake, SCH23390, F(1,14) < 1, p = 0.514, 1.15 ± 0.36 g on SCH23390 vs 0.94 ± 0.23 g on vehicle; raclopride, F(1,14) = 3.9, p = 0.068, 1.82 ± 0.42 s). Thus local dopamine blockade in NAc at these doses did not impair either normal levels of motivation to eat or the motor capacity for ingestive movements. Instead our results seem to reflect a specific role of D1 receptor dopamine signals in enabling local AMPA receptor glutamate disruptions in rostral shell to stimulate eating behavior to high levels.
Fearful behaviors elicited by local glutamate disruption depend on concurrent local D1 and D2 receptor stimulation from endogenous dopamine
In contrast, simultaneous endogenous signaling at both D1 and D2 receptors in caudal sites of medial shell appeared necessary for DNQX microinjection to generate intense fearful behaviors (Figs. 1–3). Mixing either the D1 antagonist or the D2 antagonist with DNQX effectively prevented the production of any defensive treading at caudal sites, as well as the generation of any distress calls or escape reactions to human touch that otherwise were potentiated by DNQX microinjections (Figs. 2c–e, 3b,c; defensive treading, SCH23390, F(1,10) = 7.1, p = 0.024; raclopride, F(1,10) = 5.4, p = 0.043; escape attempts and jumps, DNQX alone, 40% of rats; DNQX plus SCH23390, 0%, p = 0.031 compared to DNQX, McNemar's test; DNQX plus raclopride, 13%, p = 0.219; distress calls, DNQX alone, 73% of rats; DNQX plus SCH23390, 13% of rats, p = 0.012; DNQX plus raclopride, 20% of rats, p = 0.008). In short, all fearful behaviors remained at near-zero control levels when either dopamine antagonist was mixed with DNQX.
Ruling out general suppression by dopamine antagonist microinjections
Again, D1 and D2 receptor contributions to DNQX fear induction appeared to reflect a specific interaction of these dopamine receptors with the glutamate disruption in caudal shell, because giving microinjections of either or both dopamine antagonists in the absence of DNQX did not change defensive treading from vehicle baseline levels (treading, SCH23390, F(1,14) < 1, p = 0.913; raclopride, F(1,14) < 1, p = 0.476). However, it must be noted that vehicle levels of fearful behaviors were near zero already, raising the possibility that a floor effect could have obscured a general suppression of fearful behavior by dopamine blockade. Therefore, we turn to other evidence, which also suggests that dopamine antagonist microinjections, either with DNQX or by themselves, did not generally prevent most behaviors. For example, grooming, a nonvalenced behavior that was emitted at substantial rates after vehicle, remained unsuppressed by local blockade of D1 or D2 receptors. Dopamine antagonists alone did not suppress spontaneous grooming (average, 9.33 ± 1.35 bouts on vehicle vs 8.09 ± 1.13 on SCH23390 and 8.40 ± 1.22 on raclopride; F < 1). Likewise, adding dopamine antagonists to DNQX did not suppress grooming behavior (F < 1). Microinjections of the dopamine antagonists alone did moderately suppress locomotion expressed as rears and cage crosses by about 50% from vehicle levels, though this suppression was nowhere near as strong as the abolition of DNQX-induced elevations of eating or fearful defensive treading described above (rears, SCH23390, F(1,13) = 17.6, p = 0.001; raclopride, F(1,13) = 9.8, p = 0.008; cage crosses, SCH23390, F(1,13) = 19.3, p < 0.001; raclopride, F(1,13) = 13.1, p = 0.002). Furthermore, DNQX microinjections stimulated locomotion to double or triple vehicle levels, and adding SCH23390 or raclopride to the DNQX microinjection did not prevent that rise in cage crosses and rears (main effect of DNQX, cage crosses, F(1,33) = 12.0, p = 0.002; rears, F(1,33) = 6.8, p = 0.014; SCH23390, F < 1 for rears and cage crosses; raclopride, cage crosses, F(1,19) = 2.2, p = 0.154; rears, F(1,19) = 3.2, p = 0.091). Thus, general suppression effects of dopamine antagonists were either missing or minimal and did not appear sufficient to account for the abolition of DNQX-stimulated motivated behaviors described above.
Local mode of dopamine/glutamate interaction switches flexibly as ambience reverses motivation valence
Environmental ambience flips motivational valence
As expected, for most sites in the intermediate two-thirds of medial shell (i.e., all sites between the far rostral 20% and far caudal 20%), changing environmental ambience from dark, quiet, and familiar (similar to rats' home room) to stressfully bright and noisy (extra light and raucous music) reversed the valence of motivated behavior generated by DNQX microinjections (Reynolds and Berridge, 2008) (Fig. 4). Rats emitted almost exclusively appetitive behavior in the home environment after DNQX microinjections, but emitted substantial amounts of fearful behaviors as well when tested in the stressful environment after DNQX at the same NAc sites. The familiar, low-stimulation, and presumably comfortable conditions of the home environment (which rats have been shown to prefer to standard lab illumination condition) (Reynolds and Berridge, 2008) caused the appetitive-stimulating zone within NAc to expand from rostral sites and invade caudal sites of medial shell as well, so that 90% of all medial shell locations generated intense eating behavior and food intake (>200% of vehicle; Fig. 4a). Concomitantly, the home environment virtually eliminated DNQX-induction of fearful behaviors, such as distress vocalizations, escape attempts, or defensive treading (Fig. 4a,b; treading, DNQX, F(1,7) = 3.5, p = 0.102; drug by site interaction, F(1,7) < 1, p = 0.476). Consequently, the size of the fear-inducing zone severely shrank in the home environment, leaving most midcaudal sites unable to generate fearful reactions. Thus, only one rat (which had the farthest caudal shell site) displayed >20 s of defensive treading in the home environment or emitted a distress vocalization when touched after the test (Fig. 4b).
