Abstract
The objectives of this study were to describe the serum selenium (Se) concentrations of beef cows from herds with varying levels of reproductive success, to examine factors associated with the Se concentrations at the end of the grazing season, and to determine if there were any associations between serum Se and reproductive success or calf viability. In the fall of 2001, 781 serum samples from 66 herds were analyzed as part of a nested case-control study to investigate risk factors for fertility; 10.6% were deficient in Se (< 0.025 ppm) and 86.8% were less than adequate (< 0.08 ppm). Low serum Se was most common in thin cows where feed Se was < 0.2 ppm, and from areas with more precipitation or with black or gray soils. Serum Se at pregnancy testing was not associated with an increased risk of reproductive failure. Lower herd serum Se was associated with increased risk of identifying degenerative myopathy in the subsequent calf crop (P = 0.006).
Résumé
Statut de sélénium à la fin de la saison de pâturage, performance de reproduction et myopathie dégénérative chez des troupeaux de bovins de boucherie. Les objectifs de cette étude étaient de décrire les concentrations de sélénium (Se) sérique chez les vaches de boucherie provenant de troupeaux avec divers taux de succès pour la reproduction, d’examiner les facteurs associés aux concentrations de Se à la fin de la saison de pâturage et de déterminer s’il y avait des associations entre le Se sérique et le succès pour la reproduction ou la viabilité des veaux. À l’automne 2001, 781 échantillons de sérum provenant de 66 troupeaux ont été analysés dans le cadre d’une étude de cas-témoins nichés pour étudier les facteurs de risque pour la fertilité; 10,6 % présentaient une déficience du Se (< 0,025 ppm) et 86,8 % affichaient un taux insuffisant (< 0,08 ppm). Un faible taux de Se sérique était le plus courant chez les vaches maigres dont les aliments comportaient < 0,2 ppm de Se et dans les régions avec le plus de précipitations ou avec des sols noirs ou gris. Le Se sérique lors des tests de gestation n’était pas associé à un risque accru d’échec de la reproduction. Un taux de Se sérique inférieur dans le troupeau était associé à un risque accru d’identification d’une myopathie dégénérative chez la récolte subséquente de veaux (P = 0,006).
(Traduit par Isabelle Vallières)
Introduction
Selenium (Se) deficiency has been linked to degenerative myopathy, immune deficiencies, and a variety of reproductive concerns including poor fertility and retained placentas (1–3). In a survey of United States veterinarians and diagnostic laboratories, Se deficiencies were reported to be an important livestock problem in 46 and 37 states, respectively (4).
Many researchers have examined the effects of Se deficiency in dairy herds (2,3,5–8). Where Se deficiency has been described in beef cattle, most studies have focused on herd-level risk factors and assessment of Se status (3,9–11). Other studies in beef calves have reported the effects of supplementation on growth and immune responses in controlled trials (12–14). Selenium deficiency is often listed as a differential diagnosis when investigating outbreaks of poor pregnancy rates or excessive calf losses in cow-calf herds. However, there is almost no published research supporting these connections. Research is necessary to better describe the trace mineral status of extensively managed beef cows and the association between micronutrients and reproductive performance in cow-calf herds.
The first objective of this study was to describe serum Se concentrations in both pregnant and non-pregnant beef cows from herds with varying levels of reproductive success. The second objective was to examine individual animal, herd management, and environmental factors associated with Se concentrations at the end of the grazing season. The final objective was to determine associations between the concentration of serum Se at the end of the grazing season and pregnancy status, as well as the subsequent risks of abortion, stillbirth, retained placenta, and the birth of calves that were weak or died with evidence of degenerative myopathy.
Materials and methods
Participants were selected from a group of 200 cow-calf herds enrolled in a study of factors affecting productivity in western Canada (15). From this larger study, all herds (n = 31) with pregnancy rates < 90% in the fall of 2001 were recruited for an investigation of the factors affecting reproductive performance (16). Another 35 herds were randomly selected from the herds where the pregnancy rate was ≥ 90% using a computer- generated random numbers list (16).
Blood samples were collected at the time of pregnancy testing between September and November 2001. Samples were collected from all accessible non-pregnant cows from each herd and a systematic random sample of pregnant cows. Following analysis for infectious causes of reproductive failure (16), half of the remaining samples was then randomly allocated to this analysis. Only aliquots containing a sufficient volume of non-hemolyzed serum for Se testing were submitted to the laboratory. The selection was stratified by herd and pregnancy status. The number of samples analyzed was limited by funding.
Laboratory methods
The blood samples were allowed to clot at room temperature and then stored at 4°C until the serum was separated. The serum was frozen within 48 h of collection and stored at −70°C until analysis. Laboratory staff were blinded as to the pregnancy status of the cows.
