Abstract
Altered profiles of gene expression reflect the reprogramming of intestinal epithelial cells during their maturation along the crypt-luminal axis. To focus on genes important in this process, and how they in turn are regulated, we identified 14 transcripts commonly downregulated in expression during lineage-specific maturation of the immortalized cell lines Caco-2 (absorptive), HT29Cl16E (goblet), and HT29Cl19A (secretory) induced by contact inhibition of growth or the short-chain fatty acid butyrate. One such gene, Mybl2 (Myb-related protein B), has been linked to the stem cell phenotype, and we report is also markedly suppressed in maturing cells along the crypt-luminal axis in vivo. Mybl2 is not significantly downregulated transcriptionally during colon cell maturation, but we identified a potential micro-RNA (miRNA)-binding sequence in the Mybl2 3′-untranslated region that mediates reporter gene suppression in differentiating colon cells. Accordingly, miRNAs predicted to bind this functional target are upregulated in differentiating colon epithelial cells in vitro and in vivo; expression of one of these, hsa-miR-365 (but not hsa-324–5p), suppresses Mybl2 protein expression in proliferating Caco-2 cells. These data demonstrate that miRNA silencing plays an important role in regulating gene expression in maturing colon epithelial cells, and that utilizing a target-centered approach, rather than profiling global miRNA expression, can identify physiologically relevant, functional miRNAs.
Keywords: differentiation, intestine, micro-RNA, crypt-luminal axis
normal intestinal homeostasis is dependent on reprogramming of highly proliferative, multipotent stem/progenitor cells into enterocytic, goblet, and enteroendocrine cells as they migrate along the crypt axis toward the lumen and Paneth cells that remain at the bottom of the crypt. Stem and progenitor cells at the crypt base robustly express genes that promote cell cycling, driving high levels of cell division, while they suppress expression of phenotypic markers of mature states. As these cells migrate from the crypt toward the lumen, many signals and pathways regulate their reprogramming to exit the cell cycle and undergo lineage-specific differentiation, predominantly through changes in gene expression (42, 74, 75, 77). Therefore, identifying molecules that drive this maturation and understanding how they are, in turn, regulated will provide crucial insights into how colon epithelial cells acquire mature states and avoid acquisition of premalignant and malignant phenotypes.
Reprogramming of maturing colonic epithelial cells is recapitulated in some colon adenocarcinoma cell lines in culture. These can often be induced to undergo cell-cycle arrest and express differentiated phenotypes by the short-chain fatty acid butyrate, a product of dietary fiber fermentation present at concentrations approaching 20 mM in the colon and a physiological regulator of colonic cell maturation in vivo (4, 25, 55, 68). Human adenocarcinoma cell lines have also been isolated by selection for cells that undergo contact inhibition of growth, followed by lineage-specific differentiation along absorptive (Caco-2), goblet (HT29Cl16E), or secretory (HT29Cl19A) cell lineages. We and others have dissected changes in gene expression profiles of these cell lineages as they differentiate (20, 42, 77), and we have also identified RNA (44) and protein expression profiles (10) that characterize intestinal cell maturation in vivo by analysis of cells eluted sequentially from the crypt-villus axis of the mouse small intestine. The requirements for reprogramming these highly proliferative tumor cells into growth-arrested, differentiating cells has shed light on many mechanisms that drive maturation of normal colon epithelial cells (58, 72).
Transcriptional regulation is responsible for many gene expression changes in maturing colon epithelial cells, including Wnt target gene repression (43), Notch target gene de-repression (66), and activation of phenotypic markers, such as alkaline phosphatase (28) and dipeptidyl peptidase IV (45). Nevertheless, posttranscriptional mechanisms, in particular silencing by micro-RNAs (miRNAs), also play critical roles in reprogramming of cells. These small, noncoding RNAs direct an RNA-induced silencing complex to mRNA targets by complementary base pairing between the miRNA and sequences in the 3′-untranslated region (UTR) of the message, resulting in mRNA degradation and/or translational repression (29, 30, 73). Recently, miRNAs have also been demonstrated to relieve translational inhibition of certain genes by acting as decoys that sequester heterogeneous nuclear ribonucleoprotein E2 from target mRNAs (16). miRNAs frequently target genes in developmental pathways, and their aberrant expression has been implicated in the pathogenesis of several diseases, including cancer (27) and cardiomyopathy (76).
Here we identify a subset of genes commonly downregulated in four independent colon cell models of cell maturation: butyrate-induced growth arrest and differentiation, and spontaneous differentiation along the absorptive, goblet, and deep-crypt secretory cell lineages (Caco-2, HT29Cl16E, and HT29Cl19A cells, respectively), triggered by contact-mediated growth arrest. One of these genes, Mybl2 (Myb-related protein B), belongs to a family of transcription factors linked to both cell cycling and differentiation in multiple systems (reviewed in Refs. 53, 60), and importantly, is a transcriptional regulator highly expressed in a number of pluripotent stem cells (47) and is critical to maintenance of the stem cell state (9, 69). We demonstrate that Mybl2 is indeed regulated in expression along the crypt-villus axis in vivo and that the downregulation during maturation is driven not by transcriptional regulation, but at least in part by a miRNA-mediated mechanism involving miR-365.
MATERIALS AND METHODS
Cell culture.
The Caco-2 human adenocarcinoma cell line was maintained as described (42) in medium containing 20% fetal bovine serum. Spontaneous differentiation: cells were grown to confluence (day 0) and harvested at various time points thereafter (medium changes every 2 days). For butyrate induction, cells were treated with 5 mM sodium butyrate when ∼95% confluent (day 0) and harvested at various time points thereafter.
Microarray.
Caco-2, HT29Cl16E, and HT29Cl19A RNA isolation, probe synthesis, array hybridization and scanning, and data analysis have been reported (42, 77), utilizing cDNA arrays prepared by Albert Einstein College of Medicine microarray facility (13). For each time point, arrays were performed on two independent chips: two with 8,063 sequences (16E, 19A), or one with 8,064 and the second with 9,216 (Caco-2). Criteria for altered expression was at least 1.5-fold change in day 14–15 spontaneously differentiating, or day 2 butyrate-induced, cells relative to day 0 cells. Functional group classifications utilized gene ontology annotations (http://www.geneontology.org/).
Western blot.
Caco-2 cells, harvested by scraping from six-well plates, were sonicated on ice for two 5-s pulses with a 30-s pause (setting 2, 7.5% output; Branson 450 Sonifier, Fisher Scientific). Caco-2 lysate (20 μg), 100 μg mouse small intestine (encompassing duodenum, jejunum, and ileum) crypt-villus tissue (44), or 30 μg human colon crypt-luminal tissue was analyzed by Western blotting, as described (52) except protease inhibitor cocktail was from Sigma, and blocking and antibody dilution buffer was 5% milk/Tris-buffered saline-Tween (0.05% Tween 20, 100 mM Tris pH 7.5, 150 mM NaCl). Blots were developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific), with signal detected using film or a Kodak 4000R Image Station, and analyzed with Kodak Molecular Imaging Software, version 4.0 (Eastman Kodak). Primary antibodies were as follows: 1:10 mouse anti-Mybl2 (R. Watson, Imperial College School of Medicine, London, UK), 1:1,000 rabbit anti-alkaline phosphatase (NB 600–588, Novus Biologicals), 1:4,000 mouse anti-α-tubulin (T5168, Sigma), 1:500 mouse anti-villin (610358, BD Transduction Laboratories), 1:1,000 rabbit anti-histone H2A (07–146, Millipore; gift of Dr. Richard Chawan), or 1:1,000 rabbit anti-GAPDH (Abcam; gift of Dr. Art Skoultchi). Secondary antibodies were 1:2,000 goat anti-mouse or -rabbit IgG-horseradish peroxidase (Santa Cruz).
Quantitative RT-PCR.
