Abstract
Zebrafish (Danio rerio) have been widely used to study the molecular mechanisms of development including neurodevelopment. More recently, they have begun to be used to study neuropharmacology and neurotoxicology. Critical for this line of research are methods to study behavioral function in zebrafish. Previous studies have compared zebrafish with mammalian models to determine similarities and differences in locomotor behavior, learning and memory. Relatively little research has been conducted on stress response and anxiety behavior as well as the pharmacologic response in zebrafish. We have developed a test for zebrafish to assess stress response and anxiety: the novel tank diving test. In this short test normally zebrafish dive to the bottom of a novel tank and then gradually over the 5-min test begin exploring higher levels of the tank. Nicotine, which has anxiolytic effects in rodents and humans was found to diminish this novel tank diving response in zebrafish. The current study examined the nicotinic receptor subtype selectivity involved in the actions of nicotine. Two the nicotinic receptor subtype selective antagonists were used: MLA (an α7 antagonist) and DHβE (an α4β2 antagonist). We replicated our previous finding of the anxiolytic effect of nicotine with significantly less bottom dwelling by the fish after nicotine treatment. This nicotine-induced anxiolytic effect was reversed by both MLA and DHβE, indicating that both nicotinic α7 and α4β2 receptors are involved in the nicotinic effect on anxiety.
Keywords: Zebrafish, α7 nicotinic receptors, α4β2 nicotinic receptors, Nicotine, Anxiolysis, Anxiety, Novelty, Stress Response, Diving Response, MLA, DHβE
Introduction
Zebrafish (Danio rerio) are becoming widely used for the study of the molecular bases of neurobiology with applications in neuropharmacology and neurotoxicology [8,11]. Considerable advantage comes from the ability to visually monitor neural processes as the embryo develops because of its transparent chorion. In addition, the genome of the zebrafish is well studied and there is a wide variety of genetic mutants available as well [3]. Thus, the molecular mechanisms of neurodevelopment can be easily studied in the zebrafish model. Zebrafish also can provide an inexpensive, high-throughput test subject that is useful in gaining a greater understanding of neuropharmacological mechanisms in mammals. Neurobehavioral function can be studied in zebrafish with the recent development of behavioral assessment techniques. This aids in the study of molecular mechanisms of neurobehavioral function as well as more rapid screening for pharmacological and toxicological effects.
Many early studies focused on the embryonic development of the zebrafish and the possible connections to mammalian development. However, zebrafish studies have just recently begun to look at anxiety, learning and memory. Zebrafish are able to learn a simple spatial alternation task with food acting as a reward. These results persist over a ten-day period with no intermittent testing [17]. There is also evidence that zebrafish have similar types of neurotransmitter receptors to mammals. It has been shown that they possess comparable numbers of muscarinic acetylcholine receptors, which are important in learning and memory behaviors in vertebrate animals [16]. Additionally, studies have shown that in zebrafish, much like in rodent models, an NMDA glutamate receptor antagonist increases circling behavior, alters swimming activity and impairs place preference [14]. Furthermore, anxiety in zebrafish has been shown through patterns of swimming along the edge [12] and towards the bottom of novel environments [7], which was not seen as frequently when the fish had previously been exposed to its surroundings.
Zebrafish experiments are becoming increasingly varied, but early studies involving nicotine in zebrafish focused primarily on its role in embryonic development [13]. Now we must look to explore the role that nicotine plays on learning, memory, anxiety and other behaviors. Doses of nicotine significantly improve memory performance [8] and timing studies have also been performed to find the interval of when to administer doses of nicotine to have the greatest positive effect on memory [9], which occurred 20–40 min after dosing. In addition, the study looked at the dose-effect of nicotine on zebrafish learning and memory using a delayed alternation task and found that while high doses are inhibitory, low doses do seem to enhance memory [8]. These results are consistent with rats, monkeys and humans [10].