Figure 4.
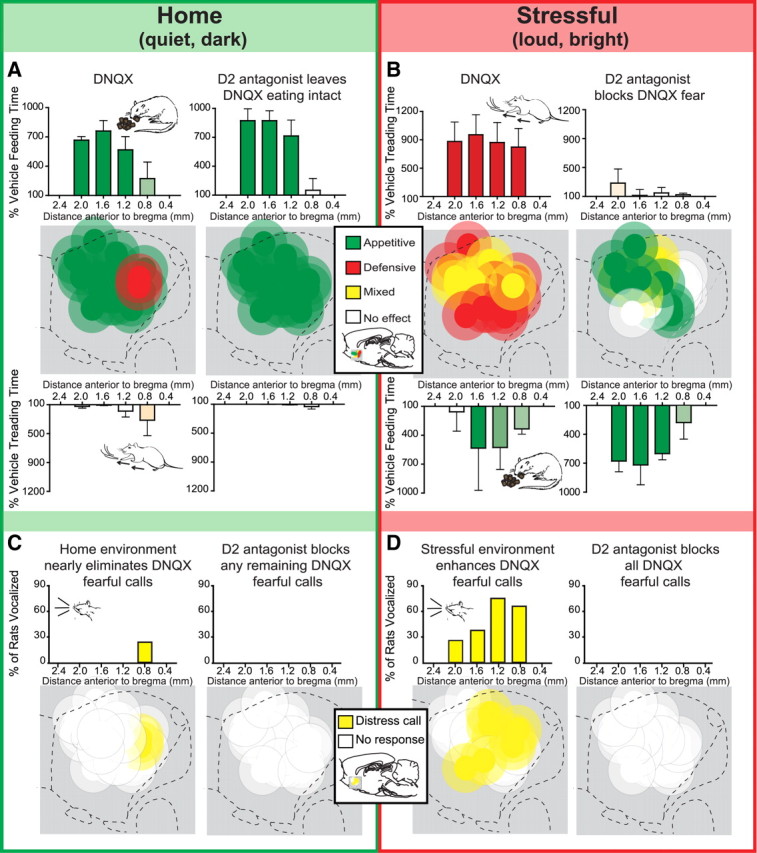
Environmental ambience shifts glutamate/dopamine interaction mode. A–D, Summary maps (n = 10) in sagittal plane show behavior produced by microinjections in the same rats of either DNQX alone or DNQX plus a D2 antagonist (raclopride), each tested both in the home environment (A) and in the stressful (B) environment. Each site was designated in a particular environment as producing primarily appetitive (green symbols), fearful (red symbols), or mixed valence (yellow symbols) motivated behavior following DNQX microinjection. Testing in the home environment caudally expanded the area in which DNQX generated purely appetitive behavior (criteria for including a site was a 200% increase in feeding behavior) and nearly eliminated the ability of DNQX to generate defensive treading behavior, distress calls, or escape attempts at any site. Conversely, testing in the stressful environment rostrally expanded the area of medial shell capable of generating intense fearful behaviors (criteria for including a site was a 500% increase in treading over vehicle levels), so that nearly every rostrocaudal location became able to generate fearful reactions after DNQX microinjections (relative to vehicle). The addition of the D2 antagonist to the microinjection blocked DNQX generation of defensive treading regardless of site. In contrast, the D2 antagonist never blocked the ability of DNQX to stimulate eating behavior and increase food intake. Histogram bars below the maps show mean behavior as a percentage of vehicle for each behavior at the rostrocaudal level as marked along the medial shell, with the dominant behavior stimulated in each environment appearing along the top (home environment, eating; stressful environment, treading; error bars indicate SEM). Maps of fearful vocalizations indicate which rats emitted audible distress calls in response to the experimenter's touch in the home environment (C) and the stressful environment (D) following DNQX alone or DNQX plus the D2 antagonist. Histograms bars above the maps show the percentage of subjects that vocalized at each rostrocaudal level.
In contrast, the loud and bright stressful environment (which rats avoid over lab conditions and quickly learn to turn off when given the opportunity) (Reynolds and Berridge, 2008) expanded the caudal fear-inducing zone to include substantial midrostral areas of medial shell and increased the levels of defensive treading stimulated by DNQX to over 600% of the corresponding levels induced in the home environment (Fig. 4b; DNQX, F(1,7) = 23.8, p = 0.002; site by drug interaction, F(1,7) < 1, p = 0.429). Similarly, the stressful environment increased the incidence of distress vocalizations generated after DNQX when the rats were touched by the experimenter at the end of the session by fivefold compared to the home environment (Fig. 4d; 50% of rats vs 10% in home environment; McNemar's test, p = 0.063). Conversely, the stressful environment eliminated pure appetitive sites in the midrostrocaudal zone, converting them into either mixed valence or purely fearful sites (Fig. 4c). The stressful environment also reduced the intensity of appetitive behaviors induced by DNQX at midrostral sites to ∼50% of home levels, even for sites that still generated any eating (average of 507 ± 142 s in the stressful environment vs 879 ± 87 s in the home environment; drug by environment interaction, eating, F(1,7) = 6.0, p = 0.044; food intake, F(1,7) = 2.9, p = 0.013).
