Abstract
Hepatic stellate cells are embedded in the loose connective tissue matrix within the space of Disse. This extracellular matrix contains several basement membrane components including laminin, but its composition changes during liver injury because of the production of extracellular matrix components found in scar tissue. These changes in extracellular matrix composition and in cell-extracellular matrix interactions may play a key role in hepatic stellate cell transdifferentiation. In this communication we used early passages of mouse hepatic stellate cells (activated HSC/myofibroblasts) to study the platelet-derived growth factor BB (PDGF-BB)-dependent expression and regulation of β-dystroglycan and its role in activated HSC/myofibroblast migration. We used Northern and Western analysis to study dystroglycan expression and confocal microscopy to investigate changes in subcellular distribution of the protein. Activated HSC migration was investigated using an in vitro wound-healing assay. PDGF-BB induced significant changes in dystroglycan regulation and subcellular distribution of the protein. Whereas steady-state levels of dystroglycan mRNA remained constant, PDGF-BB increased dystroglycan transcription but shortened the t1/2 by 50%. Moreover, PDGF-BB changed dystroglycan and α5-integrin cellular distribution. Cell migration experiments revealed that PDGF-BB-dependent migration of activated HSC/myofibroblasts was completely blocked by neutralizing antibodies to fibronectin, α5-integrin, laminin, and β-dystroglycan. Overall, these findings suggest that both laminin and fibronectin and their receptors play a key role in PDGF-BB-induced activated HSC migration.
Keywords: platelet-derived growth factor, HSC activation, fibronectin, laminin, α5-integrin
hepatic stellate cells (HSC), the main collagen producing cells of the liver, are localized to the perisinusoidal space of Disse and are embedded in a loose connective tissue matrix. This space is devoid of a continuous basement membrane but contains collagens, laminins, and fibronectins (FN) (23). Several liver cell types participate in the production of the extracellular matrix found in the space of Disse although HSC contribute to the production of type IV collagen and laminin (12). Quiescent HSC are characterized by their high content of vitamin A and triglycerides that are the basis for their isolation (28). Upon liver injury, HSC change phenotype and are transformed into myofibroblasts or activated HSC that express multiple growth factor receptors, including platelet-derived growth factor receptor-β (PDGF-βr). Similar events to those observed in vivo during HSC activation can be reproduced when culturing the cells on plastic, collagen, and/or FN-coated culture dishes (21). In contrast with these findings, culture-activated HSC/myofibroblasts on a laminin-rich gel (Matrigel) stimulates the maintenance of a quiescent state (11) and reverts the myofibroblastic phenotype (31).
Dystroglycan (DG) is a laminin-binding protein involved in cell-matrix interaction. It is produced as a large molecular-weight protein that upon proteolytic cleavage generates the two DG subunits (α and β). The β-subunit has a molecular weight of 41–43 kDa and is inserted into the plasma membrane. The α-subunit has a molecular weight of 75 kDa, and following heavy glycosylation it binds to the extracellular domain of the β-subunit. The fully glycosylated subunit has a molecular weight of 120–156 kDa (1).
It has been suggested that DG plays an important role in organizing matrix components, integrating basement membranes, and in cell-matrix interactions (15). Thus HSC may be bound to laminin within the space of Disse, and changes in the composition of the matrix after injury, namely the production of FN, could play a role in HSC activation that leads to cell migration. This is supported by the following information: 1) HSC express DG, and therefore they bind to laminin (2); 2) one of the proteins that is expressed shortly after injury is FN (22, 17), and this protein enhances HSC activation (18); 3) recent studies showed that DG is part of the focal adhesion sites (3) and that O-mannosyl phosphorylation of α-DG is required for DG interaction with laminin (35); moreover, alterations in glycosylation of DG have been found in certain human and mouse muscular dystrophies (16); 4) changes in the concentration of DG modulate the size and number of adhesions formed (33). These findings further stress the key role of DG in cell interaction with laminin and in maintaining healthy cell-matrix bonds.
As indicated previously, HSC activation is a complex process in which HSC undergo multiple changes in gene expression. Of particular interest are the expression of the PDGF-βr and the PDGF-BB-dependent stimulation of activated HSC/myofibroblast migration and proliferation. Therefore, as a first approach to study molecular events involved in activated HSC/myofibroblast migration, we investigated the role of PDGF-BB on DG expression and cellular localization. We also analyzed the effects of transforming growth factor (TGF)-β1 on the expression of DG. In this communication, we show that mouse-activated HSC express a single DG transcript of 5.4 kb and produce both α- and β-subunits of this protein. We established that PDGF-BB modulates the expression of DG mRNA by transcriptional and posttranscriptional mechanisms. It increases the transcription of the gene but shortens its half-life by 50%. We also show significant changes in DG cellular distribution. In contrast with these findings, TGF-β1 upregulates the expression of DG by a transcriptional mechanism.
MATERIALS AND METHODS
Materials.
Nonessential amino acids, kanamycin, and penicillin/streptomycin were purchased from Life Technologies (Grand Island, NY). PDGF-BB, TGF-β1, and tumor necrosis factor-α (TNF-α) were purchased from Roche Diagnostic (Indianapolis, IN). Actinomycin D and cycloheximide were purchased from Sigma Chemical (St. Louis, MO). The random priming labeling kit, [32P]-dUTP, and [32P]-dCTP were obtained from Amersham International (Arlington Heights, IL). The monoclonal antibody against the COOH terminus of α-DG and the Cy5-labeled goat anti-rabbit antibody were obtained from Chemicon International (Temecula, CA). The Cy3-labeled rabbit anti-mouse antibody was obtained from Jackson Immunoresearch Laboratories (West Grove, PA). The monoclonal antibody against α5-integrin and the polyclonal antibody against laminin-β1 were bought from Santa Cruz Biotechnologies (Santa Cruz, CA). The horseradish peroxidase-conjugated goat anti-mouse secondary antibody was obtained from Cell Signaling Technology (Danvers, MA). The Alexa Fluor 594 chicken anti-goat IgG (H&L) and the Alexa Fluor 488 anti-rabbit IgG (H&L) fluorescent antibodies were bought from Molecular Probes (Eugene, OR). The forward and reverse mouse qPCR primers used for β-DG quantification of mRNA expression were 5′-TTAGCAACTGGTGGCTTGAAC-3′ and 5′-ACAGACAGGAGGAGGAGAAAG-3′, respectively (Genbank Accession Number NM_010017.3). The forward and reverse mouse qPCR primers used for S18 were 5′-CAGAAGGATGTGAAGGATGG-3′ and 5′-CAGTGGTCTTGGTGTGCTGA, respectively (Genbank Accession Number NM_011296.2). The primers were bought from Integrated DNA Technologies (Coralville, IA).
