Abstract
Human multidrug and toxin extrusion 1 (hMATE1, SLC47A1) is a major candidate for being the molecular identity of organic cation/proton (OC/H+) exchange activity in the luminal membrane of renal proximal tubules. Although physiological function of hMATE1 supports luminal OC efflux, the kinetics of hMATE1-mediated OC transport have typically been characterized through measurement of uptake, i.e., the interaction between outward-facing hMATE1 and OCs. To examine kinetics of hMATE1-mediated transport in a more physiologically relevant direction, i.e., an interaction between inward-facing hMATE1 and cytoplasmic substrates, we measured the time course of hMATE1-mediated efflux of the prototypic MATE1 substrate, [3H]1-methyl-4-phenylpyridinium, under a variety of intra- and extracellular pH conditions, from Chinese hamster ovary cells that stably expressed the transporter. In this study, we showed that an IC50/Ki for interaction between extracellular H+ and outward-facing hMATE1 determined from conventional uptake experiments [12.9 ± 1.23 nM (pH 7.89); n = 9] and from the efflux protocol [14.7 ± 3.45 nM (pH 7.83); n = 3] was not significantly different (P = 0.6). Furthermore, kinetics of interaction between intracellular H+ and inward-facing hMATE1 determined using the efflux protocol revealed an IC50 for H+ of 11.5 nM (pH 7.91), consistent with symmetrical interactions of H+ with the inward-facing and outward-facing aspects of hMATE1.
Keywords: organic cation, transport, secretion, renal proximal tubule, pH
the designation “organic cations” (OCs) refers to a large group of organic compounds that carry net positive charge(s) at physiological pH. The majority of OCs are xenobiotic, i.e., of exogenous origin, and many are toxic. On a regular basis, individuals consume potentially toxic OCs, typically as plant products and/or prescribed medicines. Currently, ∼40% of commonly prescribed drugs can be classified as OCs (7), including metformin (anti-diabetic), cimetidine (anti-peptic ulcer), and clonidine (anti-hypertensive and analgesic). In the human body, the renal proximal tubule (RPT) is the major site for OC secretion (24). In RPT epithelial cells (RPTECs), OC secretion is a two-step process, with OC uptake across the peritubular (basolateral) membrane by a process of facilitated diffusion, and OC efflux at the luminal (apical) membrane, mainly by an OC/H+ exchange process (8).
There is broad agreement that the basolateral entry step in OC secretion by the human RPT is dominated by human organic cation transporter 2 (hOCT2, SLC22A2), which has been well-characterized both structurally and functionally (24). There is also growing consensus that the exit step, also the rate-limiting step (24), for OC secretion involves the combined activity of two members of the multidrug and toxin extrusion (MATE) family, MATE1 (SLC47A1) and MATE2-K (SLC47A2) (8, 12). Although in their proposed physiological role MATEs mediate the efflux of OCs, the characterization of MATE-mediated OC transport has, with few exceptions, relied on measurement of uptake, i.e., influx, with the tacit assumption that MATEs interact symmetrically with their substrates, including H+ (10). While this is a reasonable assumption with respect to the catalytic and energetic mechanism of transport, i.e., mediated exchange of H+ for OC (12, 19), it may well not be the case with respect to the kinetics and selectivity of substrate interaction with these transporters. In that regard, it is significant that rat Oct2 (rOct2, slc22a2) has been shown to have markedly asymmetrical interactions with (at least) selected OCs. For example, the affinity of tetrabutylammonium (TBuA) to outward-facing rOct2 is 4-fold higher than its affinity to inward-facing rOct2, whereas the affinity of corticosterone to outward-facing rOct2 is 20-fold lower than its affinity to inward-facing rOct2 (20).
To study MATE-mediated OC transport in its physiologically relevant direction, i.e., hMATE1 as an OC efflux transporter, we quantified the MATE-mediated efflux of the model OC, MPP. In this study, we show that kinetics of interaction between outward-facing hMATE1 and H+ measured using an efflux protocol are not significantly different from those obtained using conventional uptake experiments. Furthermore, for the first time, we report kinetics of interaction between inward-facing hMATE1 and H+. These results support the conclusion that, despite the likelihood of structurally distinct binding regions exposed to the cytoplasmic vs. extracellular faces of hMATE1, the kinetics of H+ interaction with each face of the transporter are similar.
MATERIALS AND METHODS
Chemicals.
[3H]1-methyl-4-phenylpyridinium ([3H]MPP; 80 Ci/mmol) and [2-(4-nitro-2,1,3-benzoxadiazol-7-yl)aminoethyl]trimethylammonium (NBD-TMA) (2) were synthesized by the Department of Chemistry and Biochemistry, University of Arizona. [14C]Mannitol (58.8 mCi/mmol) was purchased from PerkinElmer. 2′,7′-Bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl (BCECF-AM) and nigericin were obtained from Invitrogen. Tetrapentylammonium (TPeA) bromide, saponin, and other chemicals (unless otherwise noted) were purchased from Sigma.
Cell culture.
Chinese hamster ovary (CHO) Flp-In cells (Invitrogen) that stably expressed hMATE1 (CHO hMATE1 cells), created in our lab as previously described (26), were grown in Ham's F12 Kaighn's modification medium (Sigma) supplemented with 10% fetal bovine serum (HyClone) and 0.1 mg/ml of Hygromycin B (Invitrogen). These cells were maintained in an incubator (NuAire) at 37°C supplied with 5% CO2. Subculture of the cells was performed every 3 to 4 days. For transport studies, the CHO hMATE1 cells were seeded in multiwell cell culture plates (Cellstar) or in 35-mm cell culture dishes (Falcon).
hMATE1-mediated MPP uptake.
CHO hMATE1 cells were grown to confluence in 12- or 24-well cell culture plates. Before each uptake, cells were incubated in Waymouth buffer (WB; in mM: 135 NaCl, 13 HEPES, 2.5 CaCl2-2H2O, 1.2 MgCl2-6H2O, 0.8 MgSO4-7H2O, and 28 d-glucose; pH 7.4) at room temperature (between 20 to 25°C) for 30 min. After removal of WB at pH 7.4, MPP uptake was initiated by adding WB at pH 8.5 containing [3H]MPP (∼13 nM). For a time course study, [3H]MPP uptake was terminated at specific times by aspirating and rinsing each well with ice-cold (4°C) WB at pH 8.5. Cells were lysed by adding 0.5 N NaOH/1% SDS and neutralized with 1 N HCl. After being mixed with scintillation cocktail (MP Biomedicals), lysate from each sample was transferred to a liquid scintillation counter (Beckman LS6000IC) to determine the amount of [3H]MPP.
hMATE1-mediated MPP efflux.
