Abstract
The relationship between intramyocellular (IMCL) and extramyocellular lipid (EMCL) accumulation and skeletal muscle insulin resistance is complex and dynamic. We examined the effect of a short-term (7-day) low-glycemic index (LGI) diet and aerobic exercise training intervention (EX) on IMCL and insulin sensitivity in older, insulin-resistant humans. Participants (66 ± 1 yr, BMI 33 ± 1 kg/m2) were randomly assigned to a parallel, controlled feeding trial [either an LGI (LGI/EX, n = 7) or high GI (HGI/EX, n = 8) eucaloric diet] combined with supervised exercise (60 min/day, 85% HRmax). Insulin sensitivity was determined via 40 mU·m−2·min−1 hyperinsulinemic euglycemic clamp and soleus IMCL and EMCL content was assessed by 1H-MR spectroscopy with correction for fiber orientation. BMI decreased (kg/m2 −0.6 ± 0.2, LGI/EX; −0.7 ± 0.2, HGI/EX P < 0.0004) after both interventions with no interaction effect of diet composition. Clamp-derived insulin sensitivity increased by 0.91 ± 0.21 (LGI/EX) and 0.17 ± 0.55 mg·kg−1·min−1 (HGI/EX), P = 0.04 (effect of time). HOMA-IR was reduced by −1.1 ± 0.4 (LGI/EX) and −0.1 ± 0.2 (HGI/EX), P = 0.007 (effect of time), P = 0.02 (time × trial). Although both interventions increased IMCL content, (Δ: 2.3 ± 1.3, LGI/EX; 1.4 ± 0.9, HGI/EX, P = 0.03), diet composition did not significantly effect the increase. However, the LGI/EX group showed a robust increase in the [IMCL]/[EMCL] ratio compared with the HGI/EX group (Δ: 0.5 ± 0.2 LGI/EX vs. 0.07 ± 0.1, P = 0.03). The LGI/EX group also demonstrated greater reductions in [EMCL] than the HGI/EX group (Δ: −5.8 ± 3.4, LGI/EX; 2.3 ± 1.1, HGI/EX, P = 0.03). Changes in muscle lipids and insulin sensitivity were not correlated; however, the change in [IMCL]/[EMCL] was negatively associated with the change in FPI (r = −0.78, P = 0.002) and HOMA-IR (r = −0.61, P = 0.03). These data suggest that increases in the IMCL pool following a low glycemic diet and exercise intervention may represent lipid repartitioning from EMCL. The lower systemic glucose levels that prevail while eating a low glycemic diet may promote redistribution of lipid stores in the muscle.
Keywords: insulin resistance, obesity, aging, substrate utilization
continuous oversupply of lipid to skeletal muscle in the absence of regular physical activity and lipid oxidation results in excess accumulation of intramyocellular lipids (IMCL). This increased IMCL content is associated with insulin resistance and may be an early step in the development of type 2 diabetes when the level of physical activity is not a confounding factor (7, 9, 10, 20). In obese, insulin-resistant individuals, weight loss is known to increase insulin sensitivity and is accompanied by a decrease in IMCL content (31). In contrast, chronic endurance exercise training leads to both increased insulin sensitivity and increased stores of IMCL in lean, physically active individuals. This apparent paradox has been well described in the literature (1, 8, 9) and challenges the pathophysiological role of IMCL in the development of insulin resistance. Furthermore, the relationship between EMCL and insulin sensitivity is equivocal (5, 28), and the metabolic role of EMCL has not been well studied (21).
Long-term lifestyle intervention studies in insulin-resistant and diabetic subjects have consistently demonstrated a decrease in IMCL content with concomitant increases in insulin sensitivity following the intervention period (27). However, there is increasing evidence that IMCL content is extremely dynamic and responsive to both acute bouts of exercise and short-term training regimens. In lean, sedentary individuals, an early response to short-term training includes increased IMCL content and downregulation of acetyl-CoA carboxylase-2 (ACC2) gene expression, suggesting that increased IMCL content is tied to improved oxidative capacity (24–26).
We (13) have previously shown that short-term exercise training markedly improves peripheral insulin sensitivity in obese patients with type 2 diabetes. The changes in insulin action following the short-term intervention were found to be in the absence of weight loss and fat mass. These data highlight that the mechanism of improved insulin action following short-term exercise training is perhaps the result of changes in muscle substrate utilization (14).
