Abstract
Glutamine possesses gut-protective effects both clinically and in the laboratory. We have shown in a rodent model of mesenteric ischemia-reperfusion that enteral glutamine increased peroxisome proliferator-activated receptor-γ (PPAR-γ) and was associated with a reduction in mucosal injury and inflammation. The mechanism by which glutamine activates PPAR-γ is unknown, and we hypothesized that it was via a ligand-dependent mechanism. Intestinal epithelial cells, IEC-6, were co-transfected with PPAR-γ response element-luciferase promoter/reporter construct. Cells were pretreated with increasing concentrations of glutamine ± GW9662 (a specific antagonist of PPAR-γ) and analyzed for PPAR-γ response element luciferase activity as an indicator of PPAR-γ activation. PPAR-γ nuclear activity was assessed by electrophoretic mobility shift assay. Cell lysates were subjected to tandem mass spectroscopy for measurement of prostaglandin and lipoxygenase metabolites. A time- and concentration-dependent increase in PPAR-γ transcriptional activity, but not mRNA or protein, was demonstrated. Activity was abrogated by the PPAR-γ inhibitor, GW9662, and changes in activity correlated with PPAR-γ nuclear binding. Glutamine, via degradation to glutamate, activated the metabolic by-products of the lipoxygenase and linoleic acid pathways, 15-S-hydroxyeicosatetraenoic acid and dehydrogenated 13-hydroxyoctaolecadienoic acid, known endogenous PPAR-γ ligands in the small bowel. This novel mechanism may explain the gut-protective effects of enteral glutamine.
Keywords: lipoxygenase, hydroxyeicosatetraenoic acid, hydroxyoctaolecadienoic acid, glutathione, glutamate
early enteral nutrition supplemented with glutamine, arginine, omega-3 poly-unsaturated fatty acids, and nucleotides has been shown to improve outcome in severely injured and critically ill patients (22, 28). The realization that the greatest benefit in clinical outcomes was from studies utilizing specific nutrients led to the development of pharmaconutrition, which detaches nutritional support from the provision of key nutrients that may modulate the inflammatory and immune response (16). Glutamine, in particular, plays an important role in regulating the immune system and is especially important during times of stress. A meta-analysis performed by Novak et al. (30) demonstrated that, in surgical patients, glutamine supplementation may be associated with a reduction in infectious complications and shorter hospital length of stay, and, in critically ill patients, with a reduction in complications and mortality. Critical illness, in particular, leads to a marked deficiency in glutamine and is correlated with mortality in the intensive care setting (32).
Glutamine is the preferred substrate of enterocytes and is conditionally essential during times of stress. Our laboratory has demonstrated in a rodent model of mesenteric ischemia-reperfusion that glutamine preserved mucosal integrity, absorptive capacity, and ATP, as well as maintained gut barrier function (21, 20).
Importantly, glutamine's gut-protective effects were associated with an increase in peroxisome proliferator-activated receptor-γ (PPAR-γ), although the mechanism of activation is not known (34). PPAR-γ is a transcription factor that belongs to a family of nuclear receptors and plays a key role in adipocyte development and glucose homeostasis, as well as cell growth, differentiation, and apoptosis (8). Nuclear receptors, in general, and PPAR-γ, in particular, can be activated by ligand-dependent and ligand-independent mechanisms. Ligand-independent mechanisms are most often associated with kinase-dependent processes (7). Our laboratory has shown that arginine represses enterocyte PPAR-γ, and preliminary data suggest it is via a ligand-independent mechanism (4, 34).
When activated by ligands, PPAR-γ forms a heterodimer with the retoid X receptor, binds to a PPAR-γ response element (PPRE) in the promoter of target genes, and activates transcription. PPAR-γ has been recognized as an endogenous regulator of intestinal inflammation (18, 29, 41) and is an independent risk factor for longer survival in patients with colorectal cancer (31). PPAR ligands, particularly omega-3 fatty acids, have shown benefit in animal models of colitis (25). Patients with ulcerative colitis have impaired expression of PPAR-γ, while PPAR-γ has been identified as a susceptibility gene in Crohn's disease (9, 40).
Based on reversal of glutamine's protective effects by pharmacological inhibition of the PPAR-γ ligand-dependent binding site (34), we hypothesized that glutamine functioned through competition for binding to this site. Interestingly, this was not the case. Competitive binding assays demonstrated that glutamine did not bind directly to PPAR-γ. In fact, our data suggested that glutamine activated PPAR-γ via the endogenous PPAR-γ ligands, 15-S-hydroxyeicosatetraenoic acid (15-S-HETE) and dehydrogenated 13-hydroxyoctaolecadienoic acid (13-OXO-ODE), a novel mechanism for the protective effects of enteral glutamine.
MATERIALS AND METHODS
Cell culture.
Rodent intestinal epithelial cells, IEC-6, were purchased from the American Type Culture Collection (ATCC, cell line CRL-1592). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen), supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum and 2 mM glutamine. Cells were maintained in DMEM media containing 2 mM glutamine at 37°C in a humidified atmosphere of 5% CO2. Because glutamine is necessary for normal cell growth, we considered the physiological concentration found in the media as our control group. Glutamine was added to cells up to 20 mM, as previously described (3).
Reverse transcription-polymerase chain reaction.
