Abstract
Diverse lines of evidence indicate that pre-fibrillar, diffusible assemblies of the amyloid β-protein play an important role in Alzheimer’s disease pathogenesis. Although the precise molecular identity of these soluble toxins remains unsettled, recent experiments suggest that SDS-stable amyloid β-protein dimers may be the basic building blocks of Alzheimer’s disease-associated synaptotoxic assemblies and as such present an attractive target for therapeutic intervention. In the absence of sufficient amounts of highly pure cerebral amyloid β-protein dimers, we have used synthetic disulfide cross-linked dimers (free of Aβ monomer or fibrils) to generate conformation-specific monoclonal antibodies. These dimers aggregate to form kinetically trapped protofibrils, but do not readily form fibrils. We identified two antibodies, 3C6 and 4B5, which preferentially bind assemblies formed from covalent Aβ dimers, but do not bind to amyloid β-protein monomer, amyloid precursor protein, or aggregates formed by other amyloidogenic proteins. Monoclonal antibody 3C6, but not an IgM isotype-matched control antibody, ameliorated the plasticity-disrupting effects of Aβ extracted from the aqueous phase of Alzheimer’s disease brain, thus suggesting that 3C6 targets pathogenically relevant amyloid β-protein assemblies. These data prove the usefulness of covalent dimers and their assemblies as immunogens and recommend further investigation of the therapeutic and diagnostic utility of monoclonal antibodies raised to such assemblies.
Introduction
The abnormal accumulation of misfolded, β-sheet-rich, protein aggregates is associated with at least 25 disorders (Stefani, 2004; Westermark et al., 2005). Among these maladies, Alzheimer’s disease (AD) is the most common and because age is a risk factor and life expectancy is constantly increasing, so too are the number of AD cases (Davies et al., 1988; Selkoe, 2001; Ferri et al., 2005; Querfurth and LaFerla, 2010). Pathologically, AD is characterized by the presence of extracellular amyloid plaques, intraneuronal neurofibrillary tangles and synaptic loss throughout the limbic and association cortices (Alzheimer, 1906; Kidd, 1964; Khachaturian, 1985; Hardy and Allsop, 1991; Selkoe, 1991). The amyloid β-protein (Aβ) is the primary constituent of amyloid plaques and a plethora of genetic, animal modeling and biochemical data indicate that Aβ plays a central role in AD pathogenesis (Walsh and Selkoe, 2007). Numerous studies have shown that water-soluble non-fibrillar Aβ assemblies are toxic and impair disease-relevant models of synaptic form and function (Lambert et al., 1998; Walsh et al., 1999; Walsh et al., 2002; Barghorn et al., 2005; Cleary et al., 2005; Lesne et al., 2006; Lacor et al., 2007; Martins et al., 2008; Shankar et al., 2008; Noguchi et al., 2009). Although, it is not yet known which assembly form(s) of Aβ are the proximate pathogens, recent attention has focused on various forms of Aβ dimers (Shankar et al., 2008; Kok et al., 2009; Sandberg et al., 2010). Highly stable Aβ dimers are specifically found in AD brain and blood (Kuo et al., 1996; Roher et al., 1996; Mc Donald et al., 2010; Villemagne et al., 2010), and brain-derived dimers have been shown to block long-term potentiation (LTP), inhibit synapse remodeling, and impair memory consolidation (Klyubin et al., 2008; Shankar et al., 2008; Freir et al., 2011). Moreover, we have recently shown that synthetic Aβ dimers designed to mimic natural dimers can rapidly form meta-stable protofibrils that persist for prolonged intervals and potently impair synaptic plasticity (O'Nuallain et al., 2010). Similar structures are also formed by Aβ monomer, but the amount formed and the time over which they exist is dramatically extended for dimer, thus suggesting that Aβ dimers aggregate by a process distinct from monomer.
A large number of studies have demonstrated that both the active generation or passive transfer of anti-Aβ antibodies can prevent or reverse Aβ-induced cognitive impairment in APP transgenic mice (Games et al., 2006) and this has prompted several clinical trials in humans (Schenk, 2002; Gilman et al., 2005). Most forms of immunotherapy employ antibodies that recognize multiple different assembly forms of Aβ, including monomer. This approach suffers from the loss of antibody capacity due to binding to non-pathogenic forms of Aβ and removal of “useful” Aβ (Arancio and Chao, 2007; Puzzo et al., 2008). An alternate approach would be to develop antibodies that specifically recognize pathogenic forms of Aβ dimers and ameliorate their toxic activity. To address this we used a preparation of covalently stabilized Aβ (1–40)Cys26 dimers free of Aβ monomer or fibrils as an immunogen and screened hybridomas for their ability to produce antibodies that discriminate between reduced non-cross-linked monomer and covalently linked dimers. Two murine mAbs IgMs, referred to as 3C6 and 4B5, preferentially bind covalent Aβ dimer assemblies, but not Aβ monomer or fibrils formed by other amyloidogenic proteins. Notably, mAb 3C6, but not an IgM isotype-matched control antibody, ameliorated the synaptic plasticity disrupting effect of aqueous extracts of AD brain Aβ on rodent LTP. These data indicate that further investigation of the therapeutic and diagnostic utility of mAbs raised to assemblies formed from covalently stabilized Aβ dimers is warranted.
Materials and Methods
Peptides, proteins, and reagents
Human wild-type (WT) Aβ1-40 and mutant Aβ1-40S26C peptides were synthesized and purified by Dr. James I Elliott at Yale University (New Haven, CT). Human islet amyloid polypeptide (IAPP) was purchased from Quality Controlled Biochemicals (Hopkinton, MA). Mass spectrometric (MS) analysis and reverse-phase HPLC confirmed that the peptides had the correct mass and were >90% pure. Recombinant human λ6 immunoglobulin light chain variable domain, Jto (Wall et al., 1999), was a gift from Dr. Alan Solomon (University of Tennessee, Knoxville, TN). Aβ1-40 peptides contain a single tyrosine and absorption of tyrosine at 275 nm (ε275 = 1400 M−1.cm−1) was used to estimate the concentration of Aβ solutions. The concentration of IAPP and Jto were established using the MicroBCA assay (ThermoFisher Scientific, Waltham, MA). An Anti-Aβ N-terminal reactive mAb, 6E10, was from Signet (Dedham, MA). Anti-Aβ mAbs, 2G3 and 21F12, which specifically recognize Aβ terminating at residue 40 and 42, respectively (Johnson-Wood et al., 1997) were gifts from Drs. Peter Seubert and Dale Schenk (Elan Pharmaceuticals, San Francisco, CA). AW7, an anti-Aβ polyclonal antibody, was raised to aggregated synthetic Aβ1-42 and recognizes synthetic and brain-derived Aβ (Mc Donald et al., 2010). A control murine IgMκ, M1520, was from Sigma-Aldrich (Arklow, Co. Wicklow, Republic of Ireland). Unbranched dextran standards of molecular masses: 43,800; 21,400; 9890, and 4440 were purchased from Pharmacosmos (Holbaek, Denmark). Blue dextran 2000 was purchased from GE Healthcare (Bio-Sciences AB, Uppsala Sweden). Unless otherwise stated, chemicals and reagents were obtained from Sigma-Aldrich and were of the highest purity available.
Preparation of amyloidogenic conformers
Cross-linking of Aβ1-40S26C was achieved by atmospheric oxidation of ~20 µM peptide in 10 mM ammonium bicarbonate, pH 8.2, and (Aβ1-40S26C)2 dimers were isolated from unreacted monomer and higher molecular weight aggregates by SEC using a Superdex™ 75 10/300 HR or a HiLoad 16/60 Superdex™ 75 column (GE Healthcare Bio-Sciences) (Hu et al., 2008; O'Nuallain et al., 2010). An aliquot of each SEC fraction was electrophoresed on 16% polyacrylamide tris-tricine gels and peptide detected by silver staining (Schagger and von Jagow, 1987; Shevchenko et al., 1996). Fractions that contained either Aβ dimer or unreacted monomer, but not both, were immediately pooled, used, or frozen at −80 °C. WT Aβ1-40 monomers were isolated using SEC (O'Nuallain et al, 2010). Meta-stable Aβ1-40S26C protofibrils were generated by incubating (Aβ1-40S26C)2 in 20 mM sodium phosphate, pH 7.4, at 37°C for 3 d (O'Nuallain et al., 2010). WT Aβ1-40, Jto, and IAPP fibrils were prepared by incubating the ultracentrifuged disaggregated polypeptides at ~0.2–0.6 mg/ml for up to 3 weeks at 37 °C in PBS containing 0.02 % sodium azide, pH 7.4 (PBSA) (O'Nuallain and Wetzel, 2002). Jto fibrils were grown The fibrillogenesis reactions were judged to be complete when Thioflavin T (ThT) fluorescence reached plateau values. The reaction products were harvested by centrifugation at 20,200 × g for 30 min at room temperature and assessed by negative contrast electron microscopy (Walsh et al., 1997).
