Abstract
A 9-week-old kitten was diagnosed with a congenital vascular ring anomaly by means of an esophageal contrast study. At 6 mo of age, a non-selective vascular study was used to diagnose a persistent right aortic arch (PRAA). Left-sided thoracoscopic surgery was performed, using a Liga-Sure vessel sealant device to seal and transect the ligamentum arteriosum.
Résumé
Correction par thoracoscopie d’une crosse aortique droite congénitale persistante chez un jeune chat. Un chaton âgé de 9 semaines a été diagnostiqué avec une anomalie congénitale d’anneau vasculaire à l’aide d’un examen œsophagien en contraste. À l’âge de 6 mois, une étude vasculaire non sélective a été utilisée pour diagnostiquer une crosse aortique droite persistante (PRAA). Une chirurgie par thorascopie du côté gauche a été réalisée à l’aide d’un dispositif de scellage des vaisseaux Liga-Sure pour sceller et couper transversalement le ligamentum arteriosum.
(Traduit par Isabelle Vallières)
Vascular ring anomalies (VRA) result from abnormal differentiation of embryonic aortic arches into the great vessels (1,2). Several types of VRAs can occur; however, persistent right aortic arch (PRAA) is the most common cause of congenital esophageal dilation in dogs and cats (1,3). This anomaly follows development of the aorta from the embryonic right 4th aortic arch, rather than the left 4th aortic arch (2–4). The esophagus becomes entrapped between the ligamentum arteriosum (LA) dorsally, pulmonary artery on the left, aorta on the right and the heart ventrally (1–4). Esophageal constriction and proximal dilation of the esophagus result (1–4). Clinical symptoms may include stunted growth despite polyphagia, postprandial regurgitation soon after weaning, and repeated episodes of aspiration pneumonia (1,3,5,6). Diagnosis of a VRA is achieved by barium contrast radiography. If the animal has a PRAA, radiographs will show variable dilation of the esophagus proximal to an esophageal constriction at the base of the heart (3,7,8). Usually, maximal dilation is observed in the cranial mediastinal area (3). Angiocardiography is often required for definitive diagnosis of a PRAA, but in some cases, the diagnosis can only be made by surgical exploration (3,5,6). This report describes the pre-surgical management of a kitten with a PRAA, its thoracoscopic correction, and successful outcome.
Case description
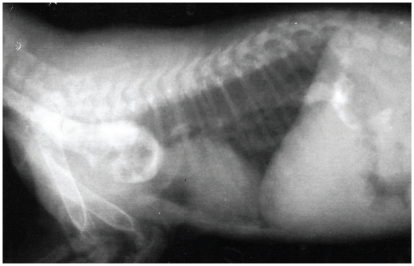
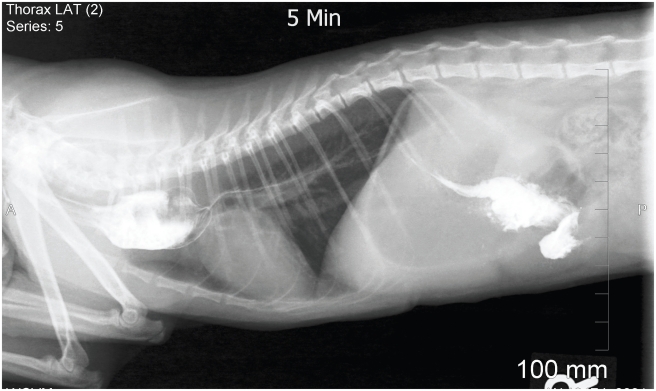
A 9-week-old female kitten was evaluated for a suspected vascular ring anomaly. Upon physical examination, the kitten was small for her age, weighed 0.36 kg, and had vital parameters that were within normal ranges. Cardiopulmonary auscultation did not identify any abnormalities. An esophageal contrast study, performed by the referring veterinarian, showed esophageal dilatation cranial to the heart base (Figure 1). Pneumonia was not observed. Regurgitation had been controlled with upright feedings of liquid gruel every 2 h. The kitten was vaccinated for feline viral rhinotracheitis, calicivirus, and panleukopenia (FVRCP).
Figure 1.
A right lateral radiograph taken during an esophageal contrast study showed dilation of the esophagus anterior to the base of the heart.