Cells were collected in Trizol (Invitrogen), and total RNA was prepared as described by the manufacturer. cDNA was synthesized from 1 μg total RNA using the iScript cDNA Sythesis Kit (Bio-Rad). Quantitative RT-PCR (qRT-PCR) utilized SYBR Green PCR Master Mix (Applied Biosystems) in 30 μl with 50 ng cDNA and 100 nM primers, with a 7900HT Sequence Detection System (Applied Biosystems). Cycling conditions were as follows: 50°C for 2 min; 95°C for 10 min; 40 cycles of 95°C for 15 s, melting temperature (Tm) for 30 s, 60°C for 30 s. For pre-mRNA and mRNA quantification, Tm = 60°C. For miRNA quantification, Tm was specific for each primer set (see miRNA quantification below). Specificity of amplification was confirmed by single peaks in dissociation curves and/or single bands on agarose gel electrophoresis. Analysis utilized SDS version 2.3 software (Applied Biosystems). GAPDH-normalized mRNA expression in differentiating cells was calculated relative to that in proliferating cells by the 2−ΔΔCt method (38). Pre-mRNA quantification utilized primers spanning intron 2/exon 3 of Mybl2, exon 4/intron 4 of cyclin D2, and intron 1/exon 2 of c-myc. Primers are listed in Supplemental Table S1. (The online version of this article contains supplemental data.)
Reporter assays.
Subconfluent/proliferating or day 10 or 12 spontaneously differentiating Caco-2 cells were transfected in 12-well plates with 1 μg/well luciferase reporter + 1 μg/well pRL-TK renilla (Promega, Madison, WI) for 48 h using Lipofectamine Plus (Invitrogen), as described by the manufacturer. The ratio of renilla-normalized luciferase activity in differentiating vs. proliferating cells was determined. Reporters were as follows: pGL2 Basic with luciferase expression driven by nucleotides −910 to −102 of the human Mybl2 promoter [R. Watson (35)] and TOP flash (33) driven by three T-cell factor binding sites upstream of a minimal c-fos promoter. Lysate (20 μl) was assayed for luciferase and renilla activity on the LMaxII dual-injection microplate reader (Molecular Devices). Data analysis utilized SoftMax Pro 5 software (Molecular Devices).
Determination of potential miRNA targets in the Mybl2 3′-UTR.
miRNAs predicted to bind the human Mybl2 3′-UTR were identified using miRBase Targets version 5 (http://microrna.sanger.ac.uk/targets/v5/), and predicted targets were compiled into 15 nonidentical sequences. RNA secondary structures were computationally modeled using GeneBee (http://www.genebee.msu.su/services/rna2_reduced.html) and Vienna (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi) algorithms. Targets forming secondary structures exhibiting negative free energies were considered further.
Assay for functional miRNA targets.
A double-stranded oligo consisting of recognition sites for the restriction endonucleases XbaI-SpeI-EcoRI-XbaI was subcloned into the XbaI site of pGL3 Control (Promega), two residues downstream of the luciferase stop codon, in forward and reverse orientations. miRNA targets, synthesized as single-stranded complementary oligos (Sigma Genosys), consisted of a dimerized Mybl2 3′-UTR potential miRNA binding site (see Fig. 6), separated by [residue A-recognition site for BamHI-residue A] and flanked by SpeI (5′) and EcoRI (3′) recognition sites. Annealed, double-stranded oligos were subcloned into pGL3 Control (forward and reverse) vectors digested with SpeI and EcoRI. The ratio of renilla-normalized luciferase activity of miRNA target (forward or reverse)-harboring vector vs. pGL3 control (forward or reverse) alone was calculated. miRNA targets suppressing parental pGL3 control activity by ≥50% were considered functional.
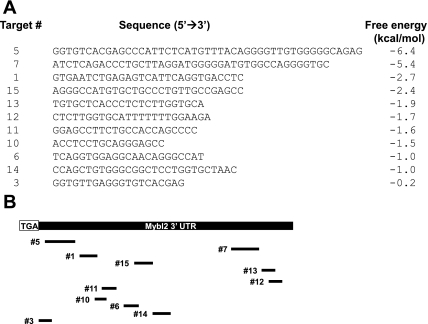
Fig. 6.
A. Putative Mybl2 3′-untranslated region (UTR) micro-RNA (miRNA) targets and predicted free energies were determined as described in materials and methods. Shown are the sequences of those targets with a free energy < 0. B: schematic showing positions of putative miRNA targets in the Mybl2 3′-UTR relative to the translational stop codon (TGA).
miRNA quantitation.
Cells were harvested in Trizol (Invitrogen), and total RNA, including those <200 nucleotides, was isolated using the mirVana miRNA Isolation Kit (Ambion), according to the manufacturer. cDNA was synthesized from 2 μg total RNA using primer RTQ, as described (57) with minor modifications. cDNA (∼10 ng) was used for qRT-PCR with a miRNA-specific primer and the universal primer RTQ-UNIr using an annealing temperature specific to each set of primers (miR-365, 588, 130a, 143, 145: Tm = 48°C; miR-30a, 362–5p, 10a, 205, 221: Tm = 50°C; miR-331–3p, 34a and let-7a1, 5S: Tm = 60°C; miR-324–5p: Tm = 75°C), and miRNA expression was normalized to that of 5S rRNA. Primers for miRNAs 588, 145, and 324–5p amplified a pre-miRNA as well as a mature form. Primer sequences are listed in Supplemental Table S1.
miRNA transfection.
Proliferating, subconfluent Caco-2 cells (∼8,333 cells/cm2) were transfected for 48 h with 100 nM miRNA (miRIDIAN miRNA mimics, Thermo Scientific Dharmacon) using Oligofectamine (Invitrogen), as described (52).
Mechanical isolation of human colon tissue along crypt-luminal axis.
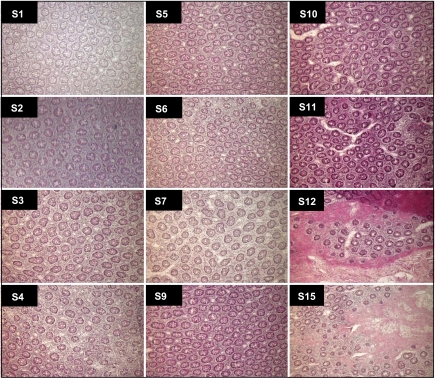
Tissue sample preparation was essentially as described (34). Briefly, tissue from a colectomy specimen removed at surgery and forwarded to Pathology at Montefiore Medical Center was obtained within ∼45 min to 1 h after resection. Four sections of morphologically normal colon mucosae, each 2.5 cm × 0.5 cm, were dissected free from underlying muscularis propria, embedded in OCT medium (Sakura Finetek) luminal face down, and frozen in liquid nitrogen. With the luminal aspect facing the blade, 10-μm-thick horizontal sections were cut sequentially from the luminal surface, such that early fractions contained cells at the top of the crypt, and later sections contained cells at the base of the crypt. Sections from ∼50-μm intervals were processed for hematoxylin and eosin staining to confirm morphology and position along the crypt-luminal axis (see Fig. 3), with intervening sections collected into 1.5-ml microfuge tubes (1 or 2 sections/tube for RNA or protein, respectively) and kept frozen at −80°C. Sections were processed for total RNA isolation using the mirVana kit, and unamplified RNA was used for qRT-PCR and miRNA quantitation, as described above. Protein lysates, prepared by sonicating two sections on ice in 250 μl at 7.5% output for two 5-s pulses with a 30-s pause, were analyzed by Western blotting, as described above. This protocol was classified as exempt, per federal regulations 45 CFR 46.101 (b), by the Montefiore Medical Center Institutional Review Board.
Fig. 3.
Sections (S1–S15) mechanically isolated from the crypt-luminal axis of the human colon, as described in materials and methods, were stained with hematoxylin and eosin. Magnification = ×10.
RESULTS
Genes commonly regulated in colon epithelial cell differentiation.