Nicotine has been shown in our previous study to reduce anxiety in zebrafish placed in a novel environment, but could then be reversed by the nonspecific nicotinic acetylcholine receptor antagonist, mecamylamine [7]. These findings, suggesting a nicotine-induced anxiolytic effect, support some earlier investigations in rodents on an elevated plus maze [2]. However, other studies have concluded that nicotine induces anxiogenic or mixed results (Reviewed in [5,7]). File et al., [6] found that nicotine injected into the dorsal hippocampus in rats had an anxiolytic effect on the elevated plus maze modeling a specific phobia, but had no effect when the elevated plus maze modeled escape components of panic disorder. In addition, they found that nicotine injected into the lateral septum had no effect in the specific phobia trial, but had an anxiogenic effect during the panic disorder trial.
The current study looked to use zebrafish to study the proposed anxiolytic effect of nicotine and further specify the role of specific nicotinic receptors in this process. Both nicotinic α7 and α4β2 receptors are thought to play a role in cognitive tasks [8]. Methyllycaconitine (MLA) and Dihydro-β-erythroidine (DHβE) are two competitive nicotinic antagonists that bind to α7 and α4β2 respectively. Studies in mice have shown that because DHβE interferes with nicotine-induced enhancement of contextual fear conditioning, that α4β2 nicotinic receptors are responsible for this form of anxiety [4]. To determine the nicotinic receptor subtype involvement with nicotine-induced anxiolytic response in a different model like novel environment we used DHβE and MLA to block α7 and α4β2 nicotinic receptors to see which would antagonize the nicotine-induced anxiolytic effect on zebrafish in a novel environment.
Materials and Methods
Subjects
Adult male and female zebrafish (Danio rerio) from Triangle Tropical Fish (Burlington, NC) were kept at approximately 28.5°C on a 12:12-h light/dark cycle (lights on at 7:00 AM). Behavioral testing of drug effects took place during the light phase between 8:00 AM and 5:00 PM. The tank water used de-ionized H2O and sea salts (Instant Ocean, 1.2g/20l of H2O). The tanks with the groups of adult fish were maintained with constant filtration and aeration. Fish were fed daily with brine shrimp or flake fish food. All the fish were drug naïve and each fish was used only once. There were 10 fish per condition.
Drug administration
Dose effect of MLA and DHBE
Methyllycaconitine (MLA) and Dihydro-β-erythroidine (DHβE) were administered by immersing the zebrafish in a beaker with concentrations of 0, 50, 100, or 200 mg/l of either antagonist for 3 min. The doses of drugs were calculated on weight of the salt form of the drug. A delay of five min was imposed between the end of dosing and the start of the trial. The fish were exposed to the antagonist in a separate beaker and then were put into a holding tank without drug for the interval between exposure and testing. Exposure to tank water without drugs added served as the control. There was no drug exposure in either the home tank or the test chamber.
Nicotine, MLA and DHBE administration
Nicotine ditartrate, methyllycaconitine (MLA) and dihydro-β-erythroidine (DHβE) were administered by immersing the zebrafish in a beaker with concentrations of 0 or 100 mg/l of nicotine and 0 or 200 mg/l of MLA or DHβE for three min. The doses of drugs were calculated on weight of the salt form of the drug. A delay of five min was imposed between the end of dosing and the beginning of the test session. The fish were exposed to the drugs in a separate beaker and then were put singly into a holding tank without drugs for the interval between exposure and testing. When multiple drugs were administered they were administered at the same time. Thus, each nicotinic antagonist was administered together with nicotine. Exposure to tank water without drugs added served as the control. As with the dose effect trials, there was no drug exposure in either the home tank or the test chamber. All the fish were drug naïve and each fish was used only once. Ten fish per treatment were used.