Two (similar) protocols were used. The first used CHO hMATE1 cells grown in 48-well cell culture plates. Cells grown to confluence were incubated in WB at pH 7.4 for 30 min at room temperature. Then, WB at pH 7.4 was replaced by WB at pH 8.5 containing [3H]MPP (∼13 nM) for 20 min to allow accumulation of labeled MPP into the cells. After the solution containing [3H]MPP was removed, the cells were briefly rinsed with WB at pH 8.5 at room temperature. Efflux of the labeled MPP was initiated by adding various buffers, according to the purpose of each experiment. The efflux was terminated by removing the buffer at a specific time, and the remaining intracellular [3H]MPP was determined by lysing the cells with 0.5 N NaOH containing 1% SDS. Radioactivity in each cell lysate was determined as described above. The amount of [3H]MPP remaining in the cells at each time point was expressed as a percentage of the amount of accumulated [3H]MPP before efflux was initiated.
The second efflux protocol measured continuous efflux from CHO hMATE1 cells grown in a 35-mm cell culture dish. After the cells were incubated in WB at pH 7.4 at room temperature for 30 min, a dish insert (Warner Instruments, RC-37FC; Supplemental Fig. S1; the online version of this article contains supplemental data) was assembled within the 35-mm dish, creating a closed chamber with a growth surface area of 1.85 cm2 and a volume of 185 μl. WB at pH 8.5 containing [3H]MPP and [14C]mannitol (∼39 nM and 10 μM, respectively) was introduced into the chamber via polyethylene (PE) tubing and [3H]MPP was allowed to accumulate for 20 min within the CHO hMATE1 cells (the mannitol served as a marker for extracellular volume). Subsequently, the buffer containing labeled solutes was removed from the chamber through PE tubing at the distal end of the chamber and hMATE1-mediated [3H]MPP efflux was initiated by introducing laminar flow of various buffers through the chamber using a syringe pump (Harvard Apparatus) at a constant rate (5 ml/min). A fraction collector was used to continuously collect buffer at the distal end of the chamber; fractions were typically collected every 6 s (usually for 2 to 2.5 min). The amounts of [3H]MPP and [14C]mannitol were measured in each sample as described earlier using a double-label counting protocol. A representative result of [3H]MPP efflux measured using the chamber protocol is shown in Supplemental Fig. S2.
Intracellular pH measurement.
CHO hMATE1 cells were incubated in WB containing 5 μM BCECF-AM at room temperature for 15 min. Using excitation wavelengths of 488 and 460 nm, the fluorescence signal from intracellular BCECF was measured at an emission wavelength of 535 nm. The ratio of fluorescence from the two excitation wavelengths was recorded over time using an InCyt Im2 imaging system (Intracellular Imaging). Intracellular pH (pHi) of CHO hMATE1 cells was calculated from a calibration curve performed after each measurement in standard pH buffers containing 10 μM nigericin (see Supplemental Fig. S3 for a representative example).
Statistics.
Data were analyzed using Prism (GraphPad Software). Statistical tools such as paired t-test or unpaired t-test were applied according to the nature of each data set.
RESULTS AND DISCUSSION
Cis inhibition by extracellular H+ of hMATE1-mediated OC uptake.
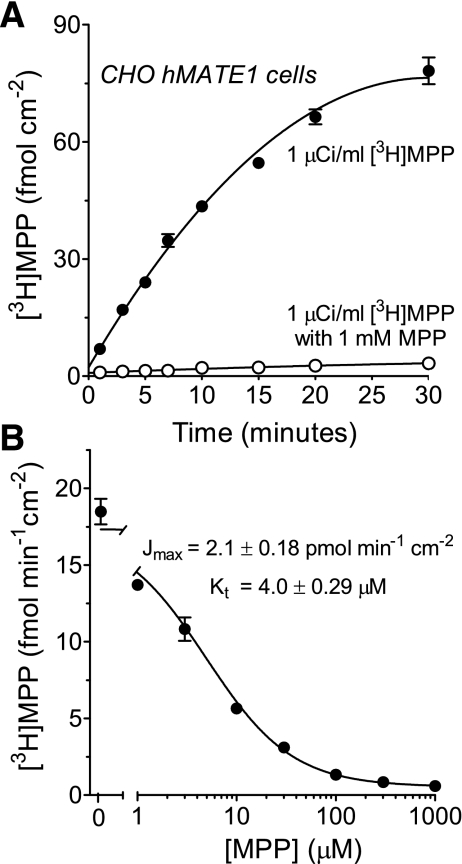
We first characterized hMATE1-mediated transport of the test substrate, MPP, in CHO cells that stably expressed the transporter. Uptake of [3H]MPP was linear for ∼10 min (Fig. 1A) and 5-min uptakes were selected for use in subsequent kinetic studies as it covered the initial rate of MPP uptake in CHO hMATE1 cells. Figure 1B shows that unlabeled MPP blocked the uptake of labeled MPP with a profile that was adequately described by the Michaelis-Menten equation for the competitive interaction of labeled and unlabeled substrate, as described previously (26):
where J* is the rate of [3H]MPP transport from a concentration equal to [MPP*]; Jmax is the maximum rate of mediated MPP transport; Kt is the MPP concentration that resulted in half-maximal transport (Michaelis constant); [MPP] is the concentration of unlabeled MPP in the uptake reaction; and D is a constant that describes the nonsaturable (first-order) component of total substrate uptake (over the range of substrate concentrations tested) that presumably reflected the combined influence of diffusive flux, nonspecific binding, and/or incomplete rinsing of the cell layer. In four separate experiments, the Jmax was 2.1 ± 0.18 pmol·min−1·cm−2 and the apparent Kt (at pH 8.5) was 4.0 ± 0.29 μM (Table 1).
Fig. 1.
Time course and kinetics of [3H]MPP uptake in Chinese hamster ovary (CHO) human multidrug and toxin extrusion 1 (hMATE1) cells. A: [3H]MPP accumulated linearly in CHO hMATE1 cells up to ∼10 min (·). [3H]MPP uptake was inhibited by coincubating the cells with 1 mM nonlabeled MPP (○). Buffer pH was 8.5. Each point is the mean (±SE) of uptake measured in 2 wells of a 24-well plate. B: uptake (5 min; pH 8.5) of [3H]MPP transport measured in the presence of increasing concentrations of unlabeled MPP (from which the kinetics of MPP transport were determined). Data shown are from a single representative experiment. Each point is the mean (±SE) of uptakes measured in 3 wells of a 24-well plate.
Table 1.
Effect of extracellular pH on the kinetics of hMATE1-mediated MPP uptake
| pHo | Kt, μM | Jmax, pmol·min−1·cm−2 |
|---|---|---|
| 8.5 ± 0.02 | 4.0 ± 0.29 | 2.1 ± 0.18 |
| 7.5 ± 0.1 | 12.3 ± 1.08* | 3.7 ± 0.36* |
Data are means ± SE. Five-minute uptakes of [3H]MPP (∼13 nM) were determined as a function of increasing concentration of unlabeled MPP at 2 values for external pH (pHo), pH 7.5 ± 0.1 (n = 4) and pH 8.5 ± 0.02 (n = 4). Both Jmax and Kt of MPP uptake in Chinese hamster ovary hMATE1 cells from these 2 pH values were significantly different (
P = 0.01).