Dietary interventions such as low-glycemic diets also have favorable effects on improving insulin sensitivity and reducing IMCL content in obese subjects (32, 35). However, the effect of lifestyle intervention strategies that combine dietary modification and aerobic exercise training on insulin sensitivity and IMCL content in populations at risk for development of type 2 diabetes such as older obese men and women with impaired glucose tolerance is not known. The purpose of this investigation was to assess the effects of a short-term diet and exercise intervention on insulin sensitivity and ICML in older obese adults and to determine the differential effects of a low-glycemic index (LGI) vs. a high-glycemic index (HGI) diet on these outcomes. We hypothesized that an LGI diet/exercise (LGI/EX) intervention would alter IMCL to a greater extent than an HGI diet/exercise (HGI/EX) intervention. We employed proton magnetic resonance spectroscopy (1H-MRS) to quantify IMCL content and used a novel fiber orientation modeling (FOM) approach to correct for bulk magnetic susceptibility and orientation of lipid strands in the extramyocellular compartment of muscle (12).
METHODS AND MATERIALS
Subjects.
Fifteen older obese sedentary volunteers (age 66 ± 1 Yr, BMI 33 ± 1 kg/m2, means ± SE) were recruited from the local population to undergo a 7-day exercise training and diet intervention. All volunteers underwent comprehensive medical screening prior to participation. Baseline subject characteristics are presented in Table 1. The study was approved by the Institutional Review Board of the Cleveland Clinic, and all subjects provided written informed consent.
Table 1.
Baseline characteristics
| LGI/EX (n = 7) | HGI/Ex (n = 8) | |
|---|---|---|
| Sex (F/M) | 4/3 | 5/3 |
| Age, yr | 68 ± 1, 61–68 | 61 ± 1, 64–75* |
| Weight, kg | 90.7 ± 6.5 | 89.7 ± 6.7 |
| BMI, kg/m2 | 32.6 ± 1.1 | 33.4 ± 1.6 |
| FM, kg | 38.8 ± 1.8 | 38.6 ± 2.8 |
| FFM, kg | 52.3 ± 5.5 | 51.0 ± 2.9 |
| FPG, mg/dl | 109.0 ± 7.5 | 97.5 ± 4.3 |
| FPI, μU/ml | 16.7 ± 1.6 | 12.1 ± 1.3 |
| Total Chol, mg/dl | 209.9 ± 15.4 | 212.9 ± 9.9 |
| HDL Chol, mg/dl | 50.7 ± 4.5 | 57.3 ± 7.4 |
| LDL Chol, mg/dl | 119.3 ± 10.5 | 133.1 ± 8.5 |
| Hb A1c, % | 5.8 ± 0.4 | 5.7 ± 0.1 |
| Vo2max, ml·kg–1·min–1 | 23.8 ± 1.1 | 22.8 ± 1.8 |
Data represent means ± SE (minimum–maximum). LGI, low-glycemic index; HGI, high-glycemic index; EX, exercise intervention; GI, glycemic index; BMI, body mass index; FM, whole body fat mass; FFM, fat-free mass; FPG, fasting plasma glucose; FPI, fasting plasma insulin; Total Chol, total cholesterol; HDL, LDL, high- and low-density lipoprotein cholesterol; Hb A1c, hemoglobin A1c; Vo2max, Maximal oxygen uptake during exhaustive exercise.
Significance P < 0.05.
Intervention.
Participants were randomized to receive either an LGI (n = 7) or an HGI (n = 8) diet for the duration of the study. In addition, all participants undertook 60-min of fully supervised aerobic exercise [EX; treadmill walking and cycle ergometry at ∼80–85% of maximum heart rate (HRmax)] each day, for 7 consecutive days, as previously described (13, 30). Caloric needs were determined by indirect calorimetry and assessment of physical activity status. All meals for the duration of the study were provided to the participants. Dietary macronutrient and fiber composition was matched between groups, and compliance was ensured via food container weigh-backs and counseling by the study dietitian. Pre- and postintervention measures were controlled during a three day in-patient stay in the Clinical Research Unit at the Cleveland Clinic.
Clinical assessments.