Fearful mode requires D2 receptor involvement, but appetitive mode does not
The most important novel finding here was that D1/D2 receptor requirements for endogenous dopamine stimulation at a given site dynamically changed with environmental ambience shifts in a manner tied to motivational valence generated by DNQX at the moment rather than to rostrocaudal location per se. Each DNQX site had two modes: appetitive and fearful, depending on external ambience of the moment. The appetitive mode (i.e., DNQX stimulation of eating induced by the dark, quiet, and familiar home environment) did not require D2 receptor activation to enhance eating, whereas the fearful mode (i.e., DNQX stimulation of defensive treading behavior and distress vocalizations induced by the loud and bright stressful environment) always required D2 receptor activation for every site to stimulate fear, regardless of rostrocaudal location (just as caudal sites had required D2 for DNQX generation of fear in the previous experiment; Fig. 4). Flips in valence mode, between appetitive and defensive, occurred for 90% of sites tested, which comprised nearly all possible intermediate rostrocaudal locations in medial shell. For the remaining 10% of sites (n = 1), DNQX microinjected into far caudal shell always generated fearful behaviors in both environments (and fearful behaviors were always eliminated by D2 blockade).
More specifically, adding the D2 antagonist to DNQX microinjection completely blocked distress calls and defensive treading behavior at all sites that otherwise generated fear after DNQX in the stressful environment (Fig. 4; rostral sites, raclopride, F(1,4) = 19.9, p = 0.021; all rats, raclopride, F(1,7) = 10.7, p = 0.022; site by drug interaction, F(1,7) < 1, p = 0.730). However, the D2 antagonist never blocked or suppressed eating behavior (i.e., appetitive motivation) generated at the same sites by DNQX in the home environment; in fact, adding the D2 antagonist actually enhanced the levels of eating behavior generated by DNQX in the stressful environment to 463% of vehicle levels and 140% of levels on DNQX alone for the same sites (Fig. 4c; average of 712 ± 178 s on DNQX plus raclopride vs 507 s on DNQX alone and 153 s on vehicle). In the stressful environment, D2 blockade magnified DNQX stimulation of eating and increased grams of food consumed, regardless of rostrocaudal location (within the intermediate zone), confirming that local D2 neurotransmission is not only unnecessary for eating enhancement but actually can oppose the generation of intense eating by local AMPA receptor blockade in medial shell (eating, raclopride, F(1,7) = 18.5, p = 0.008; site by drug interaction, F(1,7) < 1, p = 0.651; food intake, raclopride, F(1,7) = 5.6, p = 0.064; site by drug interaction, F(1,6) = 2.5, p = 0.163). While in the standard environment D2 blockade disinhibited DNQX eating only in caudal shell (Fig. 2a), the stressful environment expanded the fear-generating zone and likewise expanded the zone in which D2 blockade disinhibits DNQX eating to include midrostral zones of medial shell (Fig. 4c; eating, raclopride by environment by site interaction, F(1,25) = 6.2, p = 0.020).
Dopamine receptor roles flip reversibly between multiple transitions
In rats that displayed ambivalent (both) motivations in the stressful environment (60% of rats), DNQX-induced eating peaked in the first 15 min, whereas defensive treading peaked later in the trial (30–45 min after the microinjection; Fig. 5a). During the 20 min period of maximal overlap between appetitive and defensive behavior (minutes 10–30), most rats transitioned from appetitive to defensive only once (16%) or two to six times (50%). With relatively few transitions during the hour, any single minute was likely to consist of pure rather than mixed motivated behaviors (Fig. 5b), consistent with previous reports (Reynolds and Berridge, 2008). Dopamine D2 receptor blockade did not block eating behavior (which dominated in the first 20 min of the session), but effectively blocked defensive treading behavior (which dominated in the final 20 min).
Figure 5.
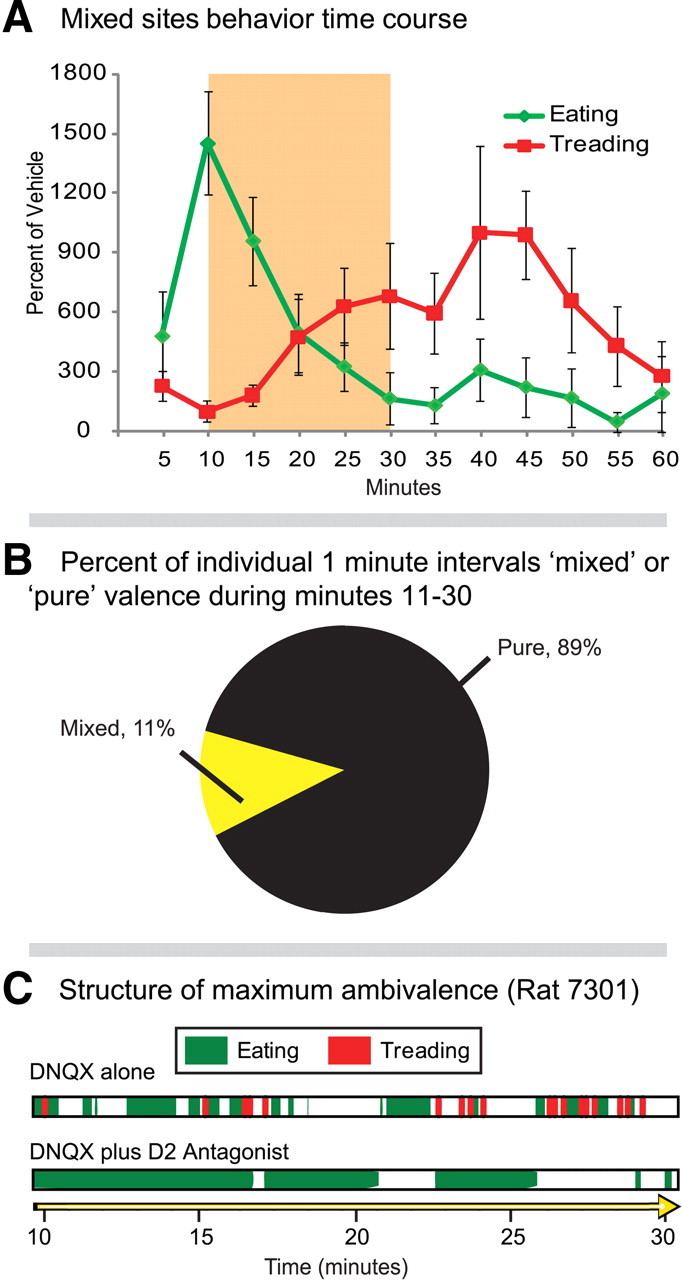
Appetitive and defensive behavior elicited from mixed valence sites in the stressful environment. A, Time course of eating and defensive treading over the 1 h trial (n = 6): eating behavior peaks early in the trial, while defensive treading emerges toward the midpoint of the trial (average percentage of vehicle; error bars indicate SEM). B, During the period of greatest overlap (minutes 11–30, highlighted in A), most individual minutes for a given rat consisted of purely appetitive or purely aversive behavior, rather than mixed, and transitions between appetitive and defensive valence tend to be limited (<10 for most rats). Yet, even for the rat that demonstrated the greatest ambivalence (C; ∼31 transitions between appetitive and defensive behaviors in the 1 h test), the addition of the D2 antagonist (raclopride) completely eliminated fearful behavior and left DNQX stimulation of eating intact.
