Abstract
The expression and activation of the Ste20-like kinase, SLK, is increased during renal development and recovery from ischemic acute renal failure. SLK promotes apoptosis, and during renal injury and repair, transcriptional induction or posttranscriptional control of SLK may, therefore, regulate cell survival. SLK contains protein interaction (coiled-coil) domains, suggesting that posttranslational homodimerization may also modulate SLK activity. We therefore expressed coiled-coil regions in the C-terminal domain of SLK as fusion proteins and demonstrated their homodimerization. By gel-filtration chromatography, endogenous and heterologously expressed SLK were detected in a macromolecular protein complex. To test the role of homodimerization in kinase activation, we constructed a fusion protein consisting of the SLK catalytic domain (amino acids 1–373) and a modified FK506 binding protein, Fv (Fv-SLK 1–373). Addition of AP20187 (an analog of FK506) enhanced the homodimerization of Fv-SLK 1–373. In an in vitro kinase assay, the dimeric Fv-SLK 1–373 displayed greater kinase activity than the monomeric form. In cells expressing Fv-SLK 1–373, homodimerization increased activation-specific phosphorylation of the proapoptotic kinases, c-Jun N-terminal kinase and p38 kinase. Compared with the monomer, dimeric Fv-SLK 1–373 enhanced the activation of a Bax promoter-luciferase reporter. Finally, expression of Fv-SLK 1–373 induced apoptosis, and the effect was increased by homodimerization. Thus the activity, downstream signaling, and functional effects of SLK are enhanced by dimerization of the kinase domain.
Keywords: apoptosis, kidney, mitogen-activated protein kinases, signal transduction
slk is a serine/threonine kinase, which belongs to the Ste20 family of kinases (14, 15). The yeast homolog Ste20p was discovered as a protein kinase in the haploid budding yeast Saccharomyces cerevisiae, and it participates in the mating pathway. Since the yeast Ste20p is believed to be a mitogen-activated protein kinase (MAPK) kinase kinase kinase (MAP4K), mammalian homologs of Ste20p may also function as MAP4Ks. In mammals, the two families of kinases related to Ste20p are the p21-activated kinases and germinal center kinases (GCK). Ste20p and the p21-activated kinases contain their kinase domain at the C termini, while the GCKs have their kinase domains at the N termini (14, 15). The GCK family has been subdivided into eight groups. Group I GCKs are situated upstream of MAP3K-1, and they activate the c-Jun N-terminal kinase (JNK) pathway, while GCKs in groups II–VIII are diverse kinases, and some may be activated in vivo by various stresses (e.g., heat shock, ischemic injury, ATP depletion). Most GCKs are expressed ubiquitously, and most do not fit into the well-defined MAPK pathways, although there are exceptions.
The pathophysiological roles of most GCKs are poorly understood. Some GCKs participate in apoptotic signaling pathways, either the induction or inhibition of apoptosis via pathways involving MAPKs (14, 15). For example, Mst1 of the GCK II subfamily can activate apoptosis through the JNK and p38 MAPK pathways (21, 28, 29). SLK is a member of the GCK V subfamily and is distantly related to Mst1 and Mst2, while sharing high homology to lymphocyte-oriented kinase, another member of the GCK V subfamily (14, 15). SLK has been shown to induce apoptosis when expressed in cultured fibroblasts (40, 41), as well as kidney tubular and glomerular epithelial cells (GECs; podocytes) in culture, and recently, podocyte-specific overexpression of SLK in transgenic mice resulted in severe podocyte injury and loss of podocytes, in keeping with apoptosis (10, 11, 13, 23). Moreover, SLK enhanced apoptosis in kidney cells after ischemia-reperfusion injury in vitro, and apoptotic signaling occurred via the JNK and p38 pathways (23), and involved p53 (10, 37). In addition to apoptosis, SLK may regulate cytoskeletal remodeling in fibroblasts and other cell lines. SLK was found to be associated with the microtubular network, and activation of SLK via focal adhesion kinase and extracellular signal-regulated kinase pathways destabilized the actin network. This process affected focal adhesion turnover, cell adhesion, spreading, and motility (3, 4, 44, 45). SLK may also be involved in the modulation of vascular tone (22).
In the kidney, SLK mRNA, protein, and kinase activity were increased during development and recovery from ischemic acute renal failure (13), which recapitulates certain aspects of kidney development (16). SLK was localized in both fetal and normal adult rat kidneys, with a strong presence in proximal and distal tubular epithelial cells, and some presence in GECs (13). Changes in SLK expression and activity have also been reported in the developing brain and in ischemic brain injury (47). Thus SLK may play a role in apoptosis incurred during the pathological response following ischemia-reperfusion injury and/or may be required for proper kidney and brain development.
The regulation of SLK catalytic activity is complex and poorly understood. Changes in activity may be associated with changes in expression (13, 18). The SLK mRNA 3′-untranslated region contains adenine and uridine-rich elements, which can destabilize the mRNA and affect protein expression (12). Posttranslational mechanisms may also regulate kinase activity. It was reported that deletion of the C-terminal domain of SLK enhanced kinase activity, and the authors suggested that the C-terminal domain may be autoinhibitory (40). SLK contains several potential phosphorylation sites, which could modulate kinase activity (4, 38), and has an extensive C-terminal domain, within which are found coiled-coils (46). Coiled-coil domains are intertwined α-helices that permit protein-protein interactions (33). Coiled-coils generally possess a pattern of heptad repeats, with hydrophobic residues in the first and fourth positions of the heptad (which can bind through van der Waals forces), and charged or polar residues in the fifth and seventh positions. The presence of the coiled-coils in the C-terminal domain suggests that SLK may undergo homodimerization or oligomerization, and previously we showed that full-length (wild-type) SLK was able to associate with a glutathione-S-transferase (GST) fusion protein, containing only the SLK C-terminal domain (23). This interaction led to enhanced SLK catalytic activity (23). Another study showed that the kinase domain of SLK (without the C-terminal domain) can dimerize in vitro, albeit dimerization was weak (38). Such dimerization facilitated autophosphorylation and kinase activation. Thus the coiled-coil domains located in the C terminus of full-length SLK may potentially enhance the effectiveness of catalytic domain dimerization to facilitate robust activation of kinase activity. By analogy, the related Ste20-like kinase Mst1 was found to homodimerize through its C-terminal region (9).
The present study addresses the regulation of SLK by homodimerization. We demonstrate that in cells, the coiled-coil regions of SLK can homodimerize and that SLK exists as a high-molecular-mass complex. To determine whether homodimerization enhances the kinase activity of SLK, we constructed a fusion protein of the SLK catalytic domain with a dimerization domain, which allows for precise control of homodimerization by the addition of a chemical compound. Using this model, we demonstrate that compared with the monomer, dimeric SLK shows greater kinase activity, stimulates various downstream signaling pathways more effectively, and enhances apoptosis.
MATERIALS AND METHODS
Materials.
Tissue culture and molecular biology reagents were purchased from Invitrogen Life Technologies (Burlington, ON) and Wisent (St. Bruno, QC). Electrophoresis and immunoblotting reagents were purchased from Bio-Rad Laboratories (Mississauga, ON) and Amersham GE Healthcare (Baie d'Urfé, QC). Myelin basic protein was purchased from Sigma-Aldrich Canada (Mississauga, ON). Mouse anti-hemagglutinin antigen epitope tag (HA) and mouse anti-GST antibody IgGs were from Santa Cruz Biotechnology (Santa Cruz, CA). [γ-33P]ATP (3,000 Ci/mmol) was purchased from PerkinElmer Canada. Protein A-coupled agarose beads were purchased from Millipore (Temecula, CA). Rabbit anti-phospho-JNK (Thr-183/Tyr-185) and rabbit anti-phospho-p38 (Thr-180/Tyr-182) IgGs were from Cell Signaling Technology (Danvers, MA). The pC4MFv2E plasmid and AP20187 were provided as contents of the Argent Regulated Homodimerization Kit (www.ariad.com/regulationkits) from ARIAD Pharmaceuticals (Cambridge, MA). The Bax promoter pGL3Bax-luciferase plasmid (35) was kindly provided by Dr. Ze'ev Ronai (Burham Institute for Medical Research, La Jolla, CA), and the p53-luciferase reporter plasmid was described previously (10). Luciferase reporter assay reagents were purchased as the Dual-Luciferase Reporter Assay System Kit from Promega (Madison, WI). Rabbit anti-SLK antibody was described previously (13, 23).
Plasmid construction.