Total RNA was isolated using SV Total RNA Isolation System (Promega), and 2.5 μg of total RNA were converted into cDNA via reverse transcription using Enhanced Avian HS RT-PCR-100 Kit (Sigma). PCR was performed using PCR Master Mix (Promega), using β-actin as an internal control. The primers used for amplification were as follows: forward 5′-ACTCCCATTCCTTTGACATC-3′, reverse 5′-TCCCCACAGACTCGGCACTC-3′ for 265-bp fragment for PPAR-γ, and forward 5′-CCGTAAAGACCTCTATGACA-3′, reverse 5′-AAGAAAGGGTGTAAAACGCA-3′ for 299-bp fragments for β-actin (10, 17). Conditions for PCR were the same for PPAR-γ and β-actin and were as follows: 3 min at 95°C, followed by 35 cycles of 95°C for 30 s, 56°C for 30 s, and 72°C for 30 s, and a final extension at 72°C for 10 min. PCR products were separated via electrophoresis on 1.5% agarose gel.
Immunoblotting.
Cells were harvested and lysed with radio-immunoprecipitation assay buffer containing protease inhibitors (Sigma) and then electrophoresed on a 10% Tris-glycine polyacrylamide gel. Equal amounts of protein were used. Proteins were transferred onto a Hybond-P membrane (Amersham Biosciences), and the membrane was blocked for 1 h in 5% nonfat dried milk in Tris-buffered saline with 0.1% Tween 20 and then incubated overnight at 4°C with antibodies against PPAR-γ (Santa Cruz), PGD2 synthase, cyclooxygenase (COX)-1 and COX-2, or β-actin (Cell Signaling). Membranes were then washed three times and incubated for 1 h at room temperature with anti-rabbit IgG, horseradish peroxidase linked whole antibody (from donkey) (Amersham Biosciences), developed with enhanced chemiluminescence plus Western blotting detection system (Amersham Biosciences), and exposed onto film.
PPAR-γ electrophoretic mobility shift assay.
Nuclear and cytoplasmic fractions were obtained with the Nuclear/Cytosol Fractionation Kit (BioVision), according to the manufacturer's protocol. The nuclear protein samples were then standardized through the Bradford protein estimation system using the Bio-Rad Protein Assay kit (Bio-Rad Laboratories). Labeled probes were prepared by end-labeling with [γ-32P]ATP (Amersham) using the T4 Polynucleotide Kinase Kit (Promega). The sequence of the double-stranded PPRE oligonucleotides probe was as follows: forward 5′-CAA AAC TAG GTC AAA GGT CA-3′. Then 10 μg of nuclear proteins were incubated with [32P]-end-labeled probes in 20 μl of the binding buffer [15 mM HEPES, 60 mM KCl, 0.5 mM EDTA, 1 mM DTT, 75 μg/ml poly (dI-dC), 100 μg/ml acetylated BSA, and 0.05% NP-40] for 20 min at room temperature. In competition assays, the 200-fold molar excess of unlabeled probe was added to the binding reaction mixture. For supershift assays, nuclear proteins were preincubated with 2 μg of rabbit anti-PPAR-γ antibody or rabbit control IgG (Santa Cruz Biotechnology) for 10 min before the addition of oligonucleotides. Protein-DNA complexes were separated by 5% polyacrylamide gel. The gels were dried and exposed on X-ray films, and the films were subjected to densitometric analysis (Optimas 6.1, Media Cybernetics, Silver Spring, MD).
Cell transfection.
One day before transfection, 5 × 104 IEC-6 cells diluted in 1 ml of DMEM media were plated in each well of a 24-well plate. When cells were at ∼95% confluency, the luciferase reporter construct PPRE X3-TK-Luc (Addgene) was transiently co-transfected with the Renilla luciferase expression vector (pRL-TK) using Lipofectamine 2000 (Invitrogen) (19). The Renilla expression vector was used as a control. Cells were incubated in DMEM medium with 1.6 μg PPRE-X3-TK-Luc, 0.3 μg Renilla expression vector, and 5.7 μl diluted Lipofectamine 2000. After 16 h, cells were treated with indicated concentrations of glutamine. For some experiments, cells were pretreated with 0–20 μM GW9662, a specific PPAR-γ antagonist. After 24 h, total cell lysates were prepared using 1× passive lysis buffer (Promega).
PPAR-γ luciferase reporter gene assay.
Luciferase assays were performed using the Dual Luciferase TM Reporter assay system (Promega, Madison, WI) in a Turner Designs TD-20/20 luminometer (Turner Designs), according to the manufacturer's instructions. Lysates were analyzed for PPRE luciferase activity as an indicator of PPAR-γ activation and normalized to Renilla luciferase expression. All experiments were performed in triplicate.
Ligand binding assay.
Three micrograms of purified PPAR-γ (GST-PPAR-γ fusion protein from Thermo Fisher Scientific), 10 nM [3H]BRL49653 (rosiglitazone) (50 Ci/mmol) (American Radiolabeled Chemicals), and 5 μM or 50 μM of glutamine were incubated with 5 μM of rosiglitazone or vehicle in a buffer containing 10 mM Tris (pH 8.0), 50 mM KCl, and 10 mM DTT at 4°C for 3 h (27). Bound and free radioactivity were separated by elution through HiTrap Desalting Column (GE Healthcare). The amount of bound [3H]rosiglitazone was determined by liquid scintillation counting. The amount of [3H]rosiglitazone bound to PPAR-γ in the presence of vehicle was assigned as 100% binding.
15-deoxy-d-Δ12,14-PGJ2 immunoassay.
IEC-6 cells cultured in 20 ml of medium in 150-mm dish at ∼95% confluence were treated with increasing doses of glutamine for 24 h. Extraction of 15-deoxy-d-Δ12,14-PGJ2 (15d-PGJ2) from culture medium supernatants and determinations of the concentrations of 15d-PGJ2 were performed by EIA (Assay Designs), according to the manufacturer's instructions.
Tandem mass spectroscopy.