Selenium concentrations were determined by a commercial laboratory (Prairie Diagnostic Services, Saskatoon, Saskatchewan) using an inductively coupled plasma spectrometer coupled with a hydride generator device to assist formation of volatile selenium hydrides [Trace Scan, Atomic Scan 16/25 Inductively Coupled Serum (ICP) spectrometer; Thermo Jarrell Ash Corporation, Franklin, Massachusetts, USA]. The detection limit for the procedure was 0.17 ppb. A standard sample of bovine liver #1577b (National Institute of Standards and Technology, Gaithersburg, Maryland, USA) was included with each batch of analyses.
Feed sample analysis
Feed samples from the 2001 growing season were collected in late spring 2002. Core samples were taken from at least 10 randomly selected bales from each separate remaining hay and greenfeed source using a manual commercial core sampler. The cores for each individual feed source were combined for submission. Multiple grab samples were also collected from each silage source. Samples from individual feed stores in each herd were submitted separately to a commercial feed test laboratory (Enviro-Test Laboratories, Saskatoon, Saskatchewan). Feed samples were digested in nitric/perchloric acid and then analyzed to determine Se concentrations by inductively coupled plasma optical emission spectroscopy (ICP-OES) (17).
Animal and herd data
Additional data were collected to measure individual and herd level risk factors that may be associated with reproductive status. Individual risk factor data included cow age, breed type, and cow body condition (BCS), scored on a 9-point scale before breeding and at pregnancy testing (18). Herd management data included vaccination status and duration of bull exposure during the breeding season. Before the breeding season, herd bulls were evaluated for breeding soundness by the herd veterinarian, using the criteria from the Western Canadian Association of Bovine Practitioners.
The pregnancy status of individual cows was determined through transrectal palpation by the herd veterinarian in the fall of 2001 (September to November). Herd risk of non-pregnancy (%) was determined as the number of females found non-pregnant (open) divided by the number of females examined for pregnancy in the fall of the year (×100).
Herd owners were asked to record the cow identification and date for every animal known to have or suspected to have aborted. An abortion was defined as an observed premature calving, judged to be at least 1 mo prior to full term, or as an assumed calf loss from a cow that was diagnosed as pregnant but which failed to calve. The herd abortion risk was defined as the number of females that aborted, expressed as a percentage of the pregnant females retained after fall pregnancy testing for spring calving. Detailed calving records at the individual animal level were also maintained by the herd owner. Herd stillbirth risk was defined as the number of calves dead at or within 1 h of birth as a proportion of the number of calves born during the period that appeared to be within 1 mo of full-term gestation.
Herd owners were asked to make every effort to locate the fetus and placenta of cows that aborted and to record the identification and date for any death losses. Postmortem examinations were to be completed by the herd owner’s veterinarian and followed a standard collection protocol for histopathologic diagnosis. A standard set of multiple tissue samples, fixed in 10% buffered formalin, were submitted to a diagnostic laboratory (Prairie Diagnostic Services). Fixed tissues were processed routinely, stained with hematoxylin and eosin, and submitted to 1 veterinary pathologist for examination. Calves were classified based on whether or not they showed any evidence of degenerative myopathy in skeletal muscles (19).
Environmental data
Land locations used for pasture, wintering and calving grounds were categorized into ecoregions defined by Environment Canada (http://sis.agr.gc.ca/cansis/publications/ecostrat/intro.html) using a GIS (ArcView GIS 3.2, ESRI, Redlands, California, USA). Soil data were provided by Agriculture and Agri-Food Canada: the Canadian Soil Information System (CanSIS) (http://sis.agr.gc.ca/cansis/index.html) and the National Soil Database (NSDB) (http://sis.agr.gc.ca/cansis/nsdb/index.html). Each herd was classified based on the dominant soil type for the pastures to which the cattle had access during the 2001 grazing season using data from the Soil Landscapes of Canada, version 2.2, of the NSDB. Information on total accumulated precipitation from the 2001 growing season was obtained for April 1 to August 31, 2001 from Drought Watch, which is maintained by Agriculture and Agrifood Canada (PFRA) (www.agr.gc.ca/pfra/drought). The precipitation data were reported and analyzed based on the categories listed on the Drought Watch map.
Statistical analysis
Measures of central tendency and spread [mean, standard deviation (s)] were determined for individual animal serum Se concentrations and for the herd average serum Se concentrations. Individual serum Se concentrations were classified as adequate or deficient based on criteria used by the commercial laboratory (20), and the proportion of animals considered deficient in each herd was summarized.