Generation of anti-Aβ dimer mAbs
Animals used to generate monoclonal antibodies (mAbs) were treated in accordance with National Institutes of Health regulations under the aegis of a protocol approved by the University of Tennessee’s Animal Care and Use Committee. Three, 5-week old BALB/c mice (Charles Rivers Laboratories, Wilmington, MA) each received three ~30 µg intraperitoneal injections of (Aβ1-40S26C)2 emulsified in aluminium and magnesium hydroxide (Imject ALUM™, Pierce, Rockford, IL). Since tissue plasminogen activator (tPA) has been implicated in the degradation of misfolded proteins, including Aβ (Kranenburg et al., 2002; Melchor et al., 2003), we reasoned that mice lacking tPA may allow higher and more prolonged circulating levels of Aβ. Therefore, we also immunized two C57BL/6 mice deficient in tPa (Carmeliet et al., 1994). Anti-Aβ dimer antibody response in the immunized animals was determined by screening sera against microtiter plate-immobilized (Aβ1-40S26C)2 using europium time-resolved fluorescence as the detection system (O'Nuallain et al., 2007). Murine sera were serially diluted in triplicate with assay buffer (1 % BSA in PBSA containing 0.05% Tween 20, pH 7.4) into microtiter plate wells (#3369, COSTAR, Corning, NY) that were coated with 400 ng of (Aβ1-40S26C)2 and blocked with 1 % BSA in PBSA. A biotinylated goat anti-mouse IgG served as the secondary antibody, and was detected with Eu3+-streptavidin and time-resolved fluorescence using a Victor2 1420 Multilabel Counter (Perkin Elmer, Waltham, MA). Antibody binding curves were fitted using a standard 3-parameter sigmoid (logistic) function (SigmaPlot 2000, version 6; Systat Software, Chicago, IL) and titer values determined as the highest serum dilution that gave a signal 3 times higher than the buffer blank.
Production of hybridomas and clonal selection using splenic B-cells was performed using standard methods (Kohler and Milstein, 1975; Yokoyama et al., 2006) with animals that had the highest anti-Aβ iters (SI). Clonal hybridoma cell lines that secreted Aβ dimer-reactive mAbs were isolated by limiting dilution of primary polyclonal hybridomas by plating ~1 and ~10 cells per well in 96-well culture plates (CELLSTAR®, Greiner Bio-One). Positive clones that recognized (Aβ1-40S26C)2 were identified using our standard europium fluorescent microtiter plate assay, subcloned and expanded for antibody production.
Antibody production, purification, and isotyping
Large scale antibody production was performed by growing hybridoma cells to high density in 350 ml CELLine bioreactor flasks (CL350, Integra Biosciences AG, Chur, Switzerland) in HyClone DMEM/F12 medium supplemented with 2.5 mM L-glutamine and 15 mM HEPES (Thermo Fisher Scientific, Logan, UT), 5% fetal bovine serum, and 100 units/ml Pen/Strep (Lonza, Valais, Switzerland). Antibodies from hybridoma supernatants were purified by a combination of water dialysis (Vollmers et al., 1996) and thiophilic affinity chromatography using HiTrap IgM columns (GE Healthcare, Uppsala, Sweden). Antibody isotyping and light chain composition, κ or λ, was determined using an IsoStrip™ mouse monoclonal antibody isotyping kit (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and silver staining was used to assess antibody purity (SFig. 1) (Shevchenko et al., 1996).
Soluble human AD brain TBS extract
Human brain tissue was obtained and used in accordance with the UCD Human Research Ethics Committee guidelines (under approval LS-E-10-10-Walsh). Frozen temporal cortex was obtained from an 85 year-old male with dementia and fulminant amyloid and tangle pathology (Braak stage =4) and was provided by Drs Dykoski and Cleary. Soluble AD brain Tris-buffered saline (TBS) extract was prepared essentially as described previously (Mc Donald et al., 2010). Briefly, ~0.9 g of temporal cortex was homogenized in 4.5 ml of freshly prepared ice-cold TBS with 25 strokes of a Dounce homogeniser (Fisher, Ottawa, Canada). The homogenate was centrifuged at 175,000 g and 4 °C (Beckman Coultour, Fullerton, CA) for 30 min, and the supernatant, referred to as the TBS extract, was carefully removed. The extract was then exchanged into 50 mM ammonium acetate, pH 8.5, using a Hi-trap (5 ml) desalting column (GE Healthcare) and stored at −80 °C. Aβ content of both the TBS extract and buffer-exchanged extracts were examined using a sensitive immunoprecipitation/Western blotting protocol and the Aβ concentration estimated made by comparison to synthetic Aβ standards (Mc Donald et al., 2010).
Amyloidogenic conformer binding
Direct and competition ELISA
Except for using immunoglobulin class specific biotinylated secondary antibodies and streptavidin-HRP (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) as the detection system, antibody binding curves against plate-immobilized Aβ conformers, Jto and IAPP fibrils were determined in the same manner as described above for our standard microtiter plate assay. The concentration of antibody that gave half-maximal binding, EC50, was determined from the fitted binding curves. To assess the ability of antibodies to recognize the solution-based structure of various amyloidogenic conformers, we developed a competition assay in which each solution-phase amyloidogenic conformer was serially diluted (0–0.1 mg/ml) into high binding microtiter plate wells (COSTAR, Corning) that were coated with 400 ng of (Aβ1-40S26C)2 and blocked with 1 % BSA in PBSA. Antibody was immediately added to each well at a concentration equal to its EC50 value, and (Aβ1-40S26C)2-bound antibody detected using our standard ELISA. A value for the concentration of a competitor that gave half-maximal inhibition of antibody binding, IC50, was determined from each sigmoidally fitted curve.
Immunoprecipitation/Western blot
Antibody binding to Aβ present in TBS extracts of human brain was investigated using our previously published immunoprecipitation protocol (Mc Donald et al., 2010; Shankar et al., 2011). Because IgMs do not bind to protein A or protein G, the procedure for immunoprecipitation using the IgM, 3C6, was adapted to include the addition of anti-IgM. Briefly, 160 µg/ml of 3C6 plus 35 µg/ml anti-IgM (µ-chain specific; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), and 30 µl of a protein A and protein G mix were added to 500 µl of AD TBS extract and incubated overnight at 4°C. Aβ-antibody complexes bound to protein A and protein G were harvested, boiled in sample buffer and electrophoresed on 16% polyacrylamide tris-tricine gels. Thereafter, proteins were transferred onto 0.2 µm nitrocellulose (Optitran, Schleicher and Schull, Germany), Aβ etected by Western blot using an equimolar mixture (1 µg/ml each) of C-terminal reactive mAbs 2G3 and 21F12, fluorochrome-coupled anti-mouse IgG (Rockland, Gilbertville, PA), and a Li-COR Odyssey near infrared imaging system (Li-COR Biosciences, Lincoln, NE) ((Mc Donald et al., 2010).
In vivo electrophysiology
In vivo studies on urethane (1.5 gm/kg i.p.) anaesthetized male Wistar rats (250–300 g) were approved by Trinity College Dublin’s ethical review committee and by the Department of Health, Republic of Ireland. Electrodes were made and implanted as described previously (Klyubin et al., 2008). Briefly, twisted-wire bipolar electrodes were constructed from Teflon-coated tungsten wires (62.5 µm inner core diameter, 75 µm external diameter). Single pathway recordings of field EPSPs were made from the stratum radiatum in the CA1 area of the right hippocampal hemisphere in response to stimulation of the ipsilateral Schaffer collateral - commissural pathway. Electrode implantation sites were identified using stereotaxic coordinates relative to bregma, with the recording site located 3.4 mm posterior to bregma and 2.5 mm right of midline, and the stimulating electrode located 4.2 mm posterior to bregma and 3.8 right of midline. The optimal depth of the wire electrodes in the stratum radiatum of the CA1 region of the dorsal hippocampus was determined using electrophysiological criteria and verified post-mortem. Test EPSPs were evoked at a frequency of 0.033 Hz and at a stimulation intensity adjusted to give an EPSP amplitude of 50% of maximum. The high frequency stimulation (HFS) protocol for inducing LTP consisted of 10 trains of 20 stimuli with an inter-stimulus interval of 5 ms (200 Hz), and an inter-train interval of 2 sec. The intensity was increased to give an EPSP of 75% of maximum amplitude during the HFS. To inject samples, a stainless-steel guide cannula (22 gauge, 0.7 mm outer diameter, 13 mm length) was implanted above the right lateral ventricle (1 mm lateral to the midline and 4 mm below the surface of the dura) just prior to electrode implantation. Intracerebroventricular (i.c.v.) injections of 5 µL (PBS vehicle or human brain extract) or 10 µL (PBS vehicle or antibody alone, or 5 µL human brain extract + 5µL PBS with antibody or vehicle) were made via an internal cannula (28 gauge, 0.36 mm outer diameter). Verification of the placement of the cannula was performed post-mortem by checking the spread of i.c.v. injected ink dye.
LTP was expressed as the mean ± s.e.m. % baseline field EPSP amplitude recorded over at least a 30 min baseline period. Similar results were obtained when the EPSP slope was measured. Statistical comparisons used paired and unpaired Student t-tests.