Due to the kitten’s small size and poor body condition, a gastrostomy tube was placed under general anesthesia via a left-sided grid approach immediately caudal to the 13th rib as previously described (9). A gastropexy around the tube was performed and the abdomen was lavaged with sterile warm saline (0.9% NaCl). The abdominal incision was closed routinely. A purse string and Chinese finger trap, using 2-0 monofilament polybutester (Novafil; Syneture, United States Surgical, Norwalk, Connecticut, USA) were used to secure the gastrostomy tube externally (10). Over the next 4 mo, the kitten was fed warmed canned food through the gastrostomy tube and weighed daily. Dietary intake was gradually increased to deliver the daily basal energy requirements for growth. The gastrostomy tube was replaced 3 mo after initial placement, due to obstruction of the tube.
At 6 mo of age and a body weight of 1.5 kg, contrast angiography was performed to determine the nature of the VRA. Pre-operative packed cell volume was 0.35 L/L and total protein was 66 g/L. Pre-anaesthetic sedation was achieved with hydromorphone hydrochloride (Sandoz Canada, Boucherville, Quebec), 0.05 mg/kg body weight (BW), IM. Anesthesia was induced with propofol (Rapinovet; Schering-Plough Animal Health, Pointe-Claire, Quebec), 6 mg/kg BW, IV and maintained with isoflurane inhalant (Abbott Laboratories, Saint-Laurent, Quebec), 2% in oxygen. Normosol-R (Hospira Healthcare, Montreal, Quebec), 10 mL/kg BW/h, IV) and cefazolin (Sandoz Canada), 22 mg/kg BW, IV were administered intraoperatively. Analgesia was provided by a continuous rate infusion of fentanyl citrate (Sandoz Canada), 2 μg/kg BW/h, IV titrated according to the patient response under general anesthesia. Hypotension and bradycardia were managed with Normosol-R (10 mL/kg BW, IV over 20 min), dextran-40 (Hospira Healthcare), 5 mL/kg boluses to a total of 15 mL/kg, IV, ephedrine (Sandoz Canada), 0.05 mg/kg BW, IV, and glycopyrrolate (Sandoz Canada), 0.01 mg/kg BW, IV. Due to the patient’s small size, a surgical approach was made to the femoral artery. However, catheterization of the femoral artery with a catheter of a sufficient size to perform a diagnostic study was unsuccessful. A non-selective vascular study was performed under fluoroscopy, using a cut-down to access the jugular vein and to administer iohexol (Omnipaque 240; GE Healthcare Canada, Mississauga, Ontario), 0.4 mL/kg BW (11,12). The study was consistent with a diagnosis of a PRAA. Buprenorphine (Vetergesic; Champion Alstoe Animal Health, Whitby, Ontario), 0.15 mg/kg BW, IV was administered post-operatively.
One week later, the kitten was anesthetized for thoracoscopic correction of the PRAA. Preoperative packed cell volume was 0.347 L/L and total protein was 65 g/L. Sedation was achieved with hydromorphone hydrochloride (Sandoz Canada), 0.1 mg/kg BW, IM. Anesthesia was induced with propofol (Rapinovet, Schering-Plough Animal Health), 6 mg/kg BW, IV and the patient was intubated and maintained with isoflurane inhalant (Abbott Laboratories), 2% in oxygen. Morphine sulfate (Morphine HP® 25; Sandoz Canada), 0.1 mg/kg BW in 0.2 mL of 0.9% NaCl, was administered by epidural injection. Cefazolin (Sandoz Canada), 22 mg/kg BW, IV, every 2 h and fluid therapy with Normosol-R (Hospira Healthcare), 10 mL/kg BW/h, IV) and 2.5% dextrose were administered. Glycopyrrolate (Sandoz Canada), 0.01 mg/kg BW, IV, fluid boluses (Normosol-R, Hospira Healthcare; 10 mL/kg BW, IV over 20 min) and dextran-40 (Hospira Healthcare), 5 mL/kg BW boluses, to a total of 15 mL/kg BW, IV were administered to treat intraoperative bradycardia (heart rate 103 beats per minute) and hypotension (mean blood pressure of 55 mmHg). Morphine sulphate (Sandoz Canada) was delivered by a continuous rate infusion, 0.1 mg/kg BW/h, IV.