The interaction between outward-facing hMATE1 and H+ was initially characterized using two experimental approaches that each involved measurement of uptake. First, the IC50 for H+ inhibition of hMATE1-mediated MPP uptake was determined (Fig. 2). The decrease in MPP uptake resulting from an increase in extracellular [H+] ([H+]o) was adequately described by the following relationship (4):
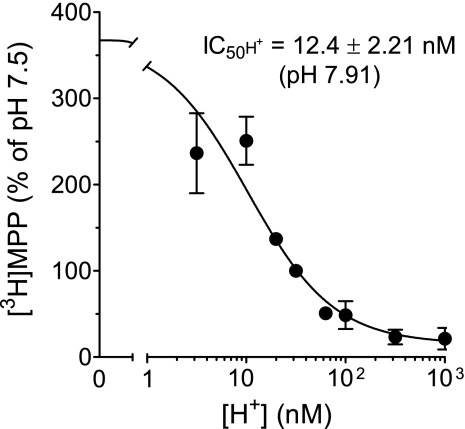
where J is the rate of [3H]MPP uptake; Japp is the product of the maximum rate of [3H]MPP uptake (Jmax) and the ratio of the Ki of H+ and Kt for MPP transport; and IC50 is the concentration of [H+]o that reduced mediated (i.e., blockable) [3H]MPP transport by 50%. The IC50 for H+-inhibited hMATE1-mediated MPP uptake was 12.4 ± 2.21 nM (pH 7.91; n = 5). Although this is the first kinetic assessment of the inhibitory effect of extracellular H+ on hMATE1-mediated transport, several previous studies showed that transport was reduced as extracellular pH was decreased below a value of 8.5 (12, 18, 19). The most detailed of these (18), when replotted as the effect of [H+]o on MATE1-mediated transport of [14C] tetraethylammonium (TEA; a prototypic OC), suggested an IC50 of ∼15 nM (pH ∼7.8), similar to the value noted here.
Fig. 2.
IC50 of extracellular H+ as an inhibitor of hMATE1-mediated MPP uptake. Five-minute uptakes of 13 nM [3H]MPP into CHO hMATE1 cells were plotted as a function of extracellular [H+]. Each point is the mean (±SE) of uptakes determined in 5 separate experiments (n = 5).
It is also generally observed that hMATE1-mediated OC transport decreases as pH values increase beyond 8.5 (12, 18), suggesting that H+ interaction with hMATE1 is unlikely to be limited to a simple competitive interaction and probably includes indirect effects of reduced [H+]o on the structure of hMATE1 and/or other proteins. Therefore, we decided to use another approach to determine an “apparent Ki” for hydrogen ion (Ki-appH), namely, the influence of extracellular [H+] on the kinetics of MPP transport. Kinetics of hMATE1-mediated [3H]MPP uptake at pH 7.5 ([H+] = 31.6 nM) and pH 8.5 ([H+] = 3.2 nM) are presented in Table 1. The increase in [H+]o resulted in a threefold increase in the apparent Kt for MPP transport. This effect was anticipated given the previous observation that extracellular H+ acts as a competitive inhibitor of OC/H+ exchange in isolated renal brush-border membrane vesicles (BBMV) (23). However, in our current experiments on [3H]MPP uptake in intact cells, it was also evident that the increase in extracellular [H+] resulted in a modest (1.8-fold) but significant (P < 0.01) increase in the Jmax for MPP transport, which is not consistent with a purely competitive interaction of H+ with MPP at the external face of hMATE1. This result was, in fact, seen in all four experiments that measured the kinetics of MPP transport at two different pHs (Table 1). Nevertheless, although these data suggest that extracellular hydrogen ion interacts with hMATE1 at several sites, resulting in a “mixed-type” inhibitory profile, we suggest that the predominant influence of H+ on apparent Kt reflected competition between H+ and MPP for a common site or (more likely) a set of mutually exclusive sites within a common binding surface.
The simplifying assumption that H+ acts predominantly as a competitive inhibitor of hMATE1-mediated MPP transport permitted calculation of Ki-appH using the relationships (4):
where
For these relationships, J and Jmax are as previously described; Kt is the Michaelis constant for hMATE1-mediated MPP transport; and Kt-app is the apparent Kt for MPP transport (reflecting the competitive influence of [H+]o). From the experiments shown in Table 1, the Ki-appH for the competitive interaction of H+ with extracellular aspect of hMATE1 was 13.5 ± 0.84 nM (pH 7.87), which was not different from the apparent IC50 for H+ inhibition of hMATE1-mediated MPP transport, noted above (P = 0.73).
Characterization of hMATE1-mediated OC efflux.
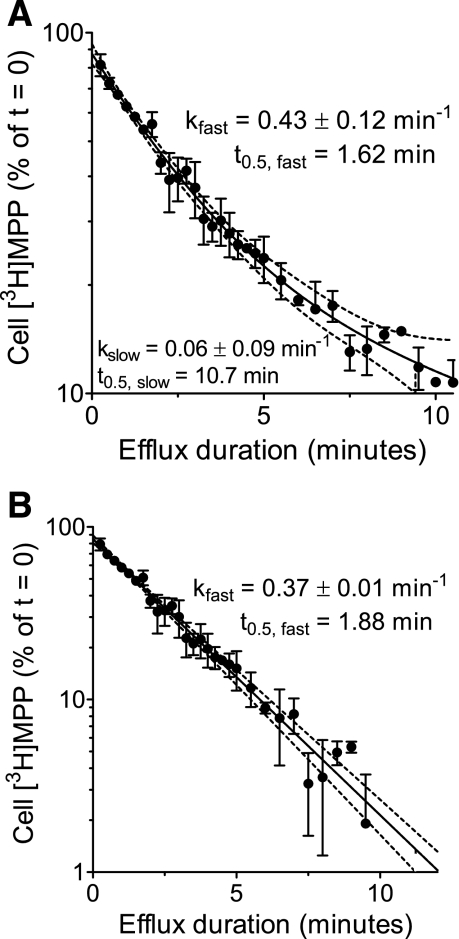
The intent of this study was development of a quantitative view of the kinetics of hMATE1-mediated transport when operating in its “physiological” direction, i.e., exporting OCs. To this end, we used two similar approaches to quantify hMATE1-mediated efflux from cells stably expressing this protein. The first involved measurement of the amount of substrate retained in cells over time after an initiation of efflux. Figure 3A shows the 10-min time course of [3H]MPP efflux from CHO hMATE1 cells obtained using this method. As expected, there was a time-dependent decrease in cell [3H]MPP and, as supported by observations described below, the loss of [3H]MPP from the cells was effectively restricted to efflux via hMATE1. Because the concentration of [3H]MPP in the cells at time 0 was likely to be well below the Kt at the cytoplasmic face of the transporter, the kinetics of efflux could have been expected to be first-order.1 This was not, however, the case, as shown by the nonlinearity of the semilog presentation of these data (Fig. 3A). The curvilinearity of the plot suggested that MPP efflux involved at least two compartments, arranged in series (e.g., an endosomal compartment and the cytoplasm) with the extracellular compartment. Furthermore, the evident curvilinearity necessitates that passage from the first (“endosomal”) into the second (cytoplasm) is rate-limiting. This is not an unexpected result in light of evidence that organic cations can, in fact, be sequestered within intracellular compartments (11), including uptake of TEA into endosomal vesicles isolated from rat renal cortex (14).
