Abstract
Recent studies in smooth muscle-specific Na+/Ca2+ exchanger-1 knockout (NCX1sm−/−) mice reveal reduced arterial pressure and impaired myogenic responses compared with heterozygous littermates. In this study, we determined renal function in male anesthetized NCX1sm−/− mice and NCX1 heterozygous (NCX1+/−) littermates before and during acute ANG II infusions. Systolic blood pressure in awake mice was lower in NCX1sm−/− mice compared with NCX1+/− mice (119 ± 4 vs. 131 ± 3 mmHg, P < 0.05). Acute ANG II infusions (5 ng·min−1·g−1 body wt) increased mean arterial pressure in anesthetized NCX1+/− (109 ± 2 to 134 ± 3 mmHg, P < 0.001, n = 8) and NCX1sm−/− (101 ± 8 to 129 ± 8 mmHg, P < 0.01, n = 6) mice to a similar extent (Δ25 ± 1 vs. Δ28 ± 4 mmHg, P > 0.05). In response to ANG II infusions, PAH clearance (CPAH) decreased from 1.39 ± 0.27 to 0.98 ± 0.22 ml·min−1·g−1 (P < 0.05) and glomerular filtration rate (GFR) was reduced from 0.50 ± 0.09 to 0.32 ± 0.06 ml·min−1·g−1 (P < 0.05) in NCX1+/− mice. In contrast, the NCX1sm−/− did not exhibit significant reductions in either CPAH (1.16 ± 0.30 to 1.22 ± 0.34 ml·min−1·g−1, P > 0.05) or GFR (0.48 ± 0.08 to 0.41 ± 0.05 ml·min−1·g−1, P > 0.05) during acute ANG II infusions. Using flometry to measure renal blood flow continuously, NCX1sm−/− mice had significantly attenuated responses to ANG II infusions (−34.2 ± 3.9%, P < 0.05) compared with those in NCX1+/− mice (−48 ± 2%) or in wild-type mice (−69 ± 7%). These data indicate that renal vascular responses to ANG II are attenuated in NCX1sm−/− mice compared with NCX1+/− mice and that NCX1 contributes to the renal vasoconstriction response to acute ANG II infusions.
Keywords: sodium/calcium exchanger, renal vascular tone regulation, arterial pressure
the na+/ca2+ exchanger (NCX) is a plasma membrane transporter expressed in various cell types including vascular smooth muscle cells (7, 14). Membrane potential and transmembrane gradients of Na+ and Ca2+ control this bidirectional exchanger (7). Depending on the prevailing Na+ and Ca2+ electrochemical gradients, the NCX can either extrude Ca2+ in parallel with the plasma membrane ATP-driven Ca2+ pump or it can mediate Ca2+ entry in parallel with various ion channels (7). Another family of plasma membrane Na+/Ca2+ exchanger proteins (NCKX) has also been identified in some mammalian tissues; NCKX mediates Ca2+ movements that are both K+ and Na+ dependent (7). The Ca2+ movement mediated by members of the NCX family are, however, only Na+ dependent (7). The mammalian NCX family comprises three isoforms that include NCX1, NCX2, and NCX3 (14, 19). While the specific roles of these three isoforms are not yet established, their levels vary considerably during murine postnatal development (18). The heart expresses NCX1.1 exclusively, and vascular tissue expresses predominantly the NCX1.3 and NCX1.7 splice variants of NCX1 (14). NCX1 has also been found in distal convoluted tubule cells (20, 33). The NCX1 gene transcript undergoes alternative splicing to produce tissue-specific isoforms with distinct functional characteristics (26).
Ca2+ entry induces muscle contraction, neurotransmitter release, and activation of various signal transduction pathways. Ca2+ that enters the cells must be extruded to avoid Ca2+ overload (16). The Ca2+ entry mode of vascular NCX1 is involved in the contractile regulation of small arteries and in the development of salt-dependent hypertension (7, 14). Heterozygous NCX1-deficient (NCX1+/−) mice have low-salt sensitivity, whereas transgenic mice that specifically overexpress NCX1.3 in smooth muscle (NCX1.3smTg/Tg) have elevated blood pressure and are hypersensitive to salt (14). These findings indicate that salt-sensitive hypertension is linked to Ca2+ entry through NCX1 in arterial smooth muscle (14). Indeed, NCX1 protein is upregulated in arteries in several types of hypertension (23, 31, 36, 39).