Isolation and culture activation of HSC.
For these experiments we used pools of livers obtained from BC6D2F2 mice (a hybrid cross derived from the C57BL/6 and DBA/2 strains). The cells were a gift from Dr. Francesco Ramirez at the Mount Sinai School of Medicine, as well as previously euthanized mice, which were used to obtain additional cells. All animal protocols were approved by the Ethics Committee at the George Washington University Medical Center. The livers were chopped into very small fragments using a sharp razor blade and washed twice with PBS to remove blood. The liver fragments were washed once with Leffert's solution containing EGTA and then twice with Leffert's buffer to remove EDTA (6). Fragments were then digested for 30 min at 37°C with a mixture of collagenase, protease, and DNaseI dissolved in Leffert's buffer containing 5 mM CaCl2. After digestion, the cell suspension was centrifuged at 500 g for 2 min to remove hepatocytes and debris. The cells remaining in the supernatant (nonparenchymal cells) were pelleted after centrifugation at 1,350 g for 5 min. The pellet was resuspended in 15 ml of a 25% Optiprep solution (Sigma), overlaid with 15 ml of a 17% solution of Optiprep followed by 10 ml of Hanks' basic salt solution. After centrifugation at 1,350 g for 20 min, the cells in the interface between the Hanks' solution and 17% Optiprep layers were aspirated and washed twice with Hanks' solution. Half the cells were frozen and used for RNA extraction as described below, and the other half were culture activated for 7 days. Total RNA from the cultured cells was extracted with Trizol (Invitrogen, Carlsbad, CA) as described (7). For other experiments, cells in their activated phenotype (between passages 5 and 7) were incubated at 37°C with 5% CO2 in MEM (Mediatech, Herndon, VA) supplemented with 10% FBS (Hyclone, Logan, UT), nonessential amino acids, kanamycin, and penicillin/streptomycin. Sixteen hours before performing the experiments, the culture medium was replaced by a serum-free medium containing 0.2% BSA fraction V (Sigma), and cells were maintained in this medium throughout the duration of the experiment. To test the effect of growth factors and cytokines on the expression of dystroglycan mRNA, culture-activated HSC/myofibroblasts were incubated with PDGF-BB (50 ng/ml), TGF-β1 (8 ng/ml), or TNF-α (20 ng/ml). In some experiments, the following inhibitors of transcription and protein synthesis were added 30 min before cytokine or growth factor administration: actinomycin D (10 μg/ml) and cycloheximide (0.3 mmol/l). Clones CFSC-8B and CFSC-2G that were cloned from an HSC isolated from a cirrhotic rat liver (14) have been previously characterized (13). Hepatocytes were isolated from rat liver by collagenase perfusion as described (26).
RNA extraction and Northern blot analysis.
Total RNA was extracted from cell cultures and isolated rat hepatocytes by the method of Chomzcynski and Sacchi (8). Northern blot assays were performed using 15 μg of total RNA per lane, electrophoresed onto 1% denatured formaldehyde-agarose gels, transferred to Gene-Screen membranes (NEN Life Science Products, Boston, MA), and cross linked with a UV Stratalinker 1800 (Stratagene, La Jolla, CA). Membrane hybridization was performed using [α-32P]-labeled cDNA probes for DG and riboprotein S14. In experiments performed with actinomycin D, an S18 probe was used because S14 mRNA was rapidly degraded by this treatment (ATCC, Rockville, MD). The cDNA probe of DG was synthesized from total mHSC RNA by RT-PCR (4). The rabbit DG sense 5′-GCCCTGGAGCCTGACTTTAAGGC-3′ and the antisense primer 5′-TCGTCCAGCTCGTCTGCAAAGA-3′, corresponding to nucleotide sequences 2252–2274 and 2565–2577, respectively, have been previously described (30). RT-PCR was performed using the ABI 2720 Thermal Cycler (Foster City, CA). The PCR product was cloned into the pCR 2.1 vector using the TA Cloning Kit (Invitrogen). Transformed bacteria were screened onto Luria broth agar plates containing 10 mg/ml ampicillin (Life Technologies) with the selection of a single positive clone. The size of the RT-PCR product and its nucleotide sequencing were identical to that expected for the sequence of mouse DG. The signals from cDNA S14 riboprotein and S18 ribosomal subunit were used to control the loading differences. Northern hybridizations were performed under stringent conditions as previously described (13, 14). Values are reported as the means of triplicate experiments ± SE.
In vitro transcription assays.
mHSC were grown in 75-cm2 flasks and serum starved, as described above. Nuclear extraction and hybridization were performed as previously described (32). Signal intensities were determined by phosphorimaging or by X-ray film analysis. Unless otherwise indicated, all the values are expressed as the means of triplicate experiments ± SE.
Western blot analysis.
Whole cell protein extracts of culture-activated mHSC/myofibroblasts were prepared with lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 1% Triton X-100, 1 mM PMSF, 1 μg/ml each leupeptin, antipain, chymostatin, and pepstatin). Protein concentration was determined using the bicinchoninic acid assay according to the manufacturer's instructions (Pierce Chemical, Rockford, IL). SDS-polyacrylamide gel (12%) electrophoresis was performed using 5 μg of protein (20) and transferred to Immobilon-P membrane (Millipore, Bedford, MA). The membranes were blocked overnight with 5% non-fat dry milk in Tris-buffered saline solution containing 0.1% Tween-20, incubated with a mouse monoclonal anti-β-DG antibody (dilution 1:750) for at least 2 h, followed by one additional hour with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (dilution 1:3,000), and developed using the Supersignal West Pico enhanced chemiluminescence system (Thermo Scientific, Waltham, MA). Membranes were stripped and reprobed with mouse monoclonal anti-β-actin primary antibody (Sigma) to normalize protein loading differences. Quantitative results were obtained by densitometric analysis using UN-Scan-It software (Silk Scientific, Orem, UT). Values are expressed as the means of triplicate experiments ± SE.
Wound healing in vitro assay.