However, two rats stood out as especially ambivalent, transitioning between appetitive and defensive behavior >25 times each within the hour after pure DNQX microinjections in the stressful environment. This represented the closest approach to simultaneous display of opposite motivations that we observed. Even in these rats, however, D2 receptor blockade consistently blocked only defensive behavior emitted under the loud and bright conditions, and never appetitive behavior (in either stressful or home environments; Fig. 5c), which continued to occur at similar levels and time points after DNQX plus D2 antagonist microinjection as after pure DNQX in the corresponding environment. Thus, motivated behavior produced by dopamine/glutamate interactions appeared to be able to shift rapidly and repeatedly between appetitive and fearful modes. When environmental conditions fostered ambivalence in a susceptible individual, a site could flip valence modes >20 times in a single hour.
Fos plume analysis: defining size of microinjection local impact
Localization of function was aided by assessing the extent of local impact of drug microinjections on nearby tissue, as reflected in Fos plumes around the microinjection center (Fig. 1b). Rats used previously for behavioral testing in the environmental shift group were assessed for Fos plumes after the end of the experiment. However, as anticipated, we confirmed that rats that had already completed behavioral testing had shrunken Fos plumes compared to the dedicated Fos group that received only a single microinjection, indicating that DNQX-induced plumes from rats that received six previous microinjections no longer represent the maximal impact radius of drug spread. DNQX produced plumes in the dedicated Fos group that were nearly four times larger in volume (nearly two times larger in radius) than in the previously behaviorally tested group (F(9,90) = 3.3, p < 0.002). Therefore, when mapping functional drug spread in all figures, we relied on plume radius data from the dedicated Fos group (matched to initial behavioral test conditions) to avoid underestimation when assessing the maximal spread of local impact for microinjections and to construct plume maps for localization of function. However, all other data besides plume radii shown in maps were obtained exclusively from the behaviorally tested group (i.e., colors and bar graphs reflecting intensities of eating and fearful behaviors induced at particular sites).
Pure DNQX microinjections produced plume centers of double the intensity of vehicle-level Fos expression in a small volume of 0.02 mm3 for the dedicated Fos group (Fig. 1b, top middle; radius, 0.18 ± 0.04 mm SEM). Rats that had received six previous microinjections had an even smaller volume center of 0.004 mm3 (radius, 0.1 mm). Surrounding plume centers, Fos expression in the maximal group had a larger halo of 0.23 mm3 volume of milder elevation >1.5 times vehicle levels (radius, 0.38 ± 0.05 mm SEM; rats tested previously six times had smaller outer halos of 0.05 mm3 volume; radius, 0.23 mm). Addition of the D1 antagonist (SCH23390) shrank plumes and attenuated the intensity of DNQX-induced elevations in local Fos expression (Fig. 1b, bottom middle; DNQX vs DNQX plus SCH23390, post hoc pairwise comparison with Sidak corrections, p < 0.01). SCH23390 shrank the total volume of DNQX Fos plumes to <0.18 mm3 (outer halo radius, 0.35 ± 0.05 mm SEM). In contrast, addition of the D2 antagonist (raclopride) expanded intense centers of Fos expression and enhanced DNQX-induced elevation in local Fos expression (Fig. 1b, bottom left; DNQX vs DNQX plus raclopride, post hoc pairwise comparisons with Sidak corrections, p < 0.05). Raclopride expanded the inner center of doubled Fos expression produced by DNQX to a volume of 0.15 mm3 (radius, 0.33 ± 0.042 mm SEM) and left unchanged the radius and intensity of the outer plume halo (of 1.5× expression). We note that the D1 antagonist apparently predominates over the D2 antagonist in effects on local Fos when both are microinjected jointly with DNQX, as DNQX Fos plumes shrink following the addition of combined D1 and D2 antagonists (Faure et al., 2008).