Fig. 3.
Time course of MPP loss from CHO hMATE1 cells. A: following a 20-min preload of 13.5 nM [3H]MPP, the amount of labeled MPP remaining in the cells was measured as a function of time after efflux was initiated in an MPP-free buffer of pH 7.4 ([H+]o = 39.8 nM). Each point is the mean [3H]MPP content (±SE) of cells in 2 wells of a 48-well plate in a single representative experiment. The semilog plot reveals that there were multiple compartments involved in [3H]MPP efflux from CHO hMATE1 cells as discussed in the text. The solid line represents 2-phase exponential decline in cell [3H]MPP (kinetic parameters listed in the figure). B: time course of the first-order (single phase) decline in cell [3H]MPP, reflecting correction for sequestered [3H]MPP (see text for discussion). The dotted lines in both graphs represent the 95% confidence interval of the fitted values (solid line).
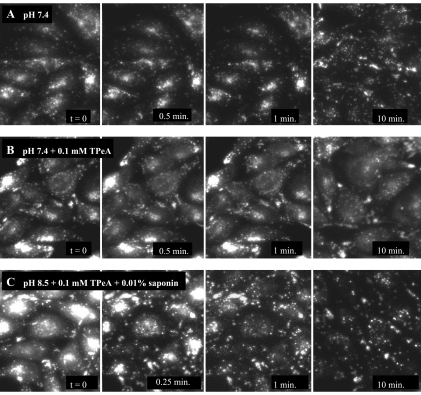
To assess, at least qualitatively, if such sequestration could account for the non-first-order efflux profile shown in Fig. 3A, we examined the time course of efflux of a fluorescent substrate for OC transporters, NBD-TMA (λex = 458 nm, λem = 530 nm) (2). NBD-TMA blocked hMATE1-mediated MPP transport in CHO cells with an IC50 of 14.8 μM (data not shown), and although NBD-TMA may well display a quantitatively distinct distribution profile in CHO cells than the structurally distinct MPP, it provided a useful probe for visualizing the complexity of substrate distribution in these cells. CHO hMATE1 cells were incubated in WB at pH 8.5 containing 0.5 mM NBD-TMA for 15 min in an incubator with 5% CO2 at 37°C. The NBD-TMA-loaded cells were then transferred to the stage of an epifluorescence microscope (Olympus IX71). Intracellular accumulation of fluorescent signal was only observed in cells that expressed hMATE1 (data not shown). After brief rinsing, NBD-TMA efflux was initiated by adding buffers without substrate. Figure 4 provides a sequence of images showing the time course of NBD-TMA efflux from CHO hMATE1 cells under several conditions.
Fig. 4.
Time course of [2-(4-nitro-2,1,3-benzoxadiazol-7-yl)aminoethyl]trimethylammonium (NBD-TMA) efflux in CHO hMATE1 cells. CHO hMATE1 cells were preloaded with 0.5 mM NBD-TMA at 37°C for 15 min. Following aspiration of the medium, the cells were transferred to a fluorescent microscope, rinsed with Waymouth buffer (WB) at pH 8.5 at which point an image was collected representing t = 0. Subsequent images were collected at the indicated time points following introduction of test media. For each condition, the cells were illuminated only during collection of the image. The 10-min images were from different fields of cells (to avoid the influence of photobleaching).
Before the efflux was initiated, i.e., at t = 0, fluorescence within CHO hMATE1 cells could be categorized into two distinct patterns: a comparatively diffuse, homogenous signal and a punctate signal. The diffuse signal was observed throughout the cells and probably represented free NBD-TMA in the cytoplasm. In contrast, the punctate signal was scattered, more intense, and probably represented NBD-TMA sequestered within cytoplasmic organelles. Our finding was similar to a result from primary rat choroid plexus cells showing that distribution of accumulated quinacrine (another fluorescent OC) includes both a diffuse and a punctate fluorescent signal (11). Furthermore, as noted earlier, endosomes isolated from the rat renal cortex accumulate TEA via TEA/H+ exchange (14). Based on these results, we suggest that the punctate distribution of NBD-TMA in CHO cells most likely represented a similar sequestration in cytoplasmic endosomes.
But is efflux of sequestered OC from this intracellular compartment slow compared with efflux from the cytoplasm across the plasma membrane? When efflux of NBD-TMA was performed at extracellular pH 7.4, the diffuse signal rapidly diminished and almost disappeared after 10 min, whereas the punctate signal was still largely retained (Fig. 4A). In contrast, when 0.1 mM TPeA, a nontransported inhibitor of OC/H+ exchange in renal BBMV (22), was present in the extracellular medium during the efflux period, both patterns of NBD-TMA signal were effectively retained for at least 10 min (Fig. 4B). A brief exposure to 0.01% saponin can selectively permeabilize cholesterol-rich membranes, such as the plasma membrane (17), and when NBD-TMA-loaded cells were exposed to saponin (coexposed with 0.1 mM TPeA), the diffuse signal cleared as early as 15 s while the punctate fluorescent signal still remained after 10 min of efflux (Fig. 4C).
These results support the contention that at the start of the efflux period, [3H]MPP was distributed in at least two compartments: a “fast” cytoplasmic compartment that should readily access hMATE1 at the plasma membrane; and a “slow” compartment consisting of [3H]MPP sequestered in various cytoplasmic organelles. The solid line fit to the data presented in Fig. 3A assumed a two-phase model for the exponential loss of [3H]MPP from CHO hMATE1 cells: a large compartment (77% of accumulated [3H]MPP]) that turned over with a half-time of 1.6 min; and a smaller compartment (23% of accumulated [3H]MPP) that turned over with a half-time of over 10 min. For this study, we focused on the early time course of [3H]MPP efflux that we suggest was dominated by the fast, cytoplasmic compartment. We found that [3H]MPP in the slow, sequestered compartment could be treated as a constant, permitting the data to be analyzed using a one-compartment model that decayed to a plateau, using the relationship: [3H]MPPt = ([3H]MPPt0 − C)e(−kt) + C, where [3H]MPPt is total labeled MPP in the cells at any time t after initiation of efflux (t0); C is sequestered [3H]MPP; and k is the first-order rate constant for hMATE1-mediated [3H]MPP efflux from the cytoplasmic compartment. Correcting total [3H]MPP in the cells for the sequestered compartment resulted in an efflux profile that was described by the relationship:[3H]MPPt = ([3H]MPPt0)e(−kt).
The adequacy of this model to describe the efflux of [3H]MPP from CHO hMATE1 cells is evident in the linearity of the semi log plot of these data presented in Fig. 3B, and the similarity in the rate constant of efflux (k) calculated after subtraction of sequestered [3H]MPP (0.37 min−1) to the rate constant of efflux from the fast compartment of the two-compartment model (0.43 min−1; Fig. 3A). For the rest of this study, each efflux data set was analyzed by a single-phase decay equation after subtraction of the estimated sequestered pool of [3H]MPP, as shown above.
