Abstract
Dysregulation of urinary sodium chloride (NaCl) excretion can result in extracellular fluid (ECF) volume expansion and hypertension. Recent studies demonstrated that urinary nucleotide excretion increases in mice ingesting a high-salt diet and that these increases in extracellular nucleotides can signal through P2Y2 receptors in the kidney collecting duct to inhibit epithelial Na+ channels (ENaC). However, under conditions of ECF volume expansion brought about by high-dietary salt intake, ENaC activity should already be suppressed. We hypothesized that alternative pathways exist by which extracellular nucleotides control renal NaCl excretion. We used an inner medullary collecting duct (mIMCD-K2) cell line in an Ussing chamber system as a model to study additional ion transport pathways that are regulated by extracellular nucleotides. When ENaC was inhibited, the addition of adenosine triphosphate (ATP) to the basal side of cell sheets activated both P2Y1 and P2Y2 receptors, inducing a transient increase in short-circuit current (Isc); addition of ATP to the apical side activated only P2Y2 receptors, inducing first a transient and then a sustained increase in Isc. The ATP-induced increases in Isc were blocked by pretreatment with a phospholipase C (PLC) inhibitor, a calcium (Ca2+) chelator, or Ca2+-activated Cl− channel (CACC) inhibitors, suggesting that ATP signals through both PLC and intracellular Ca2+ to activate CACC. We propose that P2Y1 and P2Y2 receptors operate in tandem in IMCD cells to provide an adaptive mechanism for enhancing urinary NaCl excretion in the setting of high-dietary NaCl intake.
Keywords: ATP
defects in the regulation of urinary sodium chloride (NaCl) excretion can lead to extracellular fluid (ECF) volume expansion and high blood pressure. The collecting duct (CD) of the kidney plays a critical role in fine-tuning NaCl balance and is subject to extensive hormonal regulation. For example, under conditions of ECF volume depletion, the mineralocorticoid hormone aldosterone can increase ECF volume by activating the epithelial Na+ channel (ENaC), which is the principal ion channel responsible for Na+ absorption in the kidney CD. While the regulation of ENaC-mediated Na+ transport is thought to be a major determinant of ECF volume and blood pressure, Cl− transport in the kidney CD may also contribute to the regulation of NaCl balance by a variety of pathways whose hormonal regulatory mechanisms are not well-established.
Extracellular nucleotides such as adenosine triphosphate (ATP) are becoming recognized as autocrine or paracrine hormones that can be released by renal epithelial cells in response to various environmental cues (9, 33, 36). Extracellular ATP activates plasma membrane-localized purinergic receptors belonging to one of two classes, the P2X ion channels and the P2Y guanine nucleotide-binding protein (G protein)-coupled receptors, either of which regulate a diverse set of physiological processes (9). Recent studies suggested that urinary nucleotides and the P2Y2 receptor system contribute to the regulation of NaCl homeostasis and blood pressure. High-NaCl feeding in mice stimulates an increase in urinary excretion of nucleotides, including UTP, ATP, and their hydrolytic products, which coincides with decreased ENaC activity in the cortical CD (26). ATP in the tubular fluid of the cortical CD activates P2Y2 receptors, which decrease the open probability and net activity of ENaC (25, 30). Studies of P2Y2 knockout mice further confirm that P2Y2 receptors participate in maintaining salt balance and blood pressure. P2Y2 knockout mice exhibit renal NaCl retention and hypertension (25, 30); moreover, these mice develop a greater level of salt-sensitive hypertension than do wild-type mice when exogenous mineralocorticoids are infused to maintain ENaC activity at high levels (26).
However, when wild-type mice (21) or humans (17, 23, 31) ingest a high-NaCl diet, ECF volume expands, leading to low levels of serum aldosterone and, as a consequence, diminished ENaC activity in the kidney CD. Thus, the observed increase in urinary nucleotide excretion in mice fed a high-NaCl diet suggests that ATP-mediated downregulation of ENaC may not be important when aldosterone levels are suppressed. We hypothesized that alternative pathways exist by which ATP induces urinary NaCl excretion in the inner medullary collecting duct (IMCD), a nephron segment that is known to secrete Cl− under conditions of high-NaCl intake (14, 35).
