Abstract
Peroxisome proliferator-activated receptor (PPAR) agonists are used as adjunct therapy in the treatment of diabetes mellitus. Fibrates, including fenofibrate, gemfibrozil, benzafibrate, ciprofibrate, and clofibrate act on PPAR alpha to reduce the level of hypertriglyceridemia. However, agonists (ligands) of PPAR-beta/delta receptors, such as tesaglitazar, muraglitazar, ragaglitazar, imiglitazar, aleglitazar, alter the body's energy substrate preference from glucose to lipids and hence contribute to the reduction of blood glucose level. Glitazones or thiazolidinediones on the other hand, bind to PPAR-gamma receptors located in the nuclei of cells. Activation of PPAR-gamma receptors leads to a decrease in insulin resistance and modification of adipocyte metabolism. They reduce hyperlipidaemia by increasing the level of ATP-binding cassette A1, which modifies extra-hepatic cholesterol into HDL. Dual or pan PPAR ligands stimulate two or more isoforms of PPAR and thereby reduce insulin resistance and prevent short- and long-term complications of diabetes including micro-and macroangiopathy and atherosclerosis, which are caused by deposition of cholesterol. This review examines the chemical structure, actions, side effects and future prospects of dual and pan PPAR agonists.
Keywords: Diabetes mellitus, PPAR agonists, thiazolidinediones, fibrates, medicinal chemistry.
INTRODUCTION
Diabetes mellitus is a common metabolic disease affecting millions of people worldwide. Type 1 diabetes is usually caused by immune destruction of pancreatic islet cells while type 2 is associated with insulin resistance, hyperglycemia and other metabolic conditions such as hypertension, obesity and hyperlipidemia.
Thiazolidinediones and fibrate drugs are one of the most commonly used medications in the treatment of type 2 diabetes, hyperlipidemia and insulin resistance. How do these drugs work? They bind to peroxisome proliferator-activated receptors (PPARs) which are located in cell nuclei and behave as transcription factors regulating the expression of several genes [1]. Activated PPARs play important roles in the regulation of cellular development and differentiation, and the metabolism of body fuel including carbohydrates, lipids and proteins [2,3]. PPARs increase the proliferation of peroxisomes in the hepatic cells of frogs [4]. They also bind to special regions of DNA of certain genes and are able to heterodimerize when in contact with retinoid X receptor. The biological effects of PPARs depend on the shape of their ligand-binding domain, which is modified by ligand binding protein and by scores of other co-activator and co-repressor polypeptides [5]. PPARs may bind to several endogenous ligands, including free fatty acids, eicosanoids, prostaglandins and leukotrienes [6].
TYPES OF PEROXISOME PROLIFERATOR-ACTIVATED RECEPTORS (PPARs)
Alpha, beta, gamma and delta PPARs have been identified [7]. The alpha isoform is widely expressed in the liver, kidney, cardiac, skeletal muscle and adipocytes. In contrast, the beta/delta type is mostly observed in the brain, adipose tissue, and the skin. PPARγ is found in the intestinal mucosa, adipocytes and kidney [8]. PPARδ, which was identified in humans in 1992, has a similar structure to PPARβ. The name PPARδ is widely used in the US, whereas the use of PPARβ is more widespread in Europe [9].
PPARα is located in chromosome 22q12-13.1; PPARβ/δ in chromosome 6p21.2-21.1 and PPARγ in chromosome 3p25. Mutation in any of the following areas has been attributed to various abnormalities of the metabolic syndrome [10, 11].
PPAR-alpha Receptors
PPARα receptor was the first set of PPARs discovered. It was shown that in addition to its ability to increase peroxisomal numbers in rodent liver tissue, it does improve insulin sensitivity [4]. The main ligands of PPARα receptor are fibrate drugs (fenofibrate, gemfibrozil, benzafibrate (9), ciprofibrate, and clofibrate), which were originally used as an adjunct therapy in the treatment of hypercholesterolemia. It is now widely used in the treatment of hypertriglyceridemia. This is achieved by facilitating the degradation of very low density lipoprotein (VLDL) and reduction in its production. Activation of lipoprotein lipase will result in the removal of triglycerides from VLDL. In addition, lipid reduction is achieved by stimulation of intracellular fatty acid oxidation and utilization [12]. A more recent report shows that reduction in these parameters lead to a decrease in cardiovascular events [13].