Activated mHSC/myofibroblasts were grown to confluence, and, after 24 h of incubation with a serum-free medium containing 0.2% BSA, a mechanical wound was made in the center of the culture with a 200-μl pipette tip. The monolayer was washed twice with PBS, and the cells were cultured with serum-free medium. Approximately 30 min before adding 50 ng/ml of PDGF-BB to the cells, the following antibodies were added to the culture at a 1:100 dilution: anti-β1-laminin (Santa Cruz Biotechnologies), anti-β-DG (Developmental Studies Hybridoma Bank, Iowa City, IA), anti-FN (Telios Pharmaceuticals, San Diego, CA) and anti-α5-integrin. At the various time points shown in the figures, multiple pictures of the wound were taken using a ×20 objective of the Nikon Inverted Microscope. Quantitative analysis was performed with a grid measuring the size of the wound using Adobe Photoshop Elements 6.0 software. The values represent the average of 25 measurements taken from multiple pictures of triplicate experiments. Values are expressed as percent closure compared with controls ± SE.
Dual confocal immunomicroscopy.
Activated mHSC/myofibroblasts were seeded on cover slips placed in sterile Petri dishes and cultured as described. When cells were semiconfluent, the culture medium was replaced with a serum-free medium, and the cells were cultured for an additional 16 h. PDGF-BB (50 ng/ml) was added, and mHSC were cultured for an additional hour. Cover slips were then rinsed with PBS, fixed in 4% formaldehyde for ∼10 min, rinsed with PBS, and blocked with 1% bovine serum albumin for 1 h. Cover slips were incubated overnight in a humidified chamber with either primary antibodies against α5-integrin (1:10 dilution) or FN (1:10 dilution) alone or antibodies to β-DG (1:10 dilution) and laminin (1:10 dilution). After several washes with PBS, cover slips were incubated for 1 h in the dark, at room temperature with either one of the following fluorescently tagged antibodies: for DG the antibody used was Cy3-labeled goat anti-rabbit (1:300 dilution); for laminin the antibody used was Cy5-labeled goat anti-rabbit (1:300 dilution); for FN the antibody used was Alexa Fluor 594 chicken anti-goat (1:750 dilution); and for α5-integrin the antibody used was Alexa Fluor 488 goat anti-rabbit (1:750 dilution). Cover slips were then washed several times with 0.1% Tween-20/PBS and were mounted onto glass slides with Gel-Mount (Biomeda, Foster City, CA). Images were obtained using the Zeiss LSM 510 Confocal Microscope and processed with Volocity 5.0 software (PerkinElmer, Waltham, MA).
Statistical analysis.
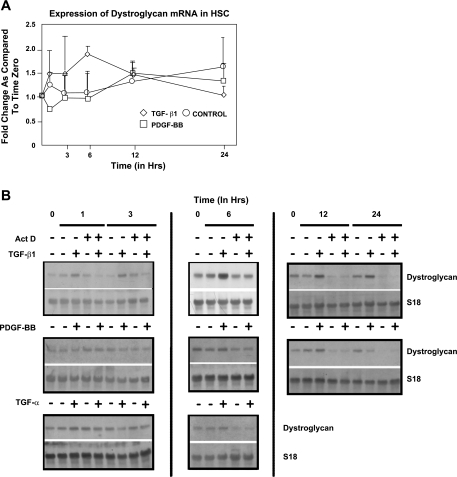
All the experiments were performed at least in triplicate, and data are expressed as means ± SE. Statistical differences between experimental groups were analyzed by Student's t-test, and values equal or smaller than P < 0.05 were considered to be significant (Microsoft Excel 2003). To determine the significance of data in Fig. 2 we used ANOVA (see the corresponding legend to Fig. 2).
Fig. 2.
A and B: transforming growth factor (TGF)-β induced a transient upregulation of β-DG expression in activated mHSC/myofibroblasts. Time-course expression of β-DG mRNA in TGF-β-treated activated HSC/myofibroblasts (◊) revealed a transient upregulation that occurred after 6 h (P = 0.037). In control HSC (○), β-DG mRNA increased slowly with time, but values did not reach statistical significance. In platelet-derived growth factor-BB (PDGF-BB)-treated activated mHSC/myofibroblasts (□), changes in mRNA expression were similar to those observed in untreated controls (P = 0.032). Signal intensities of autoradiographies were determined by densitometric analysis. Values were calculated as fold change compared with time zero and are means ± SE of 3 experiments after correcting for loading differences using expression of S14 mRNA as an internal control. *P < 0.05 was considered significant. Statistical analysis was performed using ANOVA. B: representative Northern blots. Each set corresponds to independent experiments and include time zero controls as well as the untreated controls harvested at the same time points as the cultures treated with the corresponding cytokines/growth factors. Act D, actinomycin D.
RESULTS
HSC express a single transcript of 5.4 kb.
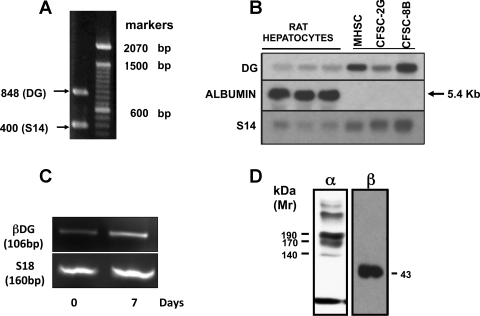
Using the primers described in materials and methods and total RNA obtained from early passages of activated mHSC/myofibroblasts, we obtained an amplicon containing 848 bp, whose nucleotide sequence corresponded to DG (Fig. 1A). Using this cDNA as a probe, we demonstrated that activated mHSC/myofibroblasts express a single transcript of 5.4 kb (Fig. 1B). Whereas activated mHSC/myofibroblasts and two rat HSC cell lines expressed DG mRNA, levels of expression in hepatocytes were very low and reflected the 5% contamination of this fraction with HSC (Fig. 1B). It has already been reported that freshly isolated human HSC express DG mRNA and protein (2). Here we show that freshly isolated mouse HSC also express DG mRNA and that its expression is upregulated after culture activation (Fig. 1C). Culture activated mouse HSC (4–5 passages) produce α- and β-DG subunits with molecular weights of 190 kDa and 43 kDa, respectively. Once established that activated mouse and rat HSC express DG mRNA, we investigated levels of protein produced as well as their cellular localization. With the use of a specific antibody to the β-DG subunit, Western blot analysis revealed that activated HSC produce the β-DG subunit with a molecular weight of 43 kDa. This molecular weight is similar to that described in many cells including rat and human HSC (2) (see Fig. 1D). Western analysis with an antibody to the α-DG subunit revealed the presence of several bands, of which the major one was 190 kDa in size (Fig. 1D). This diversity is similar to that reported by others (1). Although the nature of the various bands is unknown, the larger molecular weight bands could correspond to aggregates and/or the primary translation product before being processed into α- and β-DG subunits. Because of the multiple bands recognized by this antibody, no attempts were made to study the cellular localization or actual protein levels of the α-subunit. Therefore, unless otherwise indicated, all further studies described below were performed using activated mHSC and with antibodies to the β-DG subunit.