Body composition was measured by dual-energy X-ray absorptiometry (model iDXA; Lunar, Madison, WI) and was used to determine whole body fat mass. Aerobic fitness and cardiovascular measurements were performed using an incremental graded treadmill exercise test to determine maximal oxygen consumption (V̇o2 max). Insulin sensitivity measurements were obtained after an overnight fast by use of the euglycemic-hyperinsulinemic clamp (90 mg/dl, 40 mU·m−2·min−1). Insulin-stimulated glucose disposal rate (GDR, mg·kg−1·min−1) was determined as described previously (29, 30). Indirect calorimetry (Vmax Encore; Viasys, Yorba Linda, CA) measures were also performed prior to (basal) and during the final 30 min of the clamp procedure for determination of substrate metabolism (30). Homeostasis model assessment of insilin resistance (HOMA-IR) was also used as a marker of hepatic insulin resistance (18).
IMCL and EMCL assessments.
Magnetic resonance spectroscopy (MRS) was used to quantify lipid levels in the soleus (slow-twitch) muscle of each subject before and after the exercise intervention. The right calf of each subject was positioned in a knee coil and positioned near isocenter within a 4T Bruker Medspec MRI scanner. Following manual shimming to improve the spectral resolution of the acquisition [water linewidth (FWHM) ≤ 50 Hz (∼0.3 ppm)], a single-voxel Point-Resolved Spectroscopy (PRESS) acquisition (TR/TE = 1,500/135 ms, 128 averages, voxel size = 1 cm3, water suppression, ADC BW = 10.5 ppm) was used to acquire the MRS data. Repeatability of the voxel placement following the intervention period was facilitated with anatomic landmarks. Following the acquisition, all spectra were reconstructed and exported to Matlab (The Mathworks, Natick, MA) for quantitative analysis of the lipid peaks. Fiber orientation modeling (FOM), previously described by Khuu et al. (12), was then applied to the MRS spectra. In that seminal research, Khuu et al. demonstrated that variation in the orientation of the muscle fibers away from the main magnetic field (B0) resulted in shifts and broadening of the EMCL resonance. Thus, conventional MRS analysis (i.e., no FOM) can result in overestimation of the [IMCL]/[EMCL] ratio (12). When FOM is applied, the effect of muscle fiber orientation on [IMCL]/[EMCL] is largely mitigated. Our pilot studies (data not shown) confirmed the variation in the chemical shift and overlap of EMCL and IMCL resonances when the position of the muscle fibers deviated from B0. For the studies presented here, FOM analysis was used to compensate for these shifts and broadening to resolve and accurately measure the IMCL and EMCL peaks in human muscle (6, 12, 33). The IMCL and EMCL methylene and methyl residues were calculated, and the relative concentrations of the IMCL and EMCL triglycerides was derived using the creatine signal as a concentration standard, assuming that the skeletal muscle creatine concentration is approximately equal to 30 mmol/kg muscle wet wt (3, 4). This investigation is the first to apply the FOM analysis of muscle lipids with an exercise and diet lifestyle intervention.
Statistics.
Between-group (LGI/EX vs. HGI/EX) comparisons were analyzed using two-way (group × time) repeated-measures ANOVA, and Bonferroni post hoc tests were applied to significant group × time interactions. Baseline values for each variable were compared between groups by using unpaired t-tests. In the event of a significant t-statistic, baseline values were used as a covariate in the two-way repeated-measures ANOVA. Between-group changes (Δ) with time were also compared using one-way ANOVA and Fisher's LSD post hoc tests. In addition, univariate correlation analyses were used to identify relationships between changes (Δ) in variables. Statistical significance was accepted when P < 0.05. Analyses were carried out using StatView for Windows 5.0.1 (SAS Institute, Cary, NC), and all data are expressed as means ± SE.
RESULTS
Dietary intake and exercise training.
The LGI/EX and HGI/EX groups consumed diets that were similar in caloric and macronutrient content; however, the GI value of the diets was designed to be significantly different between groups. These data are consistent with our previous reports, and details of the dietary composition have been presented elsewhere (30).
Subject characteristics.