The purpose of our study was to characterize the specific P2Y receptor signaling pathways that control NaCl transport in the IMCD under conditions where ENaC activity is diminished. We used the mIMCD-K2 cell line as a model system to study the direct effects of nucleotides on NaCl transport across IMCD epithelia. The mIMCD-K2 cell line was originally derived from the initial segment of the IMCD and retains characteristic features of the IMCD, including the signaling and ion transport machinery that support ATP-stimulated Cl− secretion (22). Although previous studies demonstrated a role for ATP in either increasing intracellular calcium (Ca2+) concentration (4, 41) or stimulating Cl− secretion in IMCD cells (5, 22), the identity of the specific P2Y receptor subtypes involved in the integrated response to extracellular ATP has not been determined. Here, we demonstrate that ATP activates and signals through P2Y1 and P2Y2 receptors on the basal side, and P2Y2 receptors on the apical side of mIMCD-K2 cell sheets, to increase Cl− secretion through calcium-activated Cl− channels (CACC). ATP-stimulated transcellular Cl− secretion generates an electromotive force that also increases paracellular Na+ secretion, leading to net NaCl secretion. We propose that these two purinergic signaling pathways could represent another mechanism by which urinary nucleotides enhance urinary NaCl excretion under conditions of high-dietary NaCl intake.
METHODS
Cell culture.
The mIMCD-K2 cell line was kindly provided by Dr. Bruce Stanton (Dartmouth Medial School, Hanover, NH). Cells of passages 38–46 were expanded and plated on polycarbonate Snapwell inserts (Corning Costar), as described previously (28). Transepithelial voltage (Vte) and resistance (Rte) were measured with an EVOM “chopstick” voltmeter (World Precision Instruments, Sarasota, FL). Cells were in defined medium until Rte reached resistance values between 800 and 1,200 Ω·cm2 within 10–14 days.
Ussing chamber measurements.
Cell sheets were mounted between the Lucite half chambers of the Ussing chamber apparatus (Physiological Instruments, San Diego, CA) for electrophysiological studies, as described previously (27, 28). Cell sheets were bathed in Krebs-Henseleit solution (in mM: 140 NaCl, 25 NaHCO3, 5 KCl, 5 glucose, 2 CaCl2, and 1 MgCl2) and gassed with a mixture of 95% O2-5% CO2. Vte across cell sheets was clamped to 0 mV, and a set voltage pulse of 1 mV was applied across cell sheets for 200 ms every 20 s. The short-circuit current (Isc) and Rte across cell sheets were continuously recorded using Acquire and Analyze Software (Physiological Instruments).
Ion substitution experiments.
To establish the presence of Na+ secretion across cell sheets, Na+ substitution experiments were also performed, in which NaCl was replaced with N-methyl-d-glucamine chloride (NMDG-Cl) in the Krebs-Henseleit solution of the apical bath, while the concentrations of all other ions were kept constant. In these experiments, the basal bath solution contained 140 mM NaCl and 25 mM NaHCO3, whereas the apical bath solution contained 25 mM NaHCO3 and 140 mM NMDG-Cl, which created a Na+ concentration gradient in which the basal Na+ concentration was 6.6-fold higher than that in the apical bath. All Isc measurements were made after correcting for junction potentials created by the asymmetric bath solutions.
Pharmacological agents.
Cell sheets were exposed to a series of pharmacological agents once Isc and Rte stabilized in the Ussing chamber. The apical side of all cell sheets was treated with 10−5 M amiloride (Sigma, St. Louis, MO), an ENaC inhibitor. ATP (10−5 M) was then added sequentially to the apical and basal sides of cell sheets. Some cell sheets were exposed to the P2Y1 receptor agonist MRS2365 (10−6 M) or the P2Y2 receptor agonist thio-UTP (10−6 M). Other cell sheets were pretreated with the P2Y1 receptor antagonist MRS2500 (10−6 M) before addition of P2Y1 and P2Y2 receptor agonists. To rule out involvement of adenosine receptors in ATP-induced Isc (IscATP), the A2b receptor antagonist PSB603 (10−7 M) and the broad spectrum adenosine receptor antagonist DPCPX (10−4 M) were added to both sides of cell sheets, as described previously (28). All agonists and antagonists were obtained from Tocris Bioscience (Ellisville, MO).