Fig. 1.
Activated hepatic stellate cell (HSC)/myofibroblasts express a single β-dystroglycan (DG) transcript of 5.4 kb. A: expression of DG mRNA was determined by RT-PCR using total RNA obtained from early passage-activated mHSC/myofibroblasts. As expected from the position of the primers used (1228–1253 to 2102–2126), an amplicon of 848 bp was obtained. The amplicon was purified from agarose gels, sequenced, and cloned into the EcoR1 restriction site of the PCR 2.1 vector (Invitrogen). After growing the plasmid, the insert was removed with EcoR1, purified, labeled with α32P, and used as a probe for Northern analysis (see materials and methods). B: Northern analysis of DG mRNA expression in activated mHSC/myofibroblasts, 2 rat HSC cell lines (CFSC-8B and CFSC-2G), and rat hepatocytes. As shown, the expression of β-DG in isolated rat hepatocytes was very low and reflected the 5% contamination of this fraction with HSC. All the HSC assayed showed a single transcript of 5.4 kb. C: resultant gel from qPCR analysis of β-DG in quiescent and activated mouse HSC. Although fainter in quiescent HSC, both quiescent and activated HSC revealed a β-DG band of ∼106 bp in size. D: Western analysis of total protein obtained from cell extracts revealed bands of >200, 190, 170, and 140 kDa of α-DG and a 43-kDa band of β-DG.
Time-course analysis of DG mRNA expression.
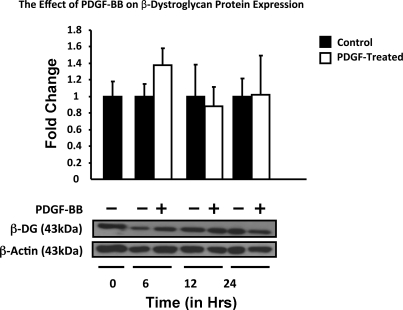
Time studies revealed that the expression of DG mRNA by culture-activated HSC/myofibroblasts is constant for roughly 6 h, after which time it steadily increased, and by 24 h values were 57 ± 0.6% (P = NS) above those found at zero time. PDGF-BB did not have a significant effect on the expression of DG mRNA and followed the levels of the untreated control, except for a fast decrease after 30 min that was recovered after 1 h (P = 0.032). TGF-β upregulated the expression of DG mRNA, transiently reaching maximum levels by 6 h (1.9 ± 0.17, P = 0.037), after which time values decreased below those found in untreated controls by 24 h [1.02 ± 0.02 (P = NS) (Fig. 2A)]. Figure 2B corresponds to the representative Northern blots. In agreement with the lack of significant changes in DG mRNA, protein levels were more or less constant (Fig. 3). However, as shown below (Fig. 7B), the subcellular localization of the protein changed after PDGF-BB administration.
Fig. 3.
Time-course analysis of β-DG protein expression in activated mouse HSC treated with PDGF-BB. Activated HSC/myofibroblasts were incubated with 50 ng/ml PDGF-BB for the times indicated, and protein was extracted and analyzed by Western blotting (5 μg protein/lane), as described in materials and methods. Representative blot and the histogram corresponding to the semiquantitative analysis of total β-DG obtained in triplicate experiments of cells. β-Actin was used as a loading control. All values are means of triplicate experiments ± SE.
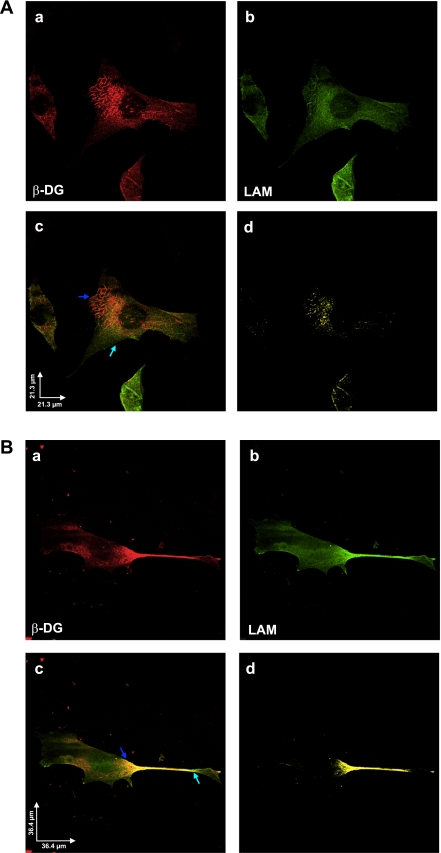
Fig. 7.
A: β-DG and laminin colocalize to the cell surface of activated HSC/myofibroblasts. Confocal images (2048 × 2048) of control untreated cells taken with ×60 objective (N.A. 1.4) at 0.110-μm pixel resolution, depicting immunolabeling for β-DG (a, red) and laminin (LAM) (b, green). c: Image obtained after merging red and green channels from a and b, respectively. Colocalization was found mainly in the cell body and at the presumptive surface of the cells (arrows). d: Pixels representing colocalization over threshold were extracted and shown in yellow. Scale bar = 21.3 μm. B: PDGF-BB treatment of activated HSC/myofibroblasts enhances the cell surface distribution of β-DG and laminin. PDGF-BB-treated activated HSC/myofibroblasts were immunolabeled with antibodies to β-DG (a, red) and laminin (b, green). This experiment was performed as described in A. c: Merged image of a and b, respectively. Colocalization pixels increased at the presumptive surface of the cells (arrows). d: Pixels representing colocalization over threshold were extracted and shown in yellow; although scattered colocalization was found in the cell body, most colocalization was present mainly within the cell protrusions. Scale bar = 36.4 μm.