Test apparatus and procedure
The zebrafish were placed in one of two 1.5-l plastic tank filled with 1350 ml of home tank water from the fish housing apparatus. Each tank was a trapezoid: 22.9 cm along the bottom, 27.9 cm at the top, 15.2 cm high and 15.9 cm along the diagonal side. It was 6.4 cm wide at the top, and tapered to 5.1 cm at the bottom. The tanks were narrow so that the distance swim by the fish could be accurately measured in two dimensions. However, there was sufficient room for the fish to easily turn around. The tanks were positioned so that the diagonal sides were facing each other, with a sheet of white paper obstructing the view into the other tank. The tanks were backlit and had a translucent white sheet of plastic serve as a background for the imaging system. They were located 88.5 cm from the Samsung 8mm Camcorder used to record the image into the Noldus Image Analysis program (Wageningen, The Netherlands). Swimming behavior was assessed by the Noldus software. Time spent in each third of the tank section horizontally as well as swim path length were the dependent measures. The choice of position (bottom vs. upper levels) was considered the index of anxiety, quite similar to the position choice of closed vs. open arms in the elevated plus maze and positions near the wall (thigmotaxis) vs. the center of an open field with rodents. As with the elevated plus maze and open field in rodents there was a separate total activity measure as well.
Data Analysis
The data were analyzed by a mixed design analysis of variance with between subjects factors of drug treatment. The same vehicle was used for all treatments and the vehicle-treated control group was used for all drug treatments. Study 1 was a simple one-way analysis of variance with vehicle-treated controls and three doses of each antagonist. The two antagonists were run contemporaneously from the same group of fish with the same control group. Study 2 was a 2 × 3 between subjects design with nicotine treatment (no nicotine vs. nicotine) and nicotinic antagonist treatment (no antagonist, MLA and DHβE). The repeated measure was minute within the 5-min session. There were two dependent measures, choice location (sec/min in the bottom third of the tank) and swimming speed (cm/min). A cut-off of p<0.05 was used as the threshold for statistical significance.
Results
Dose Effect Functions of the Nicotinic Antagonists
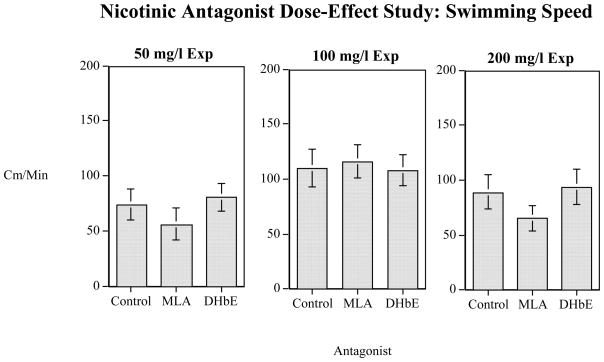
The functionally relevant dose ranges for both DHβE and MLA on anxiety behaviors in a novel environment were determined. In this dose-finding experiment, DHβE and MLA did not cause significant effects on total distance the fish swam or amount of time they spent at the bottom of the tank after dosing at 50, 100 or 200 mg/l as compared to controls (Figs. 1 and 2). There was a significant (F(4,316)=18.62, p<0.0005) main effect of increased swimming activity from the first minute to later minutes in the 5-minute trial from 56.7±5.9 cm/min during the first minute to 107.0±6.1 cm/min during the fifth minute. The interaction of minute x dose was not significant. None of the nicotinic antagonist doses tested significantly affected diving to the bottom third of the tank. There was a significant (F(4, 316)=13.96, p<0.0005) main effect of minute with the all the fish on an average spending 53.0±1.6 sec/min in the bottom third of the tank during the first minute and progressively less time in the bottom third until they averaged 42.6±2.0 sec/min in the bottom third of the tank during the fifth minute. The controls averaged 47.8±2.5 sec/min on the bottom of the tank and the MLA and DHβE treated groups all averaged within the range of 44.1 and 52.3 sec/min.
Figure 1. Nicotinic Antagonist Dose-Effect Study: Swimming Speed.
Swim activity (cm/min) after 50, 100 and 200 mg/l concentrations of MLA and DHβE and swim activity (mean±sem).
Figure 2. Nicotinic Antagonist Dose-Effect Study: Bottom-Dwelling.
Time spent (sec/min) in bottom third of novel tank after 50, 100 and 200 mg/l concentrations of MLA and DHβE (mean±sem).