To identify the classes of ion channels or transporters that respond to P2Y receptor activation, several small molecule inhibitors were used. CFTR inhibitor-172 (10−5 M, Sigma) was used to block cystic fibrosis transmembrane conductance regulator (CFTR) (20). Flufenamic acid (FFA; 10−3 M, Sigma) and niflumic acid (2 × 10−4 M, Sigma) were used to block CACC (13, 40) in mIMCD-K2 cells. FFA induced a small change in baseline Isc, which was attributed to a change in the pH of the bath solution. Diphenylamine-2-carboxylate (DPAC; 10−4 M, Sigma) was used to inhibit voltage-gated Cl− channels, and 4,4′-diisothiocyano-2, 2-stilbenedisulfonate (DIDS; 10−5 M, Sigma) was used as a broad spectrum inhibitor of Cl− channels and Cl−/HCO3− exchangers. Bumetanide (2 × 10−4 M, Sigma) was used to inhibit Na+-K+-2Cl− cotransporters, and acetazolamide (2 × 10−4 M, Sigma) was used to inhibit carbonic anhydrase. Barium chloride (BaCl2; 5 × 10−3 M, Sigma) was used as a broad spectrum K+ channel inhibitor.
To determine whether IscATP was dependent on phospholipase C (PLC) activity, cell sheets were pretreated for 10 min with U73122 (10−6 M, Tocris Bioscience). The involvement of intracellular Ca2+ in IscATP responses was analyzed by pretreating cell sheets for 15 min with the Ca2+ chelator BAPTA-AM (5 × 10−5 M). To determine whether IscATP depended on protein kinase A (PKA) activity, cell sheets were pretreated for 30 min with H-89 (10−5 M, Sigma). The involvement of protein kinase C (PKC) in IscATP responses was analyzed with GF109203X (10−6 M, Tocris Bioscience) and Go6983 (2 × 10−5 M, Tocris Bioscience).
Reverse transcriptase-PCR.
mIMCD-K2 cells were grown to resistance on permeable supports, and total RNA was harvested using an RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Mouse brain and kidney total RNA were obtained from Zyagen (San Diego, CA) to serve as positive controls. Reverse transcriptase (RT) reactions were performed according to the manufacturer's instructions (New England BioLabs, Ipswich, MA). Thermal cycling parameters were the following: incubation at 98°C for 30 s followed by 35 cycles at 98°C for 10 s, 50°C for 10 s, 72°C for 50 s. PCR primers were designed and used for detecting gene amplification of bestrophin-1 and TMEM16A (11, 24). Specificity of each set of primers was confirmed by BLAST search against GenBank. PCR products were resolved using a 1% agarose gel-dissolved Tris-Acetate EDTA buffer and visualized with ethidium bromide.
Western blot analysis.
Seventy micrograms of protein from mIMCD-K2 lysates were resolved by 10% SDS-PAGE and transferred to PVDF membranes (Bio-Rad, Hercules, CA). The membranes were immunostained with rabbit anti-human P2Y1 receptor antibody (1.5 μg/ml, Sigma) or rabbit anti-rat P2Y2 receptor antibody (4 μg/ml, Alomone Labs, Jerusalem, Israel) for detection of P2Y1 and P2Y2 receptor-immunoreactive protein, respectively. Membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences, Piscataway, NH) and processed as described previously (28).