Full-length HA-SLK and HA-SLK 1–373 expression vectors and GST-SLK(CT) (Fig. 1) were described previously (13, 23). PCR was used to subclone SLK 1–373 into pC4MFv2E (Fv-SLK 1–373; Fig. 1). Full-length human SLK cDNA was used as a template. Three PCRs were carried out, using Pwo DNA polymerase (Roche Applied Science, Mannheim, Germany). The goal of the first two PCRs was to introduce a silent mutation to abolish the XbaI site at base number 318 of human SLK. Primers 5′-CCGGAATTCGCCGCCATGTCCTTCTTCAATTTCCGTAAGA-3′ (forward-1) and 5′-TAGAAGGCATCCAGAAGCTTGACTATATTT-3′ (reverse-1) were used to create the first product of 293 base pairs, and the primers 5′-TAGTCAAGCTCCAGATGCCTTCTATTAT-3′ (forward-2) and 5′-CTAGCTAGTCTAGAGAGTTTATCTTCAGAGTTACTACGTTCTG-3′ (reverse-2) were used to create a product of 857 base pairs. The third PCR employed the first and second products as templates to produce SLK 1–373 with a product size of 1,125 base pairs, using the primers forward-1 and reverse-2 (above). The final PCR product, SLK amino acids 1–373, was ligated into the cloning vector pPCRScript Amp SK(+) (Stratagene, La Jolla, CA) and was verified by DNA sequencing. Then, the PCR product was subcloned into plasmid pC4MFv2E at the EcoRI and XbaI sites (which deleted the myristoylation signal from the vector). It should be noted that the construct also contains a C-terminal HA tag.
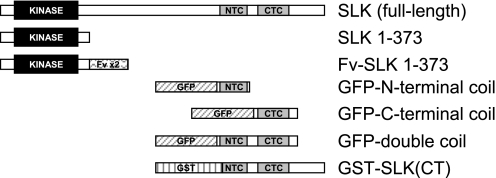
Fig. 1.
Ste20-like kinase, SLK, constructs employed in this study. See materials and methods for details. SLK (full-length; wild type), SLK 1–373, and Fv-SLK 1–373 contain hemagglutinin (HA) tags (not illustrated). CTC, C-terminal coil; NTC, N-terminal coil; GFP, green fluorescent protein; GST, glutathione S-transferase (GST).
The structure of SLK was analyzed for the presence of coiled-coils using computer programs (2, 33). Construction of green fluorescent protein (GFP)-N-terminal coil (amino acids 826–929), GFP-C-terminal coil (amino acids 942–1038), and GFP-double coil regions of SLK (amino acids 826–1038) was carried out using PCR and full-length SLK cDNA as a template (Fig. 1). Primers for the double coil were 5′-CCAGAATTCATTGTTGATGGTGTAGAAGTGAGTG-3′ (forward) and 5′-CGCGGATCCGTCTCACTGCGCTGAATCTT-3′ (reverse). For the N-terminal coil, the forward primer was the same as above, and the reverse primer was 5′-CGCGGATCCCCTTCTTTCGGTTCTTCAGC-3′. For the C-terminal coil, the forward primer was 5′-CCAGAATTCAGGAAAGAGGAGCTTGCAC-3′ and the reverse primer was the same as for the double coil. PCR products were subcloned into the vector pEGFP-C2 (Clontech, Mountain View, CA) at the EcoRI and BamHI restriction sites and were verified by DNA sequencing.
Cell culture and transfection.
Experiments were carried out in COS-1 cells (a monkey kidney cell line) and rat GECs. COS-1 cells were cultured in DMEM with 10% fetal calf serum. GECs have been characterized previously (7) and were cultured in K1 medium, which consists of DMEM, Ham F-12, with a 5% NuSerum and hormone mixture (13). Transient transfection with plasmid DNA was carried out with either Lipofectamine 2000 from Invitrogen Life Technologies or TransIT-LT1 from Mirius (Madison, WI) according to the manufacturer's instructions. Transfection with an empty vector (e.g., pRC/RSV) was used in controls. Based on transfection of GFP, the transfection efficiency in COS-1 cells was >60%, while transfection efficiency in GECs was ∼10% (10).
Immune complex kinase assays.
After treatment and incubation of cells, proteins (∼0.5 mg) were solubilized in lysis buffer containing 1% Triton X-100, 125 mM NaCl, 10 mM Tris (pH 7.50), 1 mM EGTA, 1 mM EDTA, 2 mM Na3VO4, 5 mM Na4P3O7, 25 mM NaF, 20 μM leupeptin, 10 μM pepstatin, 50 μM bestatin, 15 μM E64, 0.8 μM aprotinin, and 1 mM 4-(2-aminoethyl)benzenesulfonylfluoride (13, 23). The lysates were then centrifuged at 14,000 g for 10 min. Immunoprecipitation of proteins in the supernatants was carried out with primary (anti-HA) antibody IgG (2 h, 4°C) or nonimmune IgG as a control, followed by the absorption of complexes using protein A-coupled agarose beads (1 h, 4°C). The immunoprecipitates were then incubated with 20 mM HEPES (pH 7.20), 20 mM β-glycerophosphate, 10 mM MgCl2, 1 mM dithiothreitol, 0.5 mM Na3VO4, 0.5 mg/ml bovine brain myelin basic protein, and 20 μM [γ-33P]ATP (5 μCi). Following a 5-min incubation period at 30°C, kinase reactions were stopped with the addition of Laemmli buffer. Samples were boiled for 10 min and then used for SDS-PAGE and autoradiography. Quantification of immunoblots was performed by densitometry. Blots were scanned, and specific bands of interest were selected to measure for density using National Institutes of Health ImageJ software. Results are expressed in arbitrary units. Preliminary studies demonstrated that there was a linear relationship between densitometric measurements and the amounts of proteins loaded onto gels.
Immunoprecipitation and immunoblotting.
Cells were scraped from culture dishes into lysis buffer, and immunoprecipitation was carried out as described above (13, 23). Cell lysates or immune complexes were boiled in Laemmli buffer and were subjected to SDS-PAGE. Proteins were transferred onto nitrocellulose membranes. The membranes were blocked with 5% BSA and were incubated with primary antibody. After washing, membranes were incubated with horseradish peroxidase-coupled secondary antibody and were developed using ECL.
Analysis of proteins by gel-filtration chromatography.
Cell homogenates were prepared as described previously (13) in homogenization buffer containing 50 mM HEPES, 0.25 M sucrose, 1 mM EDTA, 1 mM EGTA, 20 μM leupeptin, 10 μM pepstatin, 50 μM bestatin, 15 μM E64, 0.8 μM aprotinin, and 1 mM 4-(2-aminoethyl)benzenesulfonylfluoride, pH 7.40. A 250-μl aliquot of the cell homogenate was applied to a Superdex 200 10/300 GL column (10-mm diameter/24-cm bed height, Amersham Biosciences) before perfusion with gel-filtration elution buffer (homogenization buffer) (20). Protein content was monitored by continuous UV absorption spectrometry at 280 nm. The flow rate was 0.25 ml/min. Proteins were collected in 0.5-ml fractions and were then detected by immunoblotting. The column was calibrated using blue dextran (2,000 kDa), thyroglobulin (669 kDa), IgG (150 kDa), and BSA (67 kDa).
Luciferase reporter assays.
Assays were performed using a Promega Dual-Luciferase Reporter Assay System Kit, according to the manufacturer's instructions (10). Luciferase activity was measured in a Berthold, Lumat LB 9507 luminometer, and the ratios between firefly luciferase activity and Renilla luciferase activity were calculated. While the firefly luciferase serves as the principal reporter, the Renilla luciferase serves as an internal control for intersample variability related to transfection.
Cell counts.
COS-1 cells were plated at 50,000 cells/35-mm cell culture dish and were transfected 24 h later. Forty-eight hours after the initial cell plating, fresh medium was added, and some samples of cells transfected with Fv-SLK 1–373 were treated with AP20187 to induce the homodimerization of Fv-SLK 1–373. Control cells (transfected with an empty vector) were also treated with AP20187. After 24 h (72 h after the initial cell plating), fresh AP20187 in fresh medium was added where applicable. The cells were harvested 96 h after the initial cell plating. Cells were detached from culture dishes with trypsin and resuspended in PBS. Cells were counted in a hemocytometer (13).
Hoechst staining.
COS-1 cells were plated, transfected, and treated with AP20187, as described above. The cells were harvested 96 h after the initial cell plating and were subjected to staining with Hoechst H33342 dye and propidium iodide. Cells with nuclei that were pyknotic and fragmented and stained negatively for propidium iodide were counted as apoptotic. Propidium iodide identifies necrotic cells (which have compromised cellular membranes), as it is impermeable to the plasma membranes of both viable and apoptotic cells (13). Analysis was performed using the University of Texas Health Science Center at San Antonio Image Tool program.