Nicotinic Antagonist Interactions with Nicotine
There was a significant (F(2,54)=6.25, p<0.005) interaction of nicotine x nicotinic antagonist treatment with regard to swim activity (Fig. 1). There is a significant increase in the swim activity of nicotine-dosed fish (F(1,54)=10.27, p<0.005). Both MLA (F(1,54)=36.52, p<0.0005) and DHβE (F(1,54)=23.86, p<0.0005) significantly blocked the increase in swimming activity caused by nicotine. MLA (200 mg/l) by itself caused a significant (F(154)=4.60, p<0.05) decrease in swimming activity relative to controls, whereas DHβE (200 mg/l) had no significant effect. Over the course of the session, there is a significant time effect of increasing distance traveled per minute as the session progressed (F(4,216)=10.24, (p<0.0005). Control fish showed relatively low activity during the first minute, but over the next few minutes, their activity more than doubled (Fig. 2). The total amount of swim activity was significantly increased in nicotine-dosed fish, with the initial swim activity nearly tripling that of controls. However, for these fish there was no significant increase of activity per minute over the five-minute trial.
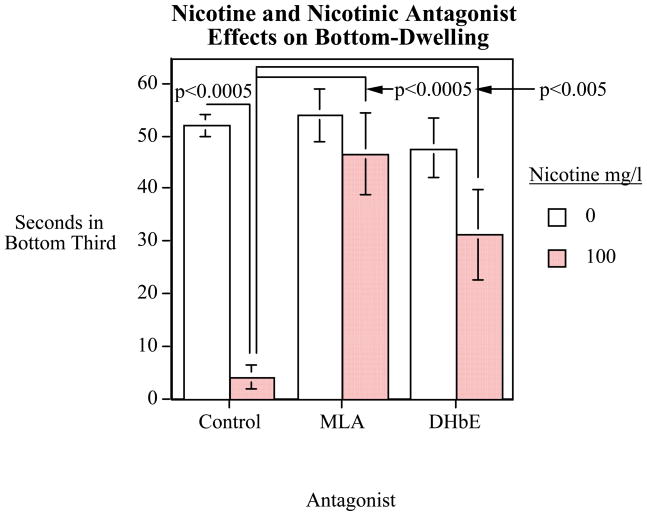
Zebrafish dosed with only nicotine spent significantly less time in the bottom of the tank (p<0.0001). As shown in figure 3, control fish spent almost 87% (52.0 sec/min) of their time in the bottom of the tank, while nicotine-dosed fish only spent about 7% (4.1 sec/min). This effect was also significant over time in the five-minute trial. The first minute that the fish is in the novel environment there was significantly (F(4,216)=7.34, p<0.0005) more swimming in the bottom third of the tank, but as time progressed, swimming in the bottom third of the tank decreased. Zebrafish dosed with only MLA spent 89% of their time in the bottom of the tank, while zebrafish dosed with nicotine and MLA spent almost 78%. There is a similar effect for DHβE, as 79% of their time is spent at the bottom, while, with the addition of nicotine, the zebrafish spent 52% of their time in the bottom third of the tank.
Figure 3. Nicotine and Nicotinic Antagonist Effects on Swimming Speed.
Swim activity (cm/min) affected by nicotine and nicotinic antagonists as measured by total distance moved per minute averaged over the 5-minute session (mean±sem). Nicotine significantly increased swimming activity. MLA significantly decreased swim activity, while DHβE at the dose used had no significant effect on its own. Both MLA and DHβE completely blocked the nicotine-induced increase in swim activity.
Discussion
In zebrafish the anxiolytic effect of nicotine can be significantly attenuated by either the α7 nicotinic antagonist MLA or α4β2 nicotinic antagonist DHβE. Both antagonists also significantly attenuated the increased swimming activity of nicotine-dosed fish. These results imply that both α7 and α4β2 nicotinic receptors play roles in the anxiolytic as well as the stimulant effects of nicotine in zebrafish.