The associations between herd management factors and Se status for individual animals were determined using linear mixed models with a random intercept to account for clustering by herd (MlwiN vers. 2.0; Centre for Multilevel Modelling, London, UK). The association between Se status of individual animals and reproductive performance (occurrence of non- pregnancy, abortion, and stillbirth) was examined using a generalized linear mixed model with a binomial distribution and logit link function and random intercept for herd. Cow age, breed, and body condition score at pregnancy testing were all considered as potential risk factors for serum Se status and as potential confounders of the relationship between serum Se concentration and individual animal reproductive performance. More complex models were explored only if more than 1 risk factor was potentially important in the model (P < 0.20).
The associations between herd level management and environmental variables and herd average Se concentrations were examined using linear regression (SAS for Windows ver 9.1; SAS Institute, Cary, North Carolina, USA). Because the environmental variables (ecoregion, soil type, and precipitation) were highly correlated only unconditional analyses were reported.
A final set of analyses was undertaken to look at associations between measured serum Se concentrations reflecting the herd status and reproductive performance of the herd. The prevalence of selenium-deficient samples from each herd and the mean serum Se concentration of the samples for each herd were each examined for associations with the reported proportion of cows in the herd that were non-pregnant, had aborted, or had a stillborn calf, retained placenta or weak calf. In this analysis, the number of animals from the herd with each outcome of interest was the numerator and the number of cows at risk of the outcome was the denominator. Generalized estimating equations were used to account for the expected clustering of reproductive outcomes within herd (PROC GENMOD, SAS for Windows ver 9.1; SAS Institute) with a binomial distribution and logit link function. A similar analysis was used to determine the association between herd Se status and whether or not a calf from the herd was diagnosed with degenerative skeletal myopathy.
Results
Study population
Blood samples were collected from 2516 cows and bred heifers selected from 12 073 females that were pregnancy tested from 66 herds in the fall of 2001 [median herd size, 173 breeding females; interquartile range (IQR): 126 to 219]. A subset of 781 samples representing all 66 herds was selected for Se testing; 380 samples from 31 herds with pregnancy rates < 90% and 401 samples from 35 herds with ≥ 90%. The median number of samples analyzed per herd was 12 (IQR: 8 to 16).
Of the 781 cows sampled, 19.5% (152) were bred heifers (1.5 y), 14.9% (116) were first calf heifers (2.5 y), 57.4% (448) were mature cows (3.5 to 9.5 y), 5.2% (41) were older cows (≥ 10 y), and the age was not reported for 3.1% (24 cows). The breeds included continental (44.9%, 351), British (40.6%, 317), or a mix of both (12.7%, 99); no breed was reported for 1.8% (14 cows). Most cows had a BCS ≥ 5 out of 9 (84.5%, 660), some had a BCS < 5 (12.3%, 96), and some had no score reported (3.2%, 25).
All bulls were examined before breeding in 61 of 66 herds; either 1 or 2 bulls were missed in the remaining 5 herds. There was a mean of 3.9 bulls for every 100 cows (range: 1.5 to 5.6). Breeding season was restricted to < 70 d in 33% of herds and from 70 to 120 d in 45%. Most herds used a BVDV and IBR vaccine in the 6-month period before breeding; 38% used a modified live vaccine, 48% used an inactivated vaccine, and the remainder (14%) used no vaccine.
For the 31 herds with pregnancy rates < 90%, the mean risk of non-pregnancy was 13.1% (range: 10.0% to 25.7%); 46% of the blood samples in this group were collected from open cows. For the randomly selected herds with pregnancy rates ≥ 90%, the mean non-pregnancy risk was 5.7% (range: 1.0% to 9.5%); 29% of the blood samples were collected from open cows.
The mean herd abortion rate was 1.5% (range: 0% to 7.3%). Of the 451 cows that were sampled, pregnant, and retained in the herd after pregnancy testing, 17 (3.8%) subsequently aborted. The herd stillbirth risk ranged from 0% to 11.1% with a mean of 2.5%. Of the 434 tested and pregnant cows that were retained in the herd until calving, 17 (3.9%) had a stillborn calf. Herd owners reported that an average of 0.6% (s = 1.2%) of cows retained their placentas and 1.1% (s = 3.9%) of calves were born weak. Of the 417 calves born alive from these cows, 20 (4.8%) died before they were 90 days of age.
During the spring of 2002, skeletal muscle was examined from 595 calves from these 66 herds (62 aborted, 192 stillborn, 136 died at ≤ 3 d, 211 died between 4 and 90 d). The median number of calves examined per herd was 8 (IQR: 5 to 13). Forty-nine (74%) herds had at least 1 diagnosis of degenerative myopathy in the calves that were examined.