This short-term diet and exercise intervention resulted in a small but significant weight loss (kg: −1.7 ± 0.6, LGI/EX; −1.9 ± 0.6, HGI/EX, P < 0.0005 for time) and decreased BMI (kg/m2: −0.6 ± 0.2, LGI/EX; −0.7 ± 0.2, HGI/EX, P < 0.0004 for time). However, the ANOVA showed no group or group × time interactions for changes in body composition. In addition, fasting plasma insulin (FPI) was reduced (μU/ml: −3.8 ± 1.6, LGI/EX; −0.3 ± 0.8, HGI/EX, P < 0.02 time × trial), whereas V̇o2 max was increased (ml·kg−1·min−1: 1.2 ± 0.7, LGI/EX; 0.5 ± 0.4, HGI/EX, P < 0.04, time × trial). This short lifestyle intervention program had no effect on total cholesterol or cholesterol subfractions.
Insulin sensitivity.
Peripheral tissue insulin sensitivity was assessed by the hyperinsulinemic-euglycemic clamp technique. Plasma glucose was maintained at 88.1 ± 1.0 mg/dl preintervention and 87.3 ± 1.0 mg/dl postintervention with coefficients of variation of 6.2 ± 1.1 and 4.6 ± 0.6%, respectively. Insulin-stimulated GDR was increased by 0.91 ± 0.21 and 0.17 ± 0.55 mg·kg−1·min−1 for the LGI/EX and HGI/EX groups, respectively (P = 0.04, effect of time). The LGI/EX program had no additional effect on improving peripheral insulin sensitivity. We also calculated HOMA-IR, a widely used index of hepatic glucose production during fasting. HOMA-IR was reduced by 1.1 ± 0.4 in the LGI/EX and 0.1 ± 0.2 in the HGI/EX group (P = 0.02, time × trial).
Skeletal muscle lipids.
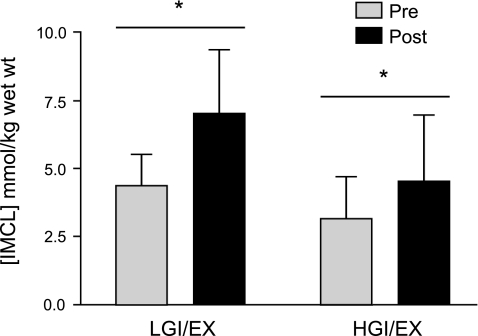
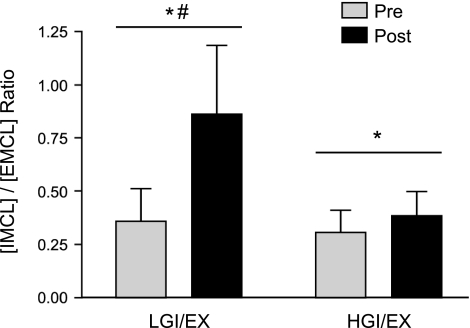
The intervention resulted in significant increases in [IMCL] (Δ, mmol/kg muscle wet wt: +2.3 ± 1.3, LGI/EX; +1.4 ± 0.9, HGI/EX), and no additional effect of diet was observed on [IMCL] (Fig. 1). However, in the LGI/EX group, the [IMCL]/[EMCL] ratio showed a robust increase above the HGI/EX condition (Δ: +0.5 ± 0.2 LGI/EX vs. 0.07 ± 0.1, P = 0.03; Fig. 2.). The LGI/EX group also demonstrated significantly greater reductions in [EMCL] compared with the HGI/EX group (LGI/EX Pre: 15.4 ± 3.9, LGI/EX Post: 9.6 ± 1.8 mmol/kg wet wt, Δ: −5.8 ± 3.4, HGI/EX Pre: 9.4 ± 1.1, HGI/EX Post: 11.7 ± 1.8 mmol/kg wet wt, Δ: 2.3 ± 1.1; P = 0.03, time × trial). No main effects were seen for muscle creatine values.
Fig. 1.
Intramyocellular lipid concentrations [IMCL] were found to increase following the 7-day diet and exercise intervention (EX) period. *Significant main effect of time, P < 0.05. No main effect of trial (LGI/EX vs. HGI/EX) or interaction was observed, P > 0.05.
Fig. 2.