Several reports reveal that Ca2+ entry via vascular NCX1 is involved in pressure-induced myogenic constriction (14, 15, 24, 35). Furthermore, arteries from smooth muscle-specific NCX1 knockout (NCX1sm−/−) mice exhibit reduced Ca2+ entry into vascular smooth muscle cells and reduced myogenic tone (25, 35). Renovascular function plays a pivotal role in salt-dependent hypertension (9, 34), and it has been reported that Na+/Ca2+ exchanger mechanisms are disrupted in salt-sensitive hypertension (2, 21, 32). In contrast, other groups observed enhanced expression of NCX1 in arterial smooth muscle in several forms of hypertension (10, 23, 39). This might imply that NCX-mediated Ca2+ transport is enhanced under these circumstances. Nevertheless, the role of NCX in mediating the renal hemodynamic responses to angiotensin II (ANG II) has not yet been assessed. We hypothesized that renal vascular responses to acute ANG II infusions are attenuated in NCX1sm−/− mice because agonist-induced vasoconstriction is attenuated in systemic (mesenteric) small arteries isolated from these mice (35). To explore this possibility, we determined renal function and renal blood flow responses in male anesthetized NCX1sm−/− and littermate NCX1+/− mice (35) before and during acute ANG II infusions. Importantly, vascular tone and blood pressure (BP) in NCX1+/− mice do not differ significantly from wild-type mice with the same C57BL/6 genetic background (35).
METHODS
Animals.
Studies were performed on 9- to 12-wk-old male NCX1sm−/− and their heterozygous NCX1+/− littermates generated and genotyped at the University of Maryland, Baltimore (35). The smooth muscle NCX1 knockout was generated by Cre-recombinase with Cre regulated by the smooth muscle myosin heavy chain promoter, but the Cre− mice are global heterozyotes (NCX1+/−) as explained previously (35). The background for all mice was C57BL/6. Unmatched normal wild-type mice (C57BL/6) were obtained from Jackson Laboratories (Bar Harbor, ME).
The mice were maintained on a 12:12-h light-dark schedule (6 AM to 6 PM) at 25°C in the animal facility at Tulane University. Normal mouse diet along with tap water was provided. The protocol was approved by the Institutional Animal Care and Use Committee of Tulane University Health Sciences Center.
Experimental protocol.
At the time of the experiments, mice obtained from Maryland weighed 39 ± 2 g and kidney weights (both kidneys) averaged 483 ± 46 mg. Wild-type mice (n = 6) were smaller weighing 26 ± 1g with an average kidney weight of 168 ± 13 mg (per kidney). Systolic BPs (SBP) in awake mice were measured by noninvasive computerized tail-cuff plethysmography (Visitech BP2000, Apex, NC). On the day of the experiment, mice were anesthetized with Inactin (thiobutabarbital sodium) injected intraperitoneally at 200 mg/kg body wt. Supplemental doses of anesthesia were administered as required to maintain a stable plane of anesthesia. Once a stable level of anesthesia was obtained, judged by heart rate and lack of toe reflex, mice were placed on a surgical table (37°C) with servo-control of temperature to maintain body temperature at 37°C. After the incision site was shaved, a tracheostomy was performed with PE-90 tubing; the exterior end of the tracheal cannula was placed inside a small plastic chamber into which humidified 95% O2-5% CO2 was continuously passed. The right carotid artery was cannulated with PE-10 tubing connected to PE-50 tubing for continuous measurement of mean arterial pressure (MAP) and blood sampling. MAP was recorded on a Grass polygraph (model 7D, Grass Instrument, Quincy, MA) through a Statham pressure transducer (model P23 Db, Statham-Gould, Oxnard, CA). The right jugular vein was catheterized with PE-10 tubing connected to PE-50 tubing for solution infusion. During surgery, isotonic saline containing 6% albumin (bovine; Sigma, St. Louis, MO) was infused at a rate of 4 μl/min. The bladder was catheterized with PE-90 tubing via a suprapubic incision for urine collections. After surgery (in the left decubitus position), the intravenous infusion solution was changed to isotonic saline containing 1% albumin, 4.5% polyfructosan [Inutest (Inulin), Laevosan, Linz/Donau, Austria], and 1.5% para-aminohippurate (PAH; Merck Sharpe & Dohme, West Point, PA), and it was infused at 4 μl/min. After a 60-min equilibration period, two 30-min control urine samples were collected. Following the two control periods (period 1 and 2), ANG II was infused at 5 ng·min−1·g−1 body wt. About 7 min were allowed after which MAP reached a peak level and urine samples were collected during the next two periods (periods 3 and 4, which lasted 20 min).
For the clearance experiments, renal plasma flow (RPF) was estimated from the clearance of PAH and glomerular filtration rate (GFR) was measured from the clearance of polyfructosan in anesthetized NCX1sm−/− (n = 6) and NCX1+/− (n = 8) mice, as described (6, 37, 38). A terminal blood sample was collected from the arterial catheter at the end of each experiment for measurement of plasma PAH, polyfructosan, and sodium concentrations.
Urine, plasma PAH, and polyfructosan measurements.
Urine and plasma PAH and polyfructosan concentrations were measured using standard colorimetric techniques as adapted for a plate reader (29). RPF was estimated from PAH clearance calculated as the ratio of urine and plasma PAH concentrations times urine flow. It is recognized that PAH clearance may not provide a definitive measure of RPF because of the uncertainty related to the PAH extraction that has not been clearly delineated in mice. GFR was calculated as the ratio of urine and plasma polyfructosan concentrations multiplied by urine flow.
Renal blood flow measurement with ultrasound flowmetry.