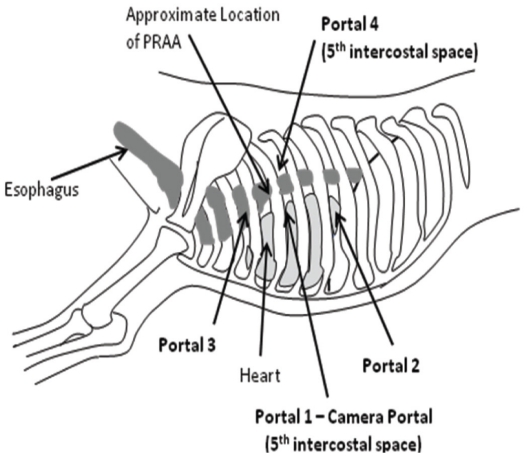
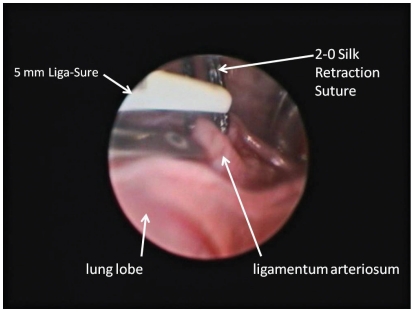
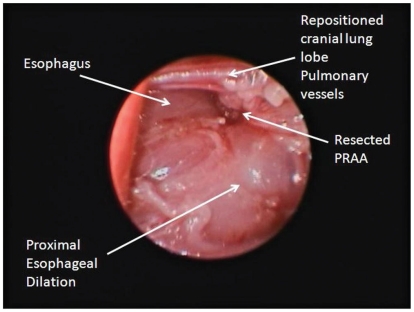
The patient was placed in right lateral recumbency and the left hemithorax clipped and prepared aseptically. Bupivicaine hydrochloride (Marcaine 0.50%; Hospira Healthcare), 2 mg/kg total, IM was injected over the 3rd, 5th, and 7th intercostal spaces. Prior to thoracoscopy, the kitten was placed on positive pressure ventilation. A 6-mm cannula was inserted at the 5th intercostal space, mid-distance between the dorsal and ventral borders of the thorax (Figure 2) for placement of a 5-mm 0 degree camera. A 2nd portal was made at the 7th intercostal space at the same level as the 1st portal (Figure 2) in order to insert a retractor. Small right angle forceps were used to retract the cranial left lung lobe, to visualize the great vessels. The 3rd portal was established at the 3rd intercostal space, slightly dorsal to the camera portal (Figure 2). A 4th portal was placed midway between the 1st portal and the dorsal aspect of the thoracic cavity at the 5th intercostal space (Figure 2). A mosquito hemostat, introduced through portals 3 and 4, was used to bluntly dissect and isolate the LA. Then 2-0 Silk (Ethicon, Somerville, New Jersey, USA) was passed through portal 3, under the LA to exit through the same portal. This was used to retract the LA cranio-laterally away from the esophagus so that the mosquito hemostat could be removed. Sterile cotton-tipped applicators were used to control minor hemorrhage and perform fine blunt dissection during the procedure. When needed, the retraction suture was also passed through portal 4 to allow dorso-lateral retraction of the LA. A 5-mm Liga-Sure vessel sealant device was introduced through portal 3 and the LA was simultaneously sealed and transected, using the silk suture to help manipulate the LA (Figure 3). The Liga-Sure was discharged twice in order to ensure complete sealing of the LA. Bands of fibrous tissue coursing across the esophagus were bluntly released using the hemostats and the Liga-Sure. A stomach tube was passed beyond the narrowed region of esophagus, to ensure all narrowing of the esophagus had been successfully released. The thoracic cavity and regions of transection were visually inspected for hemorrhage (Figure 4).
Figure 2.
Approximate location of the 4 thoracoscopic portals used for ligation of the ligamentum arteriosum during the PRAA procedure.
Figure 3.
View of the retracted ligamentum arteriosum during placement prior to firing of the 5-mm Liga-Sure device.
Figure 4.
Assessment of the esophagus after ligation and transaction of the ligamentum arteriosum and removal of the narrowing bands of fibrous tissue.