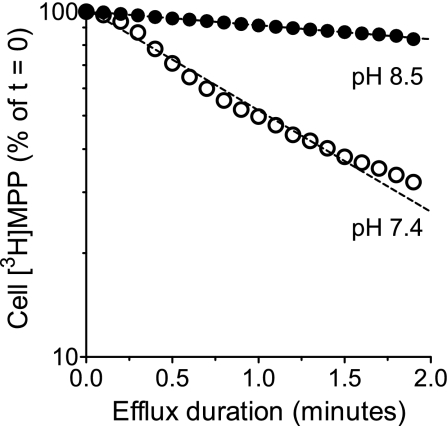
The analysis of [3H]MPP efflux from preloaded CHO hMATE1 cells also permitted a direct test of the hypothesis that an inwardly-directed H+ gradient should increase the rate of hMATE1-mediated MPP efflux. These experiments used the second of the two efflux protocols described in materials and methods, the flow chamber that permitted continuous monitoring of [3H]MPP that left the cells into the passing stream of buffer. Within the limit of resolution of the method, the efflux of MPP from the cytoplasmic compartment of CHO hMATE1 cells (i.e., total efflux corrected for the slow compartment) was adequately described by first-order kinetics (Fig. 5). In these experiments, the half-life (t0.5) of hMATE1-mediated MPP efflux in buffer of the extracellular pH 8.5 was 9.8 ± 1.43 min (n = 4), and decreasing the external pH to 7.4 (i.e., increasing [H+]o from 3.2 to 39.8 nM) resulted in a sevenfold decrease (P = 0.0004) in the t0.5, down to 1.4 ± 0.08 min (n = 5). These results confirmed previous observations (12, 19) that inwardly-directed H+ gradients trans-stimulate MATE1-mediated OC efflux and, of particular importance to the present context, provided a validation for our approach to measuring hMATE1-mediated OC efflux.
Fig. 5.
Effect of extracellular H+ on hMATE1-mediated [3H]MPP efflux. Efflux was performed at extracellular pH 8.5 ([H+]out = 3.2 nM) and at pH 7.4 ([H+]out = 39.8 nM) using the efflux chamber protocol.
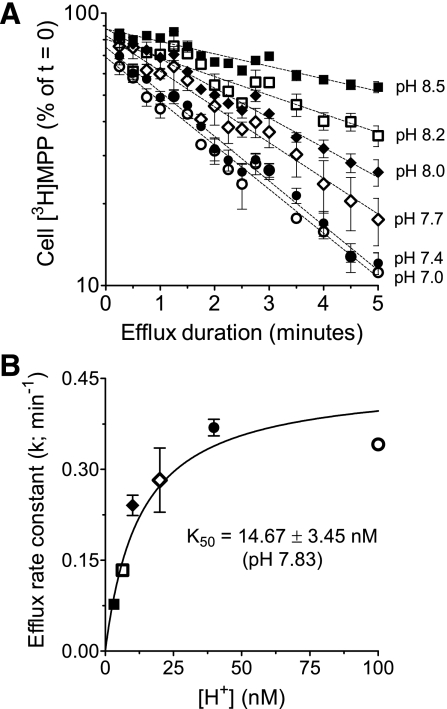
Quantification of MPP efflux also provided an alternative means for assessing the kinetics of H+ interaction with the external face of hMATE1. Figure 6A shows the effect of systematically increasing extracellular [H+] on the rate of MPP efflux. As expected, hMATE1-mediated MPP efflux was stimulated by increasing the concentration of extracellular H+, i.e., the exchangeable substrate. Furthermore, the rate constant for MPP efflux supported by each extracellular [H+], when plotted as a function of [H+] (Fig. 6B), was adequately described by the Michaelis-Menten equation; the half-maximal stimulation of MPP efflux occurred at an extracellular pH of 7.83 ([H+] = 14.7 ± 3.45 nM; n = 3), and this result was not significantly different from either the IC50 (P = 0.79) or the Ki (P = 0.63) for extracellular H+ inhibition of hMATE1-mediated MPP uptake. Note that hMATE1-mediated MPP efflux was also performed at pHo 6.5 and 6.0, but the rate constants for efflux at these values of pHo (0.37 ± 0.013 and 0.36 ± 0.017 min−1, respectively) did not differ from that measured at pHo 7.0 (0.37 ± 0.02 min−1) and, so (for clarity) were not shown in Fig. 6A.
Fig. 6.
Effect of increasing extracellular [H+] on the kinetics of hMATE1-mediated [3H]MPP efflux. A: time course of [3H]MPP efflux from the “fast” cytoplasmic compartment (as defined in the text) was determined in buffers of decreasing external pH. The value of the slow compartment used for each condition was determined from the efflux profile at pHo 7.0. Each point is the mean of results obtained in 3 separate experiments. B: rate constants for [3H]MPP efflux from the fast compartment (the slopes of the lines fit to the data in A) plotted as a function of extracellular [H+] (line fit using Michaelis-Menten equation).
Interaction of intracellular H+ with the cytoplasmic face of hMATE1.
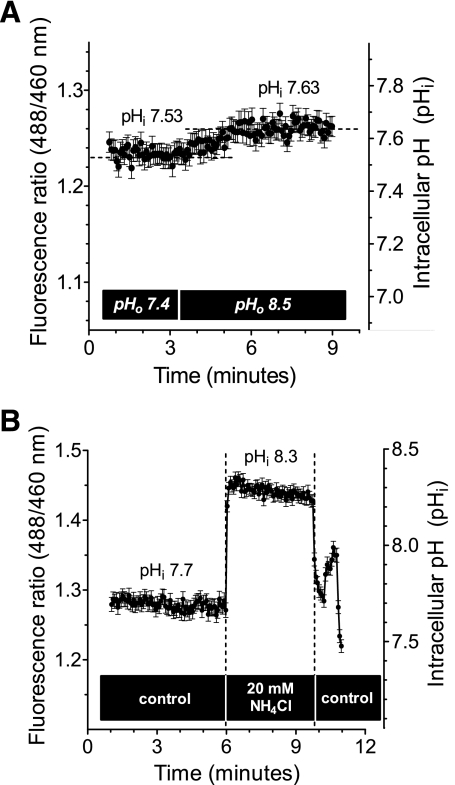
To examine kinetics of interaction between H+ and the inward-facing aspect of hMATE1, MPP efflux was determined as a function of intracellular H+ concentration. From multiple measurements at room temperature in WB at pH 7.4, CHO hMATE1 cells demonstrated a baseline pHi of 7.57 ± 0.03 ([H+]i = 27.1 ± 1.79 nM; n = 21). This baseline value varied by <0.2 pH units during a 15-min exposure to WB of different pH. Figure 7A shows a representative result of the change in pHi resulting from an acute shift from an extracellular pH of 7.4 to one of 8.5; baseline pHi increased by only 0.1 pH units (from pH 7.53 to pH 7.63). Manipulation of pHi was achieved through acute exposure of cells to buffers containing increasing concentrations of NH4Cl. Figure 7B shows the effect on the pHi of CHO hMATE1 cells of an acute exposure to 20 mM NH4Cl. Cytoplasmic pH shifted within 8 s from a steady-state value of 7.7 to 8.3, which was maintained up to 3 min. Acute exposure of these cells to 2.5, 5, 10, or 20 mM NH4Cl effectively established cytoplasmic pH values of pH 7.8 ([H+]i = 15.8 ± 0.44 nM; n = 3), pH 7.9 (12.5 ± 1.42 nM; n = 6), pH 8.1 (8.7 ± 0.76 nM; n = 3), or pH 8.2 (5.8 ± 0.35 nM; n = 4), respectively.