Supplementation practices and selenium in stored forage samples
Cows were managed on natural and tame pastures during the breeding season. Loose trace mineral supplement was provided by 27% of herd owners, 33% used trace minerals in blocks or tubs, 6% used both methods, and 44% did not provide any supplementation on summer pasture. The Se concentration in the supplements was not reported. The cows remained on pasture or were moved to graze on crop residue until pregnancy testing.
During the feeding period, subsequent to pregnancy testing and leading up to calving in 2002, 8 herd owners reported giving the cows Se injections. Loose trace mineral supplement was provided by 70% of herd owners to some part of the breeding herd, 13.6% used blocks, 3% used lick tanks, and 3% reported using pellets with supplemental trace minerals and Se.
The median number of forage samples analyzed for Se per herd (N = 52 herds) was 3 (IQR: 2 to 4). Of the 162 forage samples analyzed, 105 were below the laboratory detection limit (< 0.2 ppm) for Se; 44 herds (85%) had ≥ 1 sample with < 0.2 ppm Se.
Serum selenium concentration
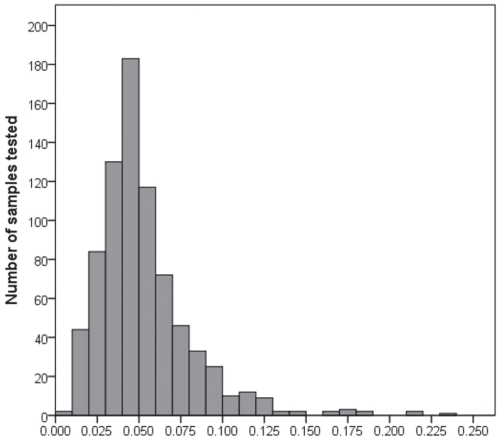
Of the 781 serum samples tested for Se at the end of the grazing season, 83 (10.6%) were deficient (< 0.025 ppm). Most samples (86.8%, 678/781) were less than adequate (< 0.08 ppm). The mean serum Se concentration was 0.052 ppm (s = 0.028) (Figure 1).
Figure 1.
Distribution of serum selenium concentrations (ppm) for samples collected from 781 breeding females from 66 beef herds at pregnancy testing in the fall of 2001.
Cows that were thin at pregnancy testing had lower serum Se levels than those with adequate body condition (P = 0.007). Age (P = 0.40) and breed (P = 0.98) were not associated with Se status. The mean serum Se for the pregnant cows was 0.051 ppm (s = 0.026) and for the non-pregnant cows was 0.053 ppm (s = 0.029).
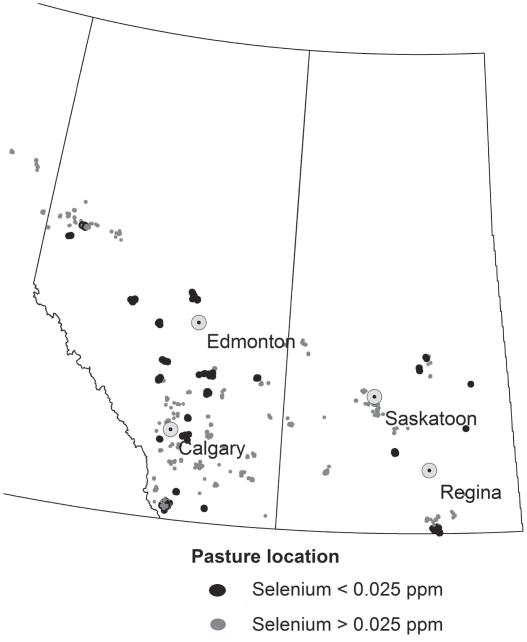
The mean herd prevalence of cows with serum Se concentration < 0.025 ppm was 0.11 (s = 0.23) and with serum Se concentration < 0.080 ppm was 0.86 (s = 0.27); 31.8% (21/66) of herds had ≥ 1 selenium-deficient animal (Figure 2). Herd average Se status varied substantially across the 66 herds (mean = 0.052 ppm, s = 0.024) with 66.6% of the total variation in serum Se accounted for by differences between herds. Although clustering by herd was very significant (P < 0.01), there was a substantial portion of variation among animals within the study herds (33.4%). Accounting for body condition at pregnancy testing did not change the proportion of variation accounted for by differences among herds (66.5%).
Figure 2.
Map of pasture locations used by 21 herds where ≥ 1 cow that had deficient serum selenium concentration (< 0.025 ppm) and 45 herds that had no cows with deficient serum selenium concentrations measured at the end of the grazing season.