Using our laboratory's published databases (Refs. 42, 77, available at www.augenlichtlab.com), gene expression profiles were compared at intermediate time points of differentiation of colon epithelial cell lines to identify sequences that potentially regulate colon cell maturation rather than those that arise as downstream markers of a mature state. Therefore, data were analyzed at 14–15 days of spontaneous differentiation in Caco-2, HT29Cl16E, and HT29Cl19A cells, which fully differentiate, respectively, along the absorptive, goblet, and deep-crypt secretory cell lineages in 20–21 days, and at 2 days of butyrate treatment of Caco-2 cells, which require 5–7 days to fully express absorptive cell markers. Comparison of >8,000 overlapping sequences in these databases identified 14 significantly downregulated (reduced ≥1.5-fold relative to proliferating cells) in all differentiating cells (Table 1). These sequences encompass functional groups involving chromosome organization, transcriptional activity, DNA replication and repair, cell cycle, RNA processing and transport, translation, protein folding and modification, and cell adhesion. Each sequence was altered during maturation of each of three distinct colon epithelial cell lineages induced by two different methods and, therefore, likely functions in the complex process of colon cell maturation.
Table 1.
Genes downregulated at least 1.5-fold in differentiating colon epithelial cells
| Fraction of Undifferentiated RNA |
|||||
|---|---|---|---|---|---|
|
Day 14–15 spontaneous |
Day 2 NaB |
||||
| Gene Name (Gene ID) | HT29 Cl16E | HT29 Cl19A | Caco-2 | Caco-2 | Functional Category |
| Cyclin A2 (CCNA2) | 0.56 | 0.64 | 0.51 | 0.35 | Cell cycle |
| Cyclin D2 (CCND2) | 0.61 | 0.66 | 0.29 | 0.61 | Cell cyle |
| Myb-related protein B (Mybl2) | 0.58 | 0.64 | 0.54 | 0.54 | Transcription factor activity |
| G1 to S phase transition 1 (GSPT1) | 0.60 | 0.55 | 0.53 | 0.60 | Cell cycle, translation |
| Forkhead box M1 (FOXM1) | 0.57 | 0.45 | 0.56 | 0.44 | Transcription factor activity |
| Minichromosome maintenance deficient 6 (MCM6) | 0.50 | 0.60 | 0.66 | 0.40 | DNA replication |
| Disks, large homolog (DLG1) | 0.45 | 0.62 | 0.56 | 0.48 | Cell-cell adhesion |
| Structural maintenance of chromosomes 2-like 1 (SMC2L1) | 0.62 | 0.49 | 0.61 | 0.62 | Mitotic chromosome condensation |
| Nibrin isoform 1 (NBS1) | 0.63 | 0.62 | 0.66 | 0.66 | DNA repair |
| Glutamyl-prolyl tRNA synthetase (EPRS) | 0.34 | 0.52 | 0.58 | 0.53 | Aminoacyl-tRNA ligase activity |
| Exosome component 9 (EXOSC9) | 0.58 | 0.66 | 0.60 | 0.45 | rRNA processing |
| tRNA exportin (XPOT) | 0.53 | 0.55 | 0.64 | 0.52 | tRNA binding, transport |
| NAT13, Mak3 homolog (MAK3) | 0.63 | 0.58 | 0.55 | 0.58 | N-acetyltransferase activity |
| Chaperonin containing TCP1, subunit 7 isoform b (CCT7) | 0.58 | 0.66 | 0.49 | 0.62 | Protein folding |
The average microarray signal intensity for each gene in day 14-15 spontaneously differentiated Caco-2, HT29Cl16E, or HT29Cl19A or day 2 butyrate-induced Caco-2 cells is indicated relative to the signal intensity, arbitrarily assigned a value of 1, for each gene in undifferentiated cells. Details of microarray analysis have been described (42, 77). Mybl2 (boldface type), a transcription factor involved in proliferation and differentiation of several cell types, was studied further. NaB, sodium butyrate.
Mybl2 is suppressed in differentiating colon cells in vitro and in vivo.
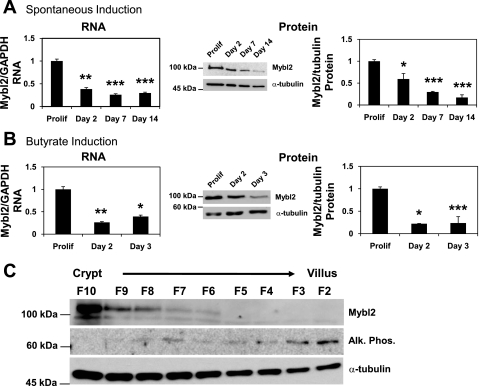
One of the 14 downregulated genes (Table 1) is Mybl2, a sequence that encodes a transcription factor with an established role in promoting cell proliferation (3, 23, 36, 37, 49, 56, 59, 63) and a putative function in regulating differentiation of a number of cell types (8, 17, 22, 54, 56). As expected from analysis of the expression array databases (above), Mybl2 conformed to this expression profile, with Mybl2 RNA and protein robustly expressed in proliferating Caco-2 cells, but significantly suppressed in cells undergoing differentiation induced by either contact-mediated growth arrest (Fig. 1A) or butyrate treatment (Fig. 1B). Importantly, Mybl2 has been identified as a marker of stemlike, pluripotent cells (9, 47).
Fig. 1.
A and B: RNA (left) or protein (right) levels of Mybl2 (Myb-related protein B) in Caco-2 cells grown to confluency and spontaneously differentiated (Sp) for 2, 7, or 14 days (A) or induced to differentiate for 2 or 3 days with 5 mM sodium butyrate (B) were measured by real-time qPCR or Western blotting. For RNA, each bar represents the average + SE of 3 independent experiments, each performed in duplicate, of Mybl2 normalized to GAPDH and expressed relative to that in subconfluent, proliferating cells (Prolif). For protein, each bar represents the average + SE of 3 independent experiments of Mybl2 normalized to α-tubulin and expressed relative to that in Prolif. Middle panels are representative Western blots. Unpaired, 2 tailed Student's t-test (relative to Prolif): *P < 0.05; **P < 0.005; ***P < 0.0005. C: Mybl2, alkaline phosphatase (ALPi), and α-tubulin protein levels in tissue fractions isolated sequentially along the crypt-villus axis of the whole mouse small intestine (encompassing duodenum, jejunum, and ileum) were measured by Western blotting.
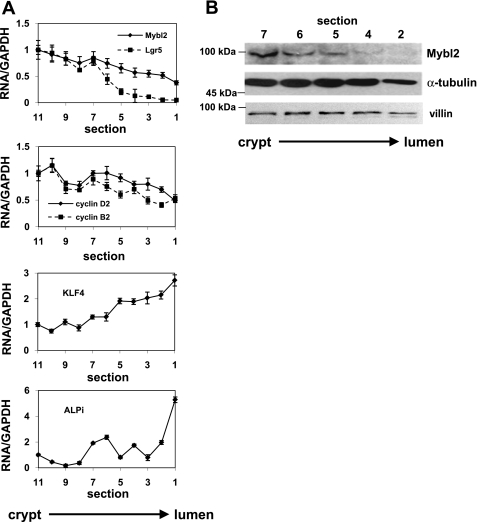
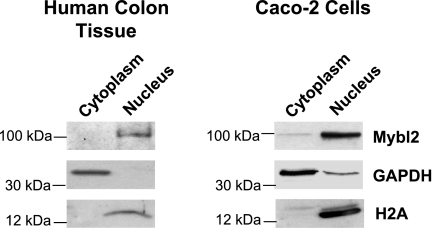
Two methods were used to determine the pattern of expression of Mybl2 along the crypt-luminal axis. First, with mouse tissue, we used sequential elution of cells from the intestinal mucosa that, as we and others have extensively validated, isolates cells as a function of their position along the crypt-villus axis of the mouse small intestine in vivo (19, 44, 64, 79). Second, we sectioned fresh frozen human mucosal samples from the luminal surface downward toward the crypts (see materials and methods). These approaches demonstrate that mouse (Fig. 1C) and human (Fig. 2B) Mybl2 protein is most highly expressed in the stem/progenitor cell compartment at the bottom of the crypt and decreases in a gradient to undetectable levels as intestinal epithelial cells undergo maturation along the crypt-luminal axis. This decreasing Mybl2 expression in these fractions as cells mature along the crypt-villus axis is similar to that of proteins, such as cyclin-dependent kinase 2 and proliferating cell nuclear antigen, which drive proliferation and is in contrast to that of differentiation markers, such as alkaline phosphatase (Fig. 1C) or villin (Fig. 2B) (44, 64). Similarly, RNA for Mybl2, the cell-cycle promoting factors cyclin B2 and cyclin D2, and the stem cell marker Lgr5 are highly expressed in human stem and progenitor cell populations at crypt bottoms and decrease markedly in differentiating cells near the lumen, whereas RNA for the differentiation-specific markers gut enriched Kruppel-like factor-4 and alkaline phosphatase exhibit the opposite expression pattern and are enriched in differentiated cells near the lumen (Fig. 2A). Hematoxylin and eosin staining confirms an ordered architecture of cells in these human colon tissues, with well-organized crypts surrounded by lamina propria throughout the crypt-luminal axis (Fig. 3). Mybl2's localization to the nuclei of these human mucosal cells (Fig. 4), similar to that in Caco2 cells (Fig. 4), confirms its role as a transcriptional regulator of key genes in the colon.