PPAR-delta Receptors
The function of PPAR-delta has been the target of many studies in recent years because it was shown that stimulation of PPAR-delta modifies the body's energy fuel preference from glucose to fat. The structure of PPARβ is almost the same as that of PPARδ [14].
PPAR-gamma Receptors
PPARγ receptor is the main target of thiazolidinediones (TZDs), medications used in the treatment of type 2 diabetes. Other ligands that stimulate PPARγ receptors include NSAIDs such as ibuprofen and indoles. Ligands that stimulate PPARγ receptors are used in the treatment of atherosclerosis induced by hyperlipidaemia because stimulation of these receptors increase the expression of ABCA1, which modifies extra-hepatic cholesterol into HDL. This results in a net decrease in the amount of circulating lipids and lower deposition of lipids in the wall of blood vessels. Three forms of PPARγ receptors have been identified. These include PPARγ1 receptor, which is expressed in many tissues including heart, muscle, colon, kidney, pancreas, and spleen; PPARγ2 receptor found mainly in adipocytes, and PPARγ3 receptor expressed predominantly in macrophages, large intestine and white adipose tissue [2].
DUAL PPAR MODULATORS
A fourth class of PPAR agonists, called dual or pan PPAR ligands, bind two or more PPAR isoforms. This class of PPAR modulators is currently under intensive investigation for the treatment of diabetes mellitus and its complications [15, 16]. Many dual-pan PPAR modulators have been identified. The best known of these compounds include tesaglitazar (1), muraglitazar (2), naveglitazar (3), ragaglitazar (4); farglitazar (5), imiglitazar (6); aleglitazar (7), and netoglitazone (Table 1)
Table 1.
Name, Chemical Structure of Selected Dual (α/γ) PPAR Modulators
| Compound | Chemical Structure |
|---|---|
| Tesaglitazar (1) | 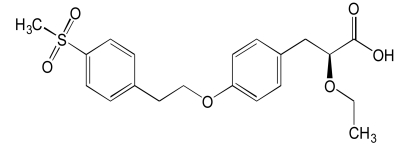 |
| Muraglitazar (2) | 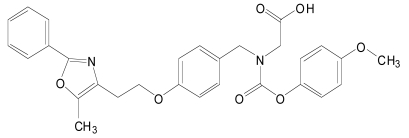 |
| Naveglitazar (3) | 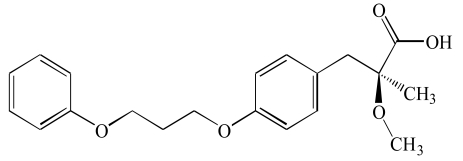 |
| Ragaglitazar (4) | 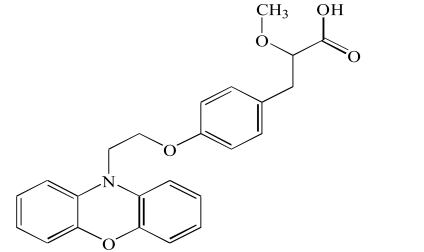 |
| Farglitazar (5) | 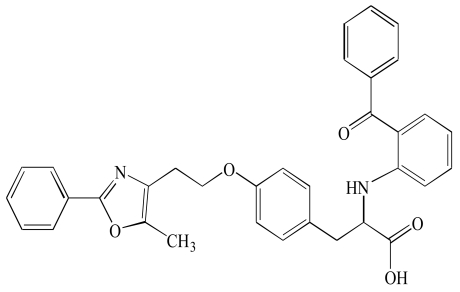 |
| Imiglitazar (6) | 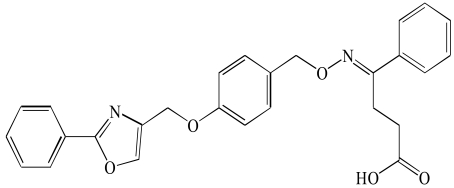 |
| Aleglitazar (7) | 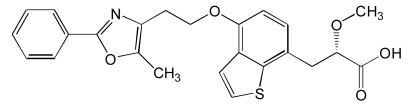 |
STRUCTURE OF DUAL/PAN PPAR MODULATORS (LIGANDS)
PPARs have modular structure with 5 functional domains including an N-terminal region; DNA-binding domain; flexible hinge region; ligand binding domain (LBD) with a large secondary structure containing 13 alpha and a beta helices. LBD is the site at which the receptor is activated or inhibited. The fifth domain is the C-terminal [17]. Most of the well known dual PPAR modulators including muraglitazar, tesaglitazar and aleglitazar have two methyl groups each. They also have hydroxyl groups (Tables 1, 2, 3).