Average Se levels did not differ between herds that did (0.053 ppm) or did not (0.048 ppm) report trace mineral supplementation on summer pasture (β, 0.005 ppm; 95% CI: −0.018 to 0.008; P= 0.47). However, the average serum Se concentrations in herds with at least 1 forage sample from the 2001 growing season that was < 0.2 ppm Se (0.047 ppm) were lower (β, −0.043 ppm; 95% CI: −0.058 to −0.028; P <0.0001) than for those in which all forage samples had ≥ 0.2 ppm Se (0.090 ppm).
Herd average Se concentrations were significantly lower in herds grazed on land that received > 250 mm of precipitation during the 2001 growing season compared with those on land that received ≤ 250 mm (P = 0.018). Herds with access to brown soils had significantly higher serum Se concentrations than herds on either black or gray soils (P < 0.001). Herds from the Mixed Grassland ecoregion had higher Se levels than those from all other areas (P < 0.001). Herds from the Moist Mixed Grassland ecoregions had higher Se levels than those from all other herds except those in the Mixed Grassland, Northern Continental Divide, and Peace Lowland ecoregions (P < 0.01).
Association between selenium status at pregnancy testing and reproductive performance
Selenium status in individual animals, measured as either the serum concentration or classified as deficient (< 0.025 ppm) or not deficient, was not associated with the risk of non-pregnancy (P > 0.26), abortion (P > 0.72), or stillbirth (P > 0.34). Accounting for BCS and age did not change the association between Se status and the risk of non-pregnancy (P > 0.24). Furthermore, herd Se status at pregnancy testing, examined as either average serum concentration of the sampled cows or the percent of the sampled cows that were deficient, was not associated with the herd risk of non-pregnancy (P > 0.19), abortion (P > 0.14), stillbirth (P > 0.20), the birth of weak calves (P > 0.11), or retained placentas (P > 0.87).
However, the 2002 calf crop from herds with lower Se status at pregnancy testing had an increased risk of at least 1 histologic diagnosis of degenerative skeletal myopathy. For every 10 ppb decrease in the herd average serum Se concentration, the odds of identifying a calf that died with degenerative skeletal myopathy increased by 1.4 times (95% CI: 1.1 to 1.7; P = 0.006), and for every 10% increase in the proportion of cows classified as deficient based on serum Se at pregnancy testing, the odds of identifying a calf with degenerative myopathy increased 1.6 times (95% CI: 1.0 to 2.6; P = 0.038).
Discussion
Low serum Se concentrations were identified in cows and heifers in herds examined at the end of the grazing season in western and central Alberta and central Saskatchewan. Deficiency was more common than reported in an earlier survey of 335 cows from 29 Alberta farms, on which 9% of the cows sampled were either marginal or deficient based on a whole blood Se of < 0.10 ppm in the fall of the year (9). The Alberta survey was consistent with Dargatz and Ross (10), who reported that 10.4% of the samples from 253 cow-calf operations in 18 states were marginally deficient in 1993 to 1994.
The present study differs from the earlier surveys in that serum Se rather than whole blood was used to assess Se status. Agreement between Se status based on serum and whole blood in an earlier study of Alberta beef herds was very good (κ = 0.79) at the herd level and good at the individual animal level (κ = 0.55) (21). While the laboratory in the present study used a different method of analysis, both laboratories had quality control programs in place with comparison to external standards. The results of the present study should be cautiously compared to the earlier reports based on whole blood Se.
Supplementation on summer pasture was not associated with Se concentrations at pregnancy testing. This finding was not unexpected as serum Se status reflects recent exposure, and herd owners were asked about supplementation practices during the breeding season. Selenium concentrations in serum or plasma are highly correlated with rate of oral or parenteral administration and respond quickly to changes in Se intake (22,23), as compared to whole blood Se which responds more slowly because most of the Se is incorporated into red blood cells during erythropoesis (24,25). However, Campbell et al (9) also reported no association between Se supplementation on summer pasture and whole blood Se concentrations.
Forage samples from the 2001 growing season were obtained for most study herds as a crude indicator of Se availability in local vegetation. Serum concentrations were significantly lower in herds where at least 1 feed sample had < 0.2 ppm. While low Se in soil and forage is recognized in many areas of western Canada (26,27), the geographic extent of the problem is not well-documented and thus recommendations for supplementation should be based on local conditions and forage analysis. The observation by Campbell et al (9) that Se concentrations were higher in cows coming off pasture from southeast Alberta than in cows from the Calgary and Edmonton areas, agreed with the present study in which none of the herds pastured in southeastern Alberta had Se deficiency.