A chest tube was placed with thoracoscopic visualization and intrapleural bupivicaine hydrochloride (Marcaine 0.50%; Hospira Healthcare), 2 mg/kg BW was administered. Each portal was closed in a similar manner: the muscles using 3-0 polydioxanone (PDS*II, Ethicon) in a cruciate pattern, subcutaneous tissue with 3-0 poliglecaprone 25 (Monocryl, Ethicon) in an inverted cruciate pattern, and the skin with a 4-0 poliglecaprone 25 (Monocryl, Ethicon) in an inverted cruciate pattern. The thoracoscopic procedure lasted 4 h and 5 min.
Immediately after thoracoscopy, the esophagus was examined endoscopically. A large mass of hair was observed within the dilated region but not removed due to difficulty advancing the endoscope into the dilated region. Difficulty directing the endoscope through the dilated region of the esophagus prevented passage into the distal esophagus, but the area appeared distended with insufflation. Hydromorphone hydrochloride (Sandoz Canada), 0.05 mg/kg BW, IM was administered on recovery from general anaesthesia, followed by buprenorphine hydrochloride (Vetergesic; Champion Alstoe Animal Health), 0.02 mg/kg BW, SQ every 6 to 8 h for 36 h. The gastrostomy tube was left in place for feeding in the initial post-surgical period. The chest tube was removed 17 h after placement. The kitten regurgitated the mass of hair 4 d after surgery.
Two weeks after correction of the PRAA, the stoma around the gastrostomy tube became painful and drained purulent material. Systemically, the kitten appeared normal. The kitten was sedated with hydromorphone hydrochloride (Sandoz Canada), 0.1 mg/kg BW, IM. The gastrostomy tube was removed and a sample of exudate submitted for culture and sensitivity. The stoma was left open for 4 to 5 d while the infection was treated. Culture yielded 1+ Escherichia coli and 4+ Streptococcus. The stoma was cleaned several times daily using 0.05% chlorhexidine solution and amoxicillin (N-Amoxi; McKesson, Richmond Hill, Ontario), 11 mg/kg BW, PO was administered every 12 h. Food continued to drain after eating, so 5 d after removal of the gastrostomy tube, general anesthesia was performed and the gastric wall was closed with 3-0 polydioxanone (PDS*II; Ethicon, Somerville, New Jersey, USA). The subcutaneous tissue and skin were left to heal by second intention.
Three days after thoracoscopic correction of the PRAA, small amounts of food were given by mouth. Regurgitation was not observed, except when associated with hairballs. A follow-up esophageal contrast study was performed 4 wk after correction of the PRAA. Rapid flow of the barium into the stomach was observed, with a residual dilation of the esophagus just cranial to the heart base (Figure 5). Despite some narrowing in the region of the PRAA, the contrast did not pool in the dilated region and the kitten had minimal signs of regurgitation.
Figure 5.
A follow-up esophageal contrast study, performed 4 weeks after correction of the PRAA, showing residual dilation of the esophagus cranial to the heart base and rapid flow of the barium into the stomach.
Four months after surgery, routine ovariohysterectomy and follow-up esophageal endoscopy were performed. Pre-anesthetic blood work showed that values were within normal limits. Hydromorphone hydrochloride (Sandoz Canada), 0.05 mg/kg BW, IV and acepromazine maleate (Atravet; Ayerst Veterinary Laboratories, Guelph, Ontario), 0.03 mg/kg BW, IV were administered. Anesthesia was induced with propofol (Rapinovet; Schering-Plough Animal Health), 6 mg/kg BW, IV, and maintained with isoflurane (1.5% to 2%) in oxygen. Glycopyrrolate (Sandoz Canada), 0.01 mg/kg BW, IV was given to treat bradycardia (95 beats per minute). Esophageal endoscopy revealed moderate dilation of the proximal esophagus but some motility was observed. The dilated region of the esophagus was empty and no constriction of the esophagus was identified. The ovariohysterectomy was performed without complications.
Twenty-seven months after the thoracoscopic ligation of the PRAA, the owner reported that the cat was doing very well. She was eating a combination of Hills Prescription diet® t/d® and Medi-Cal® preventative formula canned food. She was fed twice daily from an elevated level. Dry food was fed in small amounts from floor level. She had not regurgitated for several months. Her weight was 2.4 kg at the time of follow-up.