Table 2.
Dual PPAR Agonists (Ligands) and their Main Actions
| Dual PPAR Modulators | Indication | Dosage | Effect | Remarks |
|---|---|---|---|---|
| 1. PPAR α/γ | ||||
| Tesaglitazar(1) | Hyperlipidemia | 0.5 mg/day | reduces plasma triglycerides, increases HDL-C, stimulates fatty acid uptake and utilization in muscle cells and hepatocytes, reduces organ steatosis | Discontinued by AstraZeneca on May 26, 2006 |
| Muraglitazar(2) | 5 mg/day | Discontinued by Bristol-Myers Squibb on May 18, 2006 | ||
| Ragaglitazar(4) | 1 mg/day | Discontinued by Novo Nordisk in 2006 | ||
| Imiglitazar(6) | Suspended by Takeda Pharmaceutical in December 20, 2004 | |||
| Aleglitazar(7) | Type 2 diabetes mellitus | Phase II | ||
| 2. PPAR α/δ | ||||
| Compound T913659 | Hyperlipidemia | Under development by Tularik | ||
| 3. PPAR δ/γ | ||||
| Propionic acid derivative(8) | Type 2 diabetes mellitus, hyperlipidemia | New compound developed by Eli Lilly | ||
Table 3.
Name, Chemical Structure of Selected Dual (δ/γ) PPAR Modulators (Ligands)
| Compound | Chemical Structure |
|---|---|
| Proprionic acid (8) | 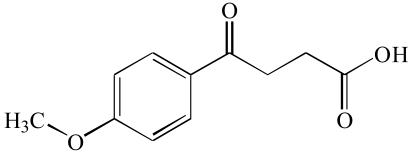 |
ACTIONS OF DUAL/PAN PPAR MODULATORS
The action of dual/pan PPAR modulators is based on the combined action of PPAR-alpha, PPAR delta/beta and/or beta gamma agonists, a quassi non-selective activation of PPAR. The main agonists of PPAR-alpha are fibrate drugs (fenofibrate, gemfibrozil, benzafibrate (9), clofibrate and ciprofibrate,) which belong to the class of amphipathic carboxylic acids. They reduce the plasma level of triglycerides and cholesterol, thus reducing atherosclerosis and other lipid-induced complications. On the other hand, PPAR- beta/delta modulators, change the body's energy source from glucose to lipids [14], thereby depleting tissue lipids, which will reduce the risks arising from hyperlipidemia. PPAR-gamma modulators, such as thiazolidinediones help to reduce insulin resistance, hypercholesterolemia and therefore contribute to the reduction of late vascular complications of diabetes mellitus (Tables 1, 2, 3).
Muraglitazar (2) has been shown to reduce HbA1C and improve lipid profile in patients with diabetic mellitus [18]. In spite of the clinical efficacy of muraglitazar (2), phase III clinical study with muraglitazar (2) was completely abolished because it was associated with oedema and heart failure [19]. However, studies in db/db mice have shown that muraglitazar (2) can prevent the onset of diabetes mellitus and its complications [20].
Another prospective dual PPAR modulator, tesaglitazar (1), was reported to be able to reduce the degree of atherosclerosis in mice with LDL receptor deficiency [21] and suppress both hyperglycemia and dyslipidemia [22]. Further trials on the use of tesaglitazar (1) have since been halted because it induces kidney failure. However, in contrast to previous studies, new reports showed that tesaglitazar (1), can indeed improve metabolic abnormalities and renal function, decreased blood pressure, and prevent glomerular and interstitial lesion in obese Zucker rats [23] and db/db mice [24].
Chiglitazar (10) is a relatively new PPAR alpha/gamma dual agonist, which has been shown to improve impaired insulin and glucose tolerance in monosodium glutamate (MSG)-obese rats. It enhances insulin sensitivity index and reduce the HOMA (Homeostatic Model Assessment) index. In addition, administration of chiglitazar (10) reduces alanine gluconeogenesis and hepatic glycogen levels in MSG obese rats. Chiglitazar (10) decreases plasma triglyceride, total cholesterol, non-esterified fatty acids and low density lipoprotein-cholesterol levels in animal models of diabetes [25] (Tables 4, 5).