Intramyocellular lipid to extramyocellular lipid ratios [IMCL]/[EMCL] were found to increase following the 7-day diet and exercise intervention period in both groups. *Significant main effect of time, P < 0.05; #significant time × trial interaction, P < 0.05. There was no main effect of trial (LGI/EX vs. HGI/EX), P > 0.05.
Correlations.
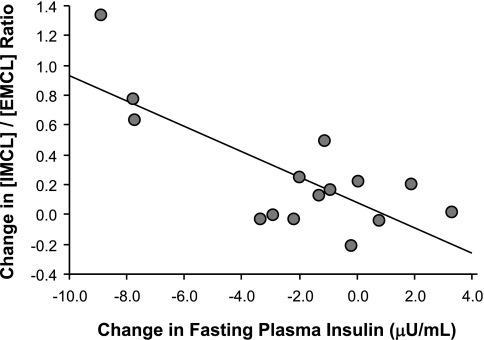
The relationship between the degree of insulin resistance and [IMCL] was explored at baseline and revealed no significant associations. Changes in the primary outcome measures of IMCL and EMCL were also explored relative to changes in insulin sensitivity and other plausible metabolic variables. No relationships were found between changes in muscle lipids and insulin sensitivity. However, the change in the [IMCL]/[EMCL] ratio was inversely correlated with the change in FPI (r = −0.76, P = 0.001; Fig. 3). This relationship ([IMCL]/[EMCL]) was extrapolated to a significant finding relative to the change in HOMA-IR (r = −0.60, P = 0.02).
Fig. 3.
The change in [IMCL]/[EMCL] ratio was negatively correlated with the change in fasting plasma insulin; r = −0.76, P = 0.001.
DISCUSSION
We found that a short-term diet and exercise program consisting of 7 consecutive days of aerobic exercise and a controlled LGI diet increased both insulin sensitivity and IMCL while reducing EMCL and fasting insulin in an older obese cohort. This study is the first to demonstrate that the combination of an LGI diet and exercise intervention increase IMCL stores while decreasing EMCL. In addition, this study is the first to apply fiber orientation modeling to muscle MR spectroscopy data following a lifestyle intervention.
The short-term diet and exercise program was effective in increasing whole body glucose disposal (mg·kg−1·min−1) by ∼40%, independently of the glycemic index of the diet. These data are consistent with our previous reports of short-term aerobic training, which demonstrated increased insulin responsiveness in obese diabetic subjects (13). Additionally, we have observed similar improvements in insulin sensitivity in a separate cohort of older obese men and women following the identical lifestyle intervention presented here (30). These data collectively demonstrate that peripheral insulin sensitivity (mainly represented by the skeletal muscle) is not acutely affected by the glycemic index of the diet, but rather by the repeated contractile stimulus induced by aerobic exercise. Numerous studies have demonstrated that short-term aerobic exercise training increases both GLUT4 mRNA and protein expression in human muscle, thus leading to increased insulin action and glycogen storage capability (11, 14, 34, 38). When either an HGI or LGI diet was added to the postexercise recovery period (12 h), there was no difference in the exercise-induced reduction of muscle glycogen, which is one of the primary factors that influence insulin sensitivity (32).
The exact mechanistic link between insulin sensitivity and IMCL has yet to be elucidated. Numerous reports have linked increased IMCL content to an increased severity of insulin resistance in type 2 diabetics; yet, restoration of mitochondrial function and increased IMCL content with exercise training in type 2 diabetics is purported to be a major factor in improving insulin sensitivity (19). In addition, consuming an HGI diet during recovery from exercise has been shown to reduce nonesterified fatty acid (NEFA) availability and increase reliance on IMCL during moderate-intensity exercise. Our data show that 1 wk of a combined LGI diet with aerobic training increases both IMCL stores and insulin sensitivity while decreasing EMCL stores in older obese men and women with impaired glucose tolerance. These findings are consistent with reports that increased IMCL content is an early response to aerobic training (25) and following acute exercise (22). Although the underlying physiology is unclear, evidence points to changes in lipid oxidation and mobilization mechanisms to support the bioenergetic demands of working muscle. Recently, ACC was implicated in driving IMCL storage, as both ACC mRNA and protein expression were elevated following short-term exercise training (24). It is still unclear whether increases in IMCL are clinically relevant, but this observation suggests that the changes in lipid utilization and storage that are occurring may be favorable to increased fatty acid oxidation. The long-term adaptations of an LGI diet and exercise intervention on IMCL content, muscle metabolism, and insulin sensitivity remain to be determined.