Extractions of intracellular eicosanoids were performed according to Yang et al (46). Before extraction, 10 μl of 15d-PGJ2-d4, 12-HETE-d8 or 15-HETE-d8, and 13-hydroxyoctaolecadienoic acid (HODE)-d4 (100 ng/ml) were added to each sample as an internal standard. Cells lysates from three separated experiments were pooled and then suspended in 0.5 ml PBS containing 1 mM CaCl2. The eicosanoids were then extracted by adding 2 ml of hexane-ethyl acetate (1:1 vol/vol) and vortex mixing for 2 min. Samples were centrifuged (1,800 g for 10 min at 4°C), and the upper organic layer was then transferred to a glass test tube on ice. The extraction step was repeated twice, and the organic phases were pooled and then dried under a stream of nitrogen. All extraction procedures were performed at minimum light levels under cold conditions (4°C). Samples were reconstituted in 200 μl methanol/10 mM ammonium acetate buffer, pH 8.5 (70:30) before liquid chromatography/tandem mass spectrometry (LC/MS/MS) analysis.
LC/MS/MS was performed as previously described using a Quattro Ultima tandem spectrometer (Micromass, Beverly, MA), equipped with an Agilent HP 1100 binary pump HPLC inlet (45). COX- and lipoxygenase (LOX)-derived lipids were chromatographically resolved using a Luna 3-μm phenyl-hexyl (2 × 150 mm) analytic column (Phenomenex, Torrance, CA). A linear methanol gradient from 50–60% for 12 min and then from 60–90% in 2 min at a flow rate of 300 μl/min was used to achieve baseline resolution for all compounds of interest. The mobile phase consisted of 10 mM ammonium acetate, pH 8.5, and methanol. The column temperature was maintained at 50°C. Injection volume was 25 μl/sample. Samples were stored at 4°C during the analysis. Individual analytes were detected using electrospray negative ionization. The identification of each lipid was confirmed by comparison to reference standards (Cayman Chemicals, Ann Arbor, MI).
Statistical analysis.
All experiments were repeated at least three times. Statistical analysis was performed by one-way analysis of variance, and individual group means were compared using Tukey's multiple-group comparison test. P values < 0.05 were considered significant. Data are expressed as means ± SE.
RESULTS
PPAR-γ DNA binding activity is increased in response to glutamine stimulation in small-bowel epithelial cells.
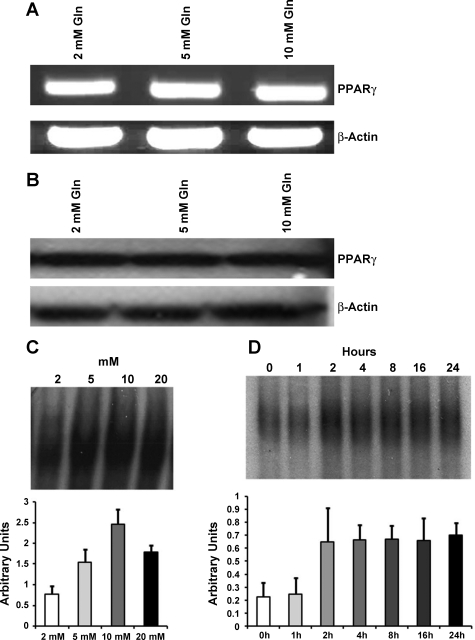
To gain preliminary insight into the role of glutamine as a potential PPAR-γ agonist in small-bowel epithelial cells, the expression of PPAR-γ mRNA in response to glutamine stimulation was analyzed. There was no change in PPAR-γ transcription with increasing doses of glutamine (Fig. 1A). Immunoblotting analysis similarly revealed no change in protein expression (Fig. 1B). As PPAR-γ is a nuclear transcription factor, DNA binding activity was measured and demonstrated a dose- and time-dependent increase in PPAR-γ (Fig. 1, C and D). Binding activity increased at 2 h, remained elevated through 24 h, and was maximal after 10 mM of glutamine.
Fig. 1.
Peroxisome proliferator-activated receptor-γ (PPAR-γ) DNA binding activity is increased by glutamine (Gln) stimulation in small bowel epithelial cells. Intestinal epithelial cells, IEC-6, were incubated in serum-free media overnight and then treated with the indicated concentrations of Gln for 24 h. A: PCR was performed for evaluation of PPAR-γ mRNA expression and showed no change in mRNA expression with increasing Gln concentration. B: Western blotting was performed for measurement of protein expression and similarly showed no change in protein expression with increasing Gln concentration. C: DNA binding activity was measured in nuclear extracts and demonstrated a dose-dependent increase in PPAR-γ DNA binding activity. The EMSA and corresponding densitometry data are shown. D: DNA binding activity also demonstrated a time-dependent increase in PPAR-γ DNA binding activity. At 24 h, activity increased 3.1-fold. The EMSA and corresponding densitometry data are shown.
Glutamine increases transcriptional activity of PPAR-γ.
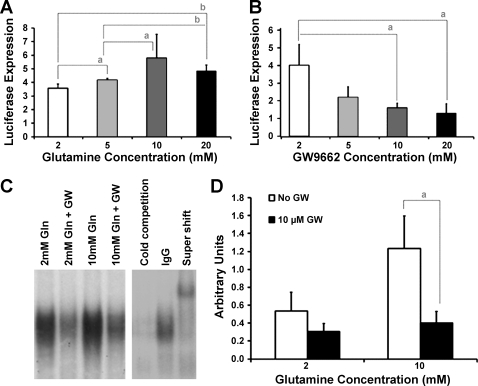
On activation by ligand, PPAR-γ binds to a PPRE in the promoter of target genes to activate transcription. A reporter plasmid assay revealed that increasing concentrations of glutamine amplified luciferase expression in a dose-response fashion (Fig. 2A), demonstrating that glutamine increased transcriptional activity of PPAR-γ. Expression was 3.6 ± 0.2 after 2 mM glutamine, 4.2 ± 0.1 after 5 mM, 6.7 ± 0.6 after 10 mM, and 6.2 ± 0.2 after 20 mM glutamine.
