Abstract
MALDI-imaging mass spectrometry (IMS) has been shown to be a powerful tool to study drug distributions in organ tissue as well as whole animal bodies. Nevertheless, not all drugs are amenable to MALDI while others may be limited by poor sensitivity poor sensitivity. The use of chemical derivatization to improve detection of small molecules by mass spectrometry techniques is well documented. To our knowledge, however, this approach has not been applied to direct tissue analysis of small organic molecules. In this manuscript, we demonstrate the use of on-tissue chemical derivatization of a small organic molecule, 3-methoxysalicylamine (3-MoSA) a scavenger of γ -ketoaldehydes. Derivatization of 3-MoSA with 1,1′-thiocarbonyldiimidazole (TCDI) results in an oxothiazolidine derivative which is detected with much greater sensitivity by MALDI than 3-MoSA itself. TCDI treatment of tissue from mice dosed with 3-MoSA allowed images to be obtained showing its spatial distribution as well as its pharmacokinetic profile in different organs. These images correlated well with results obtained from HPLC-MS/MS analyses of the same tissues. These results provide proof-of-concept that on-tissue chemical derivatization can be used to improve detection of a small organic molecule by MALDI-IMS.
Keywords: MALDI, imaging mass spectrometry, chemical derivatization, direct tissue, small molecule
INTRODUCTION
MALDI-imaging mass spectrometry (IMS) has proven to be an excellent tool in drug discovery allowing the visualization of the distribution of small molecules directly on tissue sections.[1–3] MALDI-IMS offers significant advantages over other imaging techniques such as autoradiography and fluorescence spectroscopy in that there is no need for the synthesis of radio- or fluorescently labelled compounds, which can be costly and time consuming. In addition, the native drug can be monitored as opposed to a tag, and metabolites can be easily distinguished from the parent drug.
One limitation for the application of MALDI-IMS to the analysis of small molecules is spectral interference from low molecular weight matrix ions. One way to overcome this issue is to filter the ions using collision-induced dissociation (CID), where the analyte is fragmented and imaging is performed via monitoring the major transitions to product ions. Several studies have demonstrated the great potential of this technique for the study of small molecules.[2,4–8] For example, one report described the analysis of olanzapine, an antipsychotic drug, directly from whole rat-body sections.[7] In this work, the distribution of olanzapine and two of its metabolites was visualized by monitoring the MS/MS fragmentations of the three compounds simultaneously on sagittal sections of whole rats. Another approach to overcome matrix interference in these analyses is by the use of high resolution/high mass accuracy instrumentation such as Fourier transform-ion cyclotron resonance (FT-ICR) instruments. This technique allows images to be obtained for hundreds of ions within a single MS scan, and the resulting accurate mass assignments allow matrix ions to be effectively separated from analyte ions without the need for MS/MS.[9]
In order to effectively detect small molecules directly from tissue sections, the analyte of interest must be readily ionized by the MALDI process. Sample preparation, including choice of matrix, and matrix solvent composition, are critical for overall sensitivity.[10] However, some small molecules are not suitable for analyses by MALDI directly from tissue. Chemical derivatization has been widely used as a strategy for improving the detection of poorly ionizable molecules for APCI and LC-ESI-MS/MS analysis.[11,12] However, little has been documented on the use of chemical derivatization for direct tissue MALDI analysis. Recently, work carried out by Wu et al. has shown the use of chemical derivatization for the detection of cholesterol by reactive desorption electrospray ionization (DESI).[13] In this case, the alcohol group in cholesterol is converted to its hemiacetal by reaction with betaine aldehyde which is applied to tissue via the DESI spray. Another recent study described the use of chemical derivatization to improve peptide fragmentation by MALDI directly on tissue.[14] In this study, the N-termini of tryptic peptides were derivatized with the sulfonation agents 4-SPITC and 3-SBASE to add a negative charge. The application of on-tissue chemical derivatization for the detection of drugs, however, has not yet been explored.
The molecule 3-methoxysalicylamine (3-MoSA) is a scavenger of Levuglandins (LGs).[15] Levuglandins are γ -ketoaldehydes which are derived from non-enzymatic rearrangement of prostaglandin H2, the product of the cyclooxigenases.[16] LGs are highly reactive towards primary amines, and are known to form adducts with proteins and DNA.[17,18] High levels of these LG adducts on proteins have been linked to oxidative injury, inflammation and the progression of Alzheimer's disease.[19–22] Therefore, molecules such as 3-MoSA, which can scavenge LGs and prevent the formation of LG adducts, are of great interest.