Statistics.
Data are presented as means ± SE. Significant differences between groups were established by the t-statistic. One-way ANOVA was utilized to establish significant differences among groups. Where there were significant differences, comparisons were made between groups using the t-statistic, adjusting the critical value using the Bonferroni method.
RESULTS
Homodimerization of SLK.
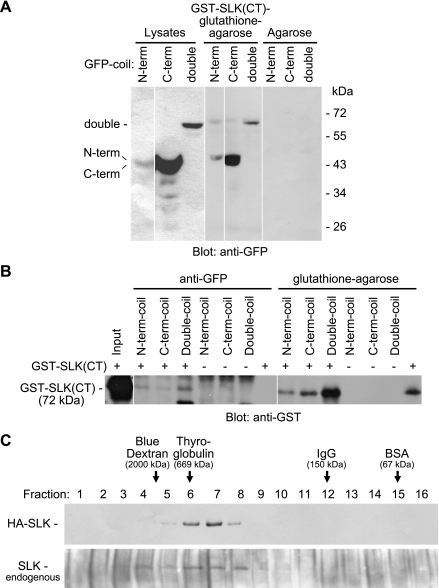
The C-terminal region of SLK contains coiled-coil structures, which may potentially be involved in homodimerization (46). In coimmunoprecipitation experiments, we previously showed that full-length (wild-type) SLK was able to associate with GST-SLK(CT) (23), implying that SLK can homodimerize via the C-terminal domain. Initially, we examined potential homotypic interactions of the coiled-coil regions of SLK. COS-1 cells were transfected with constructs containing GFP fused with the SLK N-terminal coil, C-terminal coil, or both coils (double coil) (Fig. 1). Lysates of the cells were mixed with GST-SLK(CT) and were subjected to pull-down assays or immunoprecipitation. Pull-down with GST-SLK(CT)-glutathione-agarose demonstrated associations with the SLK N-terminal, C-terminal, and double coils, and was most prominent with the C-terminal coil (Fig. 2A). The pull-downs paralleled expression levels of the coils; that is, immunoblotting of cell lysates demonstrated that after transfection of comparable amounts of the three coil cDNAs, the SLK C-terminal coil showed greatest expression, while expression of the N-terminal coil and double coil was weaker (Fig. 2A). Following expression of GFP-N-terminal coil, -C-terminal coil, or -double coil, immunoprecipitation of COS-1 cell lysates with anti-GFP antibody in the presence of exogenous GST-SLK(CT) resulted in coimmunoprecipitation of GST-SLK(CT) (Fig. 2B). Together, these results indicate that the coiled-coil regions of SLK can mediate homodimerization.
Fig. 2.
Homodimerization of SLK. A: COS-1 cells were transfected with GFP-N-terminal coil, GFP-C-terminal coil, or GFP-double coil. Lysates were mixed with GST-SLK(CT)-glutathione-agarose or agarose alone. Pull-down with GST-SLK(CT)-glutathione-agarose (but not agarose alone) demonstrated prominent association with the SLK C-terminal coil and weaker interactions with the N-terminal coil and the double coil. The lysates are presented at a lower exposure compared with the pull-downs. B: following expression of GFP-N-terminal coil, C-terminal coil, or double coil, GST-SLK(CT) was added to some of the COS-1 cell lysates (+), and the mixtures were immunoprecipitated with anti-GFP antibody, or were subjected to pull-down with glutathione-agarose. GST-SLK(CT) was recovered by both anti-GFP immunoprecipitation and glutathione-agarose pull-down. C: gel-filtration chromatography. Top: anti-HA antibody immunoblot of eluted fractions (HA-SLK-transfected COS-1 cells). Bottom: anti-SLK antibody immunoblot (untransfected cells, i.e., endogenous SLK). In cells transiently transfected with HA-SLK, SLK eluted in fractions at or just below the size of thyroglobulin (669 kDa), and there was an absence of SLK in fractions the size of IgG (150 kDa). The elution profile of endogenous SLK in COS-1 cells was similar to that of ectopic HA-SLK. In A and B (and in certain panels of the other figures below), the white spaces denote reassembly of noncontiguous gel lanes; there were no adjustments made to the digital images among the lanes that would alter the information in the panels.
To determine whether full-length SLK is present as a monomer or oligomer, we analyzed SLK-containing protein complexes by gel-filtration chromatography (Fig. 2C). Transient transfection of SLK in COS-1 cells results in about three- to fourfold overexpression compared with the endogenous SLK (23). HA-SLK expressed in COS-1 cells eluted in fractions 6–8 (∼669 kDa) but not in fraction 12 (∼150 kDa). The elution profile of endogenous SLK in COS-1 cells was similar to that of ectopically expressed HA-SLK (Fig. 2C), indicating that the elution profile was not influenced by overexpression of recombinant SLK. The calculated molecular mass of SLK is ∼140 kDa, whereas on SDS-PAGE, SLK migrates closer to ∼200 kDa. Thus the results of the gel-filtration chromatography suggest that in resting COS-1 cells, the monomeric form of SLK is not detectable. We also performed gel-filtration chromatography to examine the elution profile of endogenous SLK in GECs that were either untreated or subjected to UV stress (UV light exposure for 2 min, followed by 30-min incubation in medium). In both instances, the elution of endogenous SLK in GECs (results not shown) was identical to the results seen in COS-1 cells (Fig. 2C, bottom).
Homodimerization increases kinase activity of SLK.
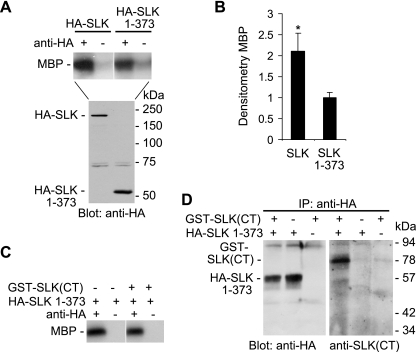
The next series of experiments addressed the role of homodimerization in modulating kinase activity. In one earlier study (40), but not another study (46), deletion of the C-terminal domain of SLK was shown to increase SLK catalytic activity, and the authors of this study suggested an autoinhibitory role for the C-terminal domain. We performed similar experiments, comparing the kinase activity of full-length SLK with that of SLK 1–373, a construct that contains only the N-terminal kinase domain (Fig. 1). In our hands, the kinase activity of full-length SLK was comparable to SLK 1–373 (Fig. 3A). However, expression of the SLK 1–373 protein appeared to be significantly greater than full-length SLK. Therefore, when kinase activity of SLK was normalized for protein expression, the activity of full-length SLK appeared to be greater compared with SLK 1–373, and certainly the activity of full-length SLK was not lower compared with SLK 1–373 (Fig. 3B). Furthermore, we added the C-terminal domain of SLK to the catalytic domain and assayed for potential interactions and kinase activity. Addition of the C-terminal domain, i.e., GST-SLK(CT), had no significant effect on the kinase activity of SLK 1–373 (Fig. 3C), although GST-SLK(CT) coimmunoprecipitated with HA-SLK 1–373 (Fig. 3D). These experiments suggest that the C-terminal domain of SLK can interact with the N-terminal catalytic domain; however, the C-terminal domain does not appear to negatively regulate SLK kinase activity. An alternate possibility is that the C-terminal domain of SLK could facilitate an increase in kinase activity by mediating homodimerization.
Fig. 3.
Effect of the C-terminal domain on the kinase activity of SLK. A and B: COS-1 cells were transiently transfected with HA-SLK (full-length) or HA-SLK 1–373 cDNAs. After 48 h, lysates were immunoprecipitated (IP) with anti-HA antibody (+) or normal IgG in controls (−) and were then subjected to a kinase assay with myelin basic protein (MBP) as substrate, or were immunoblotted with anti-HA antibody. A: representative autoradiogram of 33P incorporation into MBP and anti-HA immunoblot. B: densitometric quantification of phosphorylated MBP normalized for protein expression. Values are means ± SE of 4 experiments. *P < 0.04 SLK (full-length) vs. SLK 1–373. C and D: COS-1 cells were transiently transfected with HA-SLK 1–373 cDNA. After 48 h, cells were lysed, and GST-SLK(CT) was added to some samples (+). Samples were immunoprecipitated with anti-HA antibody (+) or normal IgG in controls (−) and were then subjected to a kinase assay with MBP as substrate (C) or were immunoblotted with anti-HA or anti-SLK(CT) antibodies (D). GST-SLK(CT) coimmunoprecitated with HA-SLK 1–373 (D) but had no effect on the kinase activity of SLK 1–373 (C).