Selenium levels were higher in herds from areas with predominantly brown soils compared with areas of black or gray soils. In western Canada, the highest frequency of selenium-deficient grains and roughages has been reported in black, dark gray, and dark-brown soil zones (26,28). Selenium is also reported to be more available to plants in areas with alkaline, well-aerated soils compared with areas of acidic and poorly drained soils (29,30). Selenium levels were also higher in herds from Mixed Grasslands or Moist Mixed Grasslands. These ecoregions correspond very closely with areas in which brown soils are most common. Selenium status was lowest in areas with the highest rainfall during the 2001 growing season. Lower levels of Se from lush, rapidly growing pasture in areas of heavy rainfall or fertilization may be the result of a dilution effect (28,29).
Pregnancy status was not associated with serum Se concentrations at the time of fall processing. Serum was collected at pregnancy testing as a matter of convenience and these samples would not reflect the Se status of the cow at the time of conception. Supplementation practices in many herds are optimized during the breeding period, but trace mineral supplementation is often less consistent in the latter part of the summer. In a recent study in Saskatchewan community pastures, researchers documented lower serum Se levels at the end of the grazing period as compared with the beginning of the breeding season (31). While fall is not the optimum time to measure trace mineral status and then examine a cause-effect association with herd fertility, this is when clinicians are most likely to collect samples to diagnose the reasons for poor pregnancy rates in beef herds.
In the present study, Se status at pregnancy testing was also not associated with the frequency of retained placentas, weak calves, abortions, or stillbirths. The effect of Se on the occurrence of retained placentas has been most consistently demonstrated in controlled studies involving supplementation (6). While deficiencies in Se or vitamin E have also been associated with weak calves at birth, clinical trials examining Se supplementation have not demonstrated decreases in the incidence of stillbirth/perinatal weak calf syndrome (14,32–34).
The reported serum status reflects Se intake on fall pasture and any free choice supplementation at the time of pregnancy testing. Most of these herds either resumed or increased the consistency of supplementation after winter feeding began in late fall and early winter. A small percentage of the herds used Se injections in the cows prior to calving. The management history suggests that the Se status should have improved before calving in these herds due to increased supplementation and, therefore, potential problems due to inadequate Se in cows coming off fall pasture might have been mitigated. Finally, the study was designed to look at differences between animals that were and were not pregnant in the fall; the power to assess the association with later reproductive events was limited due to the small number of reported outcomes. The reporting of abortions and stillbirths in the present study was verified by careful individual animal accounting; the reporting of retained placentas and weak calves could not be verified and was likely less complete.
The role of trace mineral status in abortions and stillbirths is not well understood. In a large case-control study of 2000 beef and dairy herds, Enjalbert et al (3) found increased odds of abortion and perinatal mortality in herds with a deficient or marginally deficient Se status. In earlier reports, aborted fetuses from western Canada had, on average, lower Se status than non-aborted fetuses (35,36). Aborted fetuses with lesions indicating cardiac failure also had lower Se status than aborted fetuses without these lesions.
In the present study, Se status of the herd at pregnancy testing was related to an increased risk of a histological diagnosis of degenerative myopathy in the skeletal muscles of calves that were aborted or died in the spring of 2002. The pathology data were part of a larger study examining gross postmortem and histologic findings in 203 western Canadian beef herds (19). Degenerative skeletal myopathy was a very common finding and was present in 5.6% of 178 aborted fetuses, 20.2% of 555 stillborn calves, 46.2% of 383 neonatal calves, and 40.2% of 547 older calves. Degenerative skeletal myopathy was also frequently observed in calves dying of other causes including starvation, exposure, infectious disease, and dystocia. In a comparable study, Yamini et al (37) reported that 4% of bovine abortions in the north-central United States had evidence of myopathy.
In conclusion, serum Se concentrations in cows coming off summer pasture were lowest in areas where the forage was Se deficient and in areas with gray or black soils. Cows that were thin coming off summer pasture were also at increased risk of Se deficiency. While Se status measured at pregnancy testing was not associated with an increased risk of reproductive failure in either the individual cow or herd, lower herd average serum Se concentrations were associated with a higher risk of degenerative myopathy in calves lost during the subsequent calving season.