In a separate group of mice, renal blood flow (RBF) was measured directly by ultrasound in NCX1sm−/− (n = 8) and NCX1+/− (n = 6) anesthetized male mice by using a perivascular flow probe (model 0.5 PSB, Transonic, Ithaca, NY) positioned on the left renal artery (27, 30). For comparison, RBF responses were also measured in wild-type mice (n = 6). RBF was measured using a TS420 flow meter and recorded, along with arterial pressure measured through a pressure transducer (P23XL, BD, Franklin Lakes, NJ), with a computerized recording system (MP 100A-CE, BIOPAC System, Santa Barbara, CA). After a 60-min recovery period, baseline MAP and RBF were recorded during two 30-min periods as control (period 1 and 2). Following the two control periods, ANG II was infused at 0.1, 0.25, 0.5 ng·min−1·g−1 body wt, respectively. There was a 10-min recovery period before the second and third ANG II infusions. About 7 min were allowed after which the maximum MAP and RBF responses were recorded during the ANG II infusion periods. RBF values are reported as milliliters per minute per gram kidney weight.
Urine and plasma sodium measurements.
Urine output was determined gravimetrically assuming a density of 1 g/ml. Urine and plasma sodium concentrations were measured using flame photometry (Flame Photometer IL 973, Instrumentation Laboratory, Lexington, MA). Urinary sodium excretion (UNaV) was normalized by kidney weight and urine collection time.
Statistical analysis.
The statistical analysis was performed by paired t-test using the GraphPad PRISM program (Prism 5, GraphPad, San Diego, CA) in the same individual mouse data and two-way ANOVA with Bonferroni posttests between NCX1sm−/− and NCX1+/− mice. The results are presented as means ± SE. Significance was set at P < 0.05.
RESULTS
SBP and MAP before and during acute ANG II infusions.
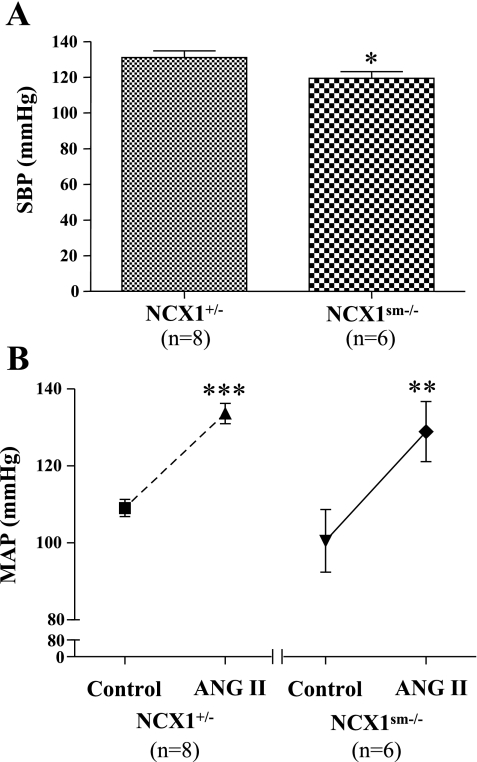
As shown in Fig. 1A and previously (35), SBP in awake mice was lower in NCX1sm−/− mice compared with NCX1+/− mice (119 ± 4 vs. 131 ± 3 mmHg, P < 0.05). In anesthetized animals under control conditions, however, MAP was not significantly lower in NCX1sm−/− mice than in NCX1+/− mice (101 ± 8 vs. 109 ± 2 mmHg, P > 0.05). Acute ANG II infusions increased MAP to 134 ± 3 mmHg in NCX1+/− (P < 0.001) and to 129 ± 8 mmHg in NCX1sm−/− mice (P < 0.01; Fig. 1B). The responses of MAP to acute ANG II infusions (5 ng·min−1·g−1 body wt) were similar between NCX1+/− and NCX1sm−/− mice (Δ25 ± 1 vs. Δ28 ± 4 mmHg, P > 0.05).
Fig. 1.
A: systolic blood pressure (SBP) measured in awake NCX1+/− and NCX1sm−/− mice measured with tail-cuff plethysmography. B: mean arterial pressure (MAP) in anesthetized mice before and during acute ANG II infusions. Values are given as means ± SE. Compared with NCX1+/−: *P < 0.05. Compared with control (before ANG II infusions): **P < 0.01, ***P < 0.001.
Effects of acute ANG II infusions on CPAH and GFR.