Results
Murine hybridomas secrete anti-Aβ dimer IgMs that preferentially bind to the surface-adsorbed conformer
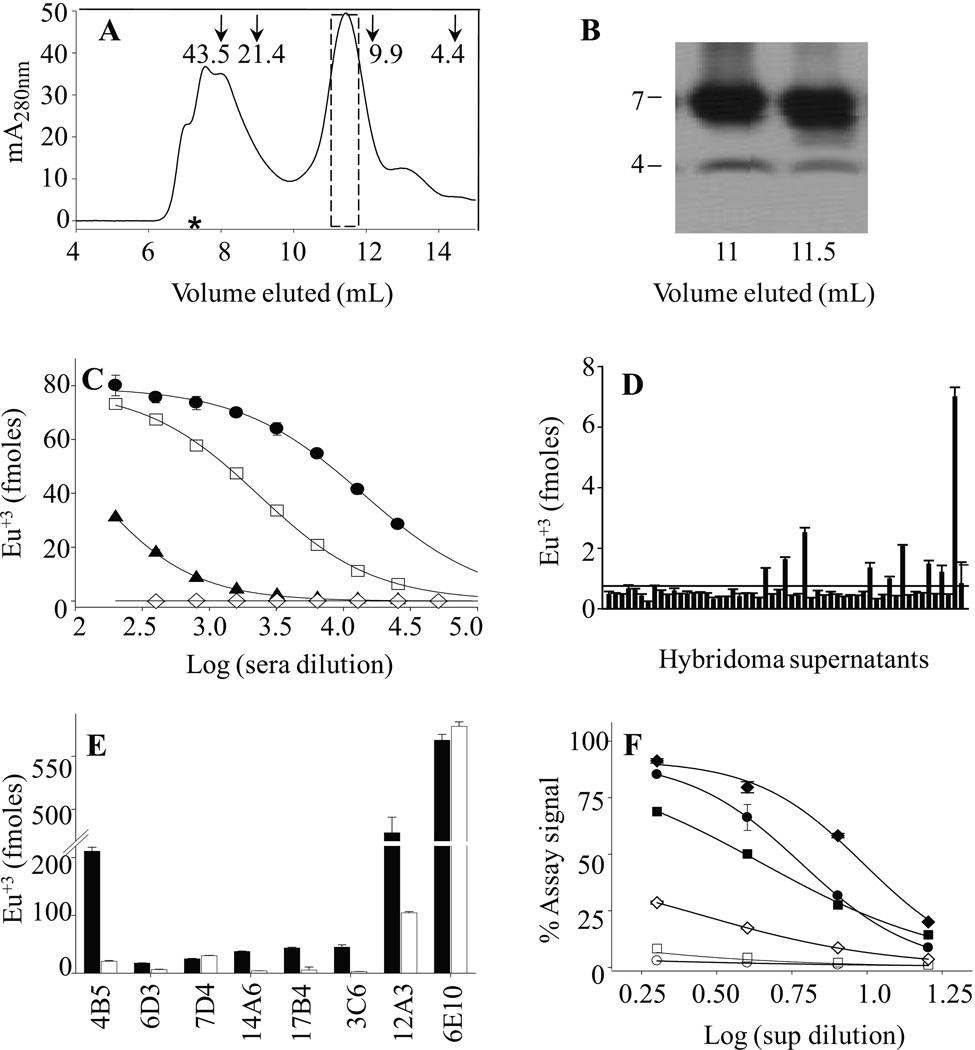
In an effort to generate novel anti-Aβ dimer mAbs we immunized 3 BALB/c mice and 2 tPA deficient mice with SEC-isolated (Aβ1-40S26C)2 (Fig. 1A, B). The latter were used because injected Aβ is much less well cleared in tPA mice (Melchor et al., 2003) thus offering the possibility of more prolonged exposure to the Aβ immunogen and the potential for a stronger immune response. An anti-Aβ dimer antibody response was elicited in all immunized mice, with sera titers in the ~3,000–100,000 range, against plate-immobilized (Aβ1-40S26C)2 (Fig. 1C & SFig. 2). The (Aβ1-40S26C)2 used for this screen had been isolated by SEC and stored frozen at −80°C for approximately one month prior to plating, and thus was likely comprised of mostly dimer plus a small percentage of pre-fibrillar dimer assemblies (O’Nuallain et al., 2010). Serum from an unimmunized mouse lacked significant amounts of anti-Aβ antibodies. Three to 4 positive hybridoma supernatants against plate-immobilized (Aβ1-40S26C)2 were identified from each of the three fusions carried out (Fig. 1D). Each fusion used splenic B-cells from one of the three animals which gave the most robust immune response. This included hybridomas from 2 BALB/c and 1 tPA knock-out immunized mice. Six of the 7 most potent hybridoma supernatants contained IgMκ Abs that preferentially bound to plate-immobilized (Aβ1-40S26C)2 relative to the reduced (monomeric) peptide (Fig. 1E), all, but one of which, 4B5, came from BALB/c hybridomas. In contrast, an IgG mAb 7D4 and a control anti-Aβ N-terminal reactive mAb, 6E10, bound dimeric and monomeric peptides equally well, whereas, a murine isotype-matched control IgM (M1520) exhibited no significant avidity (SFig. 3). Antibody binding curves for our most potent anti-Aβ dimer mAbs, 3C6, 4B5, and 12A3 against plate-immobilized (Aβ1-40S26C)2 and the reduced peptide demonstrated ~20- to 50-fold stronger binding to (Aβ1-40S26C)2 versus Aβ1-40S26C (Fig. 1F). However, since 12A3 was poorly secreted by its hybridoma, and proved difficult to purify, only 3C6 and 4B5 were characterized further.
Figure 1. Aβ dimer purification and generation of anti-Aβ dimer IgM mAbs.
Disulfide cross-linked Aβ dimers were generated by atmospheric oxidation of 20 µM Aβ1-40S26C in 20 mM ammonium bicarbonate, pH 8.5, for 5 days at room temperature. (A) The (Aβ1-40S26C)2 product was isolated by SEC using a Superdex™ 75 10/300-GL column eluted with 50 mM ammonium acetate, pH 8.5. The dashed box indicates the SEC fractions that were pooled and subsequently used as immunogen. Arrows indicate elution of unbranched dextran standards, and the hash symbol represents the void volume determined using the elution of blue dextran 2000. (B) SDS-PAGE analysis of SEC fractions from the major SEC peak confirmed the presence of disulfide cross-linked Aβ dimer. Gels have been cropped to show the region between 2 and 10 kDa because no higher migrating species were detected. (C) Representative antibody titration curves against plate-immobilized (Aβ1-40S26C)2 for three BALB/c immunized mice (●, □, ▲) had titers in the range of 3,000 to 100,000. Serum from an unimmunized mouse (◊) did not contain antibodies capable of detecting the immobilized peptide. (D) Representative initial screen of supernatants from 55 of 384 murine hybridomas against plate-immobilized (Aβ1-40S26C)2. The solid horizontal line represents the mean value of all supernatants examined. The dashed line represents the cut-off value used to select positive clones and was arbitrarily set at twice the value of the solid horizontal line. (E) Reactivity of our best hybridoma antibodies against plate-immobilized (Aβ1-40S26C)2 (■) and the same dimer reduced to monomer by β-mercaptoethanol (□). The bar chart shows that all 6 IgMκ antibodies had preference for the intact dimer. In contrast, 7D4, an IgG2bκ mAb, and an anti-Aβ N-terminal-reactive IgG mAb, 6E10, detected monomer and dimer similarly well. (F) Antibody binding curves for IgMκ's 12A3 (●, ○), 4B5 (♦, ◊), and 3C6 (■, □) against plate-immobilized (Aβ1-40S26C)2 (closed symbols) and the reduced conformer (open symbols). All antibody studies were carried out using hybridoma supernatants diluted 1:1 to 1:512 into PBSA containing 1% BSA and 0.05% Tween 20, pH 7.4.
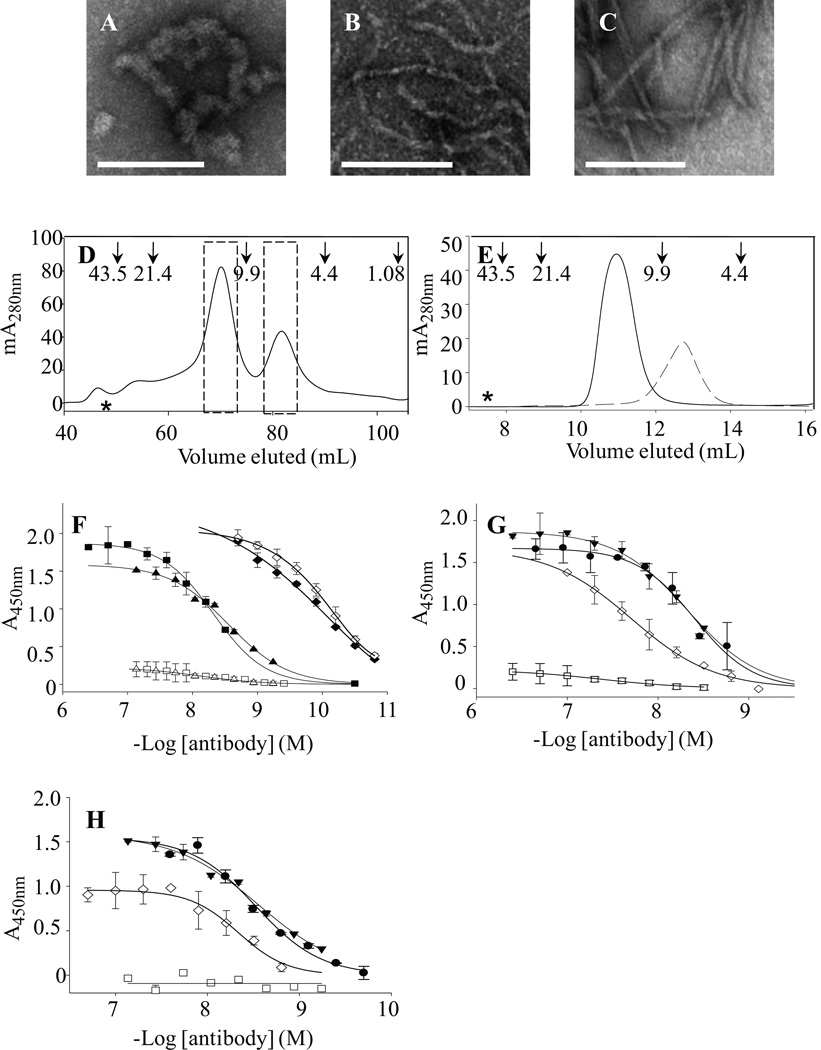
Given that SDS-PAGE and BCA assay analyses of ultracentrifuged (Aβ1-40S26C)2 with or without adjuvant (ALUM) confirmed that ~80% of the dimer immunogen was adsorbed onto the adjuvant, and the dimer is prone to form pre-fibrillar aggregates (O'Nuallain et al., 2010), we examined if mAbs 3C6 and 4B5 specifically recognized solution-phase as well as plate-immobilized (Aβ1-40S26C)2, higher aggregates of (Aβ1-40S26C)2, and WT Aβ. These studies were also motivated by our observation that freeze/thawing induced Aβ dimer aggregation into ThT positive spherical-like assemblies with diameters of 11.4 ± 1+.6 nm (O'Nuallain et al., 2010), and the immunogen and initial mAb characterization were performed using (Aβ1-40S26C)2 that was frozen for up to ~3 month, (Fig. 2A–C). To perform this analysis, we used freshly prepared highly pure SEC-isolated Aβ1-40S26C and (Aβ1-40S26C)2 (Fig. 2D). Rechromatographing SEC-isolated Aβ1-40S26C conformers on a 10/300 Superdex 75 column confirmed their high purity and the absence of higher molecular weight Aβ assemblies (Fig. 2E). To minimize the potential for spurious antibody reactivity against higher molecular weight Aβaggregates, fresh (Aβ1-40S26C)2 and monomers were stored on ice for ≤ 3 h, and the peptides coated onto microtiter plates at a concentration (10 µg/ml) that was below the critical concentration necessary for aggregation (O'Nuallain et al., 2010). Highly similar results were obtained with both fresh and frozen (Aβ1-40S26C)2, with EC50 values of ~3–5 nM and maximum signal amplitudes of ~1.5–1.9 (Fig. 2F–H, Table 1). The antibodies had essentially no binding to freshly prepared plate-immobilized Aβ1-40S26C monomers or (Aβ1-40S26C)2 that had been reduced to monomers (Fig. 2F–H, Table 1). This indicated that the mAbs specifically recognized epitopes on (AβS1-4026C)2. In contrast, the widely used anti-Aβ mAb 6E10 bound similarly to both peptide conformers.