Acknowledgments
Data were collected as part of the field research activities for the Western Canada Study of the Animal Health Effects Associated with Exposure to Emissions from Oil and Natural Gas Field Facilities. Funding support was provided through the Western Interprovincial Scientific Studies Association (WISSA). The authors acknowledge the dedicated technical support of Cindy Jelinski, Diane Sanjenko, and the staff of Prairie Diagnostic Services in managing the samples associated with this study. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Corah LR, Ives S. The effects of essential trace minerals on reproduction in beef cattle. Vet Clin North Am Food Anim Prac. 1991;7:41–57. doi: 10.1016/s0749-0720(15)30809-4. [DOI] [PubMed] [Google Scholar]
- 2.Olson JD. The role of Se and vitamin E in mastitis and reproduction of dairy cattle. Irish Vet J. 1996;49:362–364. [Google Scholar]
- 3.Enjalbert F, Lebreton P, Salat O. Effects of copper, zinc and selenium status on performance and health in commercial dairy and beef herds: Retrospective study. J Anim Physiol Anim Nutr. 2006;90:459–466. doi: 10.1111/j.1439-0396.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- 4.Edmondson AJ, Norman BB, Suther D. Survey of state veterinarians and state veterinary diagnostic laboratories for selenium deficiency and toxicosis in animals. J Am Vet Med Assoc. 1992;202:865–872. [PubMed] [Google Scholar]
- 5.Coe PH, Maas J, Retnolds J, Gardner I. Randomized field trial to determine the effects of oral selenium supplementation on milk production and reproductive performance of Holstein heifers. J Am Vet Med Assoc. 1993;202:875–881. [PubMed] [Google Scholar]
- 6.Eger S, Drori D, Kadoori I, Miller N, Schindler H. Effects of selenium and vitamin E on incidence of retained placenta. J Dairy Sci. 1985;68:2119–2122. doi: 10.3168/jds.S0022-0302(85)81077-8. [DOI] [PubMed] [Google Scholar]
- 7.Hidiroglou M, McAllister AJ, Williams CJ. Prepartum supplementation of selenium and vitamin E to dairy cows: Assessment of selenium status and reproductive performance. J Dairy Sci. 1987;70:1281–1288. doi: 10.3168/jds.S0022-0302(87)80142-X. [DOI] [PubMed] [Google Scholar]
- 8.Wichtel JJ, Keefe GP, Van Leeuwen JA, Spangler E, McNiven MA, Ogilvie TH. The selenium status of dairy herds in Prince Edward Island. Can Vet J. 2004;45:124–132. [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell JR, Jim GK, Booker CW, Guichon PT. A survey of the selenium status of beef cows in Alberta. Can Vet J. 1995;36:698–702. [PMC free article] [PubMed] [Google Scholar]
- 10.Dargatz DA, Ross PF. Blood selenium concentration in cows and heifers on 253 cow-calf operation in 18 states. J Anim Sci. 1996;74:2891–2895. doi: 10.2527/1996.74122891x. [DOI] [PubMed] [Google Scholar]
- 11.Hoff B, Schrier N, Boermans H, Faulkner H, Hussein A. Assessment of trace mineral and vitamin E status of beef cows in Ontario. Can Vet J. 2001;42:384–385. [PMC free article] [PubMed] [Google Scholar]
- 12.Swecker WS, Eversole DE, Thatcher CD, Blodgett DJ, Shurig GG, Meldrum JB. Influence of supplemental selenium on humoral immune responses in weaned beef calves. Am J Vet Res. 1989;50:1760–1763. [PubMed] [Google Scholar]
- 13.Awadeh FT, Kincaid RL, Johnson KA. Effect of level and source of dietary selenium on concentrations of thyroid hormones and immunoglobulins in beef cows and calves. J Anim Sci. 1998;76:1204–1215. doi: 10.2527/1998.7641204x. [DOI] [PubMed] [Google Scholar]
- 14.Gunter SA, Beck PA, Phillips JM. Effects of supplementary selenium source on the performance and blood measurements in beef cows and their calves. J Anim Sci. 2003;81:856–864. doi: 10.2527/2003.814856x. [DOI] [PubMed] [Google Scholar]
- 15.Waldner CL. Western Canada study of animal health effects associated with exposure to emissions from oil and natural gas field facilities. Study design and data collection I. Herd performance records and management. Arch Environ Occup Health. 2008;63:167–186. doi: 10.3200/AEOH.63.4.167-184. [DOI] [PubMed] [Google Scholar]