Different regulatory mechanisms are involved in PDGF-BB- and TGF-β1-mediated modulation of DG expression.
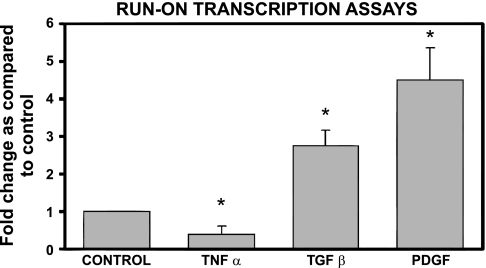
To further investigate molecular mechanisms involving PDGF-BB and TGF-β1 regulation of DG mRNA expression, we investigated whether DG was transcriptionally regulated and whether de novo protein synthesis was required for its expression. Run-on transcription assays performed 6 h after PDGF-BB administration revealed a significant increase in DG transcription [4.5 ± 0.8-fold, P < 0.05 (Fig. 4)]. This result was unexpected on the basis of the lack of a significant effect of PDGF-BB on DG steady-state mRNA levels (see Fig. 2A). As also shown in Fig. 4, TGF-β1 increased transcription of the DG gene 2.8 ± 0.4-fold (P < 0.05). As a negative control for this experiment, we tested the effect of TNF-α because this cytokine opposes some TGF-β-mediated effects. As illustrated in Fig. 4, TNF-α decreased the transcription of DG by about 50% (0.4 ± 0.2, P < 0.05).
Fig. 4.
Run-on transcription assays revealed increased transcription induced by TGF-β and PDGF-BB treatment. Run-on transcription assays were performed with nuclei obtained from control untreated, PDGF-BB-, and TGF-β1-treated activated mHSC/myofibroblasts. Tumor necrosis factor (TNF)-α was used as a negative control, as this cytokine generally exerts the opposite effects of TGF-β. Total RNA was extracted 6 h after the addition of the cytokine and processed as described in materials and methods. Signal intensities of autoradiographies were determined by densitometric analysis. Values were corrected for loading differences using signals obtained with S14 as controls. Values are expressed as fold increase compared with identical time-point controls and are means ± SE of ≥3 experiments. *P < 0.05 vs. untreated controls.
PDGF-BB shortened the half-life of DG-mRNA.
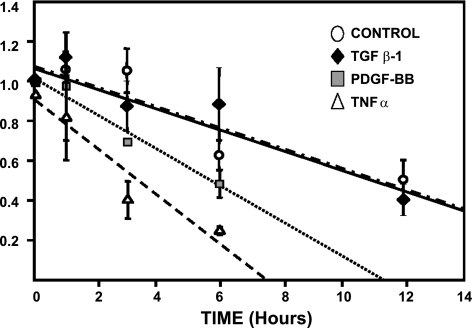
Because of the significant differences between steady-state and transcription levels, we considered it necessary to investigate whether PDGF-BB and TGF-β had any effect on DG mRNA half-life. As illustrated in Fig. 5, incubation of activated HSC/myofibroblasts with actinomycin D, an inhibitor of transcription, resulted in a steady decrease in DG mRNA levels. From the slopes of the curves obtained (see Fig. 5), we estimated that the half-life of DG mRNA in control untreated HSC was ∼11.5 h. TGF-β1 had no effect on DG mRNA half-life, and values were similar to those obtained in control cells treated with actinomycin alone. However, PDGF-BB shortened DG mRNA half-life by 50% (6 h). Because TNF-α downregulated DG transcription, we also determined the half-life of DG in TNF-α-treated HSC. As shown in Fig. 5, TNF-α shortened the half-life of DG to 3 h (75% below control levels). Representative Northern assays for these experiments are shown in Fig. 2B.
Fig. 5.
PDGF-BB but not TGF-β shortens the t1/2 of β-DG mRNA by 50%. Activated HSC/myofibroblasts were incubated for 30 min with 10 μg/ml of actinomycin D before the addition of PDGF-BB, TGF-β, or TNF-α. Changes in β-DG mRNA expression were analyzed by Northern analysis of total RNA extracted at the various time points shown. Values are means ± SE of ≥3 independent experiments. The t1/2 of DG mRNA was estimated from the slope of the double exponential curves. Representative Northern blots for these experiments are shown in Fig. 2B.
PDGF-BB and TGF-β1 upregulate DG expression by a de novo protein synthesis-dependent mechanism.
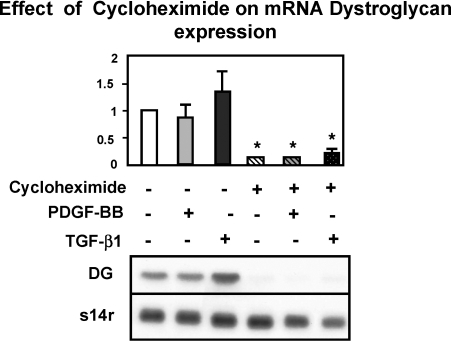
Additional experiments demonstrated that PDGF-BB- and TGF-β1-mediated upregulation of DG mRNA was de novo protein synthesis dependent. Cycloheximide, at a dose that inhibits protein synthesis in activated HSC by 95%, decreased basal expression of DG by 91.3 ± 12.2% (P < 0.01) and inhibited the PDGF-BB and TGF-β1-dependent upregulation of this message by 90 ± 10.0% (P < 0.01) and 84 ± 3.8% (P < 0.01), respectively (see Fig. 6). It is important to mention that, at the time of harvesting activated HSC/myofibroblasts, their viability was 90% as determined by Trypan blue exclusion.
Fig. 6.
Cycloheximide blocks the expression of DG mRNA. The expression of β-DG mRNA was measured in control untreated, PDGF-BB-, and TGF-β1-treated activated mHSC/myofibroblasts. Cells were treated with cycloheximide (0.3 mM) 30 min before adding the cytokine or the growth factor, and total RNA was extracted after 6 h and processed for Northern analysis (see materials and methods). As shown, cycloheximide prevented the expression of β-DG mRNA in all the conditions investigated. Data values are means ± SE of ≥3 independent experiments. *P < 0.01 and vs. untreated controls. A representative Northern blot is shown at the bottom.
PDGF-BB induces a significant change in the cellular localization of DG, laminin, FN, and α5-integrin.