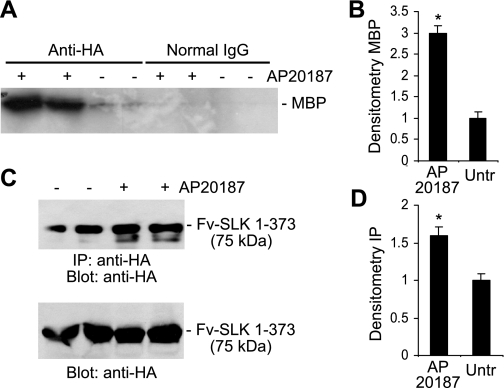
To assess whether homodimerization increases the kinase activity of SLK compared with the monomer, we adapted a system for forced dimerization of proteins in intact cells (1, 8). COS-1 cells were transiently transfected with a cDNA encoding Fv-SLK 1–373, which expresses a fusion protein of the SLK N-terminal kinase domain (SLK 1–373) and two modified FK506 binding protein domains (Fv), plus a HA tag (Fig. 1). The drug, AP20187 (an analog of FK506), binds to the Fv domains of the fusion protein, resulting in the homodimerization or oligomerization of adjacent SLK N-terminal kinase domains in a controlled fashion. Thus, to compare the kinase activities between homodimerized and monomeric Fv-SLK 1–373, cells were incubated with or without AP20187. After immunoprecipitation of Fv-SLK 1–373 with anti-HA antibody, the immune complexes were incubated with [γ-33P]ATP and myelin basic protein, and incorporation of 33P into myelin basic protein was used as a marker of kinase activity. Weak 33P incorporation was evident in cells that had not been treated with AP20187, suggesting that monomeric Fv-SLK 1–373 exhibits reduced kinase activity (Fig. 4, A and B). There was a threefold increase in 33P incorporation in cells exposed to AP20187 (i.e., dimeric SLK) compared with untreated (monomeric) (Fig. 4, A and B). Normal mouse IgG did not immunoprecipitate the fusion protein (Fig. 4A).
Fig. 4.
Homodimerization increases the kinase activity of SLK. A and B: COS-1 cells were transiently transfected with Fv-SLK 1–373 cDNA and were treated after 48 h with (+) or without (−) 1 μM AP20187 for 24 h to induce homodimerization of Fv-SLK 1–373. Lysates were immunoprecipitated with anti-HA antibody (normal IgG in controls) and were then subjected to a kinase assay with MBP as substrate. A: representative autoradiogram of phosphorylated MBP (duplicate lanes). B: densitometric quantification of phosphorylated MBP in the anti-HA antibody immunoprecipitations. Values are means ± SE of 4 experiments. *P < 0.0001 AP20187 vs. untreated. C and D: COS-1 cells were transfected with Fv-SLK 1–373 cDNA and were then treated with (+) or without (−) 1 μM AP20187 as above. Lysates were immunoprecipitated with anti-HA antibody, and the immune complexes were then immunoblotted with anti-HA antibody (C, top). Bottom: lysates immunoblotted with anti-HA antibody. D: densitometric quantification of anti-HA antibody immunoprecipitations/immunoblots. Values are means ± SE of 5 experiments. *P < 0.0001 AP20187 vs. untreated.
We also observed that COS-1 cells expressing Fv-SLK 1–373 protein and treated with AP20187 yielded a greater amount of immunoprecipitated fusion protein compared with untreated (Fig. 4, C and D). Nevertheless, the increase in kinase activity of the homodimerized Fv-SLK 1–373 was greater by about twofold compared with the monomer, even when adjusted for the amounts of immunoprecipitated proteins. The increase in the relative amounts of immunoprecipitated dimeric fusion protein may have been due to the greater abundance of HA epitopes assembled together in a more favorable conformation for binding by HA antibody, possibly due to a greater affinity of the antibody for dimerized epitopes.
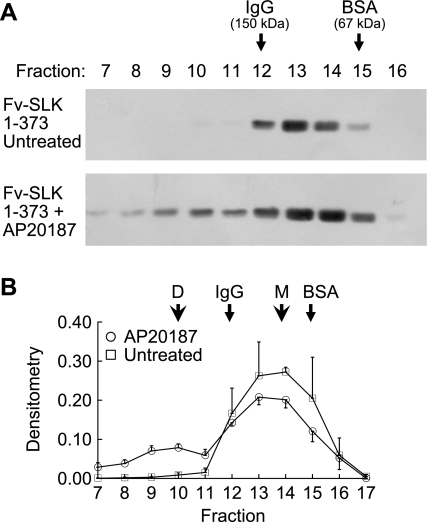
To verify the effectiveness of AP20187 in inducing homodimerization of Fv-SLK 1–373, lysates of COS-1 cells transfected with Fv-SLK 1–373, treated with or without AP20187, were subjected to gel-filtration chromatography. In untreated cells, Fv-SLK 1–373 eluted mainly in fractions 12–15 (Fig. 5, A and B), in keeping with the molecular mass of Fv-SLK 1–373 on SDS-PAGE (∼75 kDa). Densitometric quantification demonstrated that only ∼3% of total Fv-SLK 1–373 was found in fractions 7–11 (i.e., molecular mass >150 kDa) (Fig. 5B). In contrast, in AP20187-treated cells, a portion of the Fv-SLK 1–373 shifted to fractions 7–11, reflecting homodimerization (Fig. 5, A and B). Densitometric quantification indicated that AP20187 induced homodimerization of ∼30% of Fv-SLK 1–373 (Fig. 5B).
Fig. 5.
Effect of AP20187 on the homodimerization of Fv-SLK 1–373. COS-1 cells were transiently transfected with Fv-SLK 1–373 cDNA and were treated after 48 h with or without 0.1 μM AP20187 for 24 h. Lysates were subjected to gel-filtration chromatography. A: representative anti-HA antibody immunoblots. B: densitometric quantification (3–5 experiments). D, dimer; M, monomer.
Induction of p38 and JNK phosphorylation by homodimerized Fv-SLK 1–373.
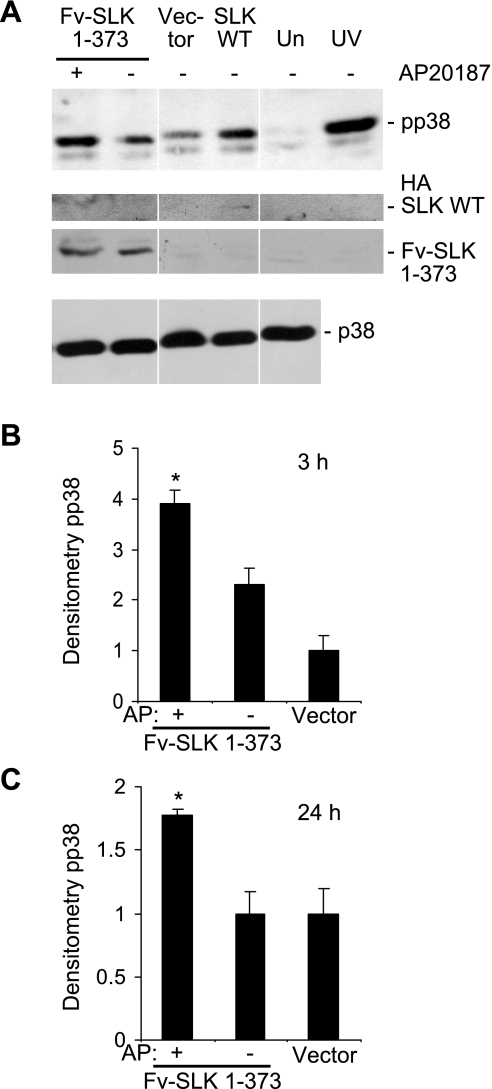
Previous studies have shown that SLK signals via the p38 and JNK pathways (10, 23, 40). To determine whether the increase in SLK activity resulting from the induction of homodimerization of Fv-SLK 1–373 can stimulate the activation of p38, lysates of transiently transfected GECs were immunoblotted with activation-specific phospho-p38 antibody. In the presence of AP20187 (3-h incubation), Fv-SLK 1–373 increased the phosphorylation of p38 by 1.7-fold compared with untreated cells (Fig. 6, A and B); the increase was 1.8-fold upon incubation with AP20187 for 24 h (Fig. 6C). In these experiments, untreated Fv-SLK 1–373 did not enhance p38 phosphorylation significantly compared with control vector transfections. Thus increased kinase activity, resulting from homodimerization of SLK, enhanced phosphorylation of p38. The expression of Fv-SLK 1–373 protein was relatively constant among the cells transfected with Fv-SLK 1–373 cDNA (Fig. 6A, anti-HA blot), and the p38 expression levels were also constant across samples (Fig. 6A). GECs transiently transfected with the control vector, treated with AP20187, and subjected to immunoblot analysis with anti-phospho-p38 antibody showed no increase in p38 phosphorylation (results not shown), indicating that AP20187 did not directly induce p38 phosphorylation.