The specificity of the anti-P2Y1 receptor antibody was previously tested against whole cell lysates from the P2Y1 receptor knockout mouse (34). The specificity of the anti-P2Y2 receptor antibody was tested by preabsorbing the antibody for 1 h with its corresponding antigen peptide at a 1:1 ratio. The membranes were then incubated with preabsorbed antigen-antibody mixture (4 μg/ml).
Statistics.
Statistical analyses for comparisons between different treatment groups of mIMCD-K2 cells were performed using paired or unpaired two-tailed Student t-tests. Differences were considered to be significant at P < 0.05.
RESULTS
ATP induces anion secretion through CACC.
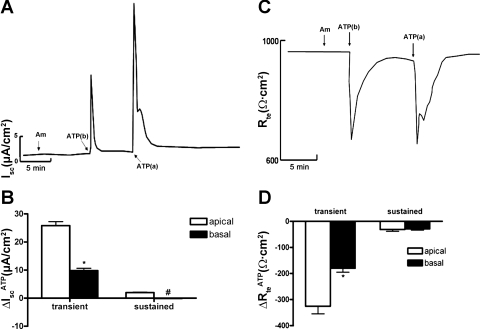
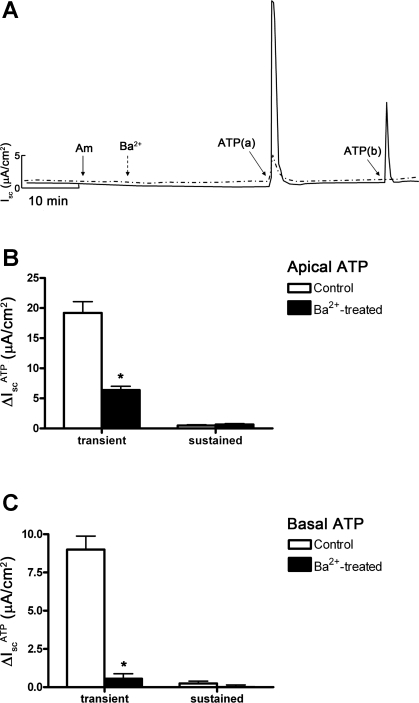
Figure 1, A and B, shows that addition of ATP (10−5 M) to the basal side of mIMCD-K2 cell sheets induced a transient increase in Isc (9.8 ± 5.4 μA/cm2) without a sustained increase in Isc, while addition of ATP (10−5 M) to the apical side caused a sharp, transient increase in Isc (25.8 ± 1.4 μA/cm2), and within 2 min, this transient current transitioned to a sustained Isc (1.98 ± 0.15 μA/cm2). Addition of ATP to either side of cell sheets also stimulated a parallel decrease in Rte, with a time course that mirrored the ATP-induced increase Isc (Fig. 1, C and D). Basal addition of ATP induced a transient decrease in Rte (−180.23 ± 4.97 Ω·cm2), while apical addition of ATP induced a larger transient decrease in Rte (−325.8 ± 29.1 Ω·cm2).
Fig. 1.
Sidedness of ATP-induced short-circuit current (Isc) in inner medullary collecting duct (mIMCD)-K2 cells. A: representative trace of Isc; Am indicates 10−5 M amiloride added to the apical bath; ATP(b) indicates 10−5 M ATP added to the basal side; ATP(a) indicates 10−5 M ATP added to the apical side. B: average Isc values in response to ATP addition (ΔIscATP) to the apical (open bars) or the basal (closed bars) sides. IscATP are categorized as transient or subsequent sustained responses. Values are represented as means ± SE (n = 42 filters). *, #Values significantly different from those induced by ATP added to the opposite side. C: representative trace of transepithelial resistance (Rte); Am indicates 10−5 M amiloride added to the apical bath; ATP(b) indicates 10−5 M ATP added to the basal side; ATP(a) indicates 10−5 M ATP added to the apical side. D: average changes in Rte values in response to ATP addition (ΔRteATP) to the apical (open bars) or the basal (closed bars) sides. ΔRteATP are categorized as transient or subsequent sustained responses. Values are represented as means ± SE (n = 48 filters). *Value significantly different from that induced by ATP added to the opposite side.