Studies of the distribution of 3-MoSA on intact tissue by MALDI-IMS are very limited by matrix ion interference and very low sensitivity, which makes this molecule a good candidate for investigating the feasibility of on-tissue chemical derivatization for improving sensitivity. In this manuscript, we report the development of a method for the derivatization of 3-MoSA directly on tissue with the reagent 1,1′-thiocarbonyldiimidazole (TCDI). The resulting 3-MoSA-TCDI derivative led to a dramatic improvement in sensitivity compared to 3-MoSA itself. MS/MS ions were easily distinguished from matrix ions and thus produced distinct images for the localization of the 3-MoSA-TCDI derivative on tissues from dosed animals. In addition, MALDI-IMS analysis of this derivative over a time course post-dose correlates well with LC-ESI-MS/MS quantitation from the same tissue homogenates. These results provide proof-of-concept that chemical derivatization directly on tissue can be used to improve the MALDI process and allow MALDI-IMS of small molecules from dosed animals.
EXPERIMENTAL
Chemicals and solutions
Ammonium bicarbonate (AMBIC), TCDI and α-cyano-4-hydroxycinnamic acid (CHCA) were purchased from Sigma-Aldrich Inc. (Milwaukee, WI). The levuglandin scavenger 3-MoSA was synthesized by Dr. Amarnath as described previously.[23] Trifluoro acetic acid (TFA) and all solvents were HPLC grade purchased from Fisher Scientific (Fair Lawn, NJ). AMBIC solution was 50 mm, pH 8.0. CHCA solution was 10 mg/ml in 60% acetonitrile and 0.2% TFA.
Animal and tissue preparation
Animal studies were carried out in accordance with the NIH Guidelines for Care and Use of Laboratory Animals. All animal protocols were approved by the Vanderbilt Institutional Animal Care and Use Committee. Mice, 12-week old males, C57BL/6N, were dosed with 3-MoSA (200 or 300 mg/kg) in PBS intraperitoneally. These high doses of 3-MoSA were chosen with the purpose of facilitating the method development by ensuring sufficient 3-MoSA is available for detection. Mice were killed and organs harvested at 15 min, 30 min, 1 h, 2 h and 4 h post-dose. Organs were flash-frozen and sectioned using a cryostat (Leica CM 3050S, Meyer Instruments, Houston, TX). Tissue sections (12 μm thick) were thaw-mounted onto a gold-coated MALDI target plate and stored in a vacuum desiccator until derivatization and MALDI analysis.
Derivatization method
A 3-MoSA-TCDI standard was prepared as reported previously in our laboratory by reaction of 3-MoSA and TCDI in a 1 : 2 molar ratio, in a 1 : 1 mixture of 1 m phosphate buffer (pH 8.0) and acetonitrile.[24] The reaction was incubated for 15 min at room temperature and 30 min at 37 °C. The derivative was extracted in ethyl acetate and the solvent evaporated to dryness. The residue was reconstituted in acetonitrile for storage until MALDI analysis. To examine the derivatization reaction on tissue, a control (non-dosed) brain tissue section was manually spotted with 1 μl of a 250 μg/ml aqueous solution of 3-MoSA standard. The spots were allowed to dry, then 1 μl of a 5 mg/ml solution of TCDI in 1 : 1 acetonitrile/AMBIC (prepared fresh and used immediately) was deposited directly on top of the 3-MoSA standard spots. The spotted tissue was kept moist by placing the MALDI target in a Petri dish containing a moist Kim-Wipe. The plate was incubated at 37 °C for 30 min. The tissue was allowed to cool and dry in a vacuum desiccator for 20 min. Matrix (CHCA, 1 μl) was manually applied to TCDI-treated areas and allowed to dry in a vacuum desiccator for 10 min. The same derivatization procedure was followed for probing the derivatization of endogenous 3-MoSA in dosed tissue. For MALDI-imaging experiments, the TCDI solution (10 mg/ml in 1 : 1 acetonitrile/AMBIC) was applied to the tissue by spray coating using a TLC reagent sprayer (a total of 40 passes). The reagent was applied as a very fine mist and never allowed to completely dry between passes. TCDI-treated plates were placed in a Petri dish containing a moist Kim-Wipe and incubated at 37 °C for 30min. The reagent was allowed to dry in a vacuum desiccator prior to matrix deposition. The matrix (CHCA) was applied to the MALDI targets by sublimation according to the procedure reported by Hankin.[25]
MALDI-image acquisition
Mass spectra were acquired using a linear ion trap mass spectrometer (Thermo MALDI LTQ XL, Waltham, MA) equipped with a nitrogen laser (337 nm) operating at 60 Hz. Data were acquired at 200 μm resolution at 108 laser shots per pixel with a target laser energy of 85 μJ; due to the presence of a neutral density filter in-line with the laser, the actual laser energy on target is much less than the target value. Spectra were accumulated at 9 microscans/step. Imaging analyses were performed in the single reaction monitoring mode m/z 196 →137 for the 3-MoSA-TCDI derivative and 154 → 137 for underivatized 3-MoSA with a normalized collision energy of 30% and 30 ms activation time. The SRM scan window was 20 Da. Images were obtained using Image Quest (Thermo) by plotting the intensity of the product ion m/z 137 normalized to the total ion current (TIC).