Discussion
In rostral shell, only endogenous dopamine signaling at D1-like receptors was needed for DNQX microinjections to stimulate fivefold increases in eating. In contrast, in caudal shell, simultaneous signaling at D1- and D2-like receptors was needed for DNQX to generate a 10-times increase in fearful reactions (distress calls, escape attempts, and active defensive treading directed at objects in cage or beyond). Yet, rostral sites in medial shell were not simply D1 dominant, nor were caudal sites D1–D2 codominant for generation of motivations by glutamate disruptions. Most intermediate sites in shell switched flexibly between generating appetitive and fearful motivations when environmental ambience changed. For those sites, D2 activity was always required for fear generation by DNQX microinjection (in the stressful environment) but never required for appetitive generation of eating (in the familiar home environment). Not only was D2 signaling unnecessary, D2 receptor blockade actually disinhibited DNQX stimulation of eating at sites when placement/environment combination otherwise facilitated fear. In short, rostrocaudal placement strongly biases the valence of motivational salience produced by glutamatergic disruptions, but dopamine interaction modes are more closely tied to appetitive/fearful valence generated at a given moment than to location per se (Reynolds and Berridge, 2008).
Mechanism of interaction between dopamine and glutamate blockade
The precise mechanism of NAc dopamine/glutamate interaction in generating intense incentive salience versus fearful salience remains a puzzle. Purely speculatively, we offer several possibilities. In the absence of glutamatergic input during AMPA blockade, NAc neurons reduce already low rates of firing, become hyperpolarized, and possibly disinhibit downstream targets in VP, LH, and VTA to stimulate motivated behaviors (Taber and Fibiger, 1997; Kelley, 1999; Meredith et al., 2008; Roitman et al., 2008; Krause et al., 2010). However, if dopamine primarily modulates glutamatergic depolarizations (Calabresi et al., 1997), then dopamine might be viewed as largely irrelevant to such hyperpolarizations.
Still, one possibility is that D2 receptor activation attenuates remaining excitatory AMPA postsynaptic impact (Cepeda et al., 1993), and so D2 blockade might prevent AMPA attenuation, disrupting local hyperpolarizations. Alternatively, D1 receptor activation may facilitate hyperpolarization in relatively inhibited neurons (Higashi et al., 1989; Pennartz et al., 1992; Moyer et al., 2007; Surmeier et al., 2007), and so D1 blockade might likewise disrupt those hyperpolarizations. Presynaptic mechanisms might also contribute, based on potential suppression of glutamate release by NAc D1 receptor activation on hippocampal or amygdala terminals, and similar presynaptic D2 suppression at prefrontal terminals (Pennartz et al., 1992; Nicola et al., 1996; Charara and Grace, 2003; Bamford et al., 2004). Presynaptic dopamine blockade might disrupt such suppressions and consequently increase glutamate release, potentially overcoming DNQX effects.
A remaining class of explanation could involve more subtle dopamine/glutamate interaction. For example, DNQX microinjections might shift AMPA/NMDA activation ratios toward NMDA, potentially relevant if NMDA receptors provide current contributions in the absence of AMPA currents (Cull-Candy and Leszkiewicz, 2004; Hull et al., 2009). Additionally, DNQX-induced local hyperpolarization may, via GABAergic connections between neighbors, laterally disinhibit surrounding neurons (Mao and Massaquoi, 2007; Faure et al., 2008; Tepper et al., 2008). Dopamine blockade could counteract both of these effects by disrupting both NMDA-mediated currents (Cepeda et al., 1993; Surmeier et al., 2007; Sun et al., 2008) and lateral inhibition (Taverna et al., 2005; Grace et al., 2007; Moyer et al., 2007; Nicola, 2007). The actual roles of these or other mechanisms in generating these phenomena will need future clarification.
Direct and indirect output pathways in D1- and D2-dependent motivation
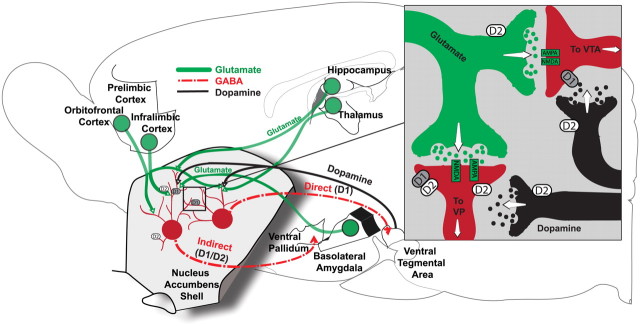
Direct and indirect pathways from shell may differentially contribute to incentive versus aversive motivation (Hikida et al., 2010). In general for striatum, D2-expressing outputs travel chiefly via the indirect pathway, and D1-expressing outputs travel via the direct pathway (Gerfen and Young, 1988; Gerfen et al., 1990; Bertran-Gonzalez et al., 2008; Matamales et al., 2009). For NAc medial shell in particular, D1-expressing neurons similarly constitute the direct output pathway to VTA, whereas equal populations of D1- and D2-dominant neurons project along the indirect pathway to the VP and LH (Fig. 6) (Haber et al., 1985; Heimer et al., 1991; Lu et al., 1998; Zhou et al., 2003; Humphries and Prescott, 2010). Additionally, 15–30% of shell neurons, likely projecting along the indirect pathway, coexpress both D1 and D2 receptors, which sometimes form a conjoined heteromer (Humphries and Prescott, 2010; Perreault et al., 2010, 2011). Speculatively, the importance of D1 receptors in enabling glutamate disruptions to generate appetitive behavior might reflect a primacy of the direct pathway from NAc to VTA. In contrast, the need for D1 and D2 coactivation for DNQX fear generation might highlight a greater contribution of the indirect pathway.