Table 4.
Name, Chemical Structure of Selected Pan (α/δ/γ) PPAR Modulators (Ligands)
| Compound | Chemical Structure |
|---|---|
| Benzafibrate (9) | 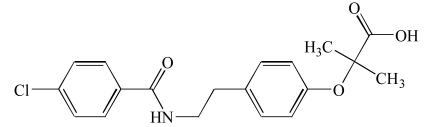 |
| Chiglitazar(10) | 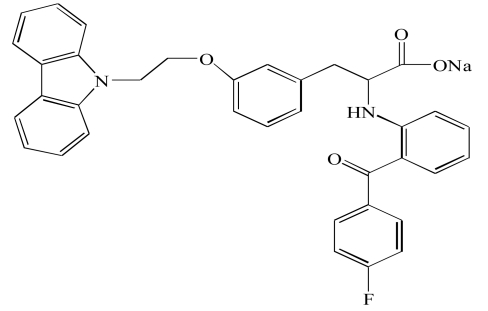 |
Table 5.
Pan PPAR Agonists (Ligands) and their Main Actions
| Pan PPAR modulators | Indication | Dosage | Effect | Remarks |
|---|---|---|---|---|
| Benzafibrate (9) | Type 2 diabetes mellitus, hyperlipidemia, atherosclerosis | increases HDL-C level, lowers blood triglyceride and glucose levels, enhances insulin sensitivity. | Marketed by Boehringer MannheimGmgH/Chong Kun Dang Pharma | |
| Chiglitazar (10) | Type 2 diabetes mellitus | Currently in Phase II by Shenzhen Chipscreen Biosciences | ||
| Netoglitazone | Obesity | In phase II. Introduced by Mitsubishi Pharma/Perlegen Sciences |
SIDE EFFECTS OF DUAL/PAN PPAR MODULATORS
Investigators working on the development of dual/pan PPAR modulators have encountered many failures with many of the agents they have developed. Benzafibrate (9) stimulates all of the three PPARs with the same efficacy. It is the first and still the only pan-PPAR agonist used therapeutically. Benzafibrate (9) increases HDL-C level and lower blood triglyceride and glucose levels. It enhances insulin sensitivity. The use and testing of many dual/pan PPAR modulators have been discontinued because of severe side effects including renal failure, fibrosarcomas, urinary cancer and anemia [26-28] (Table 6).
Table 6.
Side Effects of Dual/Pan PPAR Agonists (Ligands)
| Dual/pan PPAR Modulators | Side Effect | Cause of Side Effects | Remarks |
|---|---|---|---|
| 1. Dual (PPAR α/γ) | |||
| Tesaglitazar (1) |
Anaemia, leucopenia, renal failure, fibrosarcomas, | Stimulation of DNA synthesis [24] | Discontinued in 2006 |
| Muraglitazar (2) |
Heart failure, myocardial infarction and stroke | Blocks calcium channel [13] | Discontinued in 2006 |
| Ragaglitazar (4) |
Anaemia, oedema, and urinary tract cancer in rodents | Causes overexpresssion of early growth response-1 (EGr-1), phosphorylation of c-Jun and ribosomal S protein [25, 26] | Discontinued in 2006 |
| Imiglitazar (6) |
Liver dysfunction | Effect of cytochrome p450? | Suspended in 2004 |
| 2. Pan PPAR (α/β,δ/γ ) agonists | |||
| Benzafibrate (9) |
|||
| Chiglitazar (10) |
None reported | None reported | Currently in Phase II |
| Netoglitazone | None reported | None reported | |
FUTURE PROSPECT ON DUAL/PAN PPAR MODULATORS
Dual/pan PPAR modulators offer an efficient way of “killing two birds with one stone”, treating diabetes mellitus and preventing the complications/risk factors associated with it. The main problem with the development of dual/pan PPAR modulators is that the risks of the taking the drugs outweighs the benefit. In other to take advantage of the potential use of PPAR modulators in the treatment of metabolic diseases, the dosage regimen and the medicinal chemistry of these PPAR ligands should be re-examined.