3-MoSA saturation and TCDI treatment reproducibility
Control kidney tissue (200 mg) was mechanically homogenized in 2 ml of 20% methanol–water using a DUALL tissue grinder followed by sonication (Branson Sonifier 450). Aliquots of the tissue slurry (10 μl, 1 mg) were spiked with an appropriate amount of 3-MoSA from a 0.2 mg/ml aqueous stock solution and diluted to 20 μl with 20% methanol–water for final 3-MoSA concentrations of 200, 400, 600, 800, 1000, 1200, 1400, 1600, 1800 and 2000 ng/mg of tissue. The samples were spotted (1 μl) in triplicate on the same MALDI target plate. To test reproducibility across MALDI targets, the samples containing 600, 1000 and 1400 ng/mg were spotted six times each on the same target plate and this was repeated in triplicate on different MALDI targets. The spotted samples were allowed to dry in a vacuum desiccator and derivatization was carried out as described for tissue sections. Spectra were acquired in imaging mode over a selected area (2500×2500 μm) positioned in the center of each spotted well, with a 200 μm resolution array. Spectra were averaged across the entire sample area and average relative intensities were plotted against 3-MoSA concentration. The data was fitted to a linear regression using GraphPad Prism 4.0 (San Diego, CA).
RESULTS AND DISCUSSION
Derivatization of 3-MoSA
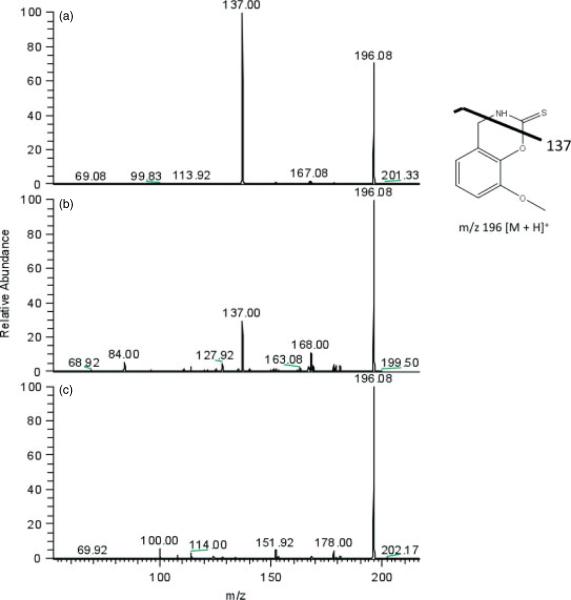
TCDI has been shown to be useful in the derivatization of cysteine and penicillamine to enhance their detection by UV absorption methods.[23] The reaction of 3-MoSA with TCDI results in an oxazine derivative with increased hydrophobicity in comparison to underivatized 3-MoSA (Scheme 1). In tissue homogenate studies carried out in our laboratory, we used this derivatization to facilitate extraction and chromatography of 3-MoSA for quantitation by LC-ESI-MS/MS methods.[24] On the basis of our success with this reaction, we investigated whether this procedure could be used to improve MALDI sensitivity. A 3-MoSA-TCDI standard was prepared by reaction of 3-MoSA and TCDI in a 1 : 2 molar ratio in phosphate buffer (pH 8.0) as described previously.[24] MALDI-MS analysis of 5 pmol of this standard resulted in a protonated 3-MoSA-TCDI molecular ion at m/z 196. MALDI-MS analysis of CHCA alone also produced an ion at m/z 196, however, this interference was alleviated by the use of MS/MS. As seen in Fig. 1(a), MS/MS analysis of the 3-MoSA-TCDI molecular ion at m/z 196 produced a prominent daughter ion at m/z 137 which was absent in the MS/MS spectra for the matrix-interfering molecular ion at m/z 196 (Fig. 1(c)). Therefore, the transition m/z 196 → 137 was utilized for MALDI-IMS experiments of 3-MoSA-TCDI.