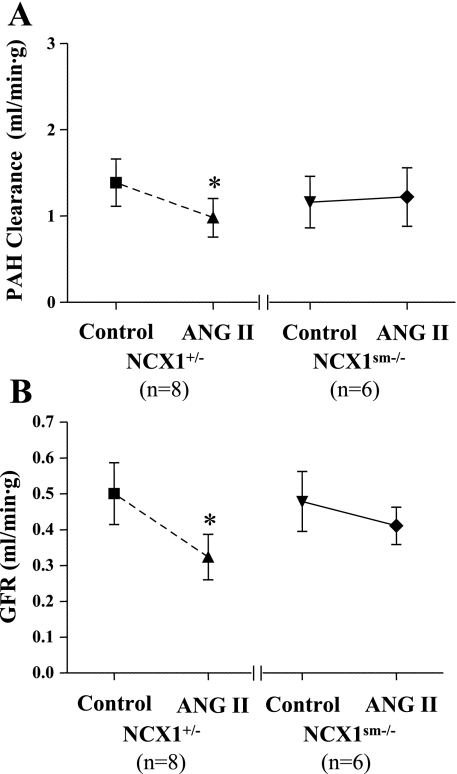
Under control conditions, neither CPAH (1.39 ± 0.27 vs. 1.16 ± 0.30 ml·min−1·g−1, P > 0.05) nor GFR (0.50 ± 0.09 vs. 0.48 ± 0.08 ml·min−1·g−1, P > 0.05) was significantly different in NCX1+/− and NCX1sm−/− mice (Fig. 2). Acute ANG II infusion reduced CPAH, a measure of RPF, from 1.39 ± 0.27 to 0.98 ± 0.22 ml·min−1·g−1 (P < 0.05) in NCX1+/− mice. GFR was also significantly reduced, from 0.50 ± 0.09 to 0.32 ± 0.06 ml·min−1·g−1 (P < 0.05), in NCX1+/− mice. In contrast, the NCX1sm−/− did not exhibit significant reductions in either CPAH (1.16 ± 0.30 to 1.22 ± 0.34 ml·min−1·g−1, P > 0.05) or GFR (0.48 ± 0.08 to 0.41 ± 0.05 ml·min−1·g−1, P > 0.05) during ANG II infusions, despite the comparable increase in MAP.
Fig. 2.
Responses of para-aminohippurate (PAH) clearance and glomerular filtration rate (GFR) to acute ANG II infusions. Values are given as means ± SE. Compared with control (before ANG II infusions): *P < 0.05.
Effect of acute ANG II infusions on RBF measured with ultrasound.
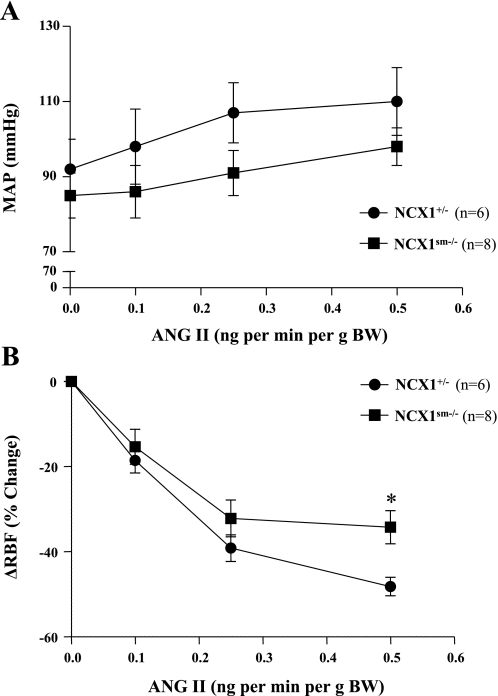
MAP and RBF responses were also evaluated in separate groups of mice using low doses of ANG II (0.1–0.5 ng·min−1·g−1 body wt) and continuous measurements. RBF values were not significantly different (3.3 ± 0.3 vs. 3.2 ± 0.3 ml·min−1·g−1, P > 0.05). In response to these lower doses of ANG II, MAP was only slightly but not significantly increased in NCX1+/− (92 ± 8 to 110 ± 9 mmHg, P > 0.05) or NCX1sm−/− (85 ± 6 to 98 ± 5 mmHg, P > 0.05) mice (Fig. 3). Interestingly, the initial RBF responses, which were used as a measure of renal vascular responsiveness, waned after a few minutes. For this analysis, the maximum responses at each ANG II dose are described. RBF was significantly decreased in NCX1+/− (3.2 ± 0.3 to 1.7 ± 0.2 ml·min−1·g−1, P < 0.001) and in NCX1sm−/− (3.3 ± 0.3 to 2.2 ± 0.4 ml·min−1·g−1, P < 0.01) mice during acute low-dose ANG II infusions (0.5 ng·min−1·g−1 body wt). The responses in the NCX1sm−/− (−34.2 ± 3.9%) were, however, significantly smaller (P < 0.05) than in the NCX1+/− mice (−48.2 ± 2.2). In wild-type mice (n = 6), MAP was significantly increased during acute ANG II infusions (76 ± 6 to 92 ± 5, 106 ± 9 and 124 ± 9 mmHg, P < 0.01). RBF significantly decreased (2.8 ± 0.2 to 0.9 ± 0.3 ml·min−1·g−1, P < 0.001). The responses of RBF in wild-type mice (−51 ± 7, −55 ± 10, and −69 ± 7%) were significantly greater than those observed in either NCX1+/− (P < 0.01) or NCX1sm−/− mice (P < 0.001). This is consistent with a NCX1 gene-dose effect on RBF, even though the wild-type mice were smaller (26 ± 6 vs. 39 ± 2 g) and more sensitive to the stress of anesthesia and surgery as reflected in lower basal BPs.