Fig. 6.
Homodimerization of Fv-SLK 1–373 increases phosphorylation of p38. A: glomerular epithelial cells were transiently transfected with Fv-SLK 1–373 cDNA, and after 48 h, were treated with (+) or without (−) 1 μM AP20187 for 3 h. Empty vector transfections were used as a control. Transfection with full-length, wild-type SLK (SLK WT), untransfected cells (Un), and exposure of cells to UV are shown for comparison. Cell lysates were immunoblotted with antibodies to phospho-p38 (pp38; top), HA (middle), and p38 (bottom). B and C: densitometric quantification of p38 phosphorylation. B: transfected cells were incubated with AP20187 (AP) for 3 h. Values are means ± SE of 6 experiments. *P = 0.0035 AP20187 vs. untreated. C: cells were incubated with AP20187 for 24 h. Values are means ± SE of 8 experiments. *P = 0.002 AP20187 vs. untreated.
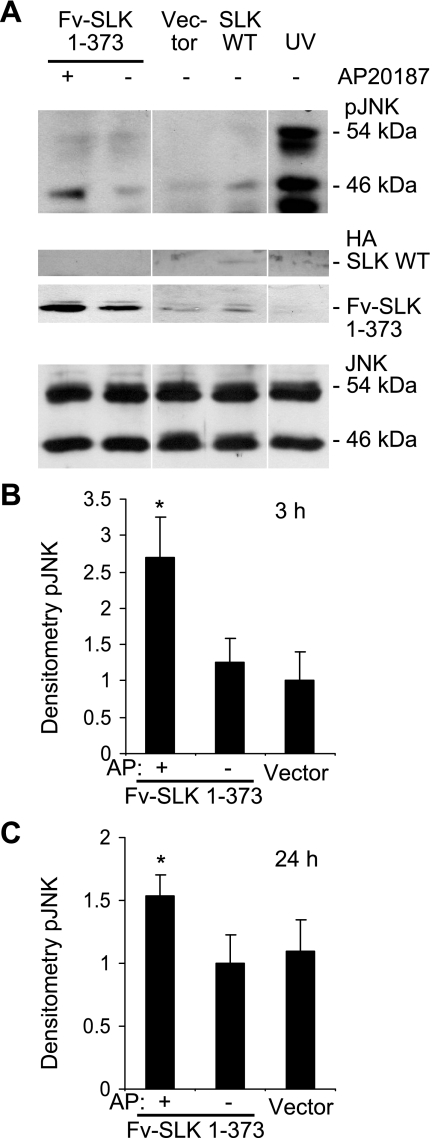
An analogous protocol was used to determine whether the increase in SLK activity after induction of homodimerization of Fv-SLK 1–373 can stimulate the phosphorylation of JNK. An increase in the activation-specific phosphorylation of the 46-kDa JNK isoform was detected in GEC transiently transfected with Fv-SLK 1–373 and treated with AP20187 compared with their untreated counterparts. Compared with the monomer, dimerized Fv-SLK 1–373 increased the phosphorylation of the 46-kDa JNK by 2.2-fold (Fig. 7, A and B) when incubation with AP20187 was for 3 h, and the increase was 1.5-fold when incubation with AP20187 was for 24 h (Fig. 7C). By analogy to phospho-p38, untreated Fv-SLK 1–373 did not enhance JNK phosphorylation significantly compared with control vector transfections, and cells transiently transfected with control vector, treated with AP20187, and subjected to immunoblot analysis with phospho-JNK antibody showed no increase in JNK phosphorylation (results not shown). The expression of Fv-SLK 1–373 protein was relatively constant among the cells transfected with Fv-SLK 1–373 cDNA, and the JNK expression levels were also constant across samples (Fig. 7A).
Fig. 7.
Homodimerization of Fv-SLK 1–373 increases phosphorylation of JNK (46-kDa isoform). A: glomerular epithelial cells were transiently transfected with Fv-SLK 1–373 cDNA, and after 48 h, were treated with (+) or without (−) 1 μM AP20187 for 3 h. Empty vector transfections were used as a control. Transfection with full-length SLK WT and exposure of cells to UV are shown for comparison. Cell lysates were immunoblotted with antibodies to phospho-JNK (pJNK; top), HA (middle), and JNK (bottom). B and C: densitometric quantification of JNK phosphorylation (46 kDa). B: transfected cells were incubated with AP20187 (AP) for 3 h. Values are means ± SE *P = 0.05 AP20187 vs. untreated, 6 experiments. C: cells were incubated with AP20187 for 24 h. Values are means ± SE of 8 experiments. *P = 0.05 AP20187 vs. untreated.
In keeping with earlier results (10), full-length (wild-type) SLK generally induced phosphorylation of both p38 and JNK (46-kDa isoform), particularly at the 3-h time point (Figs. 6A and 7A). However, by analogy to Fig. 3A, compared with Fv-SLK 1–373, expression of the full-length SLK was weak, probably due to lower transfection efficiency. Therefore, direct comparison of activation-specific phosphorylation by wild-type SLK with Fv-SLK 1–373 was not performed. UV radiation (used as a positive control) greatly induced the phosphorylation of p38 and both JNK isoforms (Figs. 6A and 7A).
Homodimerization of SLK increases apoptosis.
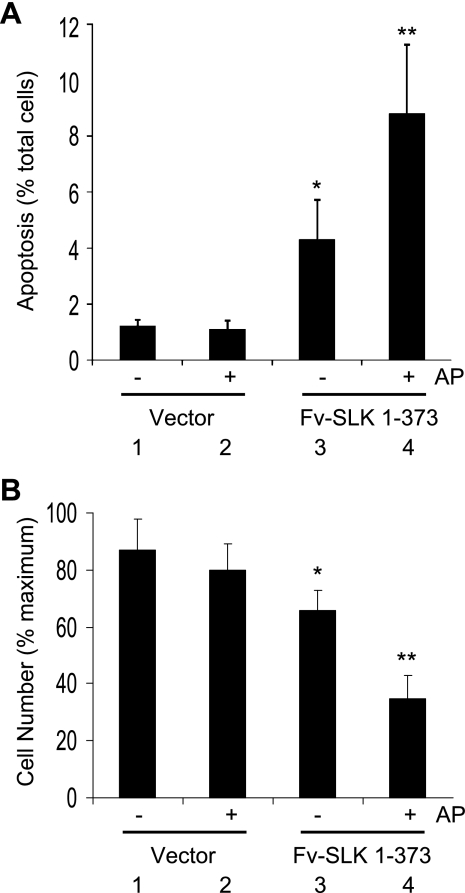
SLK promotes apoptosis in various cultured cells (including kidney cells) and in podocytes in vivo (10, 11, 13, 23, 40). To determine whether dimerization of the kinase domain affected apoptosis, COS-1 cells were transiently transfected with Fv-SLK 1–373 or control vector and were treated with or without AP20187. Expression of untreated Fv-SLK 1–373 resulted in greater apoptosis compared with control vector transfections (Fig. 8A). However, in the presence of AP20187, a greater number of Fv-SLK 1–373-transfected cells underwent apoptosis compared with untreated. AP20187 had no significant effect on apoptosis in control vector-transfected cells (Fig. 8A).
Fig. 8.
Homodimerization of Fv-SLK 1–373 enhances apoptosis. COS-1 cells were transiently transfected with Fv-SLK 1–373 cDNA or empty vector (control) and were then treated with (+) or without (−) 3 μM AP20187 (materials and methods). A: cells were analyzed by Hoechst H33342 staining. Values are means ± SE of 4 experiments. *P = 0.03 group 1 vs. group 3. **P = 0.004 group 3 vs. group 4. B: number of viable COS-1 cells. Values are means ± SE of 3 experiments. *P = 0.057 group 1 vs. group 3. **P < 0.0001 group 1 vs. group 4. **P < 0.0001 group 2 vs. group 4. **P < 0.004 group 3 vs. group 4.