Fig. 7.
Intracellular pH (pHi) in CHO hMATE1 cells. A: representative experiment showing the modest change in pHi associated with acute change of pHo; in this case, a shift from pHo of 7.4 to pHo of 8.5 increased pHi from 7.53 to 7.63. B: upon acute exposure to 20 mM NH4Cl, the pHi of CHO hMATE1 cells increased rapidly (within 8 s) from a baseline pHi value (pHi of 7.57, i.e., [H+]i = 27.1 ± 1.79 nM; n = 21) to a new value (pHi of 8.2, i.e., [H+]i = 5.8 ± 0.35 nM; n = 4) that was relatively stable for at least 3 min. Data shown are from a single representative experiment. (Each point of the fluorescence ratio in both A and B was an average value measured from 28 CHO hMATE1 cells.)
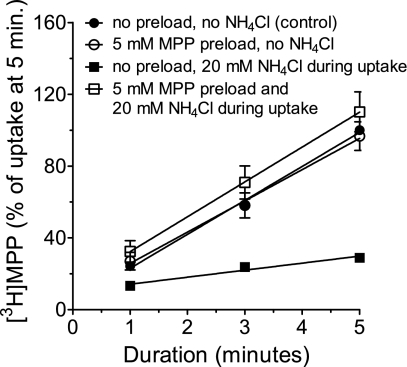
We also considered it necessary to determine whether extracellular NH4+ had a direct effect on the transport process. The presence of 20 mM NH4Cl in an uptake buffer (pH 8.5) decreased the 5-min uptake of [3H]MPP by ∼70% (Fig. 8). An inhibition of uptake was expected because of the associated decrease in exchangeable H+ in the cytoplasm noted above. However, it could also have included a direct inhibitory effect of NH4+ on the extracellular face of hMATE1. We reasoned that, if the observed decrease in MPP uptake was due simply to a decrease in intracellular [H+], then providing an alternative exchangeable substrate at the cytoplasmic face of hMATE1 should rescue the inhibitory effect of extracellular NH4+ on MPP uptake. To test this hypothesis, CHO hMATE1 cells were preloaded with a high concentration of nonlabeled MPP (5 mM MPP for 10 min) before measuring [3H]MPP uptake in WB containing 20 mM NH4Cl. Results in Fig. 8 demonstrated that [3H]MPP uptake inhibited by extracellular NH4Cl was quantitatively rescued in the cells preloaded with nonlabeled MPP, suggesting that extracellular NH4+ did not directly inhibit hMATE1-mediated MPP uptake and that observed effects of NH4Cl on transport reflected changes in the cytoplasmic [H+].
Fig. 8.
Effect of NH4Cl and MPP preload on hMATE1-mediated [3H]MPP uptake. The time course of hMATE1-mediated uptake of [3H]MPP (13.5 nM) was measured under the conditions indicated in the figure. In every case, pHo was 8.5. Each point is the mean (±SE) of uptake values (measured in triplicate) from 3 separate experiments (n = 3).
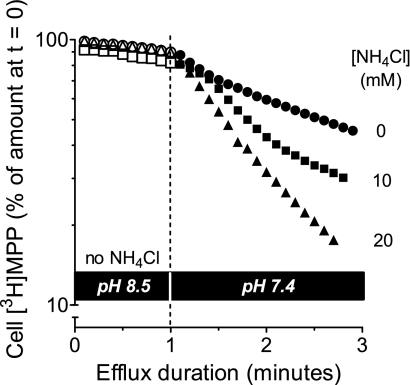
The rate of MPP efflux should increase with decreasing intracellular H+ concentration because of the associated decrease in competition between the two substrates at the cytoplasmic face of hMATE1. To test this, cells were preloaded with [3H]MPP and efflux was initiated at extracellular pH 8.5 with no NH4Cl in the extracellular medium. After 1 min, buffer was switched to WB at pH 7.4 (to stimulate efflux; see Fig. 5) containing different concentrations of NH4Cl to manipulate intracellular pH. Efflux was then continuously monitored for another 2 min. As shown in Fig. 9, decreasing the pH from 8.5 to 7.4 (i.e., increasing the concentration of extracellular H+) produced the anticipated increase (∼7-fold) in rate of [3H]MPP efflux. In this condition, with no extracellular NH4Cl, the pHi of CHO cells was 7.57 ([H+]i = 27.1 nM). As expected, alkalinization of the cytoplasm by addition of 10 mM NH4Cl (pHi = 8.1, [H+]i = 8.7 nM) or 20 mM NH4Cl (pHi = 8.2, [H+]i = 5.8 nM) did progressively increase the rate of [3H]MPP efflux.
Fig. 9.
Effect of NH4Cl on hMATE1-mediated [3H]MPP efflux. Cells were preloaded with [3H]MPP (36.8 nM) for 15 min. Efflux into a buffer of pH 8.5 was measured for 1 min, at which point the cells were exposed to a buffer of pH 7.4 containing the indicated concentrations of NH4Cl (to decrease [H+]i). Efflux into these new solutions was then determined for 2 min. Single representative examples are shown for each condition.
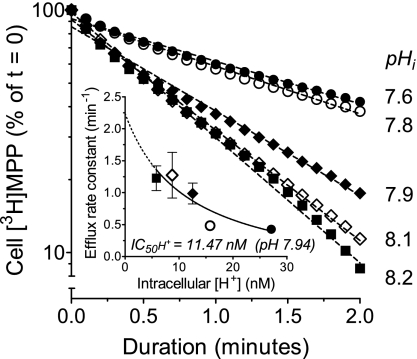
To determine the kinetics of interaction between inward-facing hMATE1 and intracellular H+, hMATE1-mediated MPP efflux was performed at an extracellular pH of 7.4 with no NH4Cl, and with 2.5, 5, 10, or 20 mM NH4Cl. As shown in Fig. 10, the efflux rate constant (k) increased in a concentration-dependent manner as the cells were exposed to increasing concentrations of NH4Cl, i.e., as intracellular [H+] was systematically decreased from 27.1 nM (pH 7.57) to 5.8 nM (pH 8.2), see Fig. 10, inset. The IC50 for cytoplasmic H+ on hMATE1-mediated MPP efflux was at pH 7.94 ([H+]i = 11.5 nM). This value did not differ substantially from the range of values (12 to 15 nM) for the apparent Ki/IC50 of H+ interaction with the extracellular face of hMATE1 described earlier. Acknowledging that the pH range upon which this estimate of the cytoplasmic IC50 was based was, of necessity, narrower than that used to study the interaction of H+ with the extracellular aspect of hMATE1, we suggest that these data support the conclusion that, within the limits of resolution of currently available methods, the intra- and extracellular faces of hMATE1 display kinetically symmetrical interactions with H+.