Discussion
Vascular ring anomalies are less common in cats than in dogs. Anomalies in dogs and cats include persistent right aortic arch with left ligamentum arteriosum, persistent right ligamentum arteriosum with a left aortic arch, aberrant left or right subclavian artery, aberrant intercostals, and double aortic arch (1). In dogs, PRAA is responsible for approximately 95% of all reported vascular ring anomalies, and German shepherd and Irish setter dogs are at greater risk (1,3). From 1960 to 2010, there were 20 reports of vascular ring anomalies in cats (2,4,6,8,13–27). Seventeen of these reports were PRAAs, 1 was a double aortic arch and 2 were left aortic arches. However, unlike dogs, no feline breed predilection has been determined, possibly due to the limited number of reports (1,25). Persians and Siamese, however, may be overrepresented (1).
Medical treatment of a PRAA is unsuccessful in most cases and is associated with a poor long-term prognosis (1,6,28). Surgical ligation and transection of the LA is recommended, removing the esophageal constriction and resulting in improvement of clinical signs, despite residual esophageal dilation (1,28). Surgical correction of the PRAA is performed through a left sided thoracotomy by ligation and transection of the ligamentum arteriosum (1–4,6–8,13,21,23,24). Any associated fibrous strands narrowing the esophagus are also transected. While this generally resolves the esophageal constriction and decreases the risk of complications associated with regurgitation (such as aspiration pneumonia), the esophageal dilation is usually permanent (1,3,28).
Surgical repair is generally recommended as soon as the VRA is diagnosed to minimize permanent dilation of the esophagus due to loss of neuromuscular function and peristalsis of the esophagus (1). In this case, surgery was delayed due to the small size of the patient; however, this did not appear to complicate the clinical outcome of surgery. The lag time between admission and thoracoscopic correction was also related to a labour dispute at the Veterinary Teaching Hospital. Ideally, surgical repair of the PRAA would have been performed at the same time as replacement of the gastrostomy tube.
A gastrostomy tube was used to manage the young cat until she was large enough to undergo the thoracoscopic procedure. The gastrostomy tube allowed basal energy requirements to be supplied, and progressive dilation of the esophagus was minimized by preventing oral feeding. Gastrostomy tubes offer immediate and long-term feeding solutions for animals, and can be maintained for weeks to months (9). In this case, the gastrostomy tube (Pezzer) was placed by an open technique. Percutaneous endoscopic guided placement was not possible due to the small patient size and the constriction present in the esophagus.
Traditional thoracotomy procedures are invasive. Potential complications include pulmonary dysfunction, infection, seroma development, lameness, and post-surgical pain (8,13,29,30). Thoracoscopy has been gaining popularity in both veterinary and human medicine, due to lower complication rates (28–33). Other benefits in both veterinary and human medicine include: decreased morbidity and post-operative pain, lower post-operative complications in high-risk patients, decreased pulmonary dysfunction and tissue trauma, shorter surgical time, decreased intra-operative hypothermia, and shorter recovery time (28–30,32–34).
In veterinary medicine, thoracoscopy has been used for exploration with biopsies, partial pericardectomy, thoracic duct ligation, occlusion of patent ductus arteriosus, lung lobe biopsy and lobectomy, and thoracoscopic correction of a PRAA in dogs (28–31,35–38). The thoracoscopic anatomy of dogs has been previously described (34) and is similar in cats. To the authors’ knowledge, thoracoscopic ligation of the PRAA has not been reported in the cat. Slight modifications in portal location were made to allow for visualization of the LA.
In this case, standard analgesic procedures were used pre- and intra-operatively. The patient was very responsive to minimal stimuli, but also sensitive to higher minimum alveolar concentrations (MAC) of isoflurane inhalant. Thus, a continuous rate infusion of fentanyl was used for its anesthetic-sparing effect as well as to help manage hypotension and maintain blood pressure in the normotensive range during anesthesia. These effects have been previously described in cats, using alfentanil to reduce the MAC of isoflurane and improve the cardiovascular status of the patient under general anesthesia (39,40). Post-operatively, the patient was comfortable with buprenorphine analgesia for a total of 36 h. Buprenorphine, due to its partial mu receptor agonist activity, slow onset, and prolonged duration of action, has been described for management of mild to moderate pain in cats (41). In this case, buprenorphine was sufficient for the post-operative analgesic management of the patient.