To substantiate the above results, we also monitored changes in cell number. Transfection of Fv-SLK 1–373 tended to reduce cell number compared with control vector transfection, and AP20187 induced a further significant reduction in cell number in the former. AP20187 had no significant effect on cell number in control vector-transfected cells (Fig. 8B).
Homodimerized SLK stimulates Bax and p53 reporter activities.
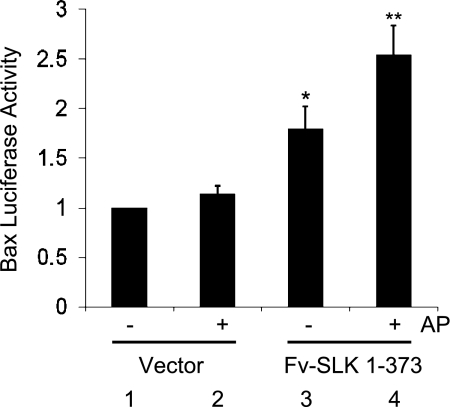
In previous studies, we showed that SLK can mediate apoptosis via JNK (10) and p38 (23), and the process involved activation of caspase-9 and release of cytochrome c, consistent with involvement of the mitochondrial apoptotic pathway (23). To confirm that the proapoptotic effect of dimeric SLK involved the mitochondrial apoptotic pathway, we examined the induction of Bax, a protein involved in the mitochondrial apoptotic pathway. In addition to p53-responsive elements (35), the Bax promoter contains an AP1 consensus sequence (34), which can be activated by the formation of Jun homodimers or Jun/Fos heterodimers mediated by the phosphorylation of c-Jun by JNK. GECs were transiently transfected with a firefly luciferase reporter plasmid that contains the Bax promoter (−680 to −317 bp) (35), along with Fv-SLK 1–373, and were treated with AP20187. Fv-SLK 1–373 increased the Bax promoter-luciferase reporter in untreated cells compared with the control vector. Fv-SLK 1–373 plus AP20187 showed a ∼50% greater Bax-luciferase reporter activity compared with the untreated Fv-SLK 1–373, indicating that homodimerization of SLK can enhance the induction of Bax and the mitochondrial apoptotic pathway (Fig. 9). We also examined the effect of SLK on the activation of the Bax-luciferase reporter in the presence or absence of JNK- or p38-directed inhibitors (SP600125 and SB203580, respectively) (10). Both inhibitors reduced luciferase activity significantly (Table 1), consistent with the involvement of JNK and p38 in the activation of Bax. Finally, we compared the magnitude of the SLK effect on the Bax-luciferase reporter with other potentially apoptogenic stimuli. In vitro ischemia-reperfusion injury (10, 13, 23), i.e., incubation of GECs with 10 μM antimycin A+10 mM 2-deoxyglucose (“chemical anoxia”; 40 min) followed by 4 h of reexposure to glucose (“recovery”) increased Bax reporter activity by 1.35 ± 0.09-fold (P = 0.01, 4 experiments), representing weaker activation, compared with SLK. Exposure of GECs to UV light, a positive control for p38 activation and apoptosis, increased the Bax reporter activity by 3.5-fold (2 experiments).
Fig. 9.
Homodimerization of Fv-SLK 1–373 enhances Bax promoter-luciferase reporter activities. Glomerular epithelial cells were transfected with a firefly luciferase reporter plasmid that contains the Bax promoter (−680 bp to −317 bp), together with Fv-SLK 1–373 or empty vector. Then, cells were treated with (+) or without (−) 2 μM AP20187 (AP). Cells were harvested 72 h after transfection. Luciferase activity is expressed in arbitrary units. Values are means ± SE of 4 experiments. *P = 0.02 group 1 vs. group 3. *P = 0.0006 group 1 vs. group 4. **P = 0.02 group 3 vs. group 4.
Table 1.
Role of JNK and p38 in the activation of the Bax-luciferase reporter by SLK
| Transfection | Bax-Luciferase Activity, arbitrary units |
|---|---|
| Vector (untreated) | 1.0 ± 0.3 |
| SLK (untreated) | 3.6 ± 1.1* |
| Vector+SP600125 | 1.1 ± 0.3 |
| SLK+SP600125 | 1.2 ± 0.3 |
| Vector+SB203580 | 0.6 ± 0.2 |
| SLK+SB203580 | 0.8 ± 0.4 |
Values are means ± SE of 3 experiments. Cells were transfected with Bax-luciferase reporter, together with hemagglutinin (HA), SLK, or control vector. The JNK-directed inhibitor SP600125 (10 μM) and the p38-directed inhibitor SB203580 (10 μM) were added after 24 h. Luciferase activity was measured after another 24 h.
P < 0.004 SLK vs. vector, P < 0.003 SLK vs. SLK+SB203580, P < 0.01 SLK vs. SLK+SP600125.
Previously, we demonstrated that SLK stimulated p53 transactivational activity (using a luciferase reporter plasmid containing a p53 cis-enhancing element) and that p53 activation was associated with apoptosis (10). In the same study, we demonstrated that activation of this p53-luciferase reporter by SLK was blocked by the JNK-directed inhibitor SP600125 or by coexpression of recombinant dominant negative JNK or p38 (10). In the present study, we used the p53 reporter assay to assess the effect of SLK dimerization on p53 activity. GECs were transfected with p53-luciferase reporter, together with a control vector or Fv-SLK 1–373. AP20187 (100 nM) was added to some cells after transfection, and luciferase activity was measured after 48 h. Compared with the vector, untreated Fv-SLK 1–373 enhanced luciferase activity 1.20 ± 0.02-fold, while Fv-SLK 1–373+AP20187 enhanced luciferase activity 1.44 ± 0.15-fold (P = 0.02 untreated vs. AP20187, 6 experiments). Although the increase in the p53-luciferase reporter was weaker compared with the Bax reporter, together the results support the view that dimerization of the SLK catalytic domain enhances proapoptotic signaling.
DISCUSSION
The present study addressed the regulation of SLK activity. Previously, it was shown that SLK expression and activity are increased in the developing kidney and in renal ischemia-reperfusion injury (13). Second, full-length SLK was able to associate with a GST fusion protein containing the SLK C terminus (23). These results, plus the presence of protein interaction (coiled-coil) domains in the C-terminal region of SLK, implied that regulation of SLK catalytic activity may involve an increase in expression and homodimerization or oligomerization. In the present study, we employed overexpression of SLK in cultured cells (which we have used to model the increased expression observed in the in vivo circumstances) to examine the role of homodimerization of SLK in detail. Two coiled-coil regions of the SLK C-terminal domain were expressed as GFP fusion proteins, and pull-down with GST-SLK(CT)-glutathione-agarose demonstrated association with the SLK coils (Fig. 2). Conversely, following expression of the GFP-N-terminal coil, C-terminal coil, or double coil, immunoprecipitation with anti-GFP antibody in the presence of exogenous GST-SLK(CT) resulted in coimmunoprecipitation of GST-SLK(CT) (Fig. 2). These results support the view that the coiled-coil regions of SLK are able to mediate homodimerization.
Gel-filtration chromatography demonstrated that full-length SLK, both ectopically expressed and endogenous forms, were present in cells as high-molecular-mass complexes, and there was an absence of SLK in fractions where the monomeric form of SLK would be predicted to appear (Fig. 2). These results indicate that in cells, SLK is unlikely to be an isolated monomer and suggest that SLK may be present in a dimeric or oligomeric form, or that the high-molecular-mass complex represents SLK in tight association with another protein(s). Further studies will be required to address these possibilities.