Scheme 1.
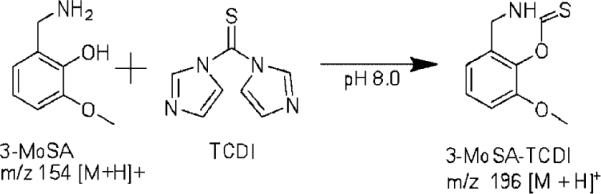
Reaction of 3-MoSA with TCDI.
Figure 1.
Full scan MS/MS of m/z 196 for 3-MoSA-TCDI derivative. (a) 3-MoSA-TCDI standard solution. (b) Brain tissue from mouse dosed with 3-MoSA (300 mg/kg), treated with TCDI and spotted with CHCA matrix. (c) Brain tissue from mouse dosed with 3-MoSA (300 mg/kg) not treated with TCDI and spotted with CHCA matrix.
Underivatized 3-MoSA produces a protonated molecular ion at m/z 154 by MALDI-MS. MS/MS of m/z 154 gives mainly a product ion at m/z 137 resulting from loss of ammonia. However, at least 200 pmol of this compound on the MALDI target are needed for detection. By comparison, the 3-MoSA-TCDI derivative can be detected in fentomole amounts. 3-MoSA undergoes significant fragmentation to m/z 137 in the MALDI source with all tested matrices. This loss of molecular ion current diminishes the sensitivity for detection of underivatized 3-MoSA. TCDI derivatization results in a more robust molecule which is less susceptible to in-source dissociation, thus increasing sensitivity. In addition to in-source dissociation, it is likely that interference with the matrix ions also contributes to the low sensitivity for the underivatized 3-MoSA. The most commonly used matrices for detection of small molecules, CHCA and 2,5-dihydroxybenzoic acid (DHB), both produce significant signal at the MS/MS transition m/z 154 → 137. Sinapinic acid (SA) does not have this interference, however, it does not result in improved detection of 3-MoSA.
Derivatization of 3-MoSA directly on tissue
To achieve derivatization of 3-MoSA on tissue, phosphate buffer was substituted by ammonium bicarbonate, as this is more compatible with MS analyses. Initially, the reaction was carried out on control tissue manually spotted with the 3-MoSA standard as described in the Experimental section. The maximum MALDI signal for the 3-MoSA-TCDI derivative (SRM 196 → 137) was achieved by incubation of the tissue with the TCDI reagent under moist conditions at 37 °C for no less than 30 min. Derivatization of endogenous 3-MoSA in dosed tissue was attained, as described in the Experimental section, by manually depositing the TCDI reagent on a brain tissue section. A positive result was obtained by MALDI-MS/MS analysis for the presence of the 3-MoSA-TCDI derivative in dosed brain (Fig. 1(b)). When the dosed brain tissue was not treated with TCDI, no signal for the 3-MoSA-TCDI derivative was detected above noise (Fig. 1(c)). Although there was matrix interference at m/z 196 as seen in all panels, the transition m/z 196 → 137 was unique to the 3-MoSA derivative (not present in panel c). The lower m/z 137/196 ratio observed in Fig. 1(b) (dosed tissue) compared with Fig. 1(a) (3-MoSA-TCDI standard) can be attributed to the fact that the concentration of 3-MoSA in the brain (Fig. 1(b)) is likely less than that in the standard solution (Fig. 1(a)).
MALDI-imaging experiments
The on-tissue derivatization method was first adapted for imaging by spray coating the TCDI reagent onto the tissue, followed by spray coating of a CHCA solution on the dry, treated tissue. However, detection of 3-MoSA-TCDI was only achieved when a very thick layer of this CHCA matrix solution was applied. Examination of the treated tissue revealed poor crystallization of the matrix. This could be due to interference by the TCDI reagent. Additionally, the large amount of matrix solution applied to the tissue lead to delocalization of the analyte off the tissue (data not shown). To circumvent this problem, matrix was instead applied by sublimation.[25] Matrix sublimation provided much greater sensitivity and retention of analyte localization. Figure 2 shows images for serial kidney sections from a mouse dosed at 300 mg/kg and sacrificed 30 min post-dose. These results show a clear image for the 3-MoSA-TCDI derivative with localization towards the center of the kidney where the renal medulla is found (Fig. 2(a)). This localization is consistent with the rapid renal clearance of 3-MoSA.[24] Fig. 2(a) and (b) show the remarkable improvement in sensitivity upon derivatization with TCDI. Despite the very high dose of 3-MoSA given to the animal, no image can be generated for 3-MoSA without TCDI treatment (Fig. 2(b)), as the transition for the underivatized compound (m/z 154 → 137) is not detected above noise level. Plotting the transition m/z 196→ 137 for tissue not treated with TCDI also resulted in no image (Fig. 2(c)), which confirms that the transition m/z 196 → 137 is unique to the 3-MoSA-TCDI derivative.