When performing thoracoscopic procedures, one-lung ventilation allows for collapse of the contralateral lung, facilitating visualization during surgery (28,31). A bronchial blocker and endoscopic confirmation of the placement of the blocker is required for one-lung ventilation. One-lung ventilation was not used as the patient was too small for available bronchoscopy, an appropriately sized bronchial blocker was not available, and retraction of the cranial lung lobe allowed for satisfactory visualization of the site.
Thoracoscopy can improve visualization of the structures within the thorax due to scope magnification (29). In dogs with PRAA’s, thoracoscopy provides improved visualization of the constriction magnifying the fibrous bands narrowing the esophagus and facilitating their complete removal (28). Subjectively, visualization via thoracoscopy was much better than would have been achieved with a small thoracotomy that a patient of this size would have allowed.
One of the benefits of thoracoscopic procedures is to potentially allow for diagnosis and treatment under 1 anesthetic procedure. In this case, a vascular study was completed to rule out less common forms of VRA’s and ensure that the correct surgical approach was performed. Due to difficulties with the femoral artery catheterization in the small patient, anesthesia was prolonged. Following the non-specific vascular study, the decision was made to recover the patient and perform the thoracoscopic procedure under a separate anesthesia. However, as surgical experience is gained, thoracoscopy may allow for diagnosis and treatment in 1 procedure.
The main disadvantages of thoracoscopy include increased equipment expense and additional training required for the surgeon (34). In this case, the surgical time for the thoracoscopic procedure was 245 min. The time difference noted between the thoracoscopic procedure and traditional thoracotomy procedures was due to the learning curve associated with the thoracoscopic procedure and the small patient size. A complete exploration of the thorax was also performed. Placement of the silk retraction suture, while time consuming, facilitated elevation of the LA for positioning of the vessel sealant device. This could have been performed with hemostats, but it would have taken up limited space in this small patient. Other techniques that could decrease surgical time include use of a 2.7-mm scope, allowing for smaller cannulas and shorter working scope length. Use of arthroscopic instrumentation may also be beneficial. As surgical experience is gained, the time taken to perform this procedure will likely improve.
Potential complications of the PRAA surgery and surgical ligation of the LA include hemorrhage, esophageal perforation, fibrotic bands that may remain across the esophagus, and post-operative pain (28). These complications can occur with either an open thoracotomy or with thoracoscopic surgery. If performing the ligation of the LA thoracoscopically, accidental penetration or perforation of the vascular structures or the lungs may occur (31). However, if complications occur during a thoracoscopic procedure and better access is required, the procedure can easily be converted to a thoracotomy (31).
In conclusion, this case report demonstrates that thoracoscopic ligation of a PRAA in a young cat of 1.5 kg is feasible and associated with minimal complications. In this case, thoracoscopy provided improved visualization via magnification of the surgical field. Thus, size should not be a deterrent to performing the procedure. The benefits offered include improved visualization in small patients and potential use of this technique for diagnosis and treatment in one anesthetic procedure.
Acknowledgments