Fig. 2.
A: RNA levels of Mybl2, leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5), cyclin B2, cyclin D2, Kruppel-like factor-4 (KLF4), and ALPi in tissue fractions isolated sequentially from the crypt-luminal axis of the human colon were measured by real-time PCR. Values shown are normalized to GAPDH and expressed relative to the level in the fraction at crypt bottom. B: Mybl2, α-tubulin, and villin protein levels in human colon crypt-luminal tissue fractions were measured by Western blotting.
Fig. 4.
Human colon tissue and Caco-2 cells were fractionated into nuclei and cytoplasm, as described in Supplemental Materials and Methods. Fractions were Western blotted with antibodies to Mybl2, GAPDH, and histone H2A.
Transcriptional repression of Mybl2 is not significant during colon cell maturation.
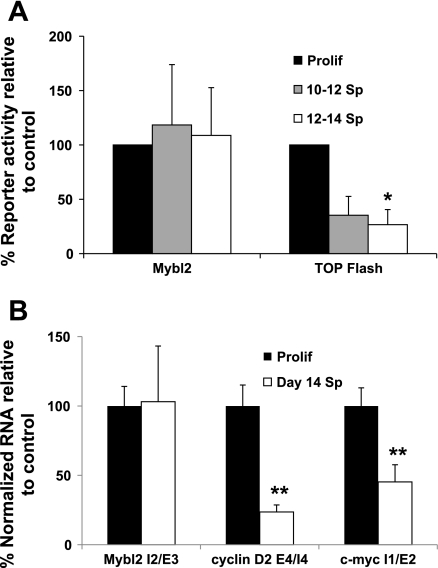
Surprisingly, there was no difference in transfected Mybl2 promoter activity in proliferating vs. spontaneously differentiated Caco-2 cells (Fig. 5A). Note the data are corrected for transfection efficiency by cotransfection with pRL-TK renilla (materials and methods), eliminating the possibility that these, as well as other, results (below) are influenced by differential transfection efficiency of cells in different physiological states. As a further control, differentiating Caco-2 cells did significantly suppress the TOP FLASH reporter [driven by a T-cell factor-responsive promoter, (33)], to 31% of the activity in proliferating cells (Fig. 5A). As confirmation of these findings, levels of Mybl2 pre-mRNA transcripts, consisting of unspliced intron/exon boundary sequences for which expression indicates ongoing transcription, are not significantly altered in day 14 spontaneously differentiated Caco-2 cells compared with proliferating cells (Fig. 5B), whereas, in contrast, pre-mRNA transcripts of cyclin D2 and c-myc, Wnt pathway target genes transcriptionally downregulated during colon cell maturation, are decreased 76% (cyclin D2) and 55% (c-myc), respectively (Fig. 5B). Therefore, transcriptional repression cannot account for the dramatic changes in expression of Mybl2 during colon cell differentiation.
Fig. 5.
A: Prolif as well as day 10 or 12 Sp Caco-2 cells were transfected with a luciferase reporter construct driven by the 808-bp human Mybl2 promoter [containing all known cis-acting elements that repress Mybl2 transcription, including the major repressive E2F binding site (7, 35, 36)] or the T-cell factor-responsive TOP flash promoter. After 48 h, luciferase reporter activity was normalized to co-transfected pRL-TK renilla reporter activity and expressed relative to that in Prolif. B: primers flanking the indicated intron/exon boundaries of Mybl2, cyclin D2, and c-myc genes were used to amplify pre-mRNA (a measure of ongoing transcription) by quantitative RT-PCR of cDNA from Prolif or day 14 Sp Caco-2 cells. pre-mRNA levels are normalized to GAPDH mRNA and expressed as a percentage of those in Prolif. Each bar represents the mean + SE of 3 independent experiments, each performed in triplicate (A) or duplicate (B). Unpaired, two-tailed Student's t-test (relative to Prolif): *P < 0.01, **P < 0.05.
Potential miRNA binding sites in the Mybl2 3′-UTR suppress reporter gene activity.
Among potential nontranscriptional mechanisms mediating Mybl2 repression in differentiating colon cells, the contribution of miRNA-mediated repression is of particular interest, since global expression profiles of miRNAs suggest that miRNA expression is closely associated with differentiated states (39). Moreover, specific miRNAs are reported to be fundamental in differentiation of hematopoietic (12, 18, 39), neuronal (41), muscle (32, 81), and adipocyte (67) cells.
Using a functional approach, rather than global screening of miRNA expression, we identified potential miRNA targets in the Mybl2 3′-UTR by searching the Sanger database, a regularly updated repository of known miRNAs. Many predicted target regions overlapped; these were combined into 15 nonidentical potential miRNA binding sites, ranging from 17 to 47 nucleotides in length and exhibiting a range of predicted free energies, as modeled using GeneBee (http://www.genebee.msu.su/services/rna2_reduced.html) and Vienna (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi) algorithms. We hypothesize that sequences exhibiting negative free energies are more stable and likely form bona fide miRNA targets. Thus, 11 of 15 Mybl2 3′-UTR sequences predicted to form secondary structures with negative free energies (Fig. 6A) were further analyzed.
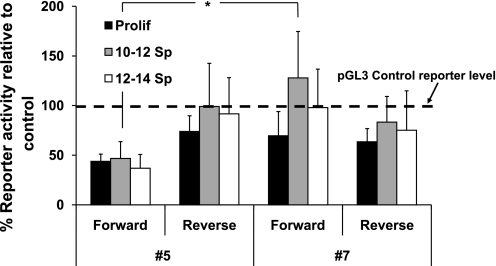
We investigated functionality of these putative miRNA targets by testing their abilities to mediate miRNA suppression of a reporter gene in spontaneously differentiating Caco-2 cells. We initially tested no. 5 and no. 7, sequences that form structures with low free energies [−6.4 (no. 5) or −5.4 (no. 7) kcal/mol] and similar lengths [47 (no. 5) or 42 (no. 7) residues], but different locations in the Mybl2 3′-UTR [10 bp (no. 5) or 296 bp (no. 7) downstream of the translational stop codon; see Fig. 6B]. Each sequence was inserted downstream of the luciferase coding region in the pGL3 control vector in either the forward or, as a negative control, reverse orientation (see materials and methods), and its ability to suppress luciferase gene expression assayed, again corrected for transfection efficiency by cotransfection of pRL-TK renilla. During spontaneous Caco-2 cell differentiation, sequence no. 5 decreased reporter gene expression by ∼60% in proliferating Caco-2 cells and by a similar, but slightly greater, extent in differentiating cells (Fig. 7). In each case, insertion of this sequence in reverse orientation either did not alter, or decreased by <50%, reporter expression (Fig. 7). In contrast, sequence no. 7 did not significantly decrease reporter expression under any condition. Thus, sequence no. 5, but not no. 7, may be a bona fide miRNA target in the Mybl2 transcript that acquires a proper configuration to bind relevant miRNAs and thus contribute to Mybl2 regulation (see discussion).