Fig. 10.
Kinetics of interaction between inward-facing hMATE1 and intracellular H+. The time course of hMATE1-mediated [3H]MPP efflux was measured for each of the indicated values of intracellular pH. Each point is the mean of values determined in 3–6 separate experiments. Inset: relationship between cytoplasmic [H+] and the calculated rate constants (means ± SE) for hMATE1-mediated [3H]MPP efflux.
It is relevant to place the kinetic profile of H+ interaction with hMATE1 into the context of OC secretion in intact RPT. In the “early” proximal tubule, pH of the glomerular filtrate is moderately alkaline, ∼7.1 (6) reflecting a plasma pH of ∼7.4. The current results suggest that, at this pH, the extracellular face of hMATE1 is ∼85% occupied by H+ in the filtrate. These protons not only facilitate an exchange for cytoplasmic OCs, but would also limit interaction between luminal OCs and outward-facing hMATE1, i.e., luminal H+ should reduce “reuptake” of secreted OCs via hMATE1. Furthermore, as a result of H+ secretion, and bicarbonate and water reabsorption, along the length of the proximal tubule, the concentration of luminal H+ increases. In the late proximal tubule, pH of the filtrate can be as acidic as pH 6.8 to pH 6.7 (1), representing a concentration of H+ that should occupy at least 92% of available hMATE1 transporters. This increase in luminal H+ would continuously reduce a reuptake of luminal OCs, the concentration of which can also be expected to increase along the length of the RPT (due to the combined influence of MATE secretory activity and water reabsorption).
The interaction of H+ with hMATE1 also underscores its role in providing the “driving force” for OC secretion in RPT. As a secondary active H+ exchanger, MATE1 can mediate export of OCs against their electrochemical gradient driven by the coupled influx of H+ moving down its electrochemical gradient. The active OC transport via the OC/H+ exchanger is frequently described as being driven by an inward H+ gradient and, indeed, is typically assessed in this way in studies using isolated renal BBMV, including our own studies (23). It is, however, evident from the previous discussion that this can give a false impression of the energetic basis of OC export mediated by MATEs in RPTEC. In the early RPT, which appears to be the primary site of OC secretion (16), the inwardly-directed chemical gradient for H+ is very modest, ∼1.25-fold, reflecting a luminal pH of ∼7.1 vs. a cytoplasmic pH of ∼7.2 (25). Nevertheless, the tubule is still poised to support a robust active transepithelial OC secretion because uptake of OC from the blood into the RPTEC across the basolateral membrane is mediated by the electrogenic activity of OCT2. Consequently, the inside negative membrane potential of these cells, −60 to −70 mV (25), can be expected to support concentrative (yet passive) accumulation of OCs to levels 10–15 times that in the blood. Importantly, the luminal OC export step, mediated by MATE1 (and/or MATE2-K), involves obligatory, electroneutral exchange (24). Thus, even in the absence of an inwardly-directed H+ chemical gradient, exchange of a proton in the filtrate for OC in the cytoplasm can permit development of a luminal OC concentration that approaches the elevated level (compared with the blood) of OC in the cytoplasm, with the result being active transepithelial secretion. The MATE1-mediated step is still the “active” element in this process because, even in the absence of a chemical gradient for H+, protons are still far from electrochemical equilibrium and kept that way through the activity of Na+/H+ exchange. The increasing acidity of the tubular filtrate along the length of the RPT further enhances the energetic effectiveness of MATE-mediated OC secretion into the tubular filtrate.
It is also relevant to discuss the potential impact on renal OC secretion of the comparatively high affinity of the cytoplasmic face of hMATE1 for H+. With an apparent Ki of 11.5 nM (pH 7.94), the cytoplasmic pH in RPTEC of pH 7.2 ([H+] = 63.1 nM) (25) suggests that the cytoplasmic face of MATE1 should be ∼85% occupied by hydrogen ion. Based on selectivity studies in our lab and others (12, 19), the affinity for OCs of the outward-facing aspect of hMATE1 is typically 10- to 1,000-times lower than that for H+, and at least in general terms this is likely to be the case for the inwardly-directed face of MATE1, as well. This would seem to “set the stage” for rather brisk competition between accumulated OCs and near-saturating levels of cytoplasmic H+. But that scenario assumes that the “bulk” pH of the cytoplasm (which is what the BCECF measurements represent) is an accurate indicator of the hydrogen ion concentration within the unstirred “microdomain” at the cytoplasmic face of the cell membrane. While this is arguably the case in the current experiments with CHO cells, in which cytoplasmic pH values were 7.5 and higher [well below the “set-point” for NHEs (13)], the same may not be the case in salt-transporting epithelia like the renal proximal tubule. Indeed, it is worth noting that there is experimental evidence that the activity of Na+/H+ exchange creates a markedly acidic microenvironment at the extracellular face of the luminal membrane of enterocytes (1). We suggest, therefore, that the activity of the major Na+/H+ exchangers (NHEs) in the luminal membrane of RPTEC, i.e., NHE3 and NHE8, could create local alkaline conditions near the cytoplasmic face of the membrane, thereby facilitating the effective interaction of accumulated OCs with the inward face of MATE1. Interestingly, results from a mathematical model of the RPT brush-border membrane indicated that Na+/H+ exchange activity is (in principle) capable of establishing a gradient of luminal H+ along the length of each microvillus, with the cytoplasmic tip of the microvillus as alkaline as pH 8.0 (9). Another consequence of the differential impact of NHE activity, i.e., local acidification of the extracellular face of the membrane vs. local alkalinization of the cytoplasmic face of the membrane, would be an effective increase in the inwardly-directed electrochemical gradient for H+ that would enhance the secretory “power” of MATE1-mediated OC export. Further studies regarding the expression profile of hMATE1 at the luminal membrane of the hRPTEC and the influence of NHEs in hMATE1-mediated OC efflux are necessary for a better understanding of this physiologically, pharmacologically, and toxicologically important process.