Figure 2. Aβ dimer aggregation, conformer purification, and antibody binding.
Negative contrast EM was performed on a 10 µM (Aβ1-40S26C)2 solution that was stored at −80 °C for 1 month in 25 mM ammonium acetate, pH 8.5 (A), and after 3 d at 37 °C in 20 mM sodium phosphate, pH 7.4 (B). For comparison, fibrils formed by incubating 30 µM monomeric WT Aβ for two weeks are also shown (C). Size bars in Panels A–C are 100 nm. (D) Cross-linked dimer and unreacted monomer from oxidized Aβ1-40S26C were isolated by SEC using a HiLoad 16/60 Superdex™ 75 column eluted with 25 mM ammonium acetate, pH 8.5. The dashed bars indicate the dimeric and monomeric fractions that were separately pooled and used in further experiments. Arrows indicate elution of unbranched dextran standards, and the hash symbol represents the void volume, which was determined using blue dextran 2000. (E) Rechromatographing of SEC-isolated (Aβ1-40S26C)2 ( ) and Aβ1-40S26C (
) and Aβ1-40S26C ( ) samples on a Superdex™ 75 10/300-GL column eluted with 25 mM ammonium acetate, pH 8.5, confirmed their high purity and the absence of higher molecular weight Aβ assemblies. (F) Antibody binding curves for purified IgMκ's 3C6 (■, □) and 4B5 (▲,Δ), and control mAb 6E10 (♦, ◊) against plate-immobilized freshly isolated (Aβ1-40S26C)2 (closed symbols) and Aβ1-40S26C (open symbols). Antibody binding curves were also determined for 3C6 (G) and 4B5 (H) binding to: freshly-isolated (Aβ1-40S26C)2 (●); (Aβ1-40S26C)2 stored for ~1 month at −80 °C in 25 mM ammonium acetate, pH 8.5, (▼); Freshly-isolated Aβ1-40S26C monomers (□), and WT Aβ1-40 fibrils (◊). Antibody binding studies were carried out in PBSA containing 1% BSA and 0.05% Tween 20, pH 7.4.
) samples on a Superdex™ 75 10/300-GL column eluted with 25 mM ammonium acetate, pH 8.5, confirmed their high purity and the absence of higher molecular weight Aβ assemblies. (F) Antibody binding curves for purified IgMκ's 3C6 (■, □) and 4B5 (▲,Δ), and control mAb 6E10 (♦, ◊) against plate-immobilized freshly isolated (Aβ1-40S26C)2 (closed symbols) and Aβ1-40S26C (open symbols). Antibody binding curves were also determined for 3C6 (G) and 4B5 (H) binding to: freshly-isolated (Aβ1-40S26C)2 (●); (Aβ1-40S26C)2 stored for ~1 month at −80 °C in 25 mM ammonium acetate, pH 8.5, (▼); Freshly-isolated Aβ1-40S26C monomers (□), and WT Aβ1-40 fibrils (◊). Antibody binding studies were carried out in PBSA containing 1% BSA and 0.05% Tween 20, pH 7.4.
Table 1. MAbs 3C6, 4B5, and 6E10 exhibit differential binding to amyloidogenic conformers.
EC50 values and maximum antibody binding signal amplitudes against plate-immobilized amyloidogenic conformers were determined from sigmoidally fitted binding curves such as those shown in Figs. 2 and 3. NB, no binding, and ND, not determined.
| Protein1 | 3C6 | 4B5 | 6E10 | |||
|---|---|---|---|---|---|---|
| EC50 (nM) |
Max. Signal (A450nm) |
EC50 (nM) |
Max. Signal (A450nm) |
EC50 (nM) |
Max. Signal (A450nm) |
|
| Fresh Aβ1-40S26C monomers | >100 | -- | >100 | -- | 0.08 ± 0.0001 | 2.0 ± 0.02 |
| Fresh (Aβ1-40S26C)2 dimers | 4.8 ± 0.01 | 1.9 ± 0.03 | 2.8 ± 0.02 | 1.6 ± 0.06 | 0.12 ± 0.001 | 1.9 ± 0.07 |
| Reduced (Aβ1-40S26C)2 | >200 | -- | >200 | -- | 0.21 ± 0.001 | 2.0 ± 0.03 |
| Frozen (Aβ1-40S26C)2 dimers | 4.0 ± 0.02 | 1.7 ± 0.06 | 3.0 ± 0.03 | 1.5 ± 0.13 | 0.09 ± 0.0001 | 2.1 ± 0.02 |
| (Aβ1-40S26C)2 protofibrils | 4.8 ± 0.01 | 1.4 ± 0.02 | 6.8 ± 0.07 | 1.3 ± 0.10 | 0.10 ± 0.0005 | 2.0 ± 0.04 |
| Fresh WT Aβ1-40 monomers | >100 | -- | >100 | -- | 0.12 ± 0.0004 | 2.3 ± 0.03 |
| Aβ1-40 fibrils | 20 ± 0.2 | 1.7 ± 0.1 | 4.6 ± 0.03 | 1.0 ± 0.03 | 0.14 ± 0.0005 | 1.7 ± 0.02 |
| IAPP fibrils | NB | NB | NB | NB | ND | ND |
| Jto fibrils | NB | NB | NB | NB | ND | ND |
WT Aβ1-40 monomers and (Aβ1-40S26C)2 dimers were freshly isolated from SEC and their identity confirmed by their elution on SEC and electrophoretic mobility on SDS-PAGE. Reduced (Aβ1-40S26C)2 dimers were treated with β–mercaptoethanol to break the stabilizing disulfide bond and quickly immobilized on ELISA plates. Frozen (Aβ1-40S26C)2 dimers were prepared as described for (Aβ1-40S26C)2 dimers, but snap frozen in liquid N2 and stored at −80 °C for up to 1 month prior to thawing at 4 °C and use. (Aβ1-40S26C)2 protofibrils were formed by incubation of (Aβ1-40S26C)2 dimers at 37 °C for 3 days and were shown to bind ThT and to appear as structures ranging from imperfect spheres of ~5 nm to flexible protofibrils of ~6–8 nm diameters and <200 nm in length.