Figure 2.
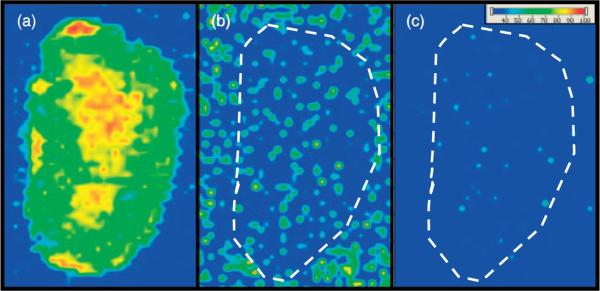
MALDI-IMS of serial kidney sections from mouse dosed with 3-MoSA at 300 mg/kg and sacrificed at 30 min post-dose. (a) TCDI-treated tissue, plotting m/z 196 → 137 for 3-MoSA-TCDI. (b) Tissue not treated with TCDI, plotting 154 → 137 (underivatized 3-MoSA). (c) Tissue not treated with TCDI, plotting 196 → 137. Images are plotted as m/z 137/TIC.
An attractive utilization of MALDI-IMS is to monitor simultaneously the tissue distribution and clearance of small molecules. To assess whether derivatization could be compatible with this application, time course post-dose experiments were carried out, and clearance of 3-MoSA in dosed animals was determined by probing the 3-MoSA-TCDI derivative by MALDI-IMS. These studies were paralleled to pharmacokinetics experiments carried out in our laboratory for the same tissues using an LC-ESI-MS/MS method. The details of the protocol used for the LC-ESI-MS/MS experiments are described for brain and plasma in a previous publication.[24] In our studies, a high dose of 3-MoSA was given to the animals (200 mg/kg), with the purpose of facilitating the method development for detection of 3-MoSA-TCDI, and MALDI-IMS analysis was performed on tissue sections from liver of dosed mice, sacrificed at different time points post-dose. The time-course imaging profile of liver tissue (Fig. 3(a)) indicates that the signal intensity for the 3-MoSA-TCDI derivative is highest at 15 min, and rapidly diminishes after 60 min. Figure 3(b) is a plot of the average signal intensity for m/z 196 → 137 over time normalized to the TIC. These results show a clear trend for rapid clearance of 3-MoSA. Despite the inherent variability of ionization suppression that can be attributed to differences in tissue biological matrices, this trend is consistent with previous LC-ESI-MS/MS quantization experiments in homogenates of small sections of these same livers (Fig. 3(c)). These results confirm that MALDI-IMS can be successfully used to probe clearance of 3-MoSA via on-tissue derivatization.
Figure 3.
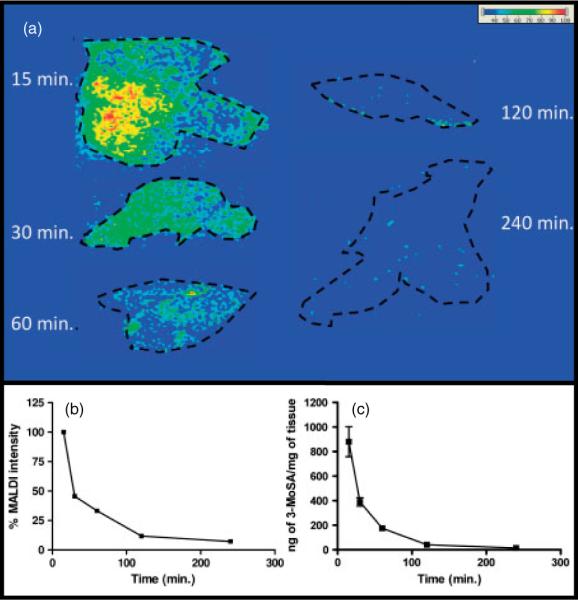
Concentration-time profile of 3-MoSA, as 3-MoSA-TCDI, in mouse liver after a single intraperitoneal injection (200 mg/kg). Tissues were harvested at 15, 30, 60, 120 and 240 min post-dose. (a) MALDI-IMS for m/z 196 → 137 of liver tissue sections from dosed mice. Images are plotted as m/z 137/TCI. (b) Average MALDI relative signal intensity for product ion m/z 137. (c) Amount of 3-MoSA in the same livers determined by LC-ESI-MS/MS of homogenates of a portion of the same livers.