Immunofluorescent confocal microscopy using antibodies to DG (red) and laminin (green) revealed that DG protein is localized around the nucleus and also in some areas corresponding to the cell surface (Fig. 7A, a). Likewise, laminin exhibits similar localization as DG. Merging of both channels showed that DG and laminin colocalized preferentially to the perimeter of the nucleus (shown in yellow) (Fig. 7A, d).
Upon treatment of activated HSC/myofibroblasts with PDGF-BB, the cells change morphology, becoming more elongated, with their main diameter decreasing in size and the cells appearing to be longer, more mobile, and emitting very long projections. In addition, as shown in Fig. 7B, a, DG changes location and accumulates on the projections with a heavier intensity in the area beneath the center of the projections. Similarly, laminin also redistributes after PDGF-BB treatment and colocalizes with DG to the projections (Fig. 7B, b, c, and d).
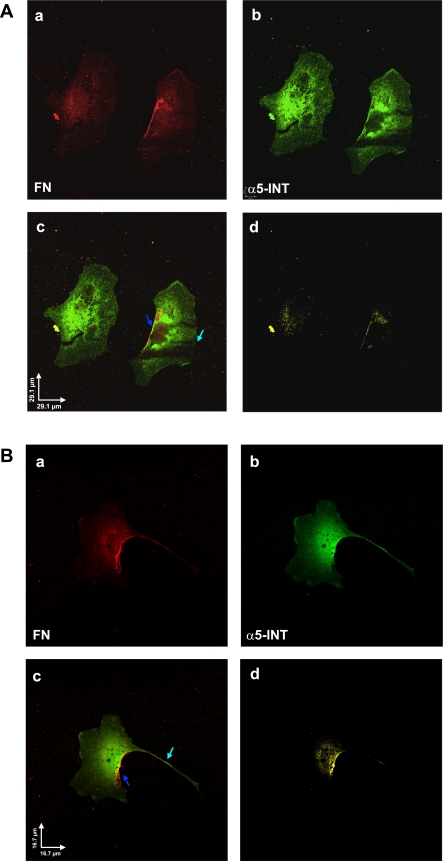
Because FN is one of the proteins deposited into the space of Disse immediately after liver injury (22), we investigated the cellular distribution of FN and its receptor, α5-integrin, in control and PDGF-BB-treated activated HSC. Immunofluorescent confocal microscopy with antibodies to FN and integrin revealed intense fluorescent staining for FN (red) and α5-integrin (green) all over the cell but especially on the lateral side (Fig. 8A, a and b, respectively). When merged, the colocalization of both proteins is clearly demonstrated (Fig. 8A, c and d).
Fig. 8.
A: fibronectin (FN) and α5-integrin (α5-INT) colocalize on the cell surface of activated HSC/myofibroblasts. Confocal images (2048 × 2048) of control untreated activated HSC/myofibroblasts taken with ×60 objective (N.A. 1.4) at 0.110-μm pixel resolution, depicting immunolabeling for FN (a, red) and α5-integrin (b, green). c: Merged red and green channels from a and b, respectively. Colocalization was found mainly at the presumptive surface of the cells. d: Pixels representing colocalization over threshold were extracted and shown in yellow. Scale bar = 29.1 μm. B: PDGF-BB enhances the colocalization of FN and α5-integrin along the cell surface of activated HSC/myofibroblasts. PDGF-BB-treated activated HSC/myofibroblasts were immunolabeled with antibodies to FN (a, red) and α5-integrin (b, green). This experiment was performed as described A. c: Merged red and green channels from a and b, respectively. Colocalization was found mainly along the presumptive surface of the cells and the long projections (arrows). d: Pixels representing colocalization over threshold were extracted and shown in yellow. Scale bar = 16.7 μm.
Upon treatment with PDGF, the two proteins redistributed and colocalized on the cell surface, showing also a light punctuated staining along the projections of the cells (Fig. 8B, a and b, respectively). This is clearly illustrated after merging of both channels (Fig. 8B, c). In contrast with the localization of DG and laminin, FN and α5-integrin staining is more intense on the lateral cell surface of the cell, whereas staining of the projections is less intense.
Activated HSC/myofibroblasts appear to migrate on a FN carpet but require laminin for attachment.
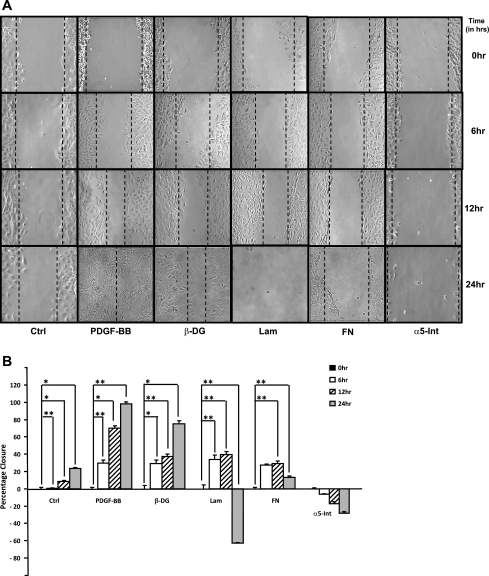
To study the role of DG in cell attachment and migration, we used the wound-healing system described in materials and methods. Migration of activated HSC/myofibroblasts into the wounded area was stimulated by the addition of 50 ng/ml of PDGF to the cultures. Negative controls were maintained in a serum-free, PDGF-free culture medium. The size of the wound was determined at the various time points indicated in the figures. The size of the wound obtained 24 h after PDGF-BB treatment was considered to be 100% closure.
To test the role of DG, laminin in cell migration, cells were incubated with the corresponding antibodies at the concentrations indicated in the legends to the figures. Control activated HSC/myofibroblasts cultured in a PDGF-BB-free medium showed some remnant migration but did not close the wound after 24–48 h (23.7% ± 1.18, P < 0.001) (see Fig. 9, A and B). In PDGF-BB-treated activated HSC/myofibroblasts, the wound was almost completely closed by 12 h (71.1% ± 2.8, P = 0.004), and by 24 h the wound was completely covered with cells (98.1% ± 2.4, P = 0.02) (see Fig. 9, A and B). Incubation with antibodies to DG inhibited wound closure by (25% ± 3.4, P = 0.001). Antibodies to laminin did not appear to inhibit cell migration during the first 12 h (39.5% ± 3.7, P = 0.01) (Fig. 9, A and B). However, by 24 h the wound was wider and the monolayer was disorganized (−62.7% ± 0.57, P = 0.02). Figure 9A shows that antibodies to FN had a similar effect as those to laminin, and, although the cells appear to migrate initially, the wound was wider after 24 h (12.9% ± 2.1, P = 0.03). Antibodies to α5-integrin completely prevented cell migration, and the wound widened by 24 h (−24.8% ± 1.5, P = 0.6). These findings suggest that antibodies to laminin, FN, and α5-integrin not only inhibited wound healing but promoted disorganization of the monolayer and cell detachment.