Fig. 3.
Responses of MAP and renal blood flow (RBF) to acute ANG II infusions. Values are given as means ± SE. Compared with NCX1+/−: *P < 0.05.
Effects of acute ANG II infusions on urine flow and UNaV.
Before acute ANG II infusions, there were no significant differences in urine flow (5.19 ± 0.68 vs. 3.93 ± 0.90 μl·min−1·g−1, P > 0.05) or urinary sodium excretion (1.38 ± 0.25 vs. 1.19 ± 0.36 μeq·min−1·g−1, P > 0.05) between NCX1+/− and NCX1sm−/− mice, respectively. Acute ANG II infusions did not significantly change urine flow (4.31 ± 0.77 μl·min−1·g−1) or sodium excretion (1.14 ± 0.22 μeq·min−1·g−1) in NCX1+/− mice. In NCX1sm−/− mice, ANG II infusions increased urine flow (5.34 ± 1.24 μl·min−1·g−1, P > 0.05) and sodium excretion rate (1.53 ± 0.34 μeq·min−1·g−1, P > 0.05).
DISCUSSION
Basal BP and the effects of ANG II on BP in NCX1+/− and NCX1sm−/− mice.
Numerous mechanisms contribute to the Ca2+ signaling and Ca2+ homeostasis that control contraction in vascular smooth muscle. Critical Ca2+ entry and Ca2+ release mechanisms that raise the cytosolic Ca2+ concentration, [Ca2+]i, and trigger vasoconstriction include voltage-gated Ca2+ channels, inositol trisphosphate (IP3)-activated channels, and receptor- and store-operated channels (ROCs and SOCs) (1, 3, 5). The latter are composed, at least in part, of C-type transient receptor potential (TRPC) channel proteins (3, 5). Additionally, NCX plays a role in smooth muscle Ca2+ homeostasis; in particular, NCX-mediated Ca2+ entry is important for the maintenance of vascular tone (1, 3, 5). Indeed, NCX1 and certain TRPC proteins, especially TRPC6, a component of ROCs, are upregulated in arterial smooth muscle in several forms of hypertension (23, 39). This has prompted the statement that, “the interrelationship among TRPC6, Na+ influx, NCX, and Ca2+ influx … is … the key to better understand the role of Na+ in hypertension” (10). To elucidate the influence of vascular NCX1 expression on renal function, we have now determined the effects of acute ANG II infusion on renovascular responses in NCXsm−/− mice and their heterozygous (NCX1+/−) siblings.
Previous reports indicate that NCX1+/− mice have normal BP and that their arteries have normal tone and function, comparable to arteries from wild-type mice, despite ∼50% reduction of NCX1 expression in vascular smooth muscle (25, 35). The NCX1+/− mice are, however, resistant to mineralocorticoid salt hypertension, whereas this treatment raises BP in wild-type mice (14). Conversely, high salt, alone, increases BP in mice that overexpress NCX1 in smooth muscle (NCX1smTg/Tg mice), but not in wild-type mice (14). The implication is that vascular NCX1 is essential for the development of salt-sensitive hypertension: it may act primarily as a Ca2+ entry pathway for regulating arterial tone, especially under Na+-retaining conditions (14, 32).
In our study, SBP was significantly lower in unanesthetized NCX1sm−/− mice than in NCX1+/− mice (Fig. 1A), as previously reported (35). Under anesthesia, basal MAP also tended to be lower, albeit not significantly, in NCX1sm−/− compared with NCX1+/− mice (Fig. 1B). These data suggest that arterial vascular tone is attenuated in NCX1sm−/− mice; the data fit with the observation that myogenic tone is attenuated in isolated, pressurized arteries from these mice (35).
GFR and RBF responses to high-dose ANG II are attenuated in NCX1sm−/− mice.
In one group of mice, we studied arterial pressure and renal function responses to acute ANG II doses (5 ng·min−1·g−1 body wt) that elicit increases in arterial pressure (37). Despite the differences in arterial tone and baseline BP, acute ANG II infusion at this high dose increased MAP by a similar extent in NCX1sm−/− and NCX1+/− mice (Fig. 1B). Moreover, under baseline conditions, both genotypes had similar GFR and similar CPAH (i.e., RBF). Even though the ANG II-induced BP increases were comparable (Fig. 1B), both the GFR and CPAH declined in response to acute, high-dose ANG II were attenuated in the NCX1sm−/− mice compared with their NCX1+/− siblings (Fig. 2). These data are consistent with attenuated Ca2+ entry into renal vascular smooth muscle cells in NCX1sm−/− mice in response to ANG II as occurs under conditions where the NCX is pharmacologically blocked (8).