3-MoSA saturation
A 3-MoSA dose saturation experiments were carried out to determine whether or not the amount of TCDI used in our derivatization experiment was sufficient to capture all of the endogenous 3-MoSA present in the tissue. In this study, a kidney tissue homogenate from a non-dosed animal was spiked with different amounts of 3-MoSA and treated with TCDI as described in the Experimental section. The use of tissue homogenate as opposed to intact tissue minimizes variability of biological matrix interference caused by tissue heterogeneity. To ascertain that the TCDI concentration used in our procedure would always be sufficient for analysis of tissues from dosed animals, we tested concentrations of 3-MoSA up to 2000 ng/mg. This concentration is higher than the maximum amount of 3-MoSA measured in kidney, liver, brain, plasma and spleen homogenates from dosed animals by the LC-ESI-MS/MS method previously reported in our laboratory.[24] Fig. 4(a) shows a plot of the relative intensities for SRM m/z 196 → 137 against 3-MoSA concentration. The absence of a plateau in this plot indicates that TCDI is still in excess at the highest 3-MoSA concentration tested. The lack of linearity of the signal at low concentration suggests that additional experiments with lower concentrations would be required to inform about the lower limit of detection.
Figure 4.
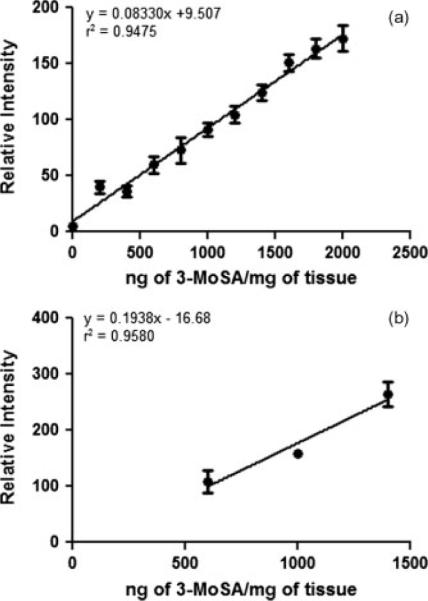
3-MoSA saturation and TCDI treatment reproducibility. (a) Plot of signal intensity for 3-MoSA-TCDI (as m/z 196 → 137) against 3-MoSA concentration in spiked kidney tissue homogenate. Samples were spotted in triplicate on the same plate and treated with TCDI in the same experiment. (b) Plot of signal intensity for 3-MoSA-TCDI against 3-MoSA concentration for three samples spotted on three different targets and treated with TCDI in three different experiments. y-axis is the average of n = 6 for each concentrations on the same MALDI target, and error bars are for three different MALDI targets. Note that the data for (b) was acquired after instrument modification which led to a higher laser intensity used in this experiment compared with (a).
Reproducibility of TCDI treatment
To determine the reproducibility of TCDI application in different experiments, the kidney tissue homogenate was spiked with three different concentrations of 3-MoSA and spotted on three different MALDI targets (n = 6 per target). TCDI was applied to each plate as described in the Experimental section. Figure 4(b) shows a plot of the average signal response for each 3-MoSA concentration across the 3 MALDI targets. These results indicate that despite the inherent potential variations that can occur when applying the TCDI reagent via manual spray coating, the overall procedure gives fairly reproducible results.
CONCLUSION AND FUTURE DIRECTIONS
The purpose of this paper is to convey proof of concept for on-tissue derivatization of 3-MoSA for MALDI-IMS studies and to provide a starting protocol applicable to other molecules. The following points are key findings of this manuscript: (1) TCDI treatment of tissues from dosed animals made it possible to detect the compound via a 3-MoSA-TCDI derivative. Without derivatization, MALDI-IMS studies of 3-MoSA would not be feasible due to the low sensitivity for detection of this compound even when high dosage is used. (2) Images of 3-MoSA-TCDI obtained for kidney, illustrate retention of spatial distribution information upon derivatization. (3) Furthermore, this work demonstrates the viability of probing the kinetics of 3-MoSA in intact tissue via on-tissue derivatization. However, further studies should include validation of the derivatization method using an internal standard in imaging experiments. This should correct for differences in ionization suppression due to variations caused by different biological matrices. Following validation, this method could be employed for studying the distribution of 3-MoSA on whole dosed animals, as has been reported in the literature for underivatized drug molecules.[7]
It is noteworthy that our manuscript provides an example of the translation of an established chemical derivatization method for quantitation of 3-MoSA by LC-ESI-MS/MS in tissue homogenates, to visualization of 3-MoSA on intact tissue by MALDI-IMS. As mentioned in the Introduction section, there are numerous methods of chemical derivatization aimed at improving detection of drug molecules by mass spectrometric techniques. There are also many drug molecules which are not amenable to MALDI-IMS studies. This example opens the door for expanding the range of drug molecules that can be studied by MALDI-IMS via adaptation of existing chemical derivatization methods.