This study confirms our earlier findings [7], that nicotine administration at 100 mg/l does have an anxiolytic effect in zebrafish as measured by the novel tank diving task. It further lends support to the notion that nicotine induces an anxiolytic response as was described by Brioni et al. [2]. However, nicotine may also play a role in motor abilities, as nicotine significantly increased swim activity an effect, which was also attenuated by both specific nicotinic antagonists. Most of the swimming of zebrafish in this environment is horizontal within a particular level of the tank with more occasional forays up or down into different levels. Future studies should explore the role nicotine plays in motor functions of zebrafish to see if they completely coincide with mammalian studies.
In another set of recent studies using the novel tank diving paradigm we tested the validity of the novel tank diving test as a model of anxiety. The diving response was examined the identical test tank under conditions when the test tank was novel vs. not novel, that is, in one condition the fish lived in a tank the same dimensions as the test tank and in the other condition the fish lived in a tank that was twice as large [1]. There was the diving response when the novel tank was unlike the home tank, but not in other fish when the same test tank was the same as the one in which they lived.
In the procedure used all fish including controls were netted for transfer from their home tank to the dosing beaker and then after dosing were transferred into a holding tank and finally into the test tank. Fish in all treatment groups were netted the same number of times at the same intervals. The repeated transfers may have added to the stress of the fish adding to their diving response in the test tank. However, this does not seem to be the principal driver of their response. In another study as described above, we found a much reduced diving response when fish housed in tanks similar to the test tank, such that it was not novel in contrast to fish housed in a larger tank and then tested in a novel-sized tank [1]. The fish in that experiment were also netted and transferred for testing.
In tests thus far with two anxiolytic drugs zebrafish have shown mixed results. We have tested individual drugs from two other classes of anxiolytics in the novel tank diving test in zebrafish. Buspirone, a serotonergic-based anxiolytic drug, produced an anxiolytic effect in zebrafish in this test, however chlordiazepoxide, a benzodiazepine-based anxiolytic drug did not [1]. The lack of identified anxiolytic effect with chlordiazepoxide was not due to insufficient dose inasmuch as the full dose effect function was tested covering no sedative effect to maximum sedation. Thus two out of three types of drugs with anxiolytic effects in humans and rats also show an anxiolytic-like effect in this zebrafish test. It may be the case that benzodiazepine receptors do not play the same role in anxiolysis in zebrafish as they do in mammals. Future studies will enlarge this library of compounds.
The study of zebrafish neurobehavioral function in general and the neurobehavioral pharmacology of nicotinic receptors in particular is at an early stage. Much remains to be done concerning the neurobehavioral pharmacology of nicotinic systems in zebrafish. For example, the characterization of nicotinic receptor subtypes in zebrafish is not entirely worked out. It is not certain that the selectivity of DHβE and MLA for zebrafish α4β2 and α7 receptors is the same as for mammalian receptors. In addition, other nicotinic receptor subtypes may be involved with the observed effects. For example, it is possible that the drug effects on bottom dwelling and swim speed are mediated through α6-containing receptors since both MLA and DHβE have affinity for α6 receptors [15]. Differential effects in zebrafish of nicotinic manipulations by sex and age also remain to be characterized. Elliot et al. [5] have worked with rats and have found that nicotine does induce the anxiolytic effect in adolescent males, but for adolescent females and all adults, there is an anxiogenic effect. The demonstration of the current behavioral results may encourage further work to completely characterize nicotinic receptor involvement in behavioral function of zebrafish.
The current study advances the characterization of nicotinic systems in zebrafish with regard to anxiolytic function and locomotor activity. It provides evidence for the involvement of both α7 and α4β2 nicotinic receptors in nicotine-induced anxiolytic and stimulant effects in zebrafish. Zebrafish can serve as inexpensive and high-throughput models for the screening of some classes of anxiolytic compounds.
Figure 4. Nicotine and Nicotinic Antagonist Effects on Swimming Speed over Minutes.
Swim activity (cm/min) affected by nicotine and nicotinic antagonists as measured by total distance moved per minute (mean±sem).
Figure 5. Nicotine and Nicotinic Antagonist Effects on Bottom-Dwelling.