The effects of ANG II on renal function result from a combination of ANG II type 1 (AT1) and ANG II type 2 (AT2) receptor-mediated events with the AT1 receptor-mediated effects generally predominating (22). Thus, ANG II administration results in net renal vasoconstriction (22). While it is possible that changes in AT1 and/or AT2 receptor expression contributed to the differences observed in the responses between the NCX1sm−/− and the NCX1+/−, it is more likely that these differences are due primarily to the knockout of the NCX1 in renal vascular smooth muscle cells. In the clearance experiments in which we evaluated sustained effects, acute ANG II infusions did not elicit significant changes in either CPAH or GFR in NCX1sm−/− mice while the NCX1+/− mice had clear reductions in both, indicating greater renal vasoconstriction.
These data demonstrate that renal vascular responses to acute ANG II infusions were attenuated in NCX1sm−/− mice. This is reflected by reduced CPAH and GFR responses compared with those observed in sibling NCX1+/− mice, and the smaller initial decreases in RBF in response to ANG II infusion. The reduced responses in the NCX1sm−/− mice indicate that NCX1 makes an important contribution to RBF in ANG II-mediated renal vasoconstriction.
RBF responses to low-dose ANG II are attenuated in NCX1sm−/− mice.
Another group of mice was used to test the effects of low (nonpressor) doses of ANG II on continuously monitored RBF using ultrasonic flowmetry. Here, too, NCX1sm−/− mice exhibited a significantly smaller reduction in RBF compared with NCX1+/− mice (Fig. 3), demonstrating that renal vascular responses to ANG II are attenuated in NCX1sm−/− mice compared with NCX1+/− mice. Thus, both the low- and high-dose ANG II infusion data lead to the same conclusion, namely, that NCX1 makes an important contribution to renal vascular tone regulation. The RBF responses in wild-type mice were greater than in either the NCX1sm−/− or NCX1+/−, suggesting a gene-dose effect. Nevertheless, the more relevant comparisons may be between the NCX1sm−/− and NCX1+/− mice. These results are consistent with the observation that KB-R7943, a NCX inhibitor, decreased the peak [Ca2+]i response to ANG II by 48%; the Ca2+ entry-mode NCX may therefore be an important Ca2+ entry pathway that mediates ANG II vasoconstriction of afferent arteriole smooth muscle cells (8). Upregulation of this mechanism may help to explain the augmented myogenic responses and enhanced phenylephrine-induced vasoconstriction in ouabain-hypertensive rat arteries as well as the increased BP (23). In contrast, Schweda et al. (28) studied the effects of the NCX blocker KB-R7943 on isolated rat kidneys perfused with an artificial perfusate. KB-7943 caused an increase in renal vascular resistance that was almost completely blocked by L-type calcium channel blockade with amlodipine. It was suggested that in this preparation, which is a high-flow/low-resistance model, NCX was extruding Ca2+ and that blockade led to an increase in intracellular Ca2+ that, in turn, activated L-type calcium channels. It is well-known that the NCX can operate in both directions depending on the ionic gradients and thus could have been contributing to Ca2+ exit in this model. Our results suggest that in an in vivo setting, ANG II stimulates NCX in renal vascular smooth muscle cells to increase Ca2+ entry, thus contributing to ANG II-mediated constriction and that this effect is attenuated or absent in the NCX1sm−/− mice. Our results are consistent with several other reports that indicate that NCX1 normally mediates Ca2+ entry in arteries with tone (14, 15, 35).
The Na+ excretion responses to ANG II infusions are complex; they depend on the dose and duration of the ANG II infusions, as well as on the magnitude of the BP responses. In this study, we did not detect significant changes in urine flow and urinary sodium excretion in NCX1+/− mice during acute ANG II infusions. Increases in MAP lead to diuresis and natriuresis (37). However, the opposing effects of decreases in CPAH and GFR observed in NCX1+/− mice opposed the pressure effects resulting in no change in urine flow and sodium excretion. In the NCX1sm−/−, the reduced Ca2+ entry into renal vascular smooth muscle cells allowed maintenance of CPAH and GFR due to attenuated renal vascular responses to acute ANG II infusions, thus allowing the effects of arterial pressure to increase urine flow and sodium excretion.
Perspectives
The data in this report demonstrate that NCX1 plays a significant role in renal vascular responses to acute ANG II infusions, as would be expected for a mechanism that contributes to the regulation of renal hemodynamics. NCX1 is also a critical link between salt and hypertension (4, 11–13). Indeed, in a Japanese general population study, NCX1 was identified as one of the genes related to susceptibility to essential hypertension (17). This raises the possibility that NCX1 contributes in a fundamental way to arterial pressure regulation. Indeed, NCX1 protein expression is upregulated in several types of human and experimental hypertension (23, 31, 36, 39). New pharmacological agents that act on NCX1 may have potential in the management of hypertension.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants HL-18426 (to L. G. Navar), HL-45215, and HL-78870 (to M. P. Blaustein), by National Institutes of Health Grant P20RR0117659 from the Institutional Development Award Program of National Center for Research Resources (to L. G. Navar), by an American Heart Association National Scientist Development Grant (to J. Zhang), and by the Tulane Mouse Phenotyping Core grant from NHLBI 1P 30 HL101285-01 (to L. G. Navar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Meng Li for managing the mouse colony and breeding and for genotyping. The NCX1 floxed mice were originally provided by Dr. K. D. Philipson (UCLA David Geffen School of Medicine, Los Angeles, CA); the smooth muscle-specific Cre mice were originally provided by Dr. M. I. Kotlikoff (Cornell University College of Veterinary Medicine, Ithaca, NY).