Fig. 7.
pGL3 control or pGL3 control harboring putative Mybl2 3′-UTR miRNA target no. 5 or no. 7 (see Fig. 6) in the 3′-UTR of luciferase were transfected into subconfluent/Prolif or day 10 or 12 Sp Caco-2 cells. After 2 days, luciferase activity from miRNA target-harboring reporter plasmids was normalized to cotransfected Renilla reporter activity and expressed relative to that from the parental pGL3 control reporter. Fifty percent of control activity was considered the threshold for significant repression. The dashed line indicates reporter activity in cells transfected with pGL3 control alone, set to 100%. Each bar is the mean + SE of at least 3 independent experiments, each performed in triplicate. Unpaired, two-tailed Student's t-test (no. 5 vs. no. 7, day 10–12): *P < 0.05.
Differentiating colon cells upregulate expression of known miRNAs targeting functional Mybl2 3′-UTR sequence no. 5 in vitro and in vivo.
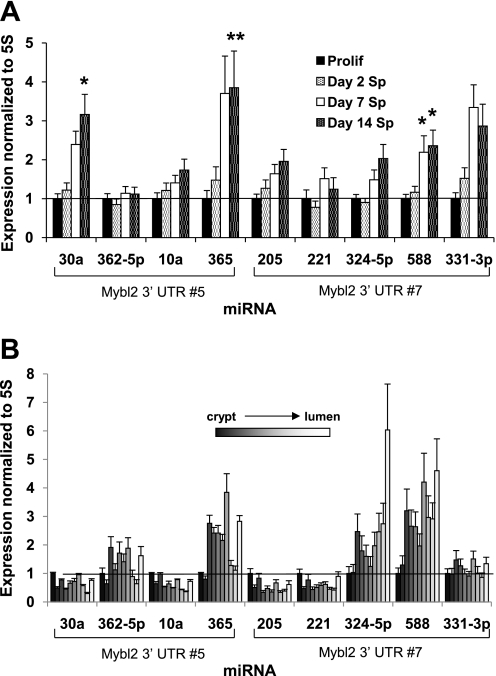
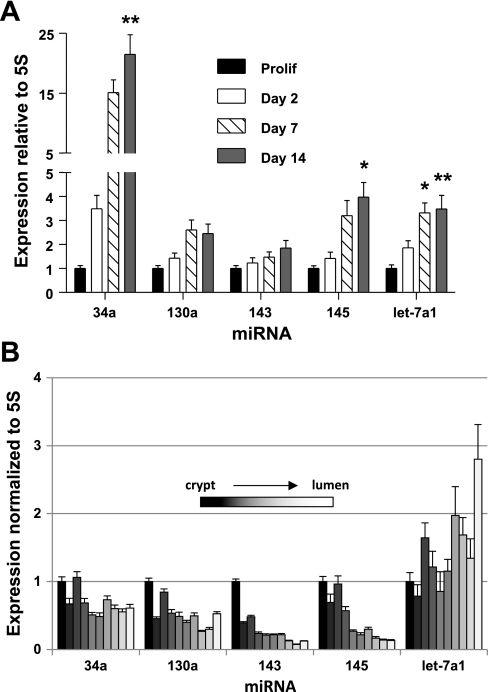
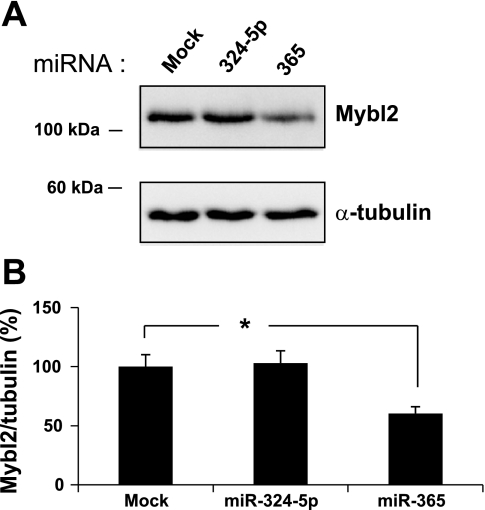
To determine whether known miRNAs contribute to Mybl2 suppression in maturing colon cells, we used qRT-PCR to assess expression of relevant miRNAs. In a subset of miRNAs predicted to bind functional Mybl2 3′-UTR sequence no. 5 (miR-30a, 362–5p, 10a, and 365), 30a and 365 substantially increased (mean 2.5-fold) in day 14 spontaneously differentiating Caco-2 cells compared with proliferating cells (Fig. 8A), with the largest increase of 3.8-fold seen for 365. There was a somewhat smaller increase in 10a, and a marginal increase in 362–5p. In addition, several miRNAs predicted to bind nonfunctional sequence no. 7 (miR-205, 221, 324–5p, 588, and 331–3p) also increased, but to a lower mean level of 2.1-fold over the level in proliferating cells (Fig. 8A). Differentiated cells along the human colon crypt-luminal axis in vivo also exhibit an increase in miR-362–5p (1.9-fold), 365 (3.8-fold), 324–5p (6.0-fold), and 588 (4.6-fold) relative to proliferating cells at the crypt bottom (Fig. 8B). Other miRNAs reported to play important roles in the colon, including miR-34a, 130, 145, and let-7a1, are also increased in differentiating Caco-2 cells (Fig. 9A). Interestingly, mature cells along the human crypt-villus axis exhibit increased let-7a1 but decreased miR-34a, 130, 143, and 145 expression (Fig. 9B). Importantly, transfection of miR-324–5p (targeting nonfunctional site no. 7) into subconfluent, proliferating Caco-2 cells had no effect on Mybl2 protein expression (Fig. 10). In contrast, miR-365 (targeting functional site no. 5) suppressed Mybl2 protein level ∼40% compared with that of mock-transfected cells (P < 0.0005, Fig. 10). Therefore, the functional miRNA binding site we identified in the Mybl2 3′-UTR indeed regulates levels of Mybl2 by one of its cognate miRNAs.
Fig. 8.
cDNAs synthesized from total RNA (including small RNAs under 200 nucleotides) isolated from Prolif and day 2, 7, and 14 Sp Caco-2 cells (A) or tissue fractions mechanically isolated sequentially along the crypt-luminal axis of the human colon (B) were used as templates for amplification of the indicated miRNAs by quantitative RT-PCR, as described in materials and methods. Expression of each miRNA was normalized to 5S RNA and plotted relative to the level in Prolif (A) or tissue at crypt bottom (B), which is assigned a value of 1. Each bar represents the mean + SE of 3 independent experiments, each performed in duplicate. Unpaired, two-tailed Student's t-test (relative to Prolif): *P < 0.05; **P < 0.005. Analysis of the Mybl2 3′-UTR (http://microrna.sanger.ac.uk/targets/v5/) indicates that miRNAs 30a, 362–5p, 10a, and 365 potentially target Mybl2 3′-UTR sequence no. 5, and that miRNAs 205, 221, 324–5p, 588, and 331–3p potentially target sequence no. 7.
Fig. 9.
Expression of the indicated miRNAs in Prolif or day 2, 7, or 14 Sp Caco-2 cells (A) or in tissue fractions mechanically isolated sequentially along the crypt-luminal axis of the human colon (B) was determined as described in Fig. 8. Unpaired, two-tailed Student's t-test (relative to Prolif): *P < 0.05; **P < 0.005.
Fig. 10.