To determine whether homodimerization of SLK can lead to an increase in its kinase activity, we employed a fusion protein consisting of the SLK N-terminal kinase domain (SLK 1–373) and two Fv domains. The drug AP20187 binds to the Fv domains, resulting in homodimerization of adjacent SLK kinase domains in a controlled fashion (1, 8). Analysis by gel-filtration chromatography demonstrated that ∼30% of Fv-SLK 1–373 underwent dimerization after treatment of cells with AP20187 (Fig. 5). Studies of other Fv fusion proteins in analogous systems have reported dimerization rates of ∼50–70% (5, 17). The relatively lower amount of Fv-SLK 1–373 dimerization may have been dependent on certain physical factors of the SLK fusion protein, such as proximity of the domains, their relative orientation, and conformational flexibility/rigidity (1). Using this dimerization system, we showed that the kinase activity of the Fv-SLK 1–373 in AP20187-treated cells is greater compared with untreated, when measured by an in vitro kinase assay (Fig. 4). These studies were extended to define the role of dimerization of the kinase domain in the function of SLK. Previously, it was demonstrated that SLK can induce apoptosis in various cell lines (including COS-1 cells, GECs, MDCK cells), and in podocytes in vivo (10, 11, 13, 23, 40, 41). In the present study, transfection of Fv-SLK 1–373 in the absence of AP20187 stimulated apoptosis compared with the control vector, whereas addition of AP20187 further enhanced apoptosis (Fig. 8). By analogy, monomeric Fv-SLK 1–373 tended to reduce cell number, while dimeric Fv-SLK 1–373 produced a substantial decrease (Fig. 8). The percent decrease in cell number exceeded the percentage of apoptotic cells (Fig. 8). The explanation for this may be that the cell count represents a cumulative loss of cells over 72 h, while the Hoechst apoptosis assay represents apoptosis at one distinct time point. Moreover, we have previously demonstrated that expression of SLK can reduce [3H]thymidine incorporation (23), in keeping with decreased proliferation. While the magnitude of apoptosis induced by SLK was relatively small (Fig. 8), it should be noted that even a small difference in the magnitude could significantly affect the ability of the kidney to recover from an apoptogenic insult in which the missing (i.e., apoptotic) cells cannot contribute to repair that partially depends upon proliferation of tubular epithelial cells (16).
Previous studies have shown that full-length SLK can induce the activation of p38 (23) and JNK (10, 40, 41) and that the activation of these stress kinase pathways leads to apoptosis. The intermediary protein kinases involved in p38 and JNK activation include apoptosis signal-regulating kinase 1 as well as MEKK1 and MKK4 (10, 40, 41). We demonstrated that dimeric Fv-SLK 1–373 induced activation-specific phosphorylation of p38 and JNK more effectively compared with the monomer (Figs. 6 and 7).
The potential involvement of the mitochondrial pathway in the apoptotic response of SLK was demonstrated in earlier work (10, 23) and was further investigated in the present study using a Bax promoter-luciferase reporter assay. Bax is a proapoptotic protein that is cytosolic in resting cells but permeates the mitochondrial membrane upon its activation, liberating mitochondrial factors, which activate caspases (24, 27). Thus the activation of Bax can act as a switch in controlling whether mitochondrial apoptosis occurs. The Bax promoter contains p53-responsive elements and an AP1 consensus sequence, which can be activated by the formation of Jun homodimers or Jun/Fos heterodimers, mediated by the phosphorylation of c-Jun by JNK (34, 35). The Bax promoter is differentially regulated by p53 in a cell type-specific manner (42), and our studies in GECs have suggested that p53 by itself is not sufficient to activate the Bax promoter (unpublished observations). In the present study, it was found that monomeric Fv-SLK 1–373 stimulated the Bax promoter-luciferase reporter, while dimeric Fv-SLK 1–373 amplified activation (Fig. 9). The activation of Bax is in keeping with earlier results (23), which supported induction of apoptosis by SLK through the mitochondrial apoptotic pathway. Bax activation may also involve posttranscriptional mechanisms, including phosphorylation by JNK or p38 (25). Thus activation of SLK and its downstream effectors, JNK and p38, could potentially contribute to Bax activation via phosphorylation.
Previously, we demonstrated that SLK stimulated p53 transactivational activity (10). Moreover, SLK activated p53 via phosphorylation on Ser-33 and Ser-315, and p53 augmented apoptosis after in vitro ischemia-reperfusion injury (10). Activation of p53 was mediated via the JNK pathway and possibly p38 (10). In the present study, we have observed that homodimerized Fv-SLK 1–373 increased p53 reporter activity more robustly compared with the monomer, providing further support for the view that dimerization of the catalytic domain enhances proapoptotic activity.
Activation through dimerization has been demonstrated for other serine/threonine protein kinases, including mixed lineage kinase 3 (32), MEKK2 (6), Ca2+/calmodulin-dependent kinase 2 (39), as well as the GCKs Mst1 (9) and Mst2 (19). Also, receptor tyrosine kinases can dimerize and become activated upon binding of extracellular ligands (31). Homodimerization of the SLK kinase domain can potentially facilitate transphosphorylation of adjacent kinase domains to induce activation. The kinase activation segment may be involved in the regulation of catalytic activity by the phosphorylation of one or more of its amino acids and consists of a magnesium-binding site (Asp-Phe-Gly or DFG motif), a short β-strand, the activation loop, and the P+1 loop (15, 36). When the activation segment phosphorylation sites are situated close together with the active site of the participating protomer, it may allow for a spatial arrangement favoring an active kinase (30, 31, 38). Moreover, in the case of SLK, phosphate moieties may stabilize the activation segment in a spatial arrangement that is favorable for the binding to substrate (38). Actually, the kinase domain of SLK can dimerize in vitro and undergo autophosphorylation and kinase activation, although dimerization is weak (38). The present study indicates that in cells, there was little detectable dimerization of the kinase domain, as Fv-SLK 1–373 was almost exclusively monomeric in the absence of AP20187 (Fig. 5); however, the monomeric SLK kinase domain possesses reduced catalytic activity (Fig. 3), signaling capabilities (Fig. 9), and proapoptotic effects (Fig. 8). Possibly, a portion of the monomeric catalytic domains, when highly expressed, spontaneously underwent dimerization, which may have resulted in a posttranslational change, such as phosphorylation. The dimerization, while transient, may have allowed for more persistent kinase activity of the monomers. Nevertheless, in cells, efficient signaling by the SLK kinase domain is enhanced by dimerization through another domain of the protein, such as the artificial Fv domain, or the native C-terminal domain, which contains coiled-coils. We detected significant p38 or JNK phosphorylation by the Fv-SLK 1–373 dimer, but not the monomer (Figs. 6 and 7); possibly, the ability of the monomer to induce signaling is related to transfection efficiency, or the assay we employed was not sufficiently sensitive.
Factors that would favor enhanced homodimerization of full-length SLK in vivo require further study. Earlier, it was proposed that the C-terminal domain of SLK fulfills an autoinhibitory function, as deletion of the C-terminal domain was reported to increase SLK activity (40, 41). This result was not confirmed in another study (46), and based on our results (Fig. 3), we conclude that the C-terminal domain of SLK may interact with the catalytic domain, but is probably not autoinhibitory. The present study indicates that in cells, SLK exists as a high-molecular-mass complex (Fig. 2), either as a dimer/oligomer or in tight association with another protein(s). The latter could potentially represent an inhibitory protein that could regulate the activity of SLK by association/dissociation. For example, one may consider the possibility that SLK binds to a scaffolding protein(s), which can assist in controlling the local concentration of SLK. There is a putative SH3 binding domain in the C-terminal region of SLK that may potentially serve as a docking site onto protein scaffolds (40). Upon ischemia-reperfusion injury, SLK could be released from protein scaffolds, which would then allow for enhanced local concentration and homodimerization via the C-terminal coiled-coil domain, leading to kinase activation. These possibilities will require further investigation, involving detailed analysis of the high-molecular-mass SLK complexes. An alternate mechanism may involve changes in SLK mRNA stability and protein expression, as shown to occur in renal ischemia-reperfusion injury or during kidney development (13). Under such conditions, increased expression of SLK would lead to a higher local concentration of SLK compared with the resting state. In turn, a higher local concentration may facilitate or strengthen homodimerization.
A better understanding of the regulation of SLK activity may be important for developing new therapeutic approaches to pathophysiological conditions involving SLK, such as ischemia-reperfusion injury, as well as wound healing, or tumor invasion. For example, it may be possible to develop pharmacological interventions directed at the homodimerization of SLK. Such drugs could target coiled-coil domain interactions and the associated van der Waals forces. Certain drugs, which could conceivably enhance homodimerization and proapoptotic effects could be applicable to suppressing tumor progression; conversely, inhibition of dimerization may be beneficial during healing from injury. In addition, the space in the dimer interface formed through activation segment exchange, close to the ATP binding site, can potentially be targeted by protein kinase inhibitors (26, 38). Finally, drugs could be designed to target the substrate binding site of SLK and inhibit downstream signaling. The modulation of tubular and glomerular cell apoptosis (16, 43) by blocking SLK activation may be a novel approach to ameliorate ischemia-reperfusion injury and acute renal failure.
GRANTS
This work was supported by Research Grants from the Canadian Institutes of Health Research (MOP-53264, MOP-84213), the Kidney Foundation of Canada, and the National Institutes of Health (R01-CA125436). A. V. Cybulsky was supported by the Catherine McLaughlin Hakim Chair.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Luigi Franchi for expert technical assistance and Dr. Louise Larose (McGill University) for helpful discussions.