REFERENCES
- 1.Michalik L, Auwerx J, Berger J P, Chatterjee V K, Glass C K, Gonzalez F J, Grimaldi P A, Kadowaki T, Lazar M A, O'Rahilly S, Palmer C N, Plutzky J, Reddy J K, Spiegelman B M, Staels B, Wahli W. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006;58:726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- 2.Berger J, Moller D E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 3.Feige J N, Gelman L, Michalik L, Desvergne B, Wahli W. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog. Lipid Res. 2006;45:120–159. doi: 10.1016/j.plipres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 5.Yu S, Reddy J K. Transcription coactivators for peroxisome proliferator-activated receptors. Biochim. Biophys. Acta. 2007;1771:936–951. doi: 10.1016/j.bbalip.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Marlow L A, Reynolds L A, Cleland A S, Cooper S J, Gumz M L, Kurakata S, Fujiwara K, Zhang Y, Sebo T, Grant C, McIver B, Wadsworth J T, Radisky D C, Smallridge R C, Copland J A. Reactivation of suppressed RhoB is a critical step for the inhibition of anaplastic thyroid cancer growth. Cancer Res. 2009;69:1536–1544. doi: 10.1158/0008-5472.CAN-08-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kliewer SA, Forman BM, Blumberg B, Ong ES, Borg-meyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyachi H. Design and synthesis of subtype- and species-selective peroxisome proliferator-activated receptor (PPAR) alpha ligands. Yakugaku Zasshi. 2004;124:803–813. doi: 10.1248/yakushi.124.803. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt A, Endo N, Rutledge S J, Vogel R, Shinar D, Rodan G A. Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acids. Mol. Endocrinol. 1992;6:1634–1641. doi: 10.1210/mend.6.10.1333051. [DOI] [PubMed] [Google Scholar]
- 10.Meirhaeghe A, Amouyel P. Impact of genetic variation of PPARgamma in humans. Mol. Genet. Metab. 2004;83:93–102. doi: 10.1016/j.ymgme.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Buzzetti R, Petrone A, Ribaudo M C, Alemanno I, Zavarella S, Mein C A, Maiani F, Tiberti C, Baroni M G, Vecci E, Arca M, Leonetti F, Di Mario U. The common PPAR-gamma2 Pro12Ala variant is associated with greater insulin sensitivity. Eur. J. Hum. Genet. 2004;12:1050–1054. doi: 10.1038/sj.ejhg.5201283. [DOI] [PubMed] [Google Scholar]
- 12.Gross B, Staels B. PPAR agonists: multimodal drugs for the treatment of type-2 diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 2007;21:687–710. doi: 10.1016/j.beem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Hiukka A, Maranghi M, Matikainen N, Taskinen MR. PARα: an emerging therapeutic target in diabetic microvascular damage. Nature Rev. Endocrinol. 2010;6:454–463. doi: 10.1038/nrendo.2010.89. [DOI] [PubMed] [Google Scholar]
- 14.Brunmair B, Staniek K, Dorig J, Szocs Z, Stadlbauer K, Marian V, Gras F, Anderwald C, Nohl H, Waldhausl W, Furnsinn C. Activation of PPAR-delta in isolated rat skeletal muscle switches fuel preference from glucose to fatty acids. Diabetologia. 2006;49:2713–2722. doi: 10.1007/s00125-006-0357-6. [DOI] [PubMed] [Google Scholar]
- 15.Balakumar P, Rose M, Ganti S S, Krishan P, Singh M. PPAR dual agonists: are they opening Pandora's Box? Pharmacol. Res. 2007;56:91–98. doi: 10.1016/j.phrs.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Fievet C, Fruchart J C, Staels B. PPARalpha and PPARgamma dual agonists for the treatment of type 2 diabetes and the metabolic syndrome. Curr. Opin. Pharmacol. 2006;6:606–614. doi: 10.1016/j.coph.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Zoete V, Grosdidier A, Michielin O. Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim. Biophys. Acta. 2007;1771:915–925. doi: 10.1016/j.bbalip.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Kendall D M, Rubin C J, Mohideen P, Ledeine J M, Belder R, Gross J, Norwood P, O'Mahony M, Sall K, Sloan G, Roberts A, Fiedorek F T, DeFronzo R A. Improvement of glycemic control, triglycerides, and HDL cholesterol levels with muraglitazar, a dual (alpha/gamma) peroxisome proliferator-activated receptor activator, in patients with type 2 diabetes inadequately controlled with metformin monotherapy: A double-blind, randomized, pioglitazone-comparative study. Diabetes Care. 2006;29:1016–1023. doi: 10.2337/diacare.2951016. [DOI] [PubMed] [Google Scholar]