Acknowledgements
This work was funded by Vanderbilt University, NIH grant HL81009 and GM 58008-10. JAO is the Thomas F. Frist, Sr., Professor of Medicine.
REFERENCES
- [1].Reyzer ML, Caprioli RM. MALDI-MS-based imaging of small molecules and proteins in tissues. Curr. Opin. Chem. Biol. 2007;11(1):29. doi: 10.1016/j.cbpa.2006.11.035. [DOI] [PubMed] [Google Scholar]
- [2].Reyzer ML, Hsieh YS, Ng K, Korfmacher WA, Caprioli RM. Direct analysis of drug candidates in tissue by matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 2003;38(10):1081. doi: 10.1002/jms.525. [DOI] [PubMed] [Google Scholar]
- [3].Sugiura Y, Setou M. Imaging mass spectrometry for visualization of drug and endogenous metabolite distribution: toward in situ pharmacometabolomes. J. Neuroimmune Pharmacol. 2010;5(1):31. doi: 10.1007/s11481-009-9162-6. [DOI] [PubMed] [Google Scholar]
- [4].Bunch J, Clench MR, Richards DS. Determination of pharmaceutical compounds in skin by imaging matrix-assisted laser desorption/ionisation mass spectrometry. Rapid Commun. Mass Spectrom. 2004;18(24):3051. doi: 10.1002/rcm.1725. [DOI] [PubMed] [Google Scholar]
- [5].Garrett TJ, Yost RA. Analysis of intact tissue by intermediate-pressure MALDI on a linear ion trap mass spectrometer. Anal. Chem. 2006;78(7):2465. doi: 10.1021/ac0522761. [DOI] [PubMed] [Google Scholar]
- [6].Hsieh Y, Casale R, Fukuda E, Chen JW, Knemeyer I, Wingate J, Morrison R, Korfmacher W. Matrix-assisted laser desorption/ionization imaging mass spectrometry for direct measurement of clozapine in rat brain tissue. Rapid Commun. Mass Spectrom. 2006;20(6):965. doi: 10.1002/rcm.2397. [DOI] [PubMed] [Google Scholar]
- [7].Khatib-Shahidi S, Andersson M, Herman JL, Gillespie TA, Caprioli RM. Direct molecular analysis of whole-body animal tissue sections by imaging MALDI mass spectrometry. Anal. Chem. 2006;78(18):6448. doi: 10.1021/ac060788p. [DOI] [PubMed] [Google Scholar]
- [8].Wang HYJ, Jackson SN, McEuen J, Woods AS. Localization and analyses of small drug molecules in rat brain tissue sections. Anal. Chem. 2005;77(20):6682. doi: 10.1021/ac050868d. [DOI] [PubMed] [Google Scholar]
- [9].Cornett DS, Frappier SL, Caprioli RM. MALDI-FTICR imaging mass spectrometry of drugs and metabolites in tissue. Anal. Chem. 2008;80(14):5648. doi: 10.1021/ac800617s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schwartz SA, Reyzer ML, Caprioli RM. Direct tissue analysis using matrix-assisted laser desorption/ionization mass spectrometry: practical aspects of sample preparation. J Mass Spectrom. 2003;38(7):699. doi: 10.1002/jms.505. [DOI] [PubMed] [Google Scholar]
- [11].Liu DQ, Hop CECA. Strategies for characterization of drug metabolites using liquid chromatography-tandem mass spectrometry in conjunction with chemical derivatization and online H/D exchange approaches. J. Pharm. Biomed. Anal. 2005;37(1):1. doi: 10.1016/j.jpba.2004.09.003. [DOI] [PubMed] [Google Scholar]
- [12].Melikian AA, O'Connor R, Prahalad AK, Hu PF, Li HY, Kagan M, Thompson S. Determination of the urinary benzene metabolites S-phenylmercapturic acid and trans, trans-muconic acid by liquid chromatography-tandem mass spectrometry. Carcinogenesis. 1999;20(4):719. doi: 10.1093/carcin/20.4.719. [DOI] [PubMed] [Google Scholar]