Fig. 2.
PPAR-γ transcriptional activity is increased by Gln, but inhibited by GW9662 (GW) in a dose-dependent fashion. A: confluent IEC-6 cells were transiently transfected with PPAR-γ response element (PPRE)-luciferase and then incubated in Gln (2–20 mM) for 24 h. Whole cell lysates were assayed for luciferase activity and normalized to Renilla luciferase expression. PPAR-γ transcriptional activity was increased by Gln in a dose-dependent fashion, reaching maximal activity after 10 mM Gln. B: confluent IEC-6 cells were transiently transfected with PPRE-luc and then preincubated with GW (0–20 μM), followed by 10 mM Gln. Whole cell lysates were assayed for luciferase activity and normalized to Renilla luciferase expression. There was an inverse correlation between PPAR-γ transcriptional activity and GW concentration, reaching maximal inhibition after 10 μM GW. C: cells were preincubated in 10 μM GW, followed by either 2 mM or 10 mM Gln, and nuclear DNA binding activity was determined. A representative EMSA is shown. D: densitometric analysis revealed that Gln increased DNA binding activity, which was significantly inhibited by GW. Changes in activity followed changes in PPAR-γ DNA binding activity. All results represent the means ± SE of triplicate determinations. aP < 0.05 and bP < 0.01.
Glutamine is not a PPAR-γ ligand.
GW9662 (2-chloro-5 nitrobenzanilide) is an irreversible antagonist that covalently modifies a cysteine residue in the ligand binding site of PPAR-γ (1). When GW9662 was added to glutamine-treated cells, there was an inverse correlation between luciferase expression and GW9662 concentration (Fig. 2B). Expression was 4.0 ± 0.6 with no inhibitor, 2.2 ± 0.4 after 5 μM GW9662, 1.6 ± 0.1 after 10 μM, and 1.3 ± 0.3 after 20 μM GW9662. Changes in activity followed changes in PPAR-γ DNA binding activity (Fig. 2, C and D), suggesting a ligand-dependent mechanism.
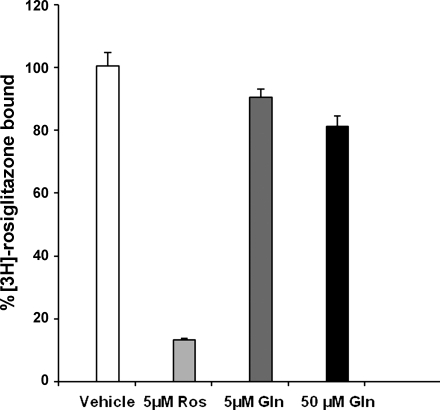
To further explore whether glutamine was indeed a PPAR-γ ligand, a ligand binding assay was performed using radiolabeled rosiglitazone, a known synthetic ligand. Only 10% of [3H]rosiglitazone was displaced by 5 μM glutamine and 19% by 50 μM glutamine (Fig. 3). Therefore, data from this competitive ligand-binding assay demonstrated that glutamine is not itself a ligand.
Fig. 3.
Gln is not a direct PPAR-γ ligand. A ligand binding assay was performed using purified PPAR-γ. [3H]rosiglitazone (Ros; PPAR-γ synthetic ligand) was incubated with either 5 μM or 50 μM of Gln, 5 μM of Ros, or vehicle. The amount of [3H]Ros bound to PPAR-γ in the presence of vehicle was assigned as 100% binding. There was no significant displacement of bound Ros by an equimolar concentration or even a 10-fold concentration of Gln, indicating that Gln is not a PPAR-γ ligand. Results represent the means ± SE of triplicate determinations.
Glutamine activates endogenous PPAR-γ ligands.
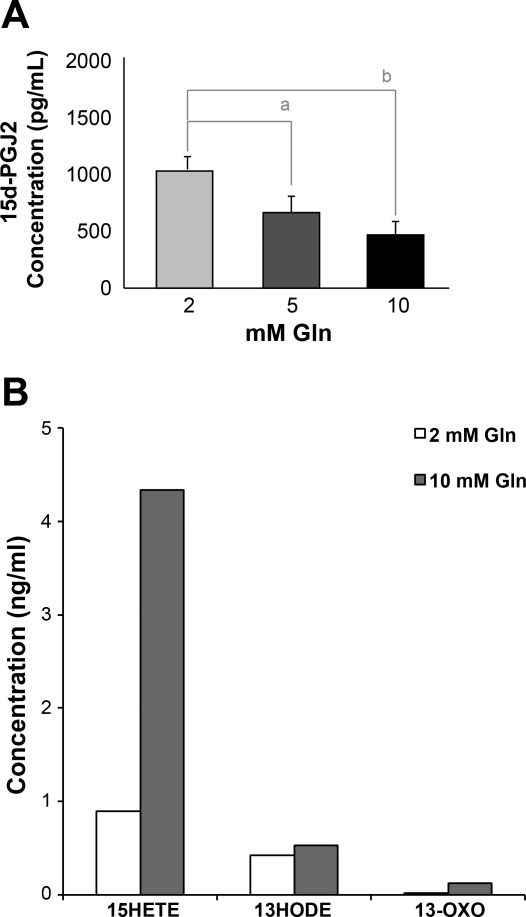
Based on reversal of glutamine's protective effects by pharmacological inhibition of the PPAR-γ ligand-dependent binding site, yet no direct ligand binding in an isolated system, we hypothesized that glutamine was activating an endogenous PPAR-γ ligand. As 15d-PGJ2 is the most potent natural ligand identified to date and has been shown to produce anti-inflammatory effects in vivo (15, 43), we measured its expression in response to increasing concentrations of glutamine. Interestingly, there was an inverse relationship between glutamine and 15d-PGJ2 (Fig. 4A). Cellular protein concentration decreased from 1,005 ± 78 pg/ml after 2 mM glutamine down to 450 ± 65 pg/ml after 10 mM glutamine. Glutamine had no effect on COX-1, COX-2, or PGD2 synthase expression, which were all upstream of 15d-PGJ2 (data not shown).