Time spent (sec/min) in bottom third of novel tank (mean±sem). Total nicotine effects on dwelling of zebrafish in the bottom third of a new tank over a 5-min trial. There was a significant decrease in bottom dwelling of nicotine-dosed fish relative to controls. Those effects were reversed by both nicotinic antagonists, MLA and DHβE.
Figure 6. Nicotine and Nicotinic Antagonist Effects on Bottom Dwelling over Minutes.
Time spent (sec/min) in bottom third of novel tank affected by nicotine and nicotinic antagonists as measured by total distance moved per minute (mean±sem).
Acknowledgments
This research was supported by grant support from NIH ES10356. The authors thank Talbia Choudhury for her assistance with testing the fish.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bencan Z, Levin ED. Chlordiazepoxide and buspirone effects in a zebrafish model of anxiety. Psychopharmacology. 2008 doi: 10.1016/j.pbb.2009.07.009. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brioni JD, O’Neill AB, Kim DJB, Buckley MJ, Decker MW, Arneric SP. Anxiolytic-like effects of the novel cholinergic channel activator ABT-418. Journal of Pharmacology and Experimental Therapeutics. 1994;271:353–361. [PubMed] [Google Scholar]
- 3.Cerutti DT, Levin ED. Cognitive impairments models using complementary species. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. CRC Press; New York: 2006. pp. 315–342. [PubMed] [Google Scholar]
- 4.Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology. 2006;184:345–352. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- 5.Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav. 2004;77:21–28. doi: 10.1016/j.pbb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 6.File SE, Cheeta S, Kenny PJ. Neurobiological mechanisms by which nicotine mediates different types of anxiety. Eur J Pharmacol. 2000;393:231–236. doi: 10.1016/s0014-2999(99)00889-4. [DOI] [PubMed] [Google Scholar]
- 7.Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiology and Behavior. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Levin ED, Chen E. Nicotinic involvement in memory function in zebrafish. Neurotoxicology and Teratology. 2004;26:731–735. doi: 10.1016/j.ntt.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology. 2006;184:547–552. doi: 10.1007/s00213-005-0162-9. [DOI] [PubMed] [Google Scholar]
- 10.Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 11.Linney E, Upchurch L, Donerly S. Zebrafish as a neurotoxicological model. Neurotoxicol Teratol. 2004;26:709–718. doi: 10.1016/j.ntt.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Pietsaro N, Kaslin J, Anichtchik OV, Panula P. Modulation of the histaminergic system and behaviour by alpha-fluoromethylhistidine in zebrafish. J Neurochem. 2003;86:432–441. doi: 10.1046/j.1471-4159.2003.01850.x. [DOI] [PubMed] [Google Scholar]
- 13.Svoboda KR, Vijayaraghavan S, Tanguay RL. Nicotinic receptors mediate changes in spinal motoneuron development and axonal pathfinding in embryonic zebrafish exposed to nicotine. J Neurosci. 2002;22:10731–10741. doi: 10.1523/JNEUROSCI.22-24-10731.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swain HA, Sigstad C, Scalzo FM. Effects of dizocilpine (MK-801) on circling behavior, swimming activity, and place preference in zebrafish (Danio rerio) Neurotoxicol Teratol. 2004;26:725–729. doi: 10.1016/j.ntt.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Vailati S, Hanke W, Bejan A, Barabino B, Longhi R, Balestra B, Moretti M, Clementi F, Gotti C. Functional 6-containing nicotinic receptors are present in chick retina. Molecular Pharmacology. 1999;56:11–19. doi: 10.1124/mol.56.1.11. [DOI] [PubMed] [Google Scholar]
- 16.Williams FE, Messer WS., Jr Muscarinic acetylcholine receptors in the brain of the zebrafish (Danio rerio) measured by radioligand binding techniques. Comparative Biochemistry & Physiology: Toxicology & Pharmacology. 2004;137:349–453. doi: 10.1016/j.cca.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Williams FE, White D, MWS A simple spatial alternation task for assessing memory function in zebrafish. Behavioural Processes. 2002;58:125–132. doi: 10.1016/s0376-6357(02)00025-6. [DOI] [PubMed] [Google Scholar]