Figure 6.
Mesocorticolimbic circuits impacted by glutamate/dopamine interactions. Close-up representation of synapses in NAc medial shell and position in larger circuits. D1 receptors are located postsynaptically on medium spiny neurons (red) that project via a direct output path to the VTA and via an indirect output path to the VP and LH. D2 receptors are shown postsynaptically on medium spiny neurons that project via the indirect output path to the VP and LH (also D1–D2 coexpressing neurons). Dopamine receptors are also shown presynaptically in NAc on dopamine (black) and glutamate (green) neurons. Glutamatergic inputs (green) are shown from medial prefrontal cortex, orbitofrontal cortex, hippocampus, thalamus, and basolateral amygdala. Dopaminergic inputs (black) to NAc shell are shown from ventral tegmental area. GABAergic output pathways are shown to the VP and LH (indirect path; D1-, D2-, and D1/D2-expressing neurons) and to the VTA (direct path; D1).
Valence mode shifts and rostrocaudal biases: mesocorticolimbic circuits
Shifts between familiar and stressful environmental ambience modulate mesocorticolimbic circuits, likely altering glutamatergic inputs to NAc from prefrontal cortex, basolateral amygdala (BLA), hippocampus, and thalamus (Swanson, 2005; Zahm, 2006; Belujon and Grace, 2008), which may interact with D1/D2 dopamine signals. For example, after theta burst firing from the BLA, rostral shell neurons can show decreased responsiveness to subsequent BLA stimulations, whereas neurons in caudal shell are more likely to increase subsequent firing to the same BLA stimulations, a difference that requires D2 receptors and that might modulate the size of appetitive versus fear-generation zones within the medial shell (Gill and Grace, 2011). Particular features of mesocorticolimbic inputs may also be important for the shell's intrinsic rostrocaudal gradient. For instance, norepinephrine from hindbrain is released chiefly in caudal regions of shell, facilitated by dopamine D1 stimulation but inhibited by D2, and might help modulate motivation valence (Berridge et al., 1997; Delfs et al., 1998; Vanderschuren et al., 1999; Schroeter et al., 2000; Park et al., 2010). Finally, point-to-point corticolimbic targeting from prefrontal cortex zones to subregions of medial shell, VP/LH and their downstream targets, permit multiple segregated loops to travel through mesocorticolimbic circuits (Thompson and Swanson, 2010), which could further contribute to localization of desire and dread generators.
Caveats regarding D1 and D2 receptors in motivated behavior
We believe our findings do not necessarily conflict with others' reports of D2/D3 involvement in incentive motivation (Bachtell et al., 2005; Bari and Pierce, 2005; Xi et al., 2006; Heidbreder et al., 2007; Gardner, 2008; Khaled et al., 2010; Song et al., 2011). As a caveat, we note that our findings are strictly limited to mechanisms that simultaneously involve (1) glutamate/dopamine interactions (2) within the NAc medial shell that (3) generate intense elevation of appetitive/fearful motivations. Although our conclusions are consistent with reports that D1 (but not D2) blockade in NAc shell prevents appetitive VTA-stimulated eating (MacDonald et al., 2004) and prevents appetitive self-stimulation via optogenetic activation of glutamatergic amygdala-NAc projections (Stuber et al., 2011), as well as reports that D2 signaling contributes to active defensive behaviors (Filibeck et al., 1988; Puglisi-Allegra and Cabib, 1988), our results do not preclude other roles for D2/D3 receptors in generating appetitive motivation in different situations. In particular, we do not contradict appetitive roles produced in different brain structures, involving different reactions (e.g., learned rather than unconditioned) or that involve deficits below normal levels of motivation. Understanding dopamine receptor roles in generating motivations will eventually require integration of all relevant facts.
GABA and metabotropic glutamate generation of motivated behavior
We suggest that rostral dopamine/glutamate interactions here generated positive incentive salience, making food perceived as more attractive to eat. In contrast, caudal or negatively valenced interactions generated fearful salience, making objects and experimenter perceived as threatening. We previously reported metabotropic glutamate blockade at sites throughout medial shell to generate fear and disgust (Richard and Berridge, 2011), and reported local GABAergic hyperpolarizations to generate rostrocaudal gradients of feeding and fear, similar to the keyboard pattern described here (Reynolds and Berridge, 2001; Faure et al., 2010). However, we do not suggest that the dopamine interactions with ionotropic glutamatergic disruptions identified here necessarily apply to metabotropic or to GABAergic NAc mechanisms of motivation. Dopamine involvement in those remains an open question. There are several neuronal differences (e.g., direct GABAergic hyperpolarizations of neurons versus glutamate blockade-mediated hyperpolarization) and functional differences (e.g., shifts in hedonic impact versus induction of motivated behavior) that could prove important.
Implications for psychopathology
Corticolimbic dopamine/glutamate interactions have been linked to both intense incentive salience and fearful salience, contributing to appetitive motivation in addiction and to intense fearful motivation in psychotic paranoia (Wang and McGinty, 1999; Barch, 2005; Taylor et al., 2005; Lapish et al., 2006; Faure et al., 2008; Jensen et al., 2008; Kalivas et al., 2009). Flips in the valence of pathologically intense motivational salience also can occur (Morrow et al., 2011). Amphetamine addicts can experience fearful “amphetamine psychosis” similar to paranoia, which may involve pathological exaggerations of fearful salience (Featherstone et al., 2007; Jensen et al., 2008; Howes and Kapur, 2009). Conversely, some schizophrenic patients exhibit higher brain activations that encode appetitive incentive salience (Elman et al., 2006; Diaconescu et al., 2011). Overall, understanding how glutamate/dopamine interactions within NAc shell create intense appetitive and/or fearful motivations may illuminate the mechanisms underlying such intense but opposite disorders of motivation.