Fig. 4.
Potential endogenous ligands of PPAR-γ in small bowel intestinal epithelial cells. IEC-6 cells were treated with the indicated concentrations of Gln for 24 h. A: 15-deoxy-d-Δ12,14-PGJ2 (15d-PGJ2) concentrations were measured, and expression decreased in a dose-dependent fashion. B: to examine lipoxygenase metabolites after exposure to Gln, cells were processed for liquid chromatography/tandem mass spectrometry. Detection was validated using deuterated standards of potential ligands. All values were normalized to cell number. Data shown are from pooled lysates from three separate experiments. There was an increase in both 15-hydroxyeicosatetraenoic acid (15-HETE) and dehydrogenated 13-hydroxyoctaolecadienoic acid (13-OXO-ODE) after increasing concentrations of Gln. 13-HODE, 13-hydroxyoctaolecadienoic acid. a P < 0.05 and b P < 0.01.
Less well-studied endogenous ligands of PPAR-γ include members of the LOX pathway and metabolic by-products of linloeic acid, such as members of the HETE and HODE family. Thus far, 13-HODE, 13-OXO-ODE (dehydrogenated 13-HODE), and 15-HETE have been identified in intestinal epithelial cells and may be important regulators of intestinal inflammation (2, 13, 24). There are no reports of an association between these LOX metabolites and glutamine. We, therefore, measured their concentrations by LC/MS/MS and demonstrated a 4.9-fold increase in 15-HETE and a 6.0-fold increase in 13-OXO-ODE (Fig. 4B). There was less than a twofold difference in the other potential ligands.
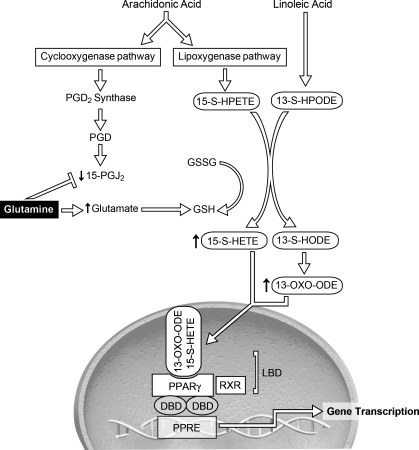
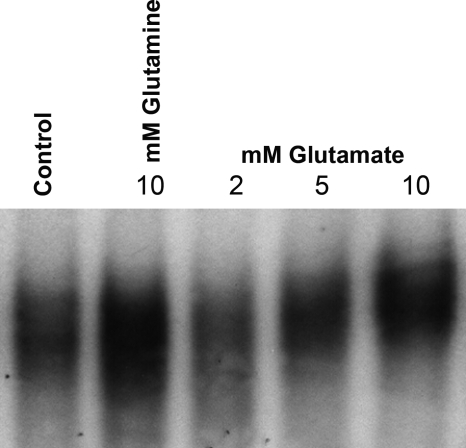
The metabolic pathways that link glutamine to these endogenous ligands and then to PPAR-γ are shown in Fig. 5 and rely on the metabolism of glutamine to glutamate in small intestinal epithelial cells. As shown in Fig. 6, increasing concentrations of glutamate increased PPAR-γ DNA binding activity in a dose-response fashion, paralleling that of glutamine.
Fig. 5.
Proposed pathway by which Gln activates PPAR-γ in small bowel intestinal epithelial cells. PPAR-γ can act as a nuclear receptor for both linoleic acid and arachidonic acid. Arachidonic acid can either be metabolized by the cyclooxygenase pathway to 15-PGJ2 or the lipoxygenase pathway. Gln stimulation resulted in a decrease in 15-PGJ2. Alternatively, arachidonic acid can be metabolized to 15-S-hydroperoxyeicosatetraenoic acid (15-S-HPETE) and then 15-S-HETE. Linoleic acid can be metabolized to 13-S-hydroperoxyoctadecadienoic acid (13-S-HPODE) and then to 13-S-HODE. 13-HODE dehydrogenase then converts 13-S-HODE into 13-OXO-ODE. The common link between 15-S-HETE and 13-OXO-ODE is glutathione (GSH), which is involved in the production of these metabolic by-products, and itself is a metabolite of Gln via glutamate. In response to Gln stimulation, 15-S-HETE and 13-OXO-ODE then enter the nucleus, bind to the ligand binding domain (LBD), and activate PPAR-γ. Once activated, PPAR-γ heterodimerizes with the retinoid X receptor (RXR), and then this complex binds to a specific DNA element, PPRE, in the promoter of target genes where it can activate transcription (8). DBD, DNA binding domain.
Fig. 6.
Glutamate increases PPAR-γ DNA binding activity in small bowel intestinal epithelial cells. IEC-6 cells were incubated in serum-free media overnight and then treated with either 10 mM Gln or the indicated concentrations of glutamate for 24 h. DNA binding activity was measured in nuclear extracts and demonstrated a dose-dependent increase in PPAR-γ DNA binding activity by glutamate.