Although our current results suggest that hMATE1 is symmetrical with respect to its kinetic interactions with intra- and extracellular H+, it is premature to assume that organic substrates will display a similar symmetry. Given the intrinsic structural asymmetry of transport proteins, in conjunction with the “alternating access” model of transporter function, it is unlikely that binding surfaces of the “translocation pathway” will be identical when accessed from the cytoplasmic vs. extracellular faces of the membrane. Whereas protons titrate single carboxyl residues, the transient, weak interactions of an organic substrate reflect interactions with multiple residues (5, 21) and even modest differences in the three-dimensional structure of binding surfaces are likely to influence binding kinetics. As noted previously, the affinities of the inner vs. outer faces of rOct2 for corticosterone and TBuA differ markedly. Importantly, the differences are in the opposite direction, whereas the inward face has a higher (×20) affinity for corticosterone and the outward face has a higher affinity (×4) for TBuA (20). We suggest that a similar degree of kinetic asymmetry can be expected for other multispecific transporters, including MATE1. In this regard, it is also appropriate to note that the kinetic profiles reported here reflect the interaction of a single substrate with MATE1, i.e., MPP. Although MPP is a widely used to characterize organic cation transporters, both OCTs (3) and MATEs (12), structurally distinct substrates for the multiselective MATE transporters could display a different kinetic interaction with H+.
In summary, the interaction between H+, both intracellular and extracellular, and the MATE1-mediated transport of MPP were examined, and the results suggested that the kinetics of interaction between inward-facing hMATE1 and intracellular H+ are not significantly different from the kinetics of the interaction between outward-facing hMATE1 and extracellular H+. Nevertheless, despite this apparent symmetry of H+ interaction with hMATE1, it can be anticipated that at least some OCs will display asymmetrical interactions with intra- vs. extracellular faces of hMATE1. The approach for assessing the kinetics of OC efflux from intact cultured cells introduced in the present study offers a potential means to address this issue.
GRANTS
The work presented here was supported, in part, by National Institutes of Health Grants DK58251, DK080801, and ES06694. During the course of this study, funding for Y. Dangprapai was partially supported by the Department of Physiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Amritlal Mandal for assistance on intracellular pH measurement.
Footnotes
The volume of CHO cells, determined using tritiated water corrected for extracellular space with [14C]mannitol, was ∼0.3 μl/cm2 of CHO cell (confluent layer; data not shown), similar to previous reports on the volume of CHO cells (15). If accumulated [3H]MPP was distributed uniformly in this volume (which, as discussed in the text, is an oversimplification), the calculated “bulk” concentration of [3H]MPP in CHO hMATE1 cells in this study was as high as ∼0.4 μM at initiation of efflux. This level of accumulated MPP is ∼35 times lower than the apparent Kt for interaction between MPP and external face of hMATE1 at pH 7.5 (12.3 ± 1.08 μM; n = 4). Although an apparent Kt for MPP interaction with cytoplasmic face of hMATE1 has not been characterized, we suggest that the [MPP]cell was sufficiently low to anticipate first-order behavior for hMATE1-mediated MPP efflux.
REFERENCES
- 1. Anderson CMH, Thwaites DT. Hijacking solute carriers for proton-coupled drug transport. Physiology (Bethesda) 25: 364–377, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Bednarczyk D, Mash EA, Aavula BR, Wright SH. NBD-TMA: a novel fluorescent substrate of the peritubular organic cation transporter of renal proximal tubules. Pflügers Arch 440: 184–192, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol 16: 871–881, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Groves CE, Evans KK, Dantzler WH, Wright SH. Peritubular organic cation transport in isolated rabbit proximal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 266: F450–F458, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Guan L, Kaback HR. Lessons from lactose permease. Annu Rev Biophys Biomol Struct 35: 67–91, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamm LL, Nakhoul N. Renal acidification. In: Brenner and Rector's The Kidney, edited by Brenner BM. Philadelphia, PA: Saunders, 2008, p. 248–267. [Google Scholar]
- 7. Kim MK, Shim CK. The transport of organic cations in the small intestine: current knowledge and emerging concepts. Arch Pharmacol Res (Seoul) 29: 605–616, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev 62: 1–96, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krahn TA, Weinstein AM. Acid/base transport in a model of the proximal tubule brush border: impact of carbonic anhydrase. Am J Physiol Renal Fluid Electrolyte Physiol 270: F344–F355, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Matsushima S, Maeda K, Inoue K, Ohta KY, Yuasa H, Kondo T, Nakayama H, Horita S, Kusuhara H, Sugiyama Y. The inhibition of human multidrug and toxin extrusion 1 is involved in the drug-drug interaction caused by cimetidine. Drug Metab Dispos 37: 555–559, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Miller DS, Villalobos AR, Pritchard JB. Organic cation transport in rat choroid plexus cells studied by fluorescence microscopy. Am J Physiol Cell Physiol 276: C955–C968, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci USA 102: 17923–17928, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ozkan P, Mutharasan R. A rapid method for measuring intracellular pH using BCECF-AM. Biochim Biophys Acta 1572: 143–148, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Pritchard JB, Sykes DB, Walden R, Miller DS. ATP-dependent transport of tetraethylammonium by endosomes isolated from rat renal cortex. Am J Physiol Renal Fluid Electrolyte Physiol 266: F966–F976, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Rotoli BM, Bussolati O, Dall'Asta V, Gazzola GC. Membrane potential and amino acid transport in a mutant Chinese hamster ovary cell line. J Cell Physiol 146: 417–424, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Schäli C, Schild L, Overney J, Roch-Ramel F. Secretion of tetraethylammonium by proximal tubules of rabbit kidneys. Am J Physiol Renal Fluid Electrolyte Physiol 245: F238–F246, 1983 [DOI] [PubMed] [Google Scholar]
- 17. Schulz I. Permeabilizing cells: some methods and applications for the study of intracellular processes. Meth Enzymol 192: 280–300, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui KI. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H+-organic cation antiporters. Biochem Pharmacol 74: 359–371, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Tsuda M, Terada T, Asaka J, Ueba M, Katsura T, Inui K. Oppositely directed H+ gradient functions as a driving force of rat H+/organic cation antiporter MATE1. Am J Physiol Renal Physiol 292: F593–F598, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Volk C, Gorboulev V, Budiman T, Nagel G, Koepsell H. Different affinities of inhibitors to the outwardly and inwardly directed substrate binding site of organic cation transporter 2. Mol Pharmacol 64: 1037–1047, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Watanabe A, Choe S, Chaptal V, Rosenberg JM, Wright EM, Grabe M, Abramson J. The mechanism of sodium and substrate release from the binding pocket of vSGLT. Nature 468: 988–991, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wright SH, Wunz TM, Wunz TP. Structure and interaction of inhibitors with the TEA/H+ exchanger of rabbit renal brush border membranes. Pflügers Arch 429: 313–324, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Wright SH, Wunz TM. Mechanism of cis- and trans-substrate interactions at the tetraethylammonium/H+ exchanger of rabbit renal brush-border membrane vesicles. J Biol Chem 263: 19494–19497, 1988 [PubMed] [Google Scholar]
- 24. Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev 84: 987–1049, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Yoshitomi K, Frömter E. Cell pH of rat renal proximal tubule in vivo and the conductive nature of peritubular HCO3− (OH−) exit. Pflügers Arch 402: 300–305, 1984 [DOI] [PubMed] [Google Scholar]
- 26. Zhang X, Cherrington NJ, Wright SH. Molecular identification and functional characterization of rabbit MATE1 and MATE2-K. Am J Physiol Renal Physiol 293: F360–F370, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.