A: Proliferating Caco-2 cells were transfected for 48 h with 100 nM miR-324–5p, 100 nM miR-365, or vehicle alone (Mock), and lysates were Western blotted for Mybl2, then for α-tubulin on the same blot. B: quantification of Western blot signals in A. Each bar represents the average + SE of 3 independent experiments and is expressed as the α-tubulin-normalized percentage of Mybl2 band intensity relative to Mock band intensity. Unpaired Student's two-tailed T-test (relative to Mock): *P < 0.01.
DISCUSSION
Exit of cells from the proliferative/progenitor cell compartment in intestinal crypts is coupled to commitment to specific lineages of differentiation. Numerous genes and pathways, including c-myc, cyclin D2, p27Kip1, Wnt signaling, and Notch signaling, regulate these transitions to mature intestinal epithelial cells. Correct functioning and homeostasis of the intestinal mucosa, including proper cessation of cell growth and commitment to differentiation, requires coordination and integration among these genes and pathways that likely contribute distinct as well as overlapping functions and regulation.
Using databases that analyze model systems of differentiation in colon epithelial cells, we identified 14 sequences commonly downregulated during maturation of intestinal epithelial cells, one of which, Mybl2, we showed to be downregulated during maturation along the crypt-luminal axes of both the mouse small intestine and the human colon in vivo (Figs. 1 and 2). Because Mybl2 is also important in differentiation of other cell types, including myeloid (8, 17, 22, 56) and neuroblastoma cells (54), and is characteristically expressed in stem/pluripotential cells (9, 47, 69, 70), it is likely that its downregulation is fundamental to intestinal cell maturation. Our recent data has, in fact, demonstrated that Mybl2 is important in linking regulation of progression through the cell cycle with commitment to differentiation (51), suggesting that, after commitment, Mybl2 suppression is important for acquisition of a fully differentiated phenotype. Therefore, in these studies, we addressed the mechanism of Mybl2 regulation, demonstrating that, during in vitro colon cell maturation, Mybl2 expression is not substantially downregulated transcriptionally (Fig. 5), consistent with the report that a posttranscriptional component contributes to regulation of this gene (56). While not excluding other possible mechanisms, we demonstrate that a potential miRNA binding site we identified in the 3′-UTR can modulate Mybl2 expression levels, that specific miRNAs predicted to recognize this site are upregulated during maturation of colon cells both in vitro and in vivo (Fig. 8), and that overexpression of one of these, miR-365, downregulates Mybl2 expression by ∼40% (Fig. 10).
Interestingly, the pattern of regulation of certain miRNAs in human colon in vivo reflects that of differentiating human colon cells in vitro. Day 14 of spontaneous induction of Caco-2 cell maturation represents an intermediate time point in the full 21-day time course of differentiation in vitro. At this time point, relative to proliferating and day 2 or 7 differentiating cells, expression of most miRNAs in Caco-2 cells is at or near their highest levels. Expression patterns of several miRNAs, including 362–5p, 365, 324–5p, 588, and let-7a1, in human colon in vivo exhibit a biphasic profile, with high levels in tissue isolated from intermediate fractions along the crypt-luminal axis (in particular fraction no. 8), likely representing colon cells at an intermediate phase of maturation, as well as in cells nearest the lumen (fraction no. 1), representing fully differentiated colon cells (Figs. 8B and 9B). These results suggest that regulation of at least certain miRNAs in Caco-2 cells in vitro reflects that of human colon cells in vivo and that miRNAs play critical roles in promoting aspects of colon cell differentiation at intermediate phases, as well as in maintaining a fully differentiated phenotype. Differences between expression patterns of miRNAs in Caco-2 cells in vitro and colon tissue in vivo, in particular for miR-30a, 10a, 205, and 331–3p, may be due to the fact that colon tissue in vivo is composed of several distinct lineages of epithelial cells, which, as a whole, may modulate individual miRNA expression differently than Caco-2 cells, which represent only the absorptive lineage. Taken together, these results suggest that there are similarities as well as differences in regulation of miRNAs in different colon cell lineages.
miRNAs likely play critical roles in colon cells, particularly in regulating pathways involved in the balance between normal cell growth and tumorigenesis. Several miRNAs, including let-7 (1, 14, 78), miR-34 (11, 26, 71), miRs −143 and −145 (2, 6, 14, 62), and miR-130a (21, 39) are repressed in colorectal tumors and human colorectal cancer cell lines relative to normal mucosa or can suppress tumor growth. Furthermore, several reports have suggested potential targets of these miRNAs in colon cells. These include Ras and c-myc for let-7 (1); cyclin E2, cyclin-dependent kinase-4, hepatocyte growth factor receptor, and E2F-1 and -3 proteins for miR-34a (26, 71); ERK-5 for miR-143 (2); and insulin receptor substrate-1 for miR-145 (62). miRNAs 143 and 145 have also been suggested to target other genes, including those involved in growth-promoting signaling pathways, by homology to known mRNA sequences (46). Moreover, a recent report indicates that miR-7 modulates the expression of the transmembrane glycoprotein CD98 during colon epithelial cell differentiation (48). These studies suggest that miRNAs play important roles in colon epithelial cells and that they may be involved in driving colon cell maturation.
Our data showing that multiple miRNAs predicted to target the functional 3′-UTR miRNA target site in Mybl2 are increased in expression during colon cell maturation are consistent with observations that miRNAs individually exert modest effects (5, 61) and act combinatorially, with a number of different miRNAs targeting the same gene. Moreover, the data support the hypothesis that factors in addition to primary sequence, including the stability of the 3′-UTR target, may influence their efficacy. Importantly, these studies demonstrate that expression profiles of miRNAs alone do not reveal their functionality. Although miR-365 and miR-324–5p are both predicted to target Mybl2 and are both increased in expression during colon epithelial cell maturation, only miR-365, which, by our analysis, is predicted to target a functional miRNA binding site in the Mybl2 3′-UTR, is able to suppress Mybl2 protein expression. miR-324–5p, predicted to bind a nonfunctional sequence in the Mybl2 3′-UTR, does not suppress Mybl2. These findings, therefore, highlight the importance of utilizing a target-centered approach to not only profile miRNA expression, but to also determine whether miRNA targets are physiologically relevant.
Several studies have documented the involvement of miR-365 in disease states, but, unlike the data herein demonstrating that miR-365 suppresses Mybl2 expression, these reports have not identified functional targets for this miRNA. miR-365 is upregulated in UVB-irradiated NIH3T3 cells (24) and human breast cancer tissue (80), and downregulated in human lupus nephritis renal tissue (15), rat vascular walls following balloon injury (31), human psoriatic skin (65), and quiescent as well as senescent (relative to replicating) human lung cells (40). miR-365 was also identified as one of 14 miRNAs upregulated in ectopic vs. eutopic endometrial tissue and predicted to target mRNAs in the c-Jun, CREB (cAMP-response element binding protein) binding protein, protein kinase b (Akt), and cyclin D1 signaling pathways (50). These studies suggest that miR-365 may play a role in fine-tuning expression of critical molecules under a number of conditions, and our data demonstrate that this encompasses Mybl2 expression during colon cell maturation.
Important to consider, however, is that the Mybl2 suppression mediated by miR-365 accounts for only part of the Mybl2 downregulation during Caco-2 cell differentiation. Because miRNAs function combinatorially, as discussed above, other upregulated miRNAs may also play a role. In this context, we have identified a number of miRNAs with documented involvement in colon cells, including miR-34a, miR-130a, miR-145, and let-7a1, as upregulated during Caco-2 cell differentiation (Fig. 9A). In particular, let-7a1 may play an important role in human colon cell differentiation, because it is also enriched in differentiating human colon tissue in vivo (Fig. 9B). Expression patterns of miR-34a, 130a, 143, and 145 in Caco-2 (absorptive) cells are very different from those (all lineages) in vivo and may indicate, as suggested above, that these miRNAs are regulated differently in distinct colon cell lineages. These, and other as yet unidentified miRNAs, may play a combinatorial role in regulating Mybl2 and other genes during colon cell maturation.