DISCUSSION
We have demonstrated that glutamine increased PPAR-γ transcriptional activity in a dose- and time-dependent fashion. Interestingly, glutamine was not a direct PPAR-γ ligand, but rather stimulated the endogenous intestinal epithelial cell PPAR-γ ligands, 15-HETE and 13-OXO-ODE. This is the first report of an association between these two LOX metabolic by-products and glutamine.
We have been interested in the molecular mechanisms governing glutamine's gut protective effects, particularly when administered to the postischemic gut. Glutamine can be metabolized by small intestinal epithelial cells, and thereby contribute to the energy supply of the enterocyte (21). Most clinical studies in severely injured patients have failed to document enhanced systemic glutamine concentrations after enteral administration, consistent with enterocyte metabolism and utilization of glutamine. The majority of glutamine absorbed by the enterocyte undergoes oxidative metabolism to glutamate and CO2. Glutamate can then enter the Kreb's cycle via 2-oxoglutarate and generate ATP, and/or glutamate can be metabolized to glutathione, a powerful antioxidant. Our data demonstrating that both glutamine and glutamate increase PPAR-γ in intestinal epithelial cells are consistent with intestinal metabolism of glutamine. Brasse-Lagnel et al. (5) also demonstrated that glutamine can effect transcriptional regulation through the production of intracellular glutamate.
The glitazones are the principal synthetic ligands for PPAR-γ (12, 37), while the most potent and well-studied endogenous PPAR-γ ligand is 15d-PGJ2. We, therefore, examined whether glutamine was activating PPAR-γ via 15d-PGJ2 and unexpectedly found that glutamine decreased 15d-PGJ2 expression. The mechanism for this observation is not clear. Glutamine had no effect on either COX-1 or COX-2 expression nor PGD2 synthase, enzymes responsible for production of PGJ2 from arachadonic acid, suggesting that glutamine or a metabolic by-product was inhibiting at the level of 15d-PGJ2 (Fig. 5). To our knowledge, there is no repressive effect on PGJ2 by metabolites of the lipooxygenase pathway on the COX pathway, although this is one possibility. Another possibility is that 15d-PGJ2 may not be functioning as a PPAR-γ ligand. Petrova et al. (33) demonstrated that 15d-PGJ2 was unable to activate a PPAR reporter gene transfected into a glial cell line. PPAR-γ-independent reactivity of 15d-PGJ2 has also been demonstrated (42, 47). As this was not the focus of the present study, no further studies were performed to investigate this finding.
Unsaturated fatty acids are more abundant in biological tissues than 15d-PGJ2. Both linoleic acid and arachidonic acid are n-6 polyunsaturated fatty acids, and PPAR-γ can act as nuclear receptors for these and other polyunsaturated fatty acids (36). As shown in Fig. 5, arachidonic acid can either be metabolized by the COX pathway to 15-PGJ2 or the LOX pathway to either 12- or 15-S-HPETE, then to 12- or 15-S-HETE via glutathione peroxidase. Only 15-HETE is found in intestinal epithelial cells and mediates transactivation of PPAR-γ via its ligand binding domain (11, 23). Subbarayan et al. (39) demonstrated that normal intestinal epithelial cells demonstrate high levels of 15-S-HETE but low levels of PPAR-γ, a relationship that was reversed in epithelial cancer cells. Our laboratory (34) has previously demonstrated low levels of PPAR-γ in normal intestinal epithelial cells and tissue that increases with injury and further increases after glutamine. As shown in the present study, this increase in PPAR-γ is associated with an increase in 15-HETE.
Linoleic acid is metabolized by 15-LOX to 13-S-hydroperoxyoctadecadienoic acid (13-S-HPODE) and then to 13-S-HODE by glutathione peroxidase (35). 13-HODE dehydrogenase converts 13-S-HODE into 13-OXO-ODE, a recently identified PPAR-γ ligand in intestinal epithelial cells with potent anti-inflammatory properties (2). Intestinal epithelial cells have high concentrations of 13-HODE dehydrogenase, which may contribute to the beneficial effects of lipid-mediated PPAR-γ activation (6). We found that 13-OXO-ODE was increased after glutamine and may contribute to glutamine's protective effects.
The common link between glutamine and 15-HETE and 13-OXO-ODE is glutathione, a metabolite of glutamine and glutamate. Glutathione participates in the conversion of 15-HETE and 13-OXO-ODE from 15-S-HETE and 13-S-HPODE, respectively. Glutathione also is thought to conjugate with 13-OXO-ODE and transport it out of the cell as a mechanism to terminate the signals generated by linoleic acid oxygenation (35). Both 15-HETE and 13-OXO-ODE are known PPAR-γ ligands and, like glutamine, increase PPAR-γ in intestinal epithelial cells. PPAR-γ has a large ligand binding site compared with other nuclear receptors, which allows versatility in ligand binding. Itoh et al. (14), using oxidized fatty acids crystallized to PPAR-γ, demonstrated that PPAR-γ can sense not just an isolated fatty acid ligand, but a pool of related ligands. The ligand binding domain also accommodated more than one copy of certain fatty acid ligands. Importantly, oxidized fatty acids coupled covalently to PPAR-γ, which stabilized the ligand binding domain and suggested that the PPAR-γ binding domain can both capture ligands at low concentrations and activate transcription longer than noncovalently bound ligands. Whether the binding domain of PPAR-γ can accommodate both 15-S-HETE and 13-OXO-ODE simultaneously is not known, but covalent bonding of these fatty acids may explain the numerous reports of gut protection afforded by glutamine. Lastly, PPAR-γ has also been identified as a major pathway through which omega-3 fatty acids exert beneficial effects (26, 38, 44). The potential additive benefit of administration of omega-3 fatty acids and glutamine warrant investigation.