EMCL lipid stores of the soleus muscle are significantly increased in obese individuals compared with nonobese, but only IMCL has been correlated with insulin resistance (15, 28). EMCL stores are adipocytes located between muscle cells but within the macrostructure of the muscle architecture. Surprisingly, previous MR spectroscopy studies of the muscle have not discussed the importance of EMCL stores, and data for EMCL go largely unreported. Furthermore, the metabolic role of EMCL in the muscle is generally unknown and the physiological role of EMCL during muscle contraction has not been fully investigated (21). The differential effect of increasing IMCL while decreasing EMCL seen herein is a novel observation. The decreases in the EMCL depot may be a positive adaptation to exercise training and are reflective of the overall reduction in body adiposity. This observation gives rise to the question of whether loss of EMCL (adiposity) or IMCL is directly responsible for the improvements in insulin sensitivity.
We observed a significant negative association between the changes in [IMCL]/[EMCL] and changes in FPI. These data suggest that insulin may be playing a role in lipid redistribution from the EMCL compartment to the IMCL. It is well known that insulin action promotes lipid storage through actions on ACC and malonyl-CoA, thus driving triglyceride synthesis. The decreases in FPI are mainly reflective of the reduced plasma glucose response from the LGI diets. It is well documented that LGI diets elicit a lower insulin response to a given carbohydrate load than HGI diets (17). Alternatively, the HGI diet may have elicited increased insulin levels throughout the day, but this was not reflected in the fasting plasma insulin levels demonstrated herein. Although this response does not appear to affect insulin sensitivity, it has been shown to markedly influence insulin secretion rates and suppress circulating NEFA (17). In the face of repeated exercise bouts, reduced NEFA will result in reduced IMCL availability and perhaps reduced IMCL content (36). Indeed, individuals with greater fasting serum insulin also had higher fasting levels of IMCL, and during hyperinsulinemia demonstrate greater serum free fatty acid concentrations (37).
MR spectroscopy is a noninvasive method that has been widely used to study lipid proton signatures in tissues such as muscle, liver, pancreas, and heart. The original concept suggested by Schick et al. (23) described the IMCL pool as spherical droplets whereas the EMCL pool is linear and parallel to the muscle fiber. These differences allowed for a ∼0.2-ppm shift in the resonance frequency, thus allowing the two pools of lipids to be differentiated. However, successful separation between the IMCL and EMCL methylene resonances are largely dependent on the parallel alignment of the muscle fibers with the main magnetic field (B0). In a muscle such as the tibialis anterior, lipid signal resolution is excellent because the fibers are longitudinal and parallel to the magnetic field. However, in this study, the soleus muscle was studied. The soleus has a bipennate fiber orientation, and the IMCL and EMCL signals begin to overlap due to magnetic susceptibility differences. Numerous approaches have been suggested to account for this phenomenon (6, 33), including mathematical modeling as described by Khuu et al. (12). We chose to employ the novel modeling approach of Khuu and colleagues known as fiber orientation modeling (FOM) to correct for the bulk magnetic susceptibility and orientation of lipid strands in the extramyocellular compartment of the muscle (12). This work elegantly describes how [IMCL]/[EMCL] is overestimated with traditional fitting algorithms such as Gaussian or Voigt line shapes as the angular dispersion from B0 increases. When FOM is applied, changes in dispersion angle had no affect on [IMCL]/[EMCL]. This accepted method effectively accounts for the quantification pitfalls in muscles such as the soleus, leading to more accurate IMCL and EMCL quantification.
This trial investigated 15 individuals, and we acknowledge that the data may be underpowered for some variables. In addition, the challenges in measuring IMCL and EMCL via MR spectroscopy cannot be overlooked and have been extensively reviewed elsewhere (2). Furthermore, the relationship between muscle lipid content and insulin resistance is further clouded by reports that age, sex, and ethnicity are the primary factors for increased IMCL stores (16). While we acknowledge that these variables may have an effect on both muscle lipid content and insulin sensitivity, this study was not designed to specifically address these subgroups. While our study has limitations, we have used a novel application of fiber orientation modeling for MR spectroscopy in human metabolic research and generated novel data related to changes in EMCL that potentially have clinically relevant implications.