Suppression of key regulatory molecules such as Mybl2 by miRNAs suggests that expression of miRNAs themselves is subject to fine control, providing a level of fine tuning in cellular reprogramming. Indeed, we have reported that Mybl2 appears to function in fine tuning progression through the cell cycle in preparing colon cells to differentiate (51). It will, therefore, be important to determine whether expression of miR-365 and other critical miRNAs is regulated by the same molecules and pathways that drive colon epithelial cell maturation, or whether distinct mechanisms are involved. Such regulation could occur at a number of levels, potentially in distinct cellular contexts: pri-miRNA transcription, nuclear processing by the Drosha protein complex, cytoplasmic processing by Dicer, incorporation into the RNA-induced silencing complex, recognition of and binding to mRNA targets, miRNA turnover, and RNA editing of miRNAs. In addition, we hypothesize that secondary structure of the miRNA target may determine whether a potential miRNA target is functional and can bind miRNAs. It is likely that the finely regulated balance in cell proliferation and allocation of cells to different lineages that establish intestinal mucosal homeostasis will be found to be dependent on multiple levels of regulation that include miRNA and other noncoding RNA components.
GRANTS
This work was supported by National Cancer Institute Grant CA137508 to M. Papetti and U54 CA100926, CA114265, CA123473, and P013330 to L. H. Augenlicht.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Katheryn E. Tanaka and Navjot Kaur for human colon patient samples, Georgia Corner for assisting with gene functional group classifications, Dr. John Mariadason for Caco-2 cell microarray data and mouse small intestine crypt-villus protein fractions, Dr. Anna Velcich for HT29 microarray data, and Elena Dhima for technical assistance.
REFERENCES
- 1. Akao Y, Nakagawa Y, Naoe T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biol Pharm Bull 29: 903–906, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Akao Y, Nakagawa Y, Naoe T. MicroRNAs 143 and 145 are possible common onco-microRNAs in human cancers. Oncol Rep 16: 845–850, 2006 [PubMed] [Google Scholar]
- 3. Arsura M, Introna M, Passerini F, Mantovani A, Golay J. B-myb antisense oligonucleotides inhibit proliferation of human hematopoietic cell lines. Blood 79: 2708–2716, 1992 [PubMed] [Google Scholar]
- 4. Augenlicht L, Velcich A, Heerdt BG. Short-chain fatty acids and molecular and cellular mechanisms of colonic cell differentiation and transformation. Adv Exp Med Biol 375: 137–148, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature 455: 64–71, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bandres E, Cubedo E, Agirre X, Malumbres R, Zarate R, Ramirez N, Abajo A, Navarro A, Moreno I, Monzo M, Garcia-Foncillas J. Identification by real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol Cancer 5: 29, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bennett JD, Farlie PG, Watson RJ. E2F binding is required but not sufficient for repression of B-myb transcription in quiescent fibroblasts. Oncogene 13: 1073–1082, 1996 [PubMed] [Google Scholar]
- 8. Bies J, Hoffman B, Amanullah A, Giese T, Wolff L. B-Myb prevents growth arrest associated with terminal differentiation of monocytic cells. Oncogene 12: 355–363, 1996 [PubMed] [Google Scholar]
- 9. Boheler KR. Stem cell pluripotency: a cellular trait that depends on transcription factors, chromatin state and a checkpoint deficient cell cycle. J Cell Physiol 221: 10–17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang J, Chance MR, Nicholas C, Ahmed N, Guilmeau S, Flandez M, Wang D, Byun DS, Nasser S, Albanese JM, Corner GA, Heerdt BG, Wilson AJ, Augenlicht LH, Mariadason JM. Proteomic changes during intestinal cell maturation in vivo. J Proteomics 71: 530–546, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 26: 745–752, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science 303: 83–86, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Cheung VG, Morley M, Aguilar F, Massimi A, Kucherlapati R, Childs G. Making and reading microarrays. Nat Genet 21: 15–19, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Cummins JM, He Y, Leary RJ, Pagliarini R, Diaz LA, Jr, Sjoblom T, Barad O, Bentwich Z, Szafranska AE, Labourier E, Raymond CK, Roberts BS, Juhl H, Kinzler KW, Vogelstein B, Velculescu VE. The colorectal microRNAome. Proc Natl Acad Sci U S A 103: 3687–3692, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dai Y, Sui W, Lan H, Yan Q, Huang H, Huang Y. Comprehensive analysis of microRNA expression patterns in renal biopsies of lupus nephritis patients. Rheumatol Int 29: 749–754, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Eiring AM, Harb JG, Neviani P, Garton C, Oaks JJ, Spizzo R, Liu S, Schwind S, Santhanam R, Hickey CJ, Becker H, Chandler JC, Andino R, Cortes J, Hokland P, Huettner CS, Bhatia R, Roy DC, Liebhaber SA, Caligiuri MA, Marcucci G, Garzon R, Croce CM, Calin GA, Perrotti D. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell 140: 652–665, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engelhard A, Campbell K, Calabretta B. B-myb alters the response of myeloid precursor cells to G-CSF. Exp Cell Res 254: 153–162, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, Nervi C, Bozzoni I. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell 123: 819–831, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Ferraris RP, Villenas SA, Diamond J. Regulation of brush-border enzyme activities and enterocyte migration rates in mouse small intestine. Am J Physiol Gastrointest Liver Physiol 262: G1047–G1059, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Fleet JC, Wang L, Vitek O, Craig BA, Edenberg HJ. Gene expression profiling of Caco-2 BBe cells suggests a role for specific signaling pathways during intestinal differentiation. Physiol Genomics 13: 57–68, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Gaur A, Jewell DA, Liang Y, Ridzon D, Moore JH, Chen C, Ambros VR, Israel MA. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res 67: 2456–2468, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Golay J, Broccoli V, Borleri GM, Erba E, Faretta M, Basilico L, Ying GG, Piccinini G, Shapiro LH, Lovric J, Nawrath M, Molling K, Rambaldi A, Introna M. Redundant functions of B-Myb and c-Myb in differentiating myeloid cells. Cell Growth Differ 8: 1305–1316, 1997 [PubMed] [Google Scholar]
- 23. Golay J, Capucci A, Arsura M, Castellano M, Rizzo V, Introna M. Expression of c-myb and B-myb, but not A-myb, correlates with proliferation in human hematopoietic cells. Blood 77: 149–158, 1991 [PubMed] [Google Scholar]
- 24. Guo L, Huang ZX, Chen XW, Deng QK, Yan W, Zhou MJ, Ou CS, Ding ZH. Differential expression profiles of microRNAs in NIH3T3 cells in response to UVB irradiation. Photochem Photobiol 85: 765–773, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med 320: 23–28, 1989 [DOI] [PubMed] [Google Scholar]
- 26. He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature 447: 1130–1134, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature 435: 828–833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hodin RA, Meng S, Archer S, Tang R. Cellular growth state differentially regulates enterocyte gene expression in butyrate-treated HT-29 cells. Cell Growth Differ 7: 647–653, 1996 [PubMed] [Google Scholar]
- 29. Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science 297: 2056–2060, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Jackson RJ, Standart N. How do microRNAs regulate gene expression? Sci STKE 2007: re1, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of microRNA in vascular neointimal lesion formation. Circ Res 100: 1579–1588, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Kim HK, Lee YS, Sivaprasad U, Malhotra A, Dutta A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol 174: 677–687, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275: 1784–1787, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, Leung SY, Chen X. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A 104: 15418–15423, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lam EW, Bennett JD, Watson RJ. Cell-cycle regulation of human B-myb transcription. Gene 160: 277–281, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Lam EW, Watson RJ. An E2F-binding site mediates cell-cycle regulated repression of mouse B-myb transcription. EMBO J 12: 2705–2713, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin D, Fiscella M, O'Connor PM, Jackman J, Chen M, Luo LL, Sala A, Travali S, Appella E, Mercer WE. Constitutive expression of B-myb can bypass p53-induced Waf1/Cip1-mediated G1 arrest. Proc Natl Acad Sci U S A 91: 10079–10083, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−DELTA DELTA C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature 435: 834–838, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Maes OC, Sarojini H, Wang E. Stepwise up-regulation of microRNA expression levels from replicating to reversible and irreversible growth arrest states in WI-38 human fibroblasts. J Cell Physiol 221: 109–119, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell 27: 435–448, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mariadason JM, Arango D, Corner GA, Aranes MJ, Hotchkiss KA, Yang W, Augenlicht LH. A gene expression profile that defines colon cell maturation in vitro. Cancer Res 62: 4791–4804, 2002 [PubMed] [Google Scholar]