Fig. 9.
A and B: antibodies to β-DG, laminin, FN, and α5-integrin interfered with activated HSC/myofibroblasts in an in vitro wound-healing system. Activated HSC/myofibroblasts were grown to confluence. After overnight culture in serum-free medium with 0.2% BSA, a linear wound was generated before the addition of each of the antibodies in a 1:100 concentration. The concentration of PDGF-BB used was 50 ng/ml. Cells were analyzed with a Nikon inverted microscope with all images taken at ×20 magnification. Multiple measurements (5 images/dish) were taken of each dish at various locations of the linear wound. Quantitative analysis was performed with a grid to measure the percent wound closure relative to the open wound using Adobe Photoshop Elements 6.0 software. B: representative graph of A. Data are expressed in terms of percentage of wound closure compared with controls. All values are the means of triplicate experiments ± SE. *P < 0.05; **P < 0.01.
DISCUSSION
The expression of PDGF-βr has been considered a hallmark of HSC activation and the expression of PDGFs as the main growth factors that induce proliferation and migration (10). However, the various extracellular matrix components to which quiescent HSC bind in the space of Disse and the receptors utilized remain to be fully elucidated. Similarly, the changes in cell adhesion and migration that occur during activation need to be further investigated.
The space of Disse contains numerous extracellular matrix components including basement membrane proteins and HSC that are embedded in this loose connective tissue (22, 23). In their quiescent state, HSC produce mainly collagen α1(III), α1(IV), and laminin B1. However, the expression of FN is not detected (12). As shown by others (2) as well as in this study, quiescent HSC express DG mRNA, albeit levels of this mRNA are low. During liver injury there are quantitative and qualitative changes in the composition of the extracellular matrix in the space of Disse (24). It has been suggested that these changes, namely the expression of laminin and DG may play a key role in the organization of a basement membrane during the capillarization of the space of Disse (2). However, as shown in this study, the expression of DG and laminin and the changes in cellular distribution could also play a role in cell migration during activation of HSC. Culture-activated HSC/myofibroblasts also express FN (9, 25), a protein known to induce the activated phenotype of HSC (25). As shown in this work, culture-activated HSC/myofibroblasts express α5-integrin, and this FN receptor colocalizes with FN. It is of significance that the cellular distribution of laminin and FN and their respective receptors DG and α5-integrin change their cellular distribution after incubation with PDGF-BB. Whereas laminin and DG are localized primarily on the long extensions of HSC, FN and α5-integrin colocalize all around the cell surface of the cells (Figs. 7 and 8). It has been suggested that PDGF-induced migration is associated with cell proliferation (29). Because PDGF-BB induces cell proliferation and migration of activated HSC/myofibroblasts, the expression and reorganization of extracellular matrix and their receptors may play a role in cell migration. As also shown in this study, antibodies to either laminin, FN, DG, and α5-integrin prevented PDGF-BB-induced cell migration and in some instances disturbed the monolayer with a significant detachment of the cells (Fig. 9).
Overall, these and other data in the literature show that PDGF-dependent migration in activated HSC/myofibroblasts is a complex process that includes several matrix components, different matrix receptors, and metalloproteinases (34, 5). It has been shown that inhibition of vitronectin expression results in the abrogation of activated HSC/myofibroblast migration (27).
Activated HSC/myofibroblasts express several metalloproteinases (19), and some of them are involved in cell migration. It has been shown that inhibition of matrix metalloproteinases 2 and 9 expression results in the inhibition of activated HSC/myofibroblast migration (34). These findings are similar to those described in malignant cells in which the same metalloproteinases are involved in tumor migration and metastasis (8).
Regulation of the expression of DG is also complex, as transcriptional and posttranscriptional mechanisms are involved in its expression. Whereas PDGF induced the transcription of the gene, it also shortened the half-life of the mRNA. Thus the increased transcription was not reflected in increased expression of DG mRNA, as the half-life of the mRNA was shortened by 50%. It is important to mention that, although PDGF-BB induced the redistribution of DG on the cell surface, it had no effect on total protein as determined by Western analysis. In contrast with these findings, TGF-β induced a transient elevation of DG mRNA that was associated with increased transcription of the gene but did not change the level of DG protein. Altogether these findings suggest that DG regulation is quite complex and includes transcriptional and posttranscriptional mechanisms that could perhaps include the rapid degradation of the DG as the cells migrate, such an event could be the trigger for the increased transcription of the DG gene observed with PDGF-BB- and TGF-β1-treated activated HSC/myofibroblasts. Altogether, these findings as well as those of others (2), show the importance of cell-matrix interactions in maintaining homeostasis. A better understanding of these interactions and the changes occurring during HSC activation could result in the development of novel targets to prevent the development of hepatic fibrosis.
GRANTS
This work was supported through NIH grant (RO1 AA1054) awarded to M. Rojkind.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Glenn E. Morris for the MANDAG2 β-DG clone, obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, Iowa. We are indebted to the Center for Microscopy and Image Analysis (GWUMC, Washington, DC) for assistance with the collection of data and the interpretation of the images obtained by dual confocal microscopy.