- 19.Nissen S E, Wolski K, Topol E J. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581–2586. doi: 10.1001/jama.294.20.joc50147. [DOI] [PubMed] [Google Scholar]
- 20.Tozzo E, Ponticiello R, Swartz J, Farrelly D, Zebo R, Welzel G, Egan D, Kunselman L, Peters A, Gu L, French M, Chen S, Devasthale P, Janovitz E, Staal A, Harrity T, Belder R, Cheng P T, Whaley J, Taylor S, Hariharan N. The dual peroxisome proliferator-activated receptor alpha/gamma activator muraglitazar prevents the natural progression of diabetes in db/db mice. J. Pharmacol. Exp. Ther. 2007;321:107–15. doi: 10.1124/jpet.106.115337. [DOI] [PubMed] [Google Scholar]
- 21.Chira E C, McMillen T S, Wang S, Haw A, 3rd, O'Brien K D, Wight T N, Chait A. Tesaglitazar a dual peroxisome proliferator-activated receptor alpha/gamma agonist, reduces atherosclerosis in female low density lipoprotein receptor deficient mice. Atherosclerosis. 2007;195:100–109. doi: 10.1016/j.atherosclerosis.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox S L. Tesaglitazar: a promising approach in type 2 diabetes. Drugs Today (Barc) 2006;42:139–146. doi: 10.1358/dot.2006.42.3.937961. [DOI] [PubMed] [Google Scholar]
- 23.Liao J, Soltani Z, Ebenezer P, Isidro-Carrion A A, Zhang R, Asghar A, Aguilar E, Francis J, Hu X, Ferder L, Reisin E. Tesaglitazar, a dual peroxisome proliferator-activated receptor agonist (PPAR alpha/gamma), improves metabolic abnormalities and reduces renal injury in obese Zucker rats. Nephron Exp. Nephrol. 2010;114:e61–68. doi: 10.1159/000254567. [DOI] [PubMed] [Google Scholar]
- 24.Cha D R, Zhang X, Zhang Y, Wu J, Su D, Han J Y, Fang X, Yu B, Breyer M D, Guan Y. Peroxisome proliferator activated receptor alpha/gamma dual agonist tesaglitazar attenuates diabetic nephropathy in db/db mice. Diabetes. 2007;56:2036–2045. doi: 10.2337/db06-1134. [DOI] [PubMed] [Google Scholar]
- 25.Hellmold H, Zhang H, Andersson U, Blomgren B, Holland T, Berg A L, Elebring M, Sjogren N, Bamberg K, Dahl B, Westerberg R, Dillner B, Tugwood J, Roberts R, Lundholm E, Camejo G, Skanberg I, Evans J. Tesaglitazar, a PPARalpha/gamma agonist, induces interstitial mesenchymal cell DNA synthesis and fibrosarcomas in subcutaneous tissues in rats. Toxicol. Sci. 2007;98:63–74. doi: 10.1093/toxsci/kfm094. [DOI] [PubMed] [Google Scholar]
- 26.Oleksiewicz M B, Thorup I, Nielsen H S, Andersen H V, Hegelund A C, Iversen L, Guldberg T S, Brinck P R, Sjogren I, Thinggaard U K, Jorgensen L, Jensen M B. Generalized cellular hypertrophy is induced by a dual-acting PPAR agonist in rat urinary bladder urothelium in vivo. Toxicol. Pathol. 2005;33:552–560. doi: 10.1080/01926230500214657. [DOI] [PubMed] [Google Scholar]
- 27.Egerod F L, Nielsen H S, Iversen L, Thorup I, Storgaard T, Oleksiewicz M B. Biomarkers for early effects of carcinogenic dual-acting PPAR agonists in rat urinary bladder urothelium in vivo. Biomarkers. 2005;10:295–309. doi: 10.1080/13547500500218682. [DOI] [PubMed] [Google Scholar]
- 28.Li P P, Shan S, Chen Y T, Ning Z Q, Sun S J, Liu Q, Lu X P, Xie M Z, Shen Z F. The PPARalpha/gamma dual agonist chiglitazar improves insulin resistance and dyslipidemia in MSG obese rats. Br. J. Pharmacol. 2006;148:610–618. doi: 10.1038/sj.bjp.0706745. [DOI] [PMC free article] [PubMed] [Google Scholar]