- 43. Mariadason JM, Bordonaro M, Aslam F, Shi L, Kuraguchi M, Velcich A, Augenlicht LH. Down-regulation of beta-catenin TCF signaling is linked to colonic epithelial cell differentiation. Cancer Res 61: 3465–3471, 2001 [PubMed] [Google Scholar]
- 44. Mariadason JM, Nicholas C, L'Italien KE, Zhuang M, Smartt HJ, Heerdt BG, Yang W, Corner GA, Wilson AJ, Klampfer L, Arango D, Augenlicht LH. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology 128: 1081–1088, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Mayo C, Lloreta J, Real FX, Mayol X. In vitro differentiation of HT-29 M6 mucus-secreting colon cancer cells involves a trychostatin A and p27(KIP1)-inducible transcriptional program of gene expression. J Cell Physiol 212: 42–50, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Michael MZSMOC, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res 1: 882–891, 2003 [PubMed] [Google Scholar]
- 47. Muller FJ, Laurent LC, Kostka D, Ulitsky I, Williams R, Lu C, Park IH, Rao MS, Shamir R, Schwartz PH, Schmidt NO, Loring JF. Regulatory networks define phenotypic classes of human stem cell lines. Nature 455: 401–405, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nguyen HT, Dalmasso G, Yan Y, Laroui H, Dahan S, Mayer L, Sitaraman SV, Merlin D. MicroRNA-7 modulates CD98 expression during intestinal epithelial cell differentiation. J Biol Chem 285: 1479–1489, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nomura N, Takahashi M, Matsui M, Ishii S, Date T, Sasamoto S, Ishizaki R. Isolation of human cDNA clones of myb-related genes, A-myb and B-myb. Nucleic Acids Res 16: 11075–11089, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG, Hull LM. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol 23: 265–275, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Papetti M, Augenlicht LH. Mybl2, a link between proliferation and differentiation in maturing colon epithelial cells. J Cell Physiol 226: 785–791, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Papetti M, Skoultchi AI. Reprogramming leukemia cells to terminal differentiation and growth arrest by RNA interference of PU.1. Mol Cancer Res 5: 1053–1062, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer 8: 523–534, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Raschella G, Negroni A, Sala A, Pucci S, Romeo A, Calabretta B. Requirement of b-myb function for survival and differentiative potential of human neuroblastoma cells. J Biol Chem 270: 8540–8545, 1995 [DOI] [PubMed] [Google Scholar]
- 55. Reddy BS. Dietary fiber and colon cancer: animal model studies. Prev Med 16: 559–565, 1987 [DOI] [PubMed] [Google Scholar]
- 56. Reiss K, Travali S, Calabretta B, Baserga R. Growth regulated expression of B-myb in fibroblasts and hematopoietic cells. J Cell Physiol 148: 338–343, 1991 [DOI] [PubMed] [Google Scholar]
- 57. Ro S, Park C, Jin J, Sanders KM, Yan W. A PCR-based method for detection and quantification of small RNAs. Biochem Biophys Res Commun 351: 756–763, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saaf AM, Halbleib JM, Chen X, Yuen ST, Leung SY, Nelson WJ, Brown PO. Parallels between global transcriptional programs of polarizing Caco-2 intestinal epithelial cells in vitro and gene expression programs in normal colon and colon cancer. Mol Biol Cell 18: 4245–4260, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sala A, Calabretta B. Regulation of BALB/c 3T3 fibroblast proliferation by B-myb is accompanied by selective activation of cdc2 and cyclin D1 expression. Proc Natl Acad Sci U S A 89: 10415–10419, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sala A, Watson R. B-Myb protein in cellular proliferation, transcription control, and cancer: latest developments. J Cell Physiol 179: 245–250, 1999 [DOI] [PubMed] [Google Scholar]
- 61. Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature 455: 58–63, 2008 [DOI] [PubMed] [Google Scholar]
- 62. Shi B, Sepp-Lorenzino L, Prisco M, Linsley P, deAngelis T, Baserga R. Micro RNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cells. J Biol Chem 282: 32582–32590, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Sitzmann J, Noben-Trauth K, Kamano H, Klempnauer KH. Expression of B-Myb during mouse embryogenesis. Oncogene 12: 1889–1894, 1996 [PubMed] [Google Scholar]
- 64. Smartt HJ, Guilmeau S, Nasser SV, Nicholas C, Bancroft L, Simpson SA, Yeh N, Yang W, Mariadason JM, Koff A, Augenlicht LH. p27kip1 Regulates cdk2 activity in the proliferating zone of the mouse intestinal epithelium: potential role in neoplasia. Gastroenterology 133: 232–243, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Sonkoly E, Wei T, Janson PC, Saaf A, Lundeberg L, Tengvall-Linder M, Norstedt G, Alenius H, Homey B, Scheynius A, Stahle M, Pivarcsi A. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One 2: e610, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci U S A 102: 12443–12448, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sun Y, Koo S, White N, Peralta E, Esau C, Dean NM, Perera RJ. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res 32: e188, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tappenden KA, Thomson AB, Wild GE, McBurney MI. Short-chain fatty acid-supplemented total parenteral nutrition enhances functional adaptation to intestinal resection in rats. Gastroenterology 112: 792–802, 1997 [DOI] [PubMed] [Google Scholar]
- 69. Tarasov KV, Tarasova YS, Tam WL, Riordon DR, Elliott ST, Kania G, Li J, Yamanaka S, Crider DG, Testa G, Li RA, Lim B, Stewart CL, Liu Y, Van Eyk JE, Wersto RP, Wobus AM, Boheler KR. B-MYB is essential for normal cell cycle progression and chromosomal stability of embryonic stem cells. PLoS One 3: e2478, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tarasov KV, Testa G, Tarasova YS, Kania G, Riordon DR, Volkova M, Anisimov SV, Wobus AM, Boheler KR. Linkage of pluripotent stem cell-associated transcripts to regulatory gene networks. Cells Tissues Organs 188: 31–45, 2008 [DOI] [PubMed] [Google Scholar]
- 71. Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci U S A 104: 15472–15477, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tremblay E, Auclair J, Delvin E, Levy E, Menard D, Pshezhetsky AV, Rivard N, Seidman EG, Sinnett D, Vachon PH, Beaulieu JF. Gene expression profiles of normal proliferating and differentiating human intestinal epithelial cells: a comparison with the Caco-2 cell model. J Cell Biochem 99: 1175–1186, 2006 [DOI] [PubMed] [Google Scholar]
- 73. Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20: 515–524, 2006 [DOI] [PubMed] [Google Scholar]
- 74. van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71: 241–260, 2009 [DOI] [PubMed] [Google Scholar]
- 75. Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H. The intestinal Wnt/TCF signature. Gastroenterology 132: 628–632, 2007 [DOI] [PubMed] [Google Scholar]
- 76. van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A 103: 18255–18260, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Velcich A, Corner G, Paul D, Zhuang M, Mariadason JM, Laboisse C, Augenlicht L. Quantitative rather than qualitative differences in gene expression predominate in intestinal cell maturation along distinct cell lineages. Exp Cell Res 304: 28–39, 2005 [DOI] [PubMed] [Google Scholar]
- 78. Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 103: 2257–2261, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Weiser MM. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem 248: 2536–2541, 1973 [PubMed] [Google Scholar]
- 80. Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, Zeng YX, Shao JY. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA 14: 2348–2360, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 436: 214–220, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.