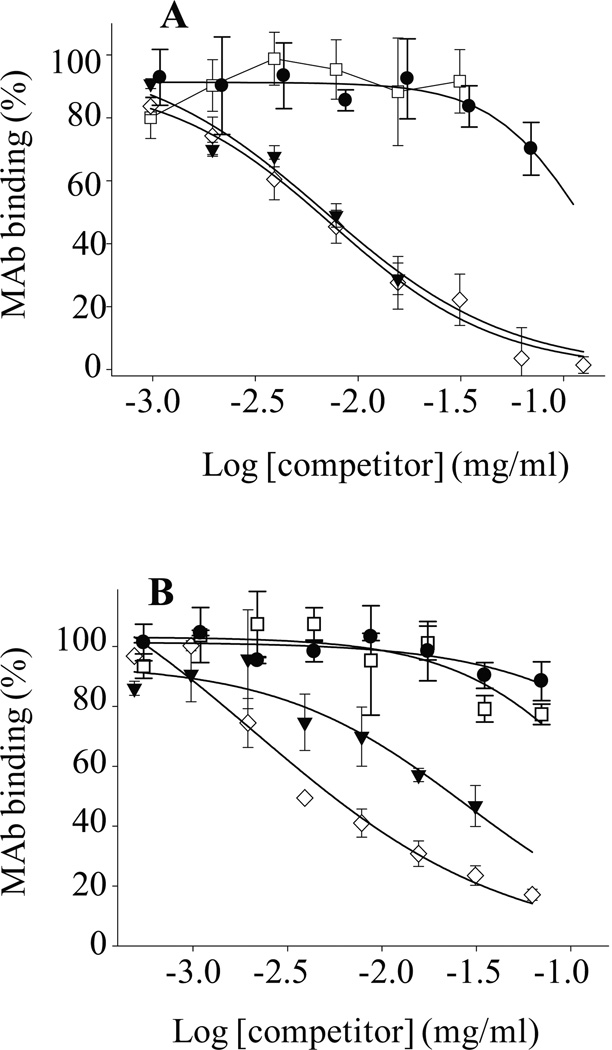
To investigate if the specificity of mAbs 3C6 and 4B5 for plate-immobilized (Aβ1-40S26C)2 was artificially induced by surface adsorption (Giacomelli and Norde, 2005; O'Nuallain et al., 2008), we employed a solution-based competition assay (O'Nuallain et al., 2006). In contrast to the results obtained for direct ELISA, the solution-based competition ELISA revealed that both 3C6 and 4B5 preferentially bound to freeze/thawed (Aβ1-40S26C)2, with IC50 values ~0.01 and 0.03 mg/ml, respectively (Fig. 3, Table 2). Like Aβ monomer, freshly prepared (Aβ1-40S26C)2 did not prevent 3C6 or 4B5 binding to the immobilized Aβ conformer, and the IC50 produced by (Aβ1-40S26C)2 in solution was >0.1 mg/ml (Fig. 3, Table 2).
Figure 3. Anti-Aβ dimer mAbs 3C6 and 4B5 binding to solution-phase Aβ conformers.
Antibody binding to solution-phase Aβ conformers was established in a competition assay whereby mAbs 3C6 (A) and 4B5 (B) binding to plate-immobilized (Aβ1-40S26C)2 was determined in the presence of: freshly-isolated (Aβ1-40S26C)2 (●); (Aβ1-40S26C)2 stored for ~1 month at −80 °C in 25 mM ammonium acetate, pH 8.5, (▼); Freshly-isolated Aβ1-40S26C monomers (□), and WT Aβ1-40 fibrils (◊). Antibody binding was carried out in PBSA containing 1% BSA and 0.05% Tween 20, pH 7.4.
Table 2. Inhibitory potencies of amyloidogenic conformers against mAbs 3C6 and 4B5 binding to plate-immobilized S26C dimers.
IC50 values and maximum binding signal amplitudes were determined from sigmoidally fit binding curves such as those shown in Fig. 3. NB, no binding.
| Competitor1 | 10−2 × IC50 (mg/ml) |
|
|---|---|---|
| 3C6 | 4B5 | |
| Fresh (Aβ1-40S26C)2 dimers | >10 | >7 |
| Frozen (Aβ1-40S26C)2 dimers | 0.70 ± 0.09 | 2.8 ± 0.2 |
| Fresh (Aβ1-40S26C)2 monomers | >7 | >7 |
| Fresh WT Aβ1-40 monomers | >7 | >7 |
| Aβ1-40 fibrils | 0.74 ± 0.03 | 0.22 ± 0.03 |
| Jto fibrils | NB | NB |
IAPP and Jto fibrils were prepared according to standard procedures and shown to bind to ThT and form typical amyloid fibrils.
Anti-Aβ dimer IgMs recognizes high-molecular weight Aβ assemblies
The 3C6 and 4B5 antibodies bound similarly to plate-immobilized (Aβ1-40S26C)2 and (Aβ1-40S26C)2 protofibrils, with EC50 values of ~3–7 nM and maximum signal amplitudes of ~1.3–1.9 (Table 1). MAb 4B5 also exhibited similar binding to WT Aβ fibrils (EC50 values of ~5 nM), but 3C6 had ~4-fold weaker reactivity, with an EC50 values of ~20 nM (Fig. 2H, I, Table 1). The similar maximum signal amplitudes for the mAbs binding to diverse Aβ conformers indicated that the same/or similar recognition sites were highly accessible on all plate-immobilized Aβ conformers studied, from dimer to fibrils. To further validate mAbs 3C6 and 4B5 reactivity with aggregated Aβ, we demonstrated that both antibodies were prevented from binding to plate-immobilized (Aβ1-40S26C)2 by solution-based (Aβ1-40S26C)2 protofibrils and WT Aβ fibrils, with IC50 values of ~0.002 mg/ml, respectively (Fig. 3, Table 2). Unlike other pan-amyloidogenic antibodies that recognize conformational epitopes on amyloidogenic assemblies independent of the protein’s sequence (Linke et al., 1973; Korth et al., 1997; Goldsteins et al., 1999; O'Nuallain and Wetzel, 2002; Kayed et al., 2003; O'Nuallain et al., 2006; Adekar et al., 2010), 3C6 and 4B5 did not bind to plate-immobilized or solution-based IAPP and LC amyloid fibrils either when plate-immobilized or in solution (Tables 1 and 2). Peptide epitope mapping, using plate-immobilized Aβ fragments, indicated that an important binding stretch for mAb 3C6 is within residues 19–35 of the Aβ peptide (SFig. 6), but both mAbs 3C6 and 4B5 bound immeasurably stronger to (Aβ1-40S26C)2. Unlike 6E10, mAb 3C6 did not bind to either full-length APP or C99 (SFig. 4), suggesting that 3C6 recognized an epitope that was both conformation- and sequence-dependent, with the conformation of the same sequence present in APP different from that in (Aβ1-40S26C)2.
MAb 3C6 specifically binds and neutralizes synaptic plasticity-disrupting AD brain-derived soluble Aβ
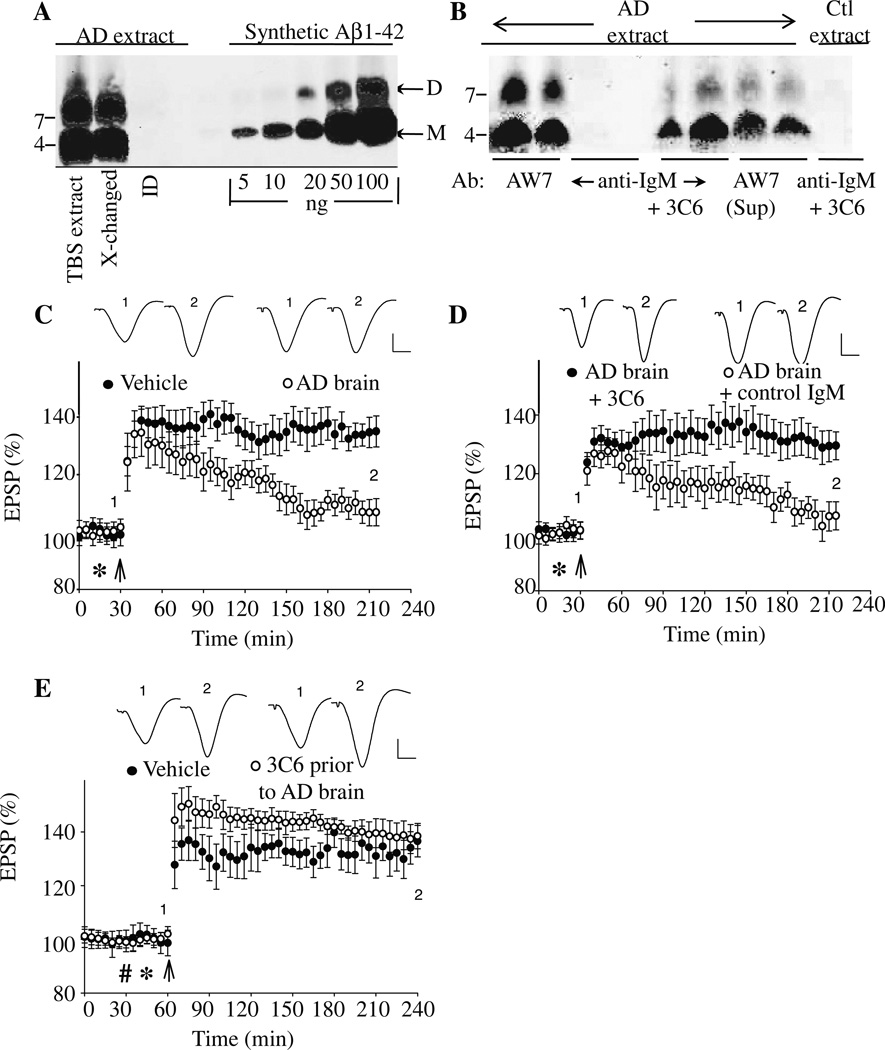
Having established 3C6 and 4B5 specific reactivities with synthetic Aβ assemblies, we determined whether one of these antibodies, 3C6, could bind to and neutralize the plasticity-disrupting activity of Aβ extracted from AD brain. We have previously shown that certain linear sequence-directed anti-Aβ antibodies can neutralize SDS-stable Aβ dimers (Klyubin et al., 2008), thus, we sought to determine if one of our conformer-specific antibodies, 3C6, could also ameliorate the inhibitory activity of Aβ. IP/Western blot analyses confirmed that mAb 3C6 specifically bound to a portion of Aβ present in AD brain TBS extract (Fig. 4A, B). However, when the supernatant from AD TBS extract IP’d with 3C6 was removed and then IP’d with AW7, additional Aβ monomer and dimer bands were detected, suggesting that only a portion of the total Aβ present in the extract was recognized by 3C6. Whether the Aβ that migrated on denaturing acrylamide gels actually existed in solution as monomer and dimer is uncertain since we have previously shown that Aβ derived from human brain elutes from SEC with a range of molecular weights, but that when subjected to SDS-PAGE only monomer and dimer are detected (Shankar et al., 2008). We take these results to mean that Aβ from the aqueous phase of human brain can exist in different sized assemblies, built up of Aβ monomer and/or SDS-stable Aβ dimer (for discussion see O’Nuallain et al., 2010, and Freir et al., 2011), and that mAb 3C6 does not recognize authentic Aβ monomer and dimer, but larger Aβ assemblies.