Footnotes
This research was supported by National Institutes of Health Grants DA015188 and MH63649 (K.C.B.) and by National Research Service Award Fellowship MH090602 (J.M.R.). We thank Stephen Burwell and Andy Deneen for assistance with histology and Brandon Aragona, Geoffrey Murphy, Joshua Berke, and Benjamin Saunders for helpful comments and discussion.
References
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophr Bull. 2005;31:875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135:959–968. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Critical role of the prefrontal cortex in the regulation of hippocampus-accumbens information flow. J Neurosci. 2008;28:9797–9805. doi: 10.1523/JNEUROSCI.2200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Stratford TL, Foote SL, Kelley AE. Distribution of dopamine beta-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse. 1997;27:230–241. doi: 10.1002/(SICI)1098-2396(199711)27:3<230::AID-SYN8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2001.04.012. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Centonze D, Bernardi G. Synaptic plasticity and physiological interactions between dopamine and glutamate in the striatum. Neurosci Biobehav Rev. 1997;21:519–523. doi: 10.1016/s0149-7634(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Thomas MJ. Biological substrates of reward and aversion: A nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci U S A. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A, Grace AA. Dopamine receptor subtypes selectively modulate excitatory afferents from the hippocampus and amygdala to rat nucleus accumbens neurons. Neuropsychopharmacology. 2003;28:1412–1421. doi: 10.1038/sj.npp.1300220. [DOI] [PubMed] [Google Scholar]
- Coss RG, Owings DH. Snake-directed behavior by snake naive and experienced California ground squirrels in a simulated burrow. Zeitschrift Fur Tierpsychologie. 1978;48:421–435. [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res. 1998;806:127–140. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- Diaconescu AO, Jensen J, Wang H, Willeit M, Menon M, Kapur S, McIntosh AR. Aberrant effective connectivity in schizophrenia patients during appetitive conditioning. Front Hum Neurosci. 2011;4:239. doi: 10.3389/fnhum.2010.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006;31:2091–2120. doi: 10.1038/sj.npp.1301051. [DOI] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J Neurosci. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Richard JM, Berridge KC. Desire and dread from the nucleus accumbens: Cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PloS One. 2010;5:e11223. doi: 10.1371/journal.pone.0011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, Kapur S, Fletcher PJ. The amphetamine-induced sensitized state as a model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1556–1571. doi: 10.1016/j.pnpbp.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Filibeck U, Cabib S, Castellano C, Puglisi-Allegra S. Chronic cocaine enhances defensive behaviour in the laboratory mouse: involvement of D2 dopamine receptors. Psychopharmacology (Berl) 1988;96:437–441. doi: 10.1007/BF02180020. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Use of animal models to develop antiaddiction medications. Curr Psychiatry Rep. 2008;10:377–384. doi: 10.1007/s11920-008-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Young WS., III Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gill KM, Grace AA. Heterogeneous processing of amygdala and hippocampal inputs in the rostral and caudal subregions of the nucleus accumbens. Int J Neuropsychopharmacol. 2011:1–14. doi: 10.1017/S1461145710001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol. 1985;235:322–335. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Andreoli M, Marcon C, Hutcheson DM, Gardner EL, Ashby CR., Jr Evidence for the role of dopamine D3 receptors in oral operant alcohol self-administration and reinstatement of alcohol-seeking behavior in mice. Addict Biol. 2007;12:35–50. doi: 10.1111/j.1369-1600.2007.00051.x. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Higashi H, Inanaga K, Nishi S, Uchimura N. Enhancement of dopamine actions on rat nucleus accumbens neurones in vitro after methamphetamine pre-treatment. J Physiol. 1989;408:587–603. doi: 10.1113/jphysiol.1989.sp017478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Isaacson JS, Scanziani M. Postsynaptic mechanisms govern the differential excitation of cortical neurons by thalamic inputs. J Neurosci. 2009;29:9127–9136. doi: 10.1523/JNEUROSCI.5971-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Jensen J, Willeit M, Zipursky RB, Savina I, Smith AJ, Menon M, Crawley AP, Kapur S. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008;33:473–479. doi: 10.1038/sj.npp.1301437. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, LaLumiere RT, Knackstedt L, Shen HW. Glutamate transmission in addiction. Neuropharmacology. 2009;56:169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Neural integrative activities of nucleus accumbens subregions in relation to learning and motivation. Psychobiology. 1999;27:198–213. [Google Scholar]
- Kelley AE, Swanson CJ. Feeding induced by blockade of AMPA and kainate receptors within the ventral striatum: a microinfusion mapping study. Behav Brain Res. 1997;89:107–113. doi: 10.1016/s0166-4328(97)00054-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Khaled MA, Farid Araki K, Li B, Coen KM, Marinelli PW, Varga J, Gaal J, Le Foll B. The selective dopamine D3 receptor antagonist SB 277011-A, but not the partial agonist BP 897, blocks cue-induced reinstatement of nicotine-seeking. Int J Neuropsychopharmacol. 2010;13:181–190. doi: 10.1017/S1461145709991064. [DOI] [PubMed] [Google Scholar]
- Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci. 2010;30:4746–4756. doi: 10.1523/JNEUROSCI.0197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapish CC, Seamans JK, Chandler LJ. Glutamate-dopamine cotransmission and reward processing in addiction. Alcohol Clin Exp Res. 2006;30:1451–1465. doi: 10.1111/j.1530-0277.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- Levita L, Dalley JW, Robbins TW. Nucleus accumbens dopamine and learned fear revisited: a review and some new findings. Behav Brain Res. 2002;137:115–127. doi: 10.1016/s0166-4328(02)00287-5. [DOI] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- MacDonald AF, Billington CJ, Levine AS. Alterations in food intake by opioid and dopamine signaling pathways between the ventral tegmental area and the shell of the nucleus accumbens. Brain Res. 2004;1018:78–85. doi: 10.1016/j.brainres.2004.05.043. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao ZH, Massaquoi SG. Dynamics of winner-take-all competition in recurrent neural networks with lateral inhibition. IEEE Trans Neural Netw. 2007;18:55–69. doi: 10.1109/TNN.2006.883724. [DOI] [PubMed] [Google Scholar]
- Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau JM, Valjent E, Herve D, Girault JA. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS One. 2009;4:e4770. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Maren S, Robinson TE. Individual variation in the propensity to attribute incentive salience to an appetitive cue predicts the propensity to attribute motivational salience to an aversive cue. Behav Brain Res. 2011;220:238–243. doi: 10.1016/j.bbr.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JT, Wolf JA, Finkel LH. Effects of dopaminergic modulation on the integrative properties of the ventral striatal medium spiny neuron. J Neurophysiol. 2007;98:3731–3748. doi: 10.1152/jn.00335.2007. [DOI] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Kombian SB, Malenka RC. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J Neurosci. 1996;16:1591–1604. doi: 10.1523/JNEUROSCI.16-05-01591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Aragona BJ, Kile BM, Carelli RM, Wightman RM. In vivo voltammetric monitoring of catecholamine release in subterritories of the nucleus accumbens shell. Neuroscience. 2010;169:132–142. doi: 10.1016/j.neuroscience.2010.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. New York: Academic; 2007. The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Dolleman-Van der Weel MJ, Kitai ST, Lopes da Silva FH. Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory limbic inputs to the shell region of the rat nucleus accumbens studied in vitro. J Neurophysiol. 1992;67:1325–1334. doi: 10.1152/jn.1992.67.5.1325. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O'Dowd BF, George SR. The dopamine D1–D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem. 2010;285:36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, O'Dowd BF, George SR. Dopamine receptor homooligomers and heterooligomers in schizophrenia. CNS Neurosci Ther. 2011;17:52–57. doi: 10.1111/j.1755-5949.2010.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Cabib S. Pharmacological evidence for a role of D2 dopamine receptors in the defensive behavior of the mouse. Behav Neural Biol. 1988;50:98–111. doi: 10.1016/s0163-1047(88)90804-7. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Metabotropic glutamate receptor blockade in nucleus accumbens shell shifts affective valence towards fear and disgust. Eur J Neurosci. 2011;33:736–747. doi: 10.1111/j.1460-9568.2010.07553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J Comp Neurol. 2000;420:211–232. [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, Li X, Gaal J, Xi ZX, Gardner EL. YQA14: a novel dopamine D(3) receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D(3) receptor-knockout mice. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf M. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Anatomy of the soul as reflected in the cerebral hemispheres: neural circuits underlying voluntary control of basic motivated behaviors. J Comp Neurol. 2005;493:122–131. doi: 10.1002/cne.20733. [DOI] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC. Feeding-evoked dopamine release in the nucleus, accumbens: regulation by glutamatergic mechanisms. Neuroscience. 1997;76:1105–1112. doi: 10.1016/s0306-4522(96)00450-2. [DOI] [PubMed] [Google Scholar]
- Taverna S, Canciani B, Pennartz CM. Dopamine D1-receptors modulate lateral inhibition between principal cells of the nucleus accumbens. J Neurophysiol. 2005;93:1816–1819. doi: 10.1152/jn.00672.2004. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Britton JC, Liberzon I. Neural response to emotional salience in schizophrenia. Neuropsychopharmacology. 2005;30:984–995. doi: 10.1038/sj.npp.1300679. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Wilson CJ, Koos T. Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Res Rev. 2008;58:272–281. doi: 10.1016/j.brainresrev.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc Natl Acad Sci U S A. 2010;107:15235–15239. doi: 10.1073/pnas.1009112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol Biochem Behav. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L, Wardeh G, De Vries TJ, Mulder AH, Schoffelmeer ANM. Opposing role of dopamine D1 and D2 receptors in modulation of rat nucleus accumbens noradrenaline release. J Neurosci. 1999;19:4123–4131. doi: 10.1523/JNEUROSCI.19-10-04123.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci U S A. 2007;104:5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Glutamate-dopamine interactions mediate the effects of psychostimulant drugs. Addict Biol. 1999;4:141–150. doi: 10.1080/13556219971641. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Cowan RL, Park S, Ansari MS, Baldwin RM, Li R, Doop M, Kessler RM, Zald DH. Correlation of individual differences in schizotypal personality traits with amphetamine-induced dopamine release in striatal and extrastriatal brain regions. Am J Psychiatry. 2011;168:418–426. doi: 10.1176/appi.ajp.2010.10020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine's rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Zahm DS. The evolving theory of basal forebrain functional–anatomical “macrosystems.”. Neurosci Biobehav Rev. 2006;30:148–172. doi: 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Zhou L, Furuta T, Kaneko T. Chemical organization of projection neurons in the rat accumbens nucleus and olfactory tubercle. Neuroscience. 2003;120:783–798. doi: 10.1016/s0306-4522(03)00326-9. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci. 2009;1156:198–210. doi: 10.1111/j.1749-6632.2009.04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]