The authors thank Drs. Peter Gilbert and Lily Su for assistance with this case. Thanks to Dr. Dan Hartney of the Blue Cross Pet Hospital, Vancouver, BC, for referral of this case. The authors also express their appreciation to all the clinicians, residents, interns, students, and support staff at the WCVM who were involved with the care of this patient. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Kyles AE. Vascular ring anomalies. In: Slatter DG, editor. Textbook of Small Animal Surgery. 3rd ed. Philadelphia, Pennsylvania: Saunders; 2003. pp. 577–582. [Google Scholar]
- 2.Berry AP, Brouwer GJ, Tennant BJ. Persistent right aortic arch in a kitten. Vet Rec. 1984;114:336–337. doi: 10.1136/vr.114.14.336. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan JW. Symposium. Thoracic surgery in the dog and cat — III: Patent ductus arteriosus and persistent right aortic arch surgery in dogs. J Small Anim Pract. 1968;9:409–428. doi: 10.1111/j.1748-5827.1968.tb04622.x. [DOI] [PubMed] [Google Scholar]
- 4.Jeffrey SC, Bataller N, Martin RA, Moon ML. Postsurgical nutritional management of megaesophagus secondary to persistent right aortic arch in a kitten. Feline Practice. 1995;23:17–23. [Google Scholar]
- 5.Holmberg DL, Presnell KR. Vascular ring anomalies: Case report and brief review. Can Vet J. 1979;20:78–81. [PMC free article] [PubMed] [Google Scholar]
- 6.Wowk BJ, Olson GA. Megaesophagus produced by persistent right aortic arch in a cat. Vet Med Small Anim Clin. 1980;75:77–83. [PubMed] [Google Scholar]
- 7.Hedlund CS. Vascular ring anomalies. In: Fossum TW, Hedlund CS, Hulse DA, editors. Small Animal Surgery. 3rd ed. St. Louis, Missouri: Mosby; 2007. pp. 405–409. [Google Scholar]
- 8.White RN, Burton CA, Hale JSH. Vascular ring anomaly with coarctation of the aorta in a cat. J Small Anim Pract. 2003;44:330–334. doi: 10.1111/j.1748-5827.2003.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 9.Gastrostomy tubes. In: Seim HB III, Willard MD, editors; Fossum TW, Hedlund CS, Hulse DA, editors. Small animal Surgery. 3rd ed. St Louis, Missouri: Mosby; 2007. pp. 100–107. [Google Scholar]
- 10.Song EK, Mann FA, Wagner-Mann CC. Comparison of different tube materials and use of Chinese finger trap or four friction suture technique for securing gastrostomy, jejunostomy, and thoracostomy tubes in dogs. Vet Surg. 2008;37:212–221. doi: 10.1111/j.1532-950X.2008.00368.x. [DOI] [PubMed] [Google Scholar]
- 11.Owens JM, Twedt DC. Nonselective angiocardiography in the cat. Vet Clin North Am. 1977;7:309–321. doi: 10.1016/s0091-0279(77)50032-9. [DOI] [PubMed] [Google Scholar]
- 12.Fox PR, Bond BR. Nonselective and selective angiocardiography. Vet Clin North Am Small Anim Pract. 1983;13:259–272. doi: 10.1016/s0195-5616(83)50029-6. [DOI] [PubMed] [Google Scholar]
- 13.Lawther WA. Diagnosis and surgical correction of persistent right aortic arch and oesophageal achalasia in the dog and cat. Aust Vet J. 1970;46:326–329. doi: 10.1111/j.1751-0813.1970.tb07912.x. [DOI] [PubMed] [Google Scholar]
- 14.Wheaton LG, Blevins WE, Weirich WE. Persistent right aortic arch associated with other vascular anomalies in two cats. J Am Vet Med Assoc. 1984;198:848–851. [PubMed] [Google Scholar]
- 15.Strathman J. What is your diagnosis? J Am Vet Med Assoc. 1989;195:650–651. [PubMed] [Google Scholar]
- 16.Reed JH, Bonasch J. The surgical correction of a persistent right aortic arch in a cat. J Am Vet Med Assoc. 1962;140:142–144. [PubMed] [Google Scholar]
- 17.Hathaway JE. Persistent right aortic arch in a cat. J Am Vet Med Assoc. 1965;147:255–259. [PubMed] [Google Scholar]
- 18.Douglas SW, Walker RG, Littlewart MCG. Persistent right aortic arch in the cat. Vet Rec. 1960;72:91–92. [Google Scholar]
- 19.Jessop L. Persistent right aortic arch in the cat causing esophageal stenosis. Vet Rec. 1960;72:46–47. [Google Scholar]
- 20.Uhrich S. Report of a persistent right aortic arch and its surgical correction in a cat. J Small Anim Prac. 1963;4:337–338. [Google Scholar]
- 21.Core SH, Dominy WR. Surgical correction of a persistent right aortic arch in a cat. Vet Med Small Anim Clin. 1979;74:822–827. [PubMed] [Google Scholar]