In conclusion, our data show that, in intestinal epithelial cells, glutamine increases transcriptional activation of PPAR-γ and suggest it is through intestinal epithelial cell endogenous ligands, 15-S-HETE and 13-OXO-ODE, which are metabolic byproducts of the LOX pathway. These novel findings shed more light onto the complex mechanisms by which glutamine exerts its gut protective effects and supports PPAR-γ as a target for pharmaconutrition.
GRANTS
This work was supported by National Institute of General Medical Sciences Grant (RO1 GM077282).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Abdelrahman M, Collin M, Thiemermann C. The peroxisome proliferator-activated receptor-γ ligand 15-deoxyΔ12,14 prostaglandin J2 reduces the organ injury in hemorrhagic shock. Shock 22: 555–561, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Altmann R, Hausmann M, Spottl T, Gruber M, Bull AW, Menzel K, Vogl D, Herfarth H, Scholmerich J, Falk W, Rogler G. 13-Oxo-ODE is an endogenous ligand for PPARγ in human colonic epithelial cells. Biochem Pharmacol 74: 612–622, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Ban K, Kozar RA. Glutamine protects against apoptosis via downregulation of Sp3 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 299: G1344–G1353, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ban K, Peng Z, Kozar RA. Arginine decreases peroxisome proliferator-activated receptor gamma activity via c-jun in both ligand dependent and independent fashions. JPEN J Parenter Enteral Nutr 35: 18, 2011 [Google Scholar]
- 5. Brasse-Lagnel C, Lavoinne A, Loeber D, Fairand A, Bôle-Feysot C, Deniel N, Husson A. Glutamine and interleukin-1 beta interact at the level of Sp1 and nuclear factor-kappa B to regulate arginosuccinate synthetase gene expression. FEBS J 274: 5250–5262, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Bull AW, Bronstein JC, Branting C, Blackburn ML, Rafter JJ. 13-HODE dehydrogenase activity as a modulator of intestinal cell differentiation, Adv Exp Med Biol 400B: 571–579, 1997 [PubMed] [Google Scholar]
- 7. Burns KA, Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta 1771: 952–960, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F. Structure of the intact PPAR-γ-RXR-α nuclear receptor complex on DNA. Nature 456: 350–356, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dubuquoy L, Jansson EA, Deeb S, Rakotobe S, Karoui M, Colombel JF, Auwerx J, Pettersson S, Desreumaux P. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology 124: 1265–1276, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Ershov AV, Bazan NG. Photoreceptor phagocytosis selectively activates PPAR gamma expression in retinal pigment epithelial cells. J Neurosci Res 60: 328–337, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Herbertsson H, Kühme T, Evertsson U, Wigren J, Hammarström S. Identification of subunits of the 650 kDa 12(S)-HETE binding complex in carcinoma cells. J Lipid Res 39: 237–244, 1998 [PubMed] [Google Scholar]
- 12. Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, Folian B, Subramaniam S, Gonzalez FJ, Glass CK, Ricote M. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest 117: 1658–1659, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Interleukin-4 dependent production of PPAR gamma ligands in macrophages by 12/15-lipoxygenase. Nature 400: 378–382, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Itoh T, Fairall L, Amin K, Inaba Y, Szanto A, Balint BL, Nagy L, Yamamoto K, Schwabe JW. Structural basis for the activation of PPARγ by oxidized fatty acids. Nat Struct Mol Biol 15: 924–931, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang CA, Ting T, Seed B. PPAR agonists inhibit production of monocyte inflammatory cytokines. Nature 391: 82–86, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Jones NE, Heyland DK. Pharmaconutrition: a new emerging paradigm. Curr Opin Gastroenterol 24: 215–222, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA, Pedersen BK. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J Physiol 528: 157–163, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kazufumi K, Wada K, Nakajiama A, Mizuguchi H, Hayakawa T, Nakagawa S, Kadowaki T, Nagai R, Kamisaki Y, Blumberg RS, Mayumi TL. A novel PPAR-γ gene therapy to control inflammation associated with inflammatory bowel disease in a murine model. Gastroenterology 24: 1315–1324, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Kim JB, Wright HM, Wright M, Spiegelman BM. ADD1/SREBP1 activates PPAR gamma through the production of endogenous ligand. Proc Natl Acad Sci U S A 95: 4333–4337, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kozar RA, Schultz SG, Bick RJ, Poindexter BJ, DeSoignie R, Moore FA. Enteral glutamine but not alanine maintains small bowel barrier function after ischemia/reperfusion injury in rats. Shock 21: 433–437, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Kozar RA, Schultz SG, Hassoun HT, DeSoignie R, Weisbrodt NW, Haber MH, Moore FA. The type of sodium-coupled solute modulates small bowel mucosal injury, transport function and ATP after Ischemia/reperfusion injury in rats. Gastroenterology 123: 810–816, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Kudsk KA, Minard G, Croce MA, Brown RO, Lowrey TS, Pritchard FE, Dickerson RN, Fabian TC. A randomized trial of isonitrogenous enteral diets after severe trauma: an immune-enhancing diet reduces septic complications. Ann Surg 224: 531–540, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee SH, Rangiah K, Williams MV, Wehr AY, DuBois RN, Blair IA. Cyclooxygenase-2-mediated metabolism of arachidonic acid to 15-oxo-eicosatetraenoic acid by rat intestinal epithelial cells. Chem Res Toxicol 20: 1665–1675, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Lee