REFERENCES
- 1. Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci 119: 199–207, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Bedossa P, Ferlicot S, Paradis V, Dargère D, Bonvoust F, Vidaud M. Dystroglycan expression in hepatic stellate cells: role in liver fibrosis. Lab Invest 82: 1053–1061, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Belkin AM, Smalheiser NR. Localization of cranin (dystroglycan) at sites of cell-matrix, and cell-cell contact: recruitment to focal adhesions is dependent upon extracellular ligands. Cell Adhes Commun 4: 281–296, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Beverley SM. Amplification of by PCR RNA. Current Protocols in Molecular Biology, vol 2, edited by FM Ausubel, R Brent, RE Kingston, DD Moore, JG Seidman, JA Smith, K Struhl. New York: Wiley Interscience, 1992, pp. 15.4.1–15.4.6 [Google Scholar]
- 5. Carloni V, Romanelli RG, Pinzani M, Laffi G, Gentilini P. Focal adhesion kinase, and phospholipase C. Gamma involvement in adhesion and migration of human hepatic stellate cells. Gastroenterology 112: 522–531, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Casini A, Cunningham M, Rojkind M, Lieber CS. Acetaldehyde increases procollagen type I, and fibronectin gene transcription in cultured rat fat-storing cells through a protein synthesis-dependent mechanism. Hepatology 13: 758–765, 1991 [PubMed] [Google Scholar]
- 7. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 8. Daniele A, Zito AF, Giannelli G, Divella R, Asselti M, Mazzocca A, Paradiso A, Quaranta M. Expression of metalloproteinases MMP-2 and MMP-9 in sentinel lymph node and serum of patients with metastatic and non-metastatic breast cancer. Anticancer Res 30: 3521–3527, 2010 [PubMed] [Google Scholar]
- 9. Dodig M, Ogunwale B, Dasarathy S, Li M, Wang B, McCullough AJ. Differences in regulation of type I collagen synthesis in primary and passaged hepatic stellate cell cultures: the role of α5β1-integrin. Am J Physiol Gastrointest Liver Physiol 293: G154–G164, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88: 125–172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friedman SL, Roll FJ, Boyles J, Arenson DM, Bissell DM. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem 264: 10756–10762, 1989 [PubMed] [Google Scholar]
- 12. Geerts A, Greenwel P, Cunningham M, De Bleser P, Rogiers V, Wisse E, Rojkind M. Identification of tissue gene transcripts in freshly isolated parenchymal, endothelial, Kupffer, and fat-storing cells by northern hybridization analysis. J Hepatol 19: 148–158, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Greenwel P, Rubin J, Schwartz M, Hertzberg EL, Rojkind M. Liver fat-storing cell clones obtained from a CCl4-cirrhotic rat are heterogeneous with regard to proliferation, expression of extracellular matrix components, interleukin-6, and connexin 43. Lab Invest 69: 210–216, 1993 [PubMed] [Google Scholar]
- 14. Greenwel P, Schwartz M, Rosas M, Peyrol S, Grimaud JA, Rojkind M. Characterization of fat-storing cell lines derived from normal, and CCl4-cirrhotic livers. Differences in the production of interleukin-6. Lab Invest 65: 644–653, 1991 [PubMed] [Google Scholar]
- 15. Henry MD, Campbell KP. A role for dystroglycan in basement membrane assembly. Cell 95: 859–870, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Hewitt JE. Abnormal glycosylation of dystroglycan in human genetic disease. Biochim Biophys Acta 1792: 853–861, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source, and biological activity of the E.I.I.I.A. segment in rat hepatic fibrogenesis. J Cell Biol 127: 2037–2048, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kato R, Kamiya S, Ueki M, Yajima H, Ishii T, Nakamura H, Katayama T, Fukai F. The fibronectin-derived antiadhesive peptides suppress the myofibroblastic conversion of rat hepatic stellate cells. Exp Cell Res 265: 54–63, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Knittel T, Mehde M, Grundmann A, Saile B, Scharf JG, Ramadori G. Expression of matrix metalloproteinases, and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol 113: 443–453, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T.4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 21. Lee KS, Buck M, Houglum K, Chojkier MJ. Activation of hepatic stellate cells by TGFalpha, and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest 96: 2461–2468, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martinez-Hernandez A. The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest 53: 166–186, 1985 [PubMed] [Google Scholar]
- 23. Martinez-Hernandez A, Amenta PS. The hepatic extracellular matrix. I. Components and distribution in normal liver. Virchows Arch A Pathol Anat Histopathol 423: 1–11, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Martinez-Hernandez A, Amenta PS. The extracellular matrix in hepatic regeneration. FASEB J 9: 1401–1410, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Milliano MT, Luxon BA. Initial signaling of the fibronectin receptor (alpha5beta1 integrin) in hepatic stellate cells is independent of tyrosine phosphorylation. J Hepatol 39: 32–37, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Neufeld DS. Isolation of rat liver hepatocytes. Methods Mol Biol 75: 145–151, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Patsenker E, Popov Y, Wiesner M, Goodman SL, Schuppan D. Pharmacological inhibition of the vitronectin receptor abrogates PDGF-BB-induced hepatic stellate cell migration, and activation in vitro. J Hepatol 46: 878–887, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Rojkind M, Reyes-Gordillo K. The Liver: Biology and Pathobiology, 5th edition, edited by Gillet J, Okabe M, Gottesman M, Arias I, Fausto N, Boyer J, Chisari F, Shafritz D. Oxford, UK: Wiley-Blackwell, 2009, pp. 407–432 [Google Scholar]
- 29. Rovida E, Navari N, Caligiuri A, Dello Sbarba P, Marra F. ERK5 differentially regulates PDGF-induced proliferation, and migration of hepatic stellate cells. J Hepatol 48: 107–115, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Shimizu H, Hosokawa H, Ninomiya H, Miner JH, Masaki T. Adhesion of cultured bovine aortic endothelial cells to laminin-1 mediated by dystroglycan. J Biol Chem 274: 11995–12000, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Sohara N, Znoyko I, Levy MT, Trojanowska M, Reuben A. Reversal of activation of human myofibroblast-like cells by culture on a basement membrane-like substrate. J Hepatol 37: 214–221, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Svegliati-Baroni G, Inagaki Y, Rincon-Sanchez AR, Else C, Saccomanno S, Benedetti A, Ramirez F, Rojkind M. Early response of alpha2(I) collagen to acetaldehyde in human hepatic stellate cells is TGF-beta independent. Hepatology 42: 343–352, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thompson O, Moore CJ, Hussain SA, Kleino I, Peckham M, Hohenester E, Ayscough KR, Saksela K, Winder SJ. Modulation of cell spreading, and cell-substrate adhesion dynamics by dystroglycan. J Cell Sci 123: 118–127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang C, Zeisberg M, Mosterman B, Sudhakar A, Yerramalla U, Holthaus K, Xu L, Eng F, Afdhal N, Kalluri R. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix, and growth factors. Gastroenterology 124: 147–159, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L, Campbell KP. Omannosyl phosphorylation of alpha-dystroglycan is required for laminin binding. Science 327: 88–92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]