REFERENCES
- 1. Amara JF, Clackson T, Rivera VM, Guo T, Keenan T, Natesan S, Pollock R, Yang W, Courage NL, Holt DA, Gilman M. A versatile synthetic dimerizer for the regulation of protein-protein interactions. Proc Natl Acad Sci USA 94: 10618–10623, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berger B, Wilson DB, Wolf E, Tonchev T, Milla M, Kim PS. Predicting coiled coils by use of pairwise residue correlations. Proc Natl Acad Sci USA 92: 8259–8263, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burakov AV, Zhapparova ON, Kovalenko OV, Zinovkina LA, Potekhina ES, Shanina NA, Weiss DG, Kuznetsov SA, Nadezhdina ES. Ste20-related protein kinase LOSK (SLK) controls microtubule radial array in interphase. Mol Biol Cell 19: 1952–1961, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaar Z, O'Reilly P, Gelman I, Sabourin LA. v-Src-dependent down-regulation of the Ste20-like kinase SLK by casein kinase II. J Biol Chem 281: 28193–28199, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Chang DW, Yang X. Activation of procaspases by FK506 binding protein-mediated oligomerization. Sci STKE 2003: PL1, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Cheng J, Zhang D, Kim K, Zhao Y, Su B. Mip1, an MEKK2-interacting protein, controls MEKK2 dimerization and activation. Mol Cell Biol 25: 5955–5964, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coers W, Reivinen J, Miettinen A, Huitema S, Vos JT, Salant DJ, Weening JJ. Characterization of a rat glomerular visceral epithelial cell line. Exp Nephrol 4: 184–192, 1996 [PubMed] [Google Scholar]
- 8. Crabtree GR, Schreiber SL. Three-part inventions: intracellular signaling and induced proximity. Trends Biochem Sci 21: 418–422, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Creasy CL, Ambrose DM, Chernoff J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J Biol Chem 271: 21049–21053, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Cybulsky AV, Takano T, Guillemette J, Papillon J, Volpini RA, Di Battista JA. The Ste20-like kinase SLK promotes p53 transactivation and apoptosis. Am J Physiol Renal Physiol 297: F971–F980, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Cybulsky AV, Takano T, Papillon J, Guillemette J, Herzenberg AM, Kennedy CR. Podocyte injury and albuminuria in mice with podocyte-specific overexpression of the Ste20-like kinase, SLK. Am J Pathol 177: 2290–2299, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cybulsky AV, Takano T, Papillon J, Hao W, Mancini A, Di Battista JA, Cybulsky MI. The 3′-untranslated region of the Ste20-like kinase SLK regulates SLK expression. Am J Physiol Renal Physiol 292: F845–F852, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Cybulsky AV, Takano T, Papillon J, Khadir A, Bijian K, Chien CC, Alpers CE, Rabb H. Renal expression and activity of the germinal center kinase SK2. Am J Physiol Renal Physiol 286: F16–F25, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol 11: 220–230, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Delpire E. The mammalian family of sterile 20p-like protein kinases. Pflügers Arch 458: 953–967, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Eggert S, Midthune B, Cottrell B, Koo EH. Induced dimerization of the amyloid precursor protein leads to decreased amyloid-beta protein production. J Biol Chem 284: 28943–28952, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ellinger-Ziegelbauer H, Karasuyama H, Yamada E, Tsujikawa K, Todokoro K, Nishida E. Ste20-like kinase (SLK), a regulatory kinase for polo-like kinase (Plk) during the G2/M transition in somatic cells. Genes Cells 5: 491–498, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Feig LA, Buchsbaum RJ. Cell signaling: life or death decisions of ras proteins. Curr Biol 12: R259–261, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Fielhaber JA, Han YS, Tan J, Xing S, Biggs CM, Joung KB, Kristof AS. Inactivation of mammalian target of rapamycin increases STAT1 nuclear content and transcriptional activity in alpha4- and protein phosphatase 2A-dependent fashion. J Biol Chem 284: 24341–24353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Graves JD, Gotoh Y, Draves KE, Ambrose D, Han DK, Wright M, Chernoff J, Clark EA, Krebs EG. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J 17: 2224–2234, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guilluy C, Rolli-Derkinderen M, Loufrani L, Bourge A, Henrion D, Sabourin L, Loirand G, Pacaud P. Ste20-related kinase SLK phosphorylates Ser188 of RhoA to induce vasodilation in response to angiotensin II type 2 receptor activation. Circ Res 102: 1265–1274, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Hao W, Takano T, Guillemette J, Papillon J, Ren G, Cybulsky AV. Induction of apoptosis by the Ste20-like kinase SLK, a germinal center kinase that activates apoptosis signal-regulating kinase and p38. J Biol Chem 281: 3075–3084, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med 361: 1570–1583, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem 281: 21256–21265, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Kufareva I, Abagyan R. Type-II kinase inhibitor docking, screening, and profiling using modified structures of active kinase states. J Med Chem 51: 7921–7932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol 15: 691–699, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81: 807–869, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Lee KK, Murakawa M, Nishida E, Tsubuki S, Kawashima S, Sakamaki K, Yonehara S. Proteolytic activation of MST/Krs, STE20-related protein kinase, by caspase during apoptosis. Oncogene 16: 3029–3037, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Lee SJ, Cobb MH, Goldsmith EJ. Crystal structure of domain-swapped STE20 OSR1 kinase domain. Protein Sci 18: 304–313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 141: 1117–1134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leung IW, Lassam N. Dimerization via tandem leucine zippers is essential for the activation of the mitogen-activated protein kinase kinase kinase, MLK-3. J Biol Chem 273: 32408–32415, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science 252: 1162–1164, 1991 [DOI] [PubMed] [Google Scholar]
- 34. Mandal M, Olson DJ, Sharma T, Vadlamudi RK, Kumar R. Butyric acid induces apoptosis by up-regulating Bax expression via stimulation of the c-Jun N-terminal kinase/activation protein-1 pathway in human colon cancer cells. Gastroenterology 120: 71–78, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80: 293–299, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Nolen B, Taylor S, Ghosh G. Regulation of protein kinases; controlling activity through activation segment conformation. Mol Cell 15: 661–675, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 10: 431–442, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Pike AC, Rellos P, Niesen FH, Turnbull A, Oliver AW, Parker SA, Turk BE, Pearl LH, Knapp S. Activation segment dimerization: a mechanism for kinase autophosphorylation of non-consensus sites. EMBO J 27: 704–714, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosenberg OS, Deindl S, Comolli LR, Hoelz A, Downing KH, Nairn AC, Kuriyan J. Oligomerization states of the association domain and the holoenzyme of Ca2+/CaM kinase II. FEBS J 273: 682–694, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Sabourin LA, Rudnicki MA. Induction of apoptosis by SLK, a Ste20-related kinase. Oncogene 18: 7566–7575, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Sabourin LA, Tamai K, Seale P, Wagner J, Rudnicki MA. Caspase 3 cleavage of the Ste20-related kinase SLK releases and activates an apoptosis-inducing kinase domain and an actin-disassembling region. Mol Cell Biol 20: 684–696, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thornborrow EC, Manfredi JJ. One mechanism for cell type-specific regulation of the bax promoter by the tumor suppressor p53 is dictated by the p53 response element. J Biol Chem 274: 33747–33756, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Wagner MC, Rhodes G, Wang E, Pruthi V, Arif E, Saleem MA, Wean SE, Garg P, Verma R, Holzman LB, Gattone V, Molitoris BA, Nihalani D. Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1. J Biol Chem 283: 35579–35589, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wagner S, Flood TA, O'Reilly P, Hume K, Sabourin LA. Association of the Ste20-like kinase (SLK) with the microtubule. Role in Rac1-mediated regulation of actin dynamics during cell adhesion and spreading. J Biol Chem 277: 37685–37692, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Wagner S, Storbeck CJ, Roovers K, Chaar ZY, Kolodziej P, McKay M, Sabourin LA. FAK/src-family dependent activation of the Ste20-like kinase SLK is required for microtubule-dependent focal adhesion turnover and cell migration. PLoS One 3: e1868, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamada E, Tsujikawa K, Itoh S, Kameda Y, Kohama Y, Yamamoto H. Molecular cloning and characterization of a novel human STE20-like kinase, hSLK. Biochim Biophys Acta 1495: 250–262, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Zhang YH, Hume K, Cadonic R, Thompson C, Hakim A, Staines W, Sabourin LA. Expression of the Ste20-like kinase SLK during embryonic development and in the murine adult central nervous system. Brain Res Dev Brain Res 139: 205–215, 2002 [DOI] [PubMed] [Google Scholar]