In summary, these data suggest that increases in the IMCL pool following a low-glycemic diet and exercise intervention may represent lipid repartitioning from EMCL. The lower systemic glucose levels that prevail while eating a low-glycemic diet, and thus lowering circulating insulin, may promote redistribution of lipid stores in the muscle. Future work will address the long-term adaptations of a low-glycemic index diet and exercise intervention on IMCL content, substrate oxidation, and changes in insulin sensitivity.
GRANTS
This research was supported by National Institutes of Health grants R01 AG-12834 (J. P. Kirwan) and T32 HL-007887 and was supported in part by the National Institutes of Health, National Center for Research Resources, CTSA 1UL1 RR-024989, Cleveland, Ohio.
DISCLOSURES
No conflicts of interest, financial or otherwise, are reported by the author(s).
ACKNOWLEDGMENTS
We thank Drs. Craig Malloy and Anthony Khuu of the Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, Texas, for use of the Fiber Orientation Model. In addition, we thank the research volunteers for their outstanding dedication and effort, and the staff of the Clinical Research Unit and the technical staff and students who helped with the implementation of the study and assisted with data collection. We also acknowledge our clinical research coordinator, Julianne Filion RN, for excellent nursing and organizational assistance.
REFERENCES
- 1. Amati F, Dube JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care 32: 1547–1549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boesch C, Machann J, Vermathen P, Schick F. Role of proton MR for the study of muscle lipid metabolism. NMR Biomed 19: 968–988, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Boesch C, Slotboom J, Hoppeler H, Kreis R. In vivo determination of intra-myocellular lipids in human muscle by means of localized 1H-MR-spectroscopy. Magn Reson Med 37: 484–493, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Bottomley PA, Lee Y, Weiss RG. Total creatine in muscle: imaging and quantification with proton MR spectroscopy. Radiology 204: 403–410, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Brechtel K, Machann J, Jacob S, Strempfer A, Schick F, Haring HU, Claussen CD. [In-vivo 1H-MR spectroscopy: the determination of the intra- and extramyocellular lipid content depending on the insulin effect in the direct offspring of type-2 diabetics]. ROFO 171: 113–120, 1999 [PubMed] [Google Scholar]
- 6. Cui MH, Hwang JH, Tomuta V, Dong Z, Stein DT. Cross contamination of intramyocellular lipid signals through loss of bulk magnetic susceptibility effect differences in human muscle using (1)H-MRSI at 4 T. J Appl Physiol 103: 1290–1298, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Dube J, Goodpaster BH. Assessment of intramuscular triglycerides: contribution to metabolic abnormalities. Curr Opin Clin Nutr Metab Care 9: 553–559, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab 294: E882–E888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Goodpaster BH, Kelley DE. Skeletal muscle triglyceride: marker or mediator of obesity-induced insulin resistance in type 2 diabetes mellitus? Curr Diab Rep 2: 216–222, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Houmard JA, Shaw CD, Hickey MS, Tanner CJ. Effect of short-term exercise training on insulin-stimulated PI 3-kinase activity in human skeletal muscle. Am J Physiol Endocrinol Metab 277: E1055–E1060, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Khuu A, Ren J, Dimitrov I, Woessner D, Murdoch J, Sherry AD, Malloy CR. Orientation of lipid strands in the extracellular compartment of muscle: effect on quantitation of intramyocellular lipids. Magn Reson Med 61: 16–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 297: E151–E156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kraniou GN, Cameron-Smith D, Hargreaves M. Effect of short-term training on GLUT-4 mRNA and protein expression in human skeletal muscle. Exp Physiol 89: 559–563, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. [erratum appears in Diabetologia 1999 Mar; 42(3):386] Diabetologia 42: 113–116, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Lawrence JC, Newcomer BR, Buchthal SD, Sirikul B, Oster RA, Hunter GR, Gower BA. Relationship of intramyocellular lipid to insulin sensitivity may differ with ethnicity in healthy girls and women. Obesity (Silver Spring) 19: 43–48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 287: 2414–2423, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 19. Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, van de Weijer T, Sels JP, Schrauwen P, Hesselink MK. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 59: 572–579, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48: 1600–1606, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Rico-Sanz J, Hajnal JV, Thomas EL, Mierisova S, Ala-Korpela M, Bell JD. Intracellular and extracellular skeletal muscle triglyceride metabolism during alternating intensity exercise in humans. J Physiol 510: 615–622, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117: 1690–1698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schick F, Eismann B, Jung WI, Bongers H, Bunse M, Lutz O. Comparison of localized proton NMR signals of skeletal muscle and fat tissue in vivo: two lipid compartments in muscle tissue. Magn Reson Med 29: 158–167, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Schrauwen-Hinderling VB, Hesselink MK, Moonen-Kornips E, Schaart G, Kooi ME, Saris WH, Schrauwen P. Short-term training is accompanied by a down regulation of ACC2 mRNA in skeletal muscle. Int J Sports Med 27: 786–791, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Schrauwen-Hinderling VB, Schrauwen P, Hesselink MK, van Engelshoven JM, Nicolay K, Saris WH, Kessels AG, Kooi ME. The increase in intramyocellular lipid content is a very early response to training. J Clin Endocrinol Metab 88: 1610–1616, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Schrauwen-Hinderling VB, van Loon LJ, Koopman R, Nicolay K, Saris WH, Kooi ME. Intramyocellular lipid content is increased after exercise in nonexercising human skeletal muscle. J Appl Physiol 95: 2328–2332, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Shaw CS, Clark J, Wagenmakers AJ. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu Rev Nutr 30: 13–34, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S. Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 51: 1022–1027, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Solomon TP, Haus JM, Kelly KR, Cook MD, Filion J, Rocco M, Kashyap SR, Watanabe RM, Barkoukis H, Kirwan JP. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr 92: 1359–1368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Solomon TP, Haus JM, Kelly KR, Cook MD, Riccardi M, Rocco M, Kashyap SR, Barkoukis H, Kirwan JP. Randomized trial on the effects of a 7-d low-glycemic diet and exercise intervention on insulin resistance in older obese humans. Am J Clin Nutr 90: 1222–1229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Solomon TP, Sistrun SN, Krishnan RK, Del Aguila LF, Marchetti CM, O'Carroll SM, O'Leary VB, Kirwan JP. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol 104: 1313–1319, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stevenson EJ, Thelwall PE, Thomas K, Smith F, Brand-Miller J, Trenell MI. Dietary glycemic index influences lipid oxidation but not muscle or liver glycogen oxidation during exercise. Am J Physiol Endocrinol Metab 296: E1140–E1147, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Szczepaniak LS, Dobbins RL, Stein DT, McGarry JD. Bulk magnetic susceptibility effects on the assessment of intra- and extramyocellular lipids in vivo. Magn Reson Med 47: 607–610, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Tanner CJ, Koves TR, Cortright RL, Pories WJ, Kim YB, Kahn BB, Dohm GL, Houmard JA. Effect of short-term exercise training on insulin-stimulated PI 3-kinase activity in middle-aged men. Am J Physiol Endocrinol Metab 282: E147–E153, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Trenell MI, Stevenson E, Stockmann K, Brand-Miller J. Effect of high and low glycaemic index recovery diets on intramuscular lipid oxidation during aerobic exercise. Br J Nutr 99: 326–332, 2008 [DOI] [PubMed] [Google Scholar]
- 36. van Loon LJ, Thomason-Hughes M, Constantin-Teodosiu D, Koopman R, Greenhaff PL, Hardie DG, Keizer HA, Saris WH, Wagenmakers AJ. Inhibition of adipose tissue lipolysis increases intramuscular lipid and glycogen use in vivo in humans. Am J Physiol Endocrinol Metab 289: E482–E493, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Virkamaki A, Korsheninnikova E, Seppala-Lindroos A, Vehkavaara S, Goto T, Halavaara J, Hakkinen AM, Yki-Jarvinen H. Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes 50: 2337–2343, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Wadley GD, Konstantopoulos N, Macaulay L, Howlett KF, Garnham A, Hargreaves M, Cameron-Smith D. Increased insulin-stimulated Akt pSer473 and cytosolic SHP2 protein abundance in human skeletal muscle following acute exercise and short-term training. J Appl Physiol 102: 1624–1631, 2007 [DOI] [PubMed] [Google Scholar]