Present address of D. Zhao: Div. of Hypertension, the Second Affiliated Hospital, Zhengzhou Univ., Zhengzhou, People's Republic of China 450014.
REFERENCES
- 1. Baryshnikov SG, Pulina MV, Zulian A, Linde CI, Golovina VA. Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes. Am J Physiol Cell Physiol 297: C1103–C1112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bell PD, Mashburn N, Unlap MT. Renal sodium/calcium exchange; a vasodilator that is defective in salt-sensitive hypertension. Acta Physiol Scand 168: 209–214, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Blaustein MP, Hamlyn JM. Signaling mechanisms that link salt retention to hypertension: endogenous ouabain, the Na+ pump, the Na+/Ca2+ exchanger and TRPC proteins. Biochim Biophys Acta 1802: 1219–1229, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blaustein MP, Zhang J, Chen L, Hamilton BP. How does salt retention raise blood pressure? Am J Physiol Regul Integr Comp Physiol 290: R514–R523, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Blaustein MP, Zhang J, Chen L, Song H, Raina H, Kinsey SP, Izuka M, Iwamoto T, Kotlikoff MI, Lingrel JB, Philipson KD, Wier WG, Hamlyn JM. The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension 53: 291–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cervenka L, Mitchell KD, Navar LG. Renal function in mice: effects of volume expansion and angiotensin II. J Am Soc Nephrol 10: 2631–2636, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Dong H, Jiang Y, Triggle CR, Li X, Lytton J. Novel role for K+-dependent Na+/Ca2+ exchangers in regulation of cytoplasmic free Ca2+ and contractility in arterial smooth muscle. Am J Physiol Heart Circ Physiol 291: H1226–H1235, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Fellner SK, Arendshorst WJ. Angiotensin II-stimulated Ca2+ entry mechanisms in afferent arterioles: role of transient receptor potential canonical channels and reverse Na+/Ca2+ exchange. Am J Physiol Renal Physiol 294: F212–F219, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Franco M, Tapia E, Santamaria J, Zafra I, Garcia-Torres R, Gordon KL, Pons H, Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Renal cortical vasoconstriction contributes to development of salt-sensitive hypertension after angiotensin II exposure. J Am Soc Nephrol 12: 2263–2271, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Giachini FR, Tostes RC. Does Na+ really play a role in Ca2+ homeostasis in hypertension? Am J Physiol Heart Circ Physiol 299: H602–H604, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Iwamoto T. Na+/Ca2+ exchange as a drug target–insights from molecular pharmacology and genetic engineering. Ann NY Acad Sci 1099: 516–528, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Iwamoto T, Kita S. Hypertension, Na+/Ca2+ exchanger, Na-ATPase. Kidney Int 69: 2148–2154, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Iwamoto T, Kita S, Katsuragi T. Salt-sensitive hypertension, Na+/Ca2+ exchanger, and vascular smooth muscle. Trends Cardiovasc Med 15: 273–277, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Iwamoto T, Kita S, Zhang J, Blaustein MP, Arai Y, Yoshida S, Wakimoto K, Komuro I, Katsuragi T. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med 10: 1193–1199, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Kashihara T, Nakayama K, Matsuda T, Baba A, Ishikawa T. Role of Na+/Ca2+ exchanger-mediated Ca2+ entry in pressure-induced myogenic constriction in rat posterior cerebral arteries. J Pharm Sci 110: 218–222, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Kimura J, Ono T, Sakamoto K, Ito E, Watanabe S, Maeda S, Shikama Y, Yatabe MS, Matsuoka I. Na+-Ca2+ exchanger expression and its modulation. Biol Pharm Bull 32: 325–331, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Kokubo Y, Inamoto N, Tomoike H, Kamide K, Takiuchi S, Kawano Y, Tanaka C, Katanosaka Y, Wakabayashi S, Shigekawa M, Hishikawa O, Miyata T. Association of genetic polymorphisms of sodium-calcium exchanger 1 gene, NCX1, with hypertension in a Japanese general population. Hypertens Res 27: 697–702, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Levitsky DO. Three types of muscles express three sodium-calcium exchanger isoforms. Ann NY Acad Sci 1099: 221–225, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Linck B, Qiu Z, He Z, Tong Q, Hilgemann DW, Philipson KD. Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3). Am J Physiol Cell Physiol 274: C415–C423, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Magyar CE, White KE, Rojas R, Apodaca G, Friedman PA. Plasma membrane Ca2+-ATPase and NCX1 Na+/Ca2+ exchanger expression in distal convoluted tubule cells. Am J Physiol Renal Physiol 283: F29–F40, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Nelson LD, Unlap MT, Lewis JL, Bell PD. Renal arteriolar Na+/Ca2+ exchange in salt-sensitive hypertension. Am J Physiol Renal Physiol 276: F567–F573, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Prieto-Carrasquero MC, Kobori H, Navar LG. The intrarenal renin-angiotensin system. In: Hypertension and Hormone Mechanisms, edited by Carey RM. Totowa, NJ: Humana, 2007, p. 3–22 [Google Scholar]