Figure 4. MAb 3C6 binds Aβ and ameliorates the block of LTP induced by AD brain extract.
A TBS extract of AD brain was examined by immunoprecipitation (IP)/Western blotting using the polyclonal anti-Aβ antibody, AW8, for IP and a combination of anti-Aβ mAbs, 2G3 and 21F12, for Western blotting (A). The first two lanes of the Western blot show that the untreated TBS extract and buffer-exchanged extract contained highly similar amounts of Aβ monomer and SDS-stable Aβ dimers. The third lane shows that the first round of IP had effectively depleted the extract of all detectable Aβ. Molecular weight standards are indicated on the left, and Aβ monomers (M) and dimers (D) labeled with arrows on the right. Based on standard synthetic Aβ1-42 included on the blot, the test samples contained 50 ng/ml and 70 ng/ml Aβ dimer and monomer, respectively (B). Western blot of Aβ IP’d from AD brain TBS extract by the anti-Aβ polyclonal IgG, AW7; anti-IgM alone, and mAb 3C6 are shown in duplicate in lanes 1–6. To determine if 3C6 bound all the Aβ present in the AD TBS extract, the material not IP’d by 3C6 was used for IP with AW7 (lanes 7+8). Similarly, to determine the specificity of the bands detected by 3C6, this antibody was also used to IP a TBS extract from a non-demented control (lane 9). The blot shows that both 3C6 and AW7, but not the control IgM, IP’d Aβ assemblies that migrated on polyacrylamide gels as monomers and SDS-stable dimers. (C) Intracerebroventricular (i.c.v.) injection (*) of AD TBS brain extract inhibited high frequency stimulation (HFS, arrow) induced LTP (107 ± 5 %, n = 4, baseline, p>0.05 compared with pre-HFS baseline, and p<0.05 compared with vehicle injected controls (135 ± 5 %, n = 8)). (D) Co-injection of 5 µg of 3C6 prevented the inhibition of LTP by AD TBS extract (130 ± 5 %, n = 5, compared p<0.05 compared with AD TBS alone, n = 5), whereas co-injection of a control IgMκ, M1520, did not (105± 5 %, p<0.05, n = 5, compared with AD TBS extract + 3C6, p>0.05 compared with AD TBS extract alone). (E) LTP was not impaired when 10 µg of 3C6 (hash symbol) was i.c.v. injected 15 min before AD TBS brain extract (138 ± 5 % (n= 4) baseline, p<0.05 compared with 136 ± 6 % (n = 5) vehicle injected controls). Insets show representative EPSP traces at the times indicated. Calibration bars: vertical, 1 mV; horizontal, 10 ms.
Acute i.c.v. injection of AD TBS extract (containing 50 ng/ml and 70 ng/ml Aβ dimer and monomer, respectively) into anesthetized rats completely inhibited hippocampal LTP (107 ± 5 % baseline, n= 4, at 3 h post-HFS, p>0.05 compared with pre-HFS baseline; p<0.05 compared with 135 ± 5 % for vehicle alone, n= 8, p<0.05) (Fig. 4C), but specific removal of all Aβ produced a TBS extract that was no longer capable of impairing LTP (131 ± 6, n = 6; p < 0.05 compared with baseline; p > 0.05 compared with vehicle injected controls at 3 h) (SFig. 5). Similarly, co-injection of 5 µg of mAb 3C6 abrogated the plasticity impairing effect of the AD TBS extract with robust LTP detected up to 3 h post-HFS (Fig. 4D; 130 ± 5 %, n= 5, p<0.05 compared with pre-HFS baseline or AD TBS extract alone). The beneficial effect of mAb 3C6 appeared to be related to its ability to bind and neutralize synaptotoxic Aβ since an unrelated IgM isotype-matched antibody, M1520 (8 µg), could not ameliorate the effect of the Aβ-containing AD TBS extract (105 ± 5 %, n= 5, p<0.05 compared with TBS extract + 3C6; p>0.05 compared with pre-HFS baseline or AD TBS extract alone).
Having established mAb 3C6’s ability to abrogate the synaptotoxic effect of soluble AD brain extract on rodent LTP when co-injected, we determined if the antibody had prophylactic properties. Panel E of Figure 4 shows that i.c.v. administration of mAb 3C6 15 min prior to AD TBS extract prevented impairment of LTP (138 ± 5 %, n = 4, p >0.05 compared with AD vehicle injected controls).
Discussion
Immunotherapy against Aβ is a promising approach for the treatment of AD, but the optimal assembly form of Aβ to target, and the window for therapy have yet to be defined (Gandy, 2010). An array of water-soluble non-fibrillar Aβ assemblies are thought to be pathogenic (Shankar and Walsh, 2009) and growing evidence suggests that SDS-stable Aβ dimer assemblies may be the basic building blocks of AD-associated synaptotoxicity (Vigo-Pelfrey et al., 1993; Roher et al., 1996; Mc Donald et al., 2010; O'Nuallain et al., 2010; Villemagne et al., 2010). Consequently, targeting dimers may deliver maximum therapeutic benefit and detection of dimers may prove useful for diagnosis of AD (Villemagne et al., 2010). In the absence of sufficient amounts of highly pure brain-derived Aβ dimers, we used synthetic disulfide cross-linked Aβ dimers to generate conformation-specific mAbs.
Two such antibodies, 3C6 and 4B5, bound robustly to aggregated (Aβ1-40S26C)2, when plate-immobilized or in solution (Table 3). In contrast, the same antibodies only reacted strongly with (Aβ1-40S26C)2 when plate-immobilized and not in solution. This difference may be due to epitope exposure on the dimeric peptide as a consequence of a subtle conformational change, aggregation, and/or an increase of binding avidity when the peptide is surface adsorbed (Giacomelli and Norde, 2005; O'Nuallain et al., 2008). Notably, Aβ dimer adsorption onto surfaces and the subsequent exposure of cryptic conformational epitopes may be physiologically relevant since in vivo Aβ is thought to be bound to proteins such as albumin (Biere et al., 1996), and the blood-borne dimeric peptide is purported to be exclusively present on cellular membranes (Villemagne et al., 2010).
Table 3. MAbs 3C6 and 4B5 binding to plate-immobilized and solution-phase amyloidogenic conformers.
NB, no binding, and ND, not determined
| Aβ Conformer | 3C6 | 4B5 | ||
|---|---|---|---|---|
| Plate | Solution | Plate | Solution | |
| (Aβ1-40S26C)2 monomers | X | X | X | X |
| (Aβ1-40S26C)2 dimers | √ | X | √ | X |
| Reduced (Aβ1-40S26C)2 dimers | X | ND | X | ND |
| (Aβ1-40S26C)2 protofibrils | √ | √ | √ | √ |
| WT Aβ1-40 monomers | X | X | X | X |
| Aβ1-40 fibrils | √ | √ | √ | √ |
| IAPP fibrils | NB | ND | NB | ND |
| Jto fibrils | NB | ND | NB | ND |
The inability of 3C6 and 4B5 to react with native Aβ dimers in solution may reflect the lack of ordered structure in the dimer and/or that the smallest Aβ assembly that the epitope is exposed on is aggregated dimers (i.e. tetramers and larger) (O'Nuallain et al., 2010). In contrast, strong antibody interactions with aggregated (Aβ1-40S26C)2 presumably reflect the exposure of conformational epitopes that are stabilized on these high-ordered assemblies (O'Nuallain et al., 2010). On first appearance, it seems surprising that antibodies generated against (Aβ1-40S26C)2 only bound to the conformer when aggregated or surface adsorbed. However, it is important to reiterate that a significant portion of (Aβ1-40S26C)2 that was used to generate the anti-Aβ dimer mAbs was adsorbed onto adjuvant. Also, since the sample had been frozen prior to use and freeze/thawing induces dimer aggregation (O'Nuallain et al., 2010), it is likely that at least a small portion of the immunogen existed as pre-fibrillar aggregates.