SH, Williams MV, Dubois RN, Blair IA. Cyclooxygenase-2-mediated DNA damage. J Biol Chem 280: 28337–28346, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Letellier RML, Dechelotte P, Lacucci M, Ghosh S. Dietary modulation of peroxisome proliferator-activated receptor gamma. Gut 58: 586–593, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, Moorhead JF, Varghese Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR gamma-dependent mechanism. Kidney Int 67: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Masugi J, Tamori Y, Kasuga M. Inhibition of adipogenesis by a COOH-terminally truncated mutant of PPAR gamma2 in 3T3-L1 cells. Biochem Biophys Res Commun 264: 93–99, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Mendez C, Jurkovich GJ, Garcia I, Davis D, Parker A, Maier RV. Effects of an immune-enhancing diet in critically injured patients. J Trauma 42: 933–940, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Nakajiama A, Wada K, Miki H, Kubota N, Nakajima N, Terauchi Y, Ohnishi S, Saubermann LJ, Kadowaki T, Blumberg RS, Nagai R, Matsuhashi N. Endogenous PPAR-γ mediates anti-inflammatory activity in murine ischemia-reperfusion injury. Gastroenterology 120: 460–469 2001 [DOI] [PubMed] [Google Scholar]
- 30. Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med 30: 2022–2029, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Ogino S, Shima K, Baba Y, Nosho K, Irahara N, Kure S, Chen L, Toyoda S, Kirkner GJ, Wang YL, Giovannucci EL, Fuchs CS. Colorectal cancer expression of peroxisome proliferator-activated receptor γ is associated with good prognosis. Gastroenterology 136: 1242–1250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oudemans-van Straaten HM, Bosman RJ, Treskes M, van der Spoel HJ, Zandstra DF. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med 27: 84–90, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down-regulation of inducible nitric-oxide synthase by 15-deoxy-Delta12,14-prostaglandin J2. Proc Natl Acad Sci USA 96: 4668–4673, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sato N, Moore FA, Smith MA, Zou L, Olufemi-Moore S, Schultz SG, Kozar RA. Differential induction of PPARγ by luminal glutamine and iNOS by luminal arginine in the rodent post ischemic small bowel. Am J Physiol Gastrointest Liver Physiol 290: G616–G623, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Seeley SK, Poposki JA, Maksimchuk J, Tebbe J, Gaudreau J, Mannervik B, Bull AW. Metabolism of oxidized linoleic acid by glutathione transferases: peroxidase activity toward 13-hydroperoxyoctadecadienoic acid. Biochim Biophys Acta 1760: 1064–1070, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Shureiqi I, Jiang W, Zuo X, Wu Y, Stimmel JB, Leesnitzer LM, Morris JS, Fan HZ, Fischer SM, Lippman SM. The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells. Proc Natl Acad Sci U S A 100: 9968–9973, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sorrentino SA, Bahlmann FH, Besler C, Muller M, Schulz S, Kirchhoff N, Doerries C, Horvath T, Limbourg A, Limbourg F, Fliser D, Haller H, Drexler H, Landmesser U. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation 116: 163–173, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Stahl A, Sapieha P, Connor KM, Sangiovanni JP, Chen J, Aderman CM, Willett KL, Krah NM, Dennison RJ, Seaward MR, Guerin KI, Hua J, Smith LE. Short Communication: PPARγ mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circ Res 107: 495–500, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Subbarayan V, Xu XC, Kim J, Yang P, Hoque A, Sabichi AL, Llansa N, Mendoza G, Logothetis CJ, Newman RA, Lippman SM, Menter DG. Inverse relationship between 15-lipoxygenase-2 and PPAR-gamma gene expression in normal epithelia compared with tumor epithelia. Neoplasia 7: 280–293, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sugawara K, Olson TS, Moskaluk CA, Stevens BK, Hoang S, Kozaiwa K, Cominelli F, Ley KF, McDuffie M. Linkage to peroxisome proliferator-activated receptor-γ in SAMP1/YitFc mice and in human Crohn's disease. Gastroenterology 128: 351–360, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Voltan S, Martines D, Elli M, Brun P, Longo S, Porzionato A, Macchi V, D'Incà R, Scarpa M, Palù G, Sturniolo GC, Morelli L, Castagliuolo I. Lactobacillus crispatus M247-derived H2O2 acts as a signal transducing molecule activating peroxisome proliferator activated receptor-γ in the intestinal mucosa. Gastroenterology 135: 1216–1227, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Weber SM, Scarim AL, Corbett JA. PPAR gamma is not required for the inhibitory actions of PGJ2 on cytokine signaling in pancreatic beta-cells. Am J Physiol Endocrinol Metab 286: E329–E336, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Willoughby DA, Moore AR, Colville-Nash PR. Cyclopentenone prostaglandins–new allies in the war on inflammation. Nat Med 6: 137–138, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell 3: 397–403, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Yang P, Chan D, Felix E, Madden T, Klein RD, Shureiqi I, Chen X, Dannenberg AJ, Newman RA. Determination of endogenous tissue inflammatory profiles by LC/MS/MS:COX- and LOX-derived bioactive lipids. Prostaglandins Leukot Essent Fatty Acids 75: 385–395, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Yang P, Felix E, Madden T, Fischer SM, Newman RA. Quantitative high performance liquid chromatography/electrospray ionization tandem mass spectrometric analysis of 2- and 3-series prostaglandins in cultured tumor cells. Anal Biochem 308: 168–177, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-gamma is a new therapeutic target in sepsis and inflammation. Shock 23: 393–399, 2005 [DOI] [PubMed] [Google Scholar]