- 23. Pulina MV, Zulian A, Berra-Romani R, Beskina O, Mazzocco-Spezzia A, Baryshnikov SG, Papparella I, Hamlyn JM, Blaustein MP, Golovina VA. Upregulation of Na+ and Ca2+ transporters in arterial smooth muscle from ouabain-induced hypertensive rats. Am J Physiol Heart Circ Physiol 298: H263–H274, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raina H, Ella SR, Hill MA. Decreased activity of the smooth muscle Na+/Ca2+ exchanger impairs arteriolar myogenic reactivity. J Physiol 586: 1669–1681, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ren C, Zhang J, Philipson KD, Kotlikoff MI, Blaustein MP, Matteson DR. Activation of L-type Ca2+ channels by protein kinase C is reduced in smooth muscle-specific Na+/Ca2+ exchanger knockout mice. Am J Physiol Heart Circ Physiol 298: H1484–H1491, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruknudin A, He S, Lederer WJ, Schulze DH. Functional differences between cardiac and renal isoforms of the rat Na+-Ca2+ exchanger NCX1 expressed in Xenopus oocytes. J Physiol 529: 599–610, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ryan MJ, Jernigan NL, Drummond HA, McLemore GR, Jr, Rimoldi JM, Poreddy SR, Gadepalli RS, Stec DE. Renal vascular responses to CORM-A1 in the mouse. Pharmacol Res 54: 24–29, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Schweda F, Seebauer H, Kramer BK, Kurtz A. Functional role of sodium-calcium exchange in the regulation of renal vascular resistance. Am J Physiol Renal Physiol 280: F155–F161, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Shi SJ, Vellaichamy E, Chin SY, Smithies O, Navar LG, Pandey KN. Natriuretic peptide receptor A mediates renal sodium excretory responses to blood volume expansion. Am J Physiol Renal Physiol 285: F694–F702, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Stec DE, Vera T, Storm MV, McLemore GR, Jr, Ryan MJ. Blood pressure and renal blow flow responses in heme oxygenase-2 knockout mice. Am J Physiol Regul Integr Comp Physiol 297: R1822–R1828, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taniguchi S, Furukawa K, Sasamura S, Ohizumi Y, Seya K, Motomura S. Gene expression and functional activity of sodium/calcium exchanger enhanced in vascular smooth muscle cells of spontaneously hypertensive rats. J Cardiovasc Pharmacol 43: 629–637, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Unlap MT, Bates E, Williams C, Komlosi P, Williams I, Kovacs G, Siroky B, Bell PD. Na+/Ca2+ exchanger: target for oxidative stress in salt-sensitive hypertension. Hypertension 42: 363–368, 2003 [DOI] [PubMed] [Google Scholar]
- 33. White KE, Gesek FA, Reilly RF, Friedman PA. NCX1 Na/Ca exchanger inhibition by antisense oligonucleotides in mouse distal convoluted tubule cells. Kidney Int 54: 897–906, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Williams DE, Prieto MC, Mullins JJ, Navar LG, Mitchell KD. AT1 receptor blockade prevents the increase in blood pressure and the augmentation of intrarenal ANG II levels in hypertensive Cyp1a1-Ren2 transgenic rats fed with a high-salt diet. Am J Med Sci 339: 356–361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang J, Ren C, Chen L, Navedo MF, Antos LK, Kinsey SP, Iwamoto T, Philipson KD, Kotlikoff MI, Santana LF, Wier WG, Matteson DR, Blaustein MP. Knockout of Na+/Ca2+ exchanger in smooth muscle attenuates vasoconstriction and L-type Ca2+ channel current and lowers blood pressure. Am J Physiol Heart Circ Physiol 298: H1472–H1483, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang S, Dong H, Rubin LJ, Yuan JX. Upregulation of Na+/Ca2+ exchanger contributes to the enhanced Ca2+ entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol 292: C2297–C2305, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Zhao D, Navar LG. Acute angiotensin II infusions elicit pressure natriuresis in mice and reduce distal fractional sodium reabsorption. Hypertension 52: 137–142, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension 54: 120–126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zulian A, Baryshnikov SG, Linde CI, Hamlyn JM, Ferrari P, Golovina VA. Upregulation of Na+/Ca2+ exchanger and TRPC6 contributes to abnormal Ca2+ homeostasis in arterial smooth muscle cells from Milan hypertensive rats. Am J Physiol Heart Circ Physiol 299: H624–H633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]