- [13].Wu CP, Ifa DR, Manicke NE, Cooks RG. Rapid, direct analysis of cholesterol by charge labeling in reactive desorption electrospray ionization. Anal. Chem. 2009;81(18):7618. doi: 10.1021/ac901003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Franck J, El Ayed M, Wisztorski M, Salzet M, Fournier I. On-tissue N-terminal peptide derivatizations for enhancing protein identification in MALDI mass spectrometric imaging strategies. Anal. Chem. 2009;81(20):8305. doi: 10.1021/ac901043n. [DOI] [PubMed] [Google Scholar]
- [15].Zagol-Ikapitte I, Amarnath V, Bala M, Roberts LJ, Oates JA, Boutaud O. Characterization of scavengers of gamma-ketoaldehydes that do not inhibit prostaglandin biosynthesis. Chem. Res. Toxicol. 2010;23(1):240. doi: 10.1021/tx900407a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Salomon RG. Distinguishing levuglandins produced through the cyclooxygenase and isoprostane pathways. Chem. Phys. Lipids. 2005;134(1):1. doi: 10.1016/j.chemphyslip.2004.12.003. [DOI] [PubMed] [Google Scholar]
- [17].Boutaud O, Li JY, Zagol I, Shipp EA, Davies SS, Roberts LJ, Oates JA. Levuglandinyl adducts of proteins are formed via a prostaglandin H-2 synthase-dependent pathway after platelet activation. J. Biol. Chem. 2003;278(19):16926. doi: 10.1074/jbc.M300940200. [DOI] [PubMed] [Google Scholar]
- [18].Carrier EJ, Amarnath V, Oates JA, Boutaud O. Characterization of covalent adducts of nucleosides and DNA formed by reaction with levuglandin. Biochemistry. 2009;48(45):10775. doi: 10.1021/bi9015132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Boutaud O, Montine TJ, Chang L, Klein WL, Oates JA. PGH(2)-derived levuglandin adducts increase the neurotoxicity of amyloid beta 1–42. J. Neurochem. 2006;96(4):917. doi: 10.1111/j.1471-4159.2005.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Davies SS, Amarnath V, Brame CJ, Boutaud O, Roberts LJ. Measurement of chronic oxidative and inflammatory stress by quantification of isoketal/levuglandin gamma-ketoaldehyde protein adducts using liquid chromatography tandem mass spectrometry. Nat. Protoc. 2007;2(9):2079. doi: 10.1038/nprot.2007.298. [DOI] [PubMed] [Google Scholar]
- [21].Salomon RG, Batyreva E, Kaur K, Sprecher DL, Schreiber MJ, Crabb JW, Penn MS, DiCorleto AM, Hazen SL, Podrez EA. Isolevuglandin-protein adducts in humans: products of free radical-induced lipid oxidation through the isoprostane pathway. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2000;1485(2–3):225. doi: 10.1016/s1388-1981(00)00038-x. [DOI] [PubMed] [Google Scholar]
- [22].Zagol-Ikapitte I, Masterson TS, Amarnath V, Montine TJ, Andreasson KI, Boutaud O, Oates JA. Prostaglandin H-2-derived adducts of proteins correlate with Alzheimer's disease severity. J. Neurochem. 2005;94(4):1140. doi: 10.1111/j.1471-4159.2005.03264.x. [DOI] [PubMed] [Google Scholar]
- [23].Amarnath V, Amarnath K. Specific determination of cysteine and penicillamine through cyclization to 2-thioxothiazolidine-4-carboxylic acids. Talanta. 2002;56(4):745. doi: 10.1016/s0039-9140(01)00631-2. [DOI] [PubMed] [Google Scholar]
- [24].Zagol-Ikapitte I, Amarnath V, Jadhav S, Oates JA, Boutaud O. Determination of 3-methoxysalicylamine levels in mouse plasma and tissue by liquid chromatography-tandem mass spectrometry: application to in vivo pharmacokinetics studies. J. Chromatogr. B. 2010;879:1098. doi: 10.1016/j.jchromb.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hankin JA, Barkley RM, Murphy RC. Sublimation as a method of matrix application for mass spectrometric imaging. J. Am. Soc. Mass Spectrom. 2007;18(9):1646. doi: 10.1016/j.jasms.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]