- 16.Waldner CL. Serological status for N. caninum, bovine viral diarrhea virus, and infectious bovine rhinotracheitis virus at pregnancy testing and reproductive performance in beef herds. Anim Repro Sci. 2005;90:219–242. doi: 10.1016/j.anireprosci.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Association of Official Analytical Chemists (AOAH) Minerals in animal feed. (968.08D) Official methods of analysis. 15th ed. 1990. [Google Scholar]
- 18.Rice LE. The effects of nutrition on reproductive performance of beef cattle. Vet Clin North Am Food Anim Pract. 1991;7:1–26. doi: 10.1016/s0749-0720(15)30807-0. [DOI] [PubMed] [Google Scholar]
- 19.Waldner CL, Kennedy RI, Rosengren LB, Pollock CM, Clark EG. A description of gross postmortem and histologic examination findings from abortion losses and calf mortalities in western Canadian beef herds. Can Vet J. 2010;51:1227–1238. [PMC free article] [PubMed] [Google Scholar]
- 20.Puls R. Mineral Levels In Animal Health: Diagnostic Data. 2nd ed. Clearbrook, British Columbia: Sherpa International; 1994. [Google Scholar]
- 21.Waldner C, Campbell J, Jim KG, Guichon PT, Booker CW. Comparison of 3 methods of selenium assessment in cattle. Can Vet J. 1998;39:225–231. [PMC free article] [PubMed] [Google Scholar]
- 22.Stowe HD, Herdt TH. Clinical assessment of selenium status of livestock. J Anim Sci. 1992;70:3928–3933. doi: 10.2527/1992.70123928x. [DOI] [PubMed] [Google Scholar]
- 23.Walburger KJ, DelCurto T, Pulsipher GD, Hathaway RL, Pirelli GJ. The effect of fertilizing forage with sodium selenate on selenium concentration of hay, drain water and serum selenium concentrations in beef heifers and calves. Can J Anim Sci. 2007;88:79–83. [Google Scholar]
- 24.Enjalbert F, Lebreton P, Salat O, Schelcher F. Effects of pre- or post-partum selenium supplementation on selenium status in beef cows and their calves. J Anim Sci. 1999;77:223–229. doi: 10.2527/1999.771223x. [DOI] [PubMed] [Google Scholar]
- 25.Scholtz RW, Hutchinson LJ. Distribution of glutathione peroxidase activity and selenium in the blood of dairy cows. Am J Vet Res. 1979;40:245–249. [PubMed] [Google Scholar]
- 26.Owen BD, Sosulski F, Wu KK, Farmer MJ. Variation in mineral content of Saskatchewan feed grains. Can J Anim Sci. 1977:679–687. [Google Scholar]
- 27.Boila RJ, Stothers SC, Campbell LD. The concentrations of selenium in the grain from wheat, barley and oats grown at selected locations throughout Manitoba. Can J Anim Sci. 1993;73:217–221. [Google Scholar]
- 28.Smart ME, Gudmondson J, Christensen DA. Trace mineral deficiencies in cattle: A review. Can Vet J. 1981;22:372–376. [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta UC, Watkinson JH. Agricultural significance of selenium. Outlook on Agric. 1985;14:183–189. [Google Scholar]
- 30.Berger LL. Variation in the trace mineral content of feedstuffs. The Prof Anim Scientist. 1996;12:1–5. [Google Scholar]
- 31.Van De Weyer LM, Hendrick S, Waldner CL. Serum micronutrient concentrations in beef cows before and after the summer grazing season. Can J Anim Sci. 2010;90:563–574. [Google Scholar]
- 32.Logan EF, Rice DA, Smyth JA, Ellis WA. Weak calf syndrome and parenteral selenium supplementation. Vet Rec. 1990;126:163–164. [PubMed] [Google Scholar]
- 33.Logan EF, Smyth JA, Kennedy DG, Rice DA, Ellis WA. Stillbirth and perinatal weak calf syndrome. Vet Rec. 1991;129:9. doi: 10.1136/vr.129.5.99. [DOI] [PubMed] [Google Scholar]
- 34.McCoy MA, Smyth JA, Ellis WA, Kennedy DG. Parenteral iodine and selenium supplementation in stillbirth/perinatal weak calf syndrome. Vet Rec. 1995;136:124–126. doi: 10.1136/vr.136.5.124. [DOI] [PubMed] [Google Scholar]
- 35.Orr JP, Blakely BR. Investigation of vitamin E and selenium status of aborted calves with myocardial degeneration. Saskatoon: Saskatchewan; 1994. Report No.: 920001. [Google Scholar]
- 36.Orr JP, Blakley BR. Investigation of the selenium status of aborted calves with cardiac failure and myocardial necrosis. J Vet Diag Invest. 1997;9:172–179. doi: 10.1177/104063879700900211. [DOI] [PubMed] [Google Scholar]
- 37.Yamini B, Mullaney TP, Patterson JS, Fitzgerald SD, Steficek BA, Kennedy F. Causes of bovine abortion in the north-central United States: Survey of 1618 Cases (1983–2001) Bov Pract. 2004;38:59–64. [Google Scholar]