- 22.Richmond BT. A case of persistent right aortic arch in the cat. Vet Rec. 1968;83:169. [Google Scholar]
- 23.Evans I, Keahs WH. Persistent right aortic arch in a cat. Vet Med Small Anim Clin. 1971;66:1090–1093. [PubMed] [Google Scholar]
- 24.Vögtili T, Weber U, Vögtli-Bürger R, Lang J. Persistent right aortic arch in a cat: Case report. Schweiz Archi Tierheilkd. 1994;136:335–339. [PubMed] [Google Scholar]
- 25.Yarim M, Gultiken ME, Ozturk S, et al. Double aortic arch in a Siamese cat. Vet Pathol. 1999;36:340–341. doi: 10.1354/vp.36-4-340. [DOI] [PubMed] [Google Scholar]
- 26.McCandlish IAP, Nash AS, Peggram A. Unusual vascular ring in a cat: Left aortic arch with right ligamentum arteriosum. Vet Rec. 1984;114:338–340. doi: 10.1136/vr.114.14.338. [DOI] [PubMed] [Google Scholar]
- 27.Van Der Linde-Sipman JS, Van Der Gaag I. Vascular ring caused by a left aortic arch, right ligamentum arteriosum and part of the right dorsal aorta in a cat. Zentralbl Veterinaermed A. 1981;28:569–573. doi: 10.1111/j.1439-0442.1981.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 28.MacPhail CM, Monnet E, Twedt DC. Thoracoscopic correction of persistent right aortic arch in a dog. J Am Anim Hosp Assoc. 2001;37:577–581. doi: 10.5326/15473317-37-6-577. [DOI] [PubMed] [Google Scholar]
- 29.Walsh PJ, Remedios AM, Ferguson JF, et al. Thoracoscopic versus open partial pericardectomy in dogs: Comparison of postoperative pain and morbidity. Vet Surg. 1999;28:472–479. doi: 10.1111/j.1532-950x.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- 30.Jackson J, Richter KP, Launer DP. Thoracoscopic partial pericardiectomy in 13 dogs. J Vet Intern Med. 1999;13:529–533. doi: 10.1892/0891-6640(1999)013<0529:tppid>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Isakow K, Fowler D, Walsh P. Video-assisted thoracoscopic division of the ligamentum arteriosum in two dogs with persistent right aortic arch. J Am Vet Med Assoc. 2000;217:1333–1336. doi: 10.2460/javma.2000.217.1333. [DOI] [PubMed] [Google Scholar]
- 32.Landreneau RJ, Hazelrigg SR, Mack MJ, et al. Postoperative pain-related morbidity: Video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993;56:1285–1289. doi: 10.1016/0003-4975(93)90667-7. [DOI] [PubMed] [Google Scholar]
- 33.Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg. 2008;85:231–235. doi: 10.1016/j.athoracsur.2007.07.080. [DOI] [PubMed] [Google Scholar]
- 34.De Rycke LM, Giele IM, Polis I, et al. Thoracoscopic anatomy of dogs positioned in lateral recumbency. J Am Anim Hosp Assoc. 2001;37:543–548. doi: 10.5326/15473317-37-6-543. [DOI] [PubMed] [Google Scholar]
- 35.Swanson SJ, Batirel HF. Video-assisted thoracic surgery (VATS) resection for lung cancer. Surg Clin North Am. 2002;82:541–559. doi: 10.1016/s0039-6109(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 36.Radlinsky MG, Mason DE, Biller DS, et al. Thoracoscopic visualization and ligation of the thoracic duct in dogs. Vet Surg. 2002;31:138–146. doi: 10.1053/jvet.2002.31062. [DOI] [PubMed] [Google Scholar]
- 37.Borenstein N, Behr L, Chetboul V, et al. Minimally invasive patent ductus arteriosus occlusion in 5 dogs. Vet Surg. 2004;33:309–313. doi: 10.1111/j.1532-950X.2004.04045.x. [DOI] [PubMed] [Google Scholar]
- 38.Radlinsky MG. Complications and need for conversion from thoracoscopy to thoracotomy in small animals. Vet Clin Small Anim. 2009;39:977–984. doi: 10.1016/j.cvsm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Ilkiw JE, Pascoe PJ, Fisher LD. Effect of alfentanil on the minimum alveaolar concentration of isoflurane in cats. Am J Vet Res. 1997;58:1274–1279. [PubMed] [Google Scholar]
- 40.Pascoe PJ, Ilkiw JE, Fisher LD. Cardiovascular effects of equipotent isoflurane and alfentanil/isoflurane minimum alveolar concentration multiple in cats. Am J Vet Res. 1997;58:1267–1273. [PubMed] [Google Scholar]
- 41.Lamont LA. Feline perioperative apin management. Vet Clin Small Anim. 2002;32:747–763. doi: 10.1016/s0195-5616(02)00028-1. [DOI] [PubMed] [Google Scholar]