Given the enormous interest in Aβ-specific antibodies as therapeutic, diagnostic, and basic research reagents, numerous antibodies have been generated and these can be grouped into 4 classes based on their ability to recognize: (1) linear epitopes (Seubert et al., 1992; Solomon et al., 1996; Jensen et al., 2000), (2) conformational epitopes exposed on all aggregated Aβ species (Lambert et al., 2001; Lee et al., 2006; Englund et al., 2007; Lambert et al., 2007), (3) epitopes on only a subset of Aβ assemblies (Barghorn et al., 2005; Lambert et al., 2007; Lafaye et al., 2009; Hillen et al., 2010), or (4) generic amyloidogenic epitopes (O'Nuallain and Wetzel, 2002; Kayed et al., 2003). Antibodies from all four groups have shown therapeutic potential in animals of AD (Games et al., 2006; Gandy, 2010; Hillen et al., 2010) and results from our studies suggest that pre-clinical assessment of (Aβ1-40S26C)2 for active immunization is merited. Specifically, mAbs 3C6 and 4B5 recognize conformational epitopes on synthetic assemblies formed from Aβ dimers. Moreover, such antibodies that recognize epitopes conserved on pathogenic Aβ assemblies without binding to the non-pathogenic “useful” peptide should have a clear therapeutic advantage over antibodies that bind to all Aβ species. Antibody binding to conformational epitopes involves interactions with sequentially discontinuous segments that are close together in three-dimensional space (Barlow et al., 1986; O'Nuallain et al., 2007). Although, it is not yet known which regions of Aβ are involved in 3C6- and 4B5-binding, preliminary studies using plate-immobilized Aβ fragments indicated that a binding stretch for mAb 3C6 lies within residues 19–35 of the Aβ peptide (SFig. 6). Such recognition may at least partly account for 3C6’s specificity for Aβ aggregates. The fact that 3C6 bound immeasurably stronger to (Aβ1-40S26C)2 than any fragment peptide, and did not bind APP or C99, indicates that these conformational epitopes are cryptic in nature. Further insights into the molecular basis of such Aβ-antibody interactions may be gleaned using random peptide phage display-generated consensus sequences (O'Nuallain et al., 2007).
Like 3C6, the other two “dimer assembly-specific” antibodies identified in the current study were IgMs. Why we only obtained anti-dimer specific IgMs and not any IgGs is unclear, but given that the utility of IgMs is more limited than is the case for IgGs our results should be regarded as providing a proof of principle of the usefulness of covalent dimers as immunogens, rather than the identification of lead therapeutic or diagnostic agents. Nevertheless, in a recent study the anti-Aβ IgM antibody, L11.3, was shown to cross the blood brain barrier and to reverse Aβ-related learning impairments (Banks et al., 2007). The potential disadvantage of using an anti-Aβ IgM versus an IgG is that these molecules are bulky, have generally low affinity for their targets, and are inherently more polyreactive than IgGs (Notkins, 2004). In addition, IgMs are less useful for ELISA and immunoprecipitation assays. Thus the development of anti-Aβ IgG antibodies with similar specificity to 3C6 should yield agents more suited for diagnostic and therapeutic use.
The pan-Aβ aggregate specificity of 3C6 and its ability to neutralize toxic human brain-derived Aβ is akin to the anti-amyloidogenic activity of previously described antibodies (Lambert et al., 2001; Lee et al., 2006; Englund et al., 2007; Lambert et al., 2007). However, mAb 3C6 has demonstrated unique reactivity since: (1) it binds to a conformational epitope present on surface adsorbed Aβ dimers, solution-phase Aβ dimer aggregates, as well as fibrils and pre-fibrillar aggregates and (2) it is specific for Aβ but does not recognize APP or C99. Moreover, the antibody’s ability to abolish the synaptic plasticity disrupting effects of soluble AD brain-derived Aβ by binding to only a portion of the extracted peptide suggests that the IgM specifically targeted pathogenically relevant Aβ assemblies. Indeed we are not aware of any other conformation-specific antibodies that have been shown to bind and ameliorate the toxic effects of water-soluble AD brain-derived Aβ.
Despite progress in understanding the underlying disease mechanisms of AD, there remains an urgent need to develop methods for use in ante-mortem diagnosis. This is a problem, not only from a clinical standpoint, but also because it affects the integrity of clinical trials and epidemiological research. Thus the development of a simple test to aid clinical diagnosis of AD is of great importance and antibodies with specificity similar to 3C6 could be of significant utility. Indeed, insights into the molecular basis of the cryptic epitope(s) that these antibodies recognize could prove invaluable for the design of novel peptide immunogens and the development of improved second generation mAbs or identification of small molecules that specifically target synaptotoxic Aβ species.
Supplementary Material
Acknowledgements
We thank Teresa Fernández Zafra for initial antibody characterization, and Mark Lyons for 6E10 binding experiments. This work was supported by funding from the Health Research Board (Grant RP/2008/30 to DMW and MJR), Science Foundation Ireland (MJR) and the National Institutes of Health (grant IRO1AGO27443 to DMW).
Abbreviations
- AD
Alzheimer’s disease
- Aβ
amyloid β-protein
- BSA
bovine serum albumin
- PBS
phosphate-buffered saline
- ELISA
enzyme-linked immunoassay
- HFS
high frequency stimulation
- HPLC
high performance liquid chromatography
- i.c.v.
intracerebroventricular
- IAPP
islet amyloid polypeptide
- LTP
long-term potentiation
- SDS
sodium dodecyl sulfate
- ThT
Thioflavin T
- tPA
tissue plasminogen activator
- TBS
Tris-buffered saline
Footnotes
Conflict of interest: DMW is a consultant and a member of the scientific advisory board of Senexis, plc., a consultant for Merck Sharp and Dohme, and Eisai Inc. BO’N is a consultant for Baxter Innovations GmbH.
References
- Adekar SP, Klyubin I, Macy S, Rowan MJ, Solomon A, Dessain SK, O'Nuallain B. Inherent anti-amyloidogenic activity of human immunoglobulin gamma heavy chains. J Biol Chem. 2010;285:1066–1074. doi: 10.1074/jbc.M109.044321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer A. Über einen eigenartigen schweren Erkrankungsproze! der Hirnrinde. Neurologisches Centralblatt. 1906;23:1129–1136. [Google Scholar]
- Arancio O, Chao MV. Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol. 2007;17:325–330. doi: 10.1016/j.conb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE, Wolf KM, Geylis V, Steinitz M. Anti-amyloid beta protein antibody passage across the blood-brain barrier in the SAMP8 mouse model of Alzheimer's disease: an age-related selective uptake with reversal of learning impairment. Exp Neurol. 2007;206:248–256. doi: 10.1016/j.expneurol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hillen H. Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer's disease. J Neurochem. 2005;95:834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- Barlow DJ, Edwards MS, Thornton JM. Continuous and discontinuous protein antigenic determinants. Nature. 1986;322:747–748. doi: 10.1038/322747a0. [DOI] [PubMed] [Google Scholar]
- Biere AL, Ostaszewski B, Stimson ER, Hyman BT, Maggio JE, Selkoe DJ. Amyloid beta-peptide is transported on lipoproteins and albumin in human plasma. J Biol Chem. 1996;271:32916–32922. doi: 10.1074/jbc.271.51.32916. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, De Vos R, van den Oord JJ, Collen D, Mulligan RC. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Davies L, Wolska B, Hilbich C, Multhaup G, Martins R, Simms G, Beyreuther K, Masters CL. A4 amyloid protein deposition and the diagnosis of Alzheimer's disease: prevalence in aged brains determined by immunocytochemistry compared with conventional neuropathologic techniques. Neurology. 1988;38:1688–1693. doi: 10.1212/wnl.38.11.1688. [DOI] [PubMed] [Google Scholar]
- Englund H, Sehlin D, Johansson AS, Nilsson LN, Gellerfors P, Paulie S, Lannfelt L, Pettersson FE. Sensitive ELISA detection of amyloid-beta protofibrils in biological samples. J Neurochem. 2007;103:334–345. doi: 10.1111/j.1471-4159.2007.04759.x. [DOI] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freir DB, Nicoll AJ, Klyubin I, Panico S, Mc Donald JM, Risse E, Asante EA, Farrow MA, Sessions RB, Saibil HR, Clarke AR, Rowan MJ, Walsh DM, Collinge J. Interaction between prion protein and toxic amyloid beta assemblies can be therapeutically targeted at multiple sites. Nat Commun. 2011;2:336. doi: 10.1038/ncomms1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Buttini M, Kobayashi D, Schenk D, Seubert P. Mice as models: transgenic approaches and Alzheimer's disease. J Alzheimers Dis. 2006;9:133–149. doi: 10.3233/jad-2006-9s316. [DOI] [PubMed] [Google Scholar]
- Gandy S. Testing the amyloid hypothesis of Alzheimer's disease in vivo. Lancet Neurol. 2010;9:333–335. doi: 10.1016/S1474-4422(10)70055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli CE, Norde W. Conformational changes of the amyloid beta-peptide (1–40) adsorbed on solid surfaces. Macromol Biosci. 2005;5:401–407. doi: 10.1002/mabi.200400189. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Goldsteins G, Persson H, Andersson K, Olofsson A, Dacklin I, Edvinsson A, Saraiva MJ, Lundgren E. Exposure of cryptic epitopes on transthyretin only in amyloid and in amyloidogenic mutants. Proc Natl Acad Sci U S A. 1999;96:3108–3113. doi: 10.1073/pnas.96.6.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Hillen H, Barghorn S, Striebinger A, Labkovsky B, Muller R, Nimmrich V, Nolte MW, Perez-Cruz C, van der Auwera I, van Leuven F, van Gaalen M, Bespalov AY, Schoemaker H, Sullivan JP, Ebert U. Generation and therapeutic efficacy of highly oligomer-specific beta-amyloid antibodies. J Neurosci. 2010;30:10369–10379. doi: 10.1523/JNEUROSCI.5721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M, Hartmann T, Engvall B, Wang R, Uljon SN, Sennvik K, Naslund J, Muehlhauser F, Nordstedt C, Beyreuther K, Lannfelt L. Quantification of Alzheimer amyloid beta peptides ending at residues 40 and 42 by novel ELISA systems. Mol Med. 2000;6:291–302. [PMC free article] [PubMed] [Google Scholar]
- Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, Lieberburg I, Schenk D, Seubert P, McConlogue L. Amyloid precursor protein processing and A beta42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci U S A. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Khachaturian ZS. Diagnosis of Alzheimer's disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kidd M. Alzheimer's disease - An electron microscopical study. Brain. 1964;87:307–320. doi: 10.1093/brain/87.2.307. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, Lemere CA, Cullen WK, Peng Y, Wisniewski T, Selkoe DJ, Anwyl R, Walsh DM, Rowan MJ. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci. 2008;28:4231–4237. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Kok WM, Scanlon DB, Karas JA, Miles LA, Tew DJ, Parker MW, Barnham KJ, Hutton CA. Solid-phase synthesis of homodimeric peptides: preparation of covalently-linked dimers of amyloid beta peptide. Chem Commun (Camb) 2009:6228–6230. doi: 10.1039/b912784d. [DOI] [PubMed] [Google Scholar]
- Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wuthrich K, Oesch B. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature. 1997;390:74–77. doi: 10.1038/36337. [DOI] [PubMed] [Google Scholar]
- Kranenburg O, Bouma B, Kroon-Batenburg LM, Reijerkerk A, Wu YP, Voest EE, Gebbink MF. Tissue-type plasminogen activator is a multiligand cross-beta structure receptor. Curr Biol. 2002;12:1833–1839. doi: 10.1016/s0960-9822(02)01224-1. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, Ball MJ, Roher AE. Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem. 1996;271:4077–4081. doi: 10.1074/jbc.271.8.4077. [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaye P, Achour I, England P, Duyckaerts C, Rougeon F. Single-domain antibodies recognize selectively small oligomeric forms of amyloid beta, prevent Abeta-induced neurotoxicity and inhibit fibril formation. Mol Immunol. 2009;46:695–704. doi: 10.1016/j.molimm.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Viola KL, Chromy BA, Chang L, Morgan TE, Yu J, Venton DL, Krafft GA, Finch CE, Klein WL. Vaccination with soluble Abeta oligomers generates toxicity-neutralizing antibodies. J Neurochem. 2001;79:595–605. doi: 10.1046/j.1471-4159.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Velasco PT, Chang L, Viola KL, Fernandez S, Lacor PN, Khuon D, Gong Y, Bigio EH, Shaw P, De Felice FG, Krafft GA, Klein WL. Monoclonal antibodies that target pathological assemblies of Abeta. J Neurochem. 2007;100:23–35. doi: 10.1111/j.1471-4159.2006.04157.x. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, Lee VM. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol Chem. 2006;281:4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Linke RP, Zucker-Franklin D, Franklin ED. Morphologic, chemical, and immunologic studies of amyloid-like fibrils formed from Bence Jones Proteins by proteolysis. J Immunol. 1973;111:10–23. [PubMed] [Google Scholar]
- Martins IC, Kuperstein I, Wilkinson H, Maes E, Vanbrabant M, Jonckheere W, Van Gelder P, Hartmann D, D'Hooge R, De Strooper B, Schymkowitz J, Rousseau F. Lipids revert inert Abeta amyloid fibrils to neurotoxic protofibrils that affect learning in mice. EMBO J. 2008;27:224–233. doi: 10.1038/sj.emboj.7601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Donald JM, Savva GM, Brayne C, Welzel AT, Forster G, Shankar GM, Selkoe DJ, Ince PG, Walsh DM. The presence of sodium dodecyl sulphate-stable Abeta dimers is strongly associated with Alzheimer-type dementia. Brain. 2010;133:1328–1341. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchor JP, Pawlak R, Strickland S. The tissue plasminogen activator-plasminogen proteolytic cascade accelerates amyloid-beta (Abeta) degradation and inhibits Abeta-induced neurodegeneration. J Neurosci. 2003;23:8867–8871. doi: 10.1523/JNEUROSCI.23-26-08867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi A, et al. Isolation and characterization of patient-derived, toxic, high mass amyloid beta-protein (Abeta) assembly from Alzheimer disease brains. J Biol Chem. 2009;284:32895–32905. doi: 10.1074/jbc.M109.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkins AL. Polyreactivity of antibody molecules. Trends in immunology. 2004;25:174–179. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- O'Nuallain B, Wetzel R. Conformational Abs recognizing a generic amyloid fibril epitope. Proc Natl Acad Sci USA. 2002;99:1485–1490. doi: 10.1073/pnas.022662599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Nuallain B, Hrncic R, Wall JS, Weiss DT, Solomon A. Diagnostic and therapeutic potential of amyloid-reactive IgG antibodies contained in human sera. J Immunol. 2006;176:7071–7078. doi: 10.4049/jimmunol.176.11.7071. [DOI] [PubMed] [Google Scholar]
- O'Nuallain B, Allen A, Ataman D, Weiss DT, Solomon A, Wall JS. Phage display and peptide mapping of an immunoglobulin light chain fibril-related conformational epitope. Biochemistry. 2007;46:13049–13058. doi: 10.1021/bi701255m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Nuallain B, Freir DB, Nicoll AJ, Risse E, Ferguson N, Herron CE, Collinge J, Walsh DM. Amyloid beta-protein dimers rapidly form stable synaptotoxic protofibrils. J Neurosci. 2010;30:14411–14419. doi: 10.1523/JNEUROSCI.3537-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Nuallain B, Acero L, Williams AD, Koeppen HP, Weber A, Schwarz HP, Wall JS, Weiss DT, Solomon A. Human plasma contains cross-reactive Abeta conformer-specific IgG antibodies. Biochemistry. 2008;47:12254–12256. doi: 10.1021/bi801767k. [DOI] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Roher AE, Chaney MO, Kuo YM, Webster SD, Stine WB, Haverkamp LJ, Woods AS, Cotter RJ, Tuohy JM, Krafft GA, Bonnell BS, Emmerling MR. Morphology and toxicity of Abeta-(1–42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer's disease. J Biol Chem. 1996;271:20631–20635. doi: 10.1074/jbc.271.34.20631. [DOI] [PubMed] [Google Scholar]
- Sandberg A, Luheshi LM, Sollvander S, Pereira de Barros T, Macao B, Knowles TP, Biverstal H, Lendel C, Ekholm-Petterson F, Dubnovitsky A, Lannfelt L, Dobson CM, Hard T. Stabilization of neurotoxic Alzheimer amyloid-{beta} oligomers by protein engineering. Proc Natl Acad Sci USA. 2010 doi: 10.1073/pnas.1001740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Schenk D. Amyloid-beta immunotherapy for Alzheimer's disease: the end of the beginning. Nat Rev Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Walsh DM. Alzheimer's disease: synaptic dysfunction and Abeta. Mol Neurodegener. 2009;4:48. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Welzel AT, McDonald JM, Selkoe DJ, Walsh DM. Isolation of low-n amyloid beta-protein oligomers from cultured cells, CSF, and brain. Methods Mol Biol. 2011;670:33–44. doi: 10.1007/978-1-60761-744-0_3. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li SM, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Solomon B, Koppel R, Hanan E, Katzav T. Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer beta-amyloid peptide. Proc Natl Acad Sci USA. 1996;93:452–455. doi: 10.1073/pnas.93.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani M. Protein misfolding and aggregation: new examples in medicine and biology of the dark side of the protein world. Biochim Biophys Acta. 2004;1739:5–25. doi: 10.1016/j.bbadis.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Vigo-Pelfrey C, Lee D, Keim P, Lieberburg I, Schenk DB. Characterization of beta-amyloid peptide from human cerebrospinal fluid. J Neurochem. 1993;61:1965–1968. doi: 10.1111/j.1471-4159.1993.tb09841.x. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Perez KA, Pike KE, Kok WM, Rowe CC, White AR, Bourgeat P, Salvado O, Bedo J, Hutton CA, Faux NG, Masters CL, Barnham KJ. Blood-borne amyloid-beta dimer correlates with clinical markers of Alzheimer's disease. J Neurosci. 2010;30:6315–6322. doi: 10.1523/JNEUROSCI.5180-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmers HP, Wozniak E, Stepien-Botsch E, Zimmermann U, Muller-Hermelink HK. A rapid method for purification of monoclonal human IgM from mass culture. Hum Antibodies Hybridomas. 1996;7:37–41. [PubMed] [Google Scholar]
- Wall J, Schell M, Murphy C, Hrncic R, Stevens FJ, Solomon A. Thermodynamic instability of human lambda 6 light chains: correlation with fibrillogenicity. Biochemistry. 1999;38:14101–14108. doi: 10.1021/bi991131j. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J Biol Chem. 1997;272:22364–22372. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Fadeeva JV, LaVoie MJ, Paliga K, Eggert S, Kimberly WT, Wasco W, Selkoe DJ. gamma-Secretase cleavage and binding to FE65 regulate the nuclear translocation of the intracellular C-terminal domain (ICD) of the APP family of proteins. Biochemistry. 2003;42:6664–6673. doi: 10.1021/bi027375c. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999;274:25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- Westermark P, Benson MD, Buxbaum JN, Cohen AS, Frangione B, Ikeda S, Masters CL, Merlini G, Saraiva MJ, Sipe JD. Amyloid: toward terminology clarification. Report from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2005;12:1–4. doi: 10.1080/13506120500032196. [DOI] [PubMed] [Google Scholar]
- Yokoyama WM, Christensen M, Dos Santos G, Miller D. Curremt Protocols in Immunology. John Wiley & Sons, Inc.; 2006. Production of Monoclonal Antibodies; pp. 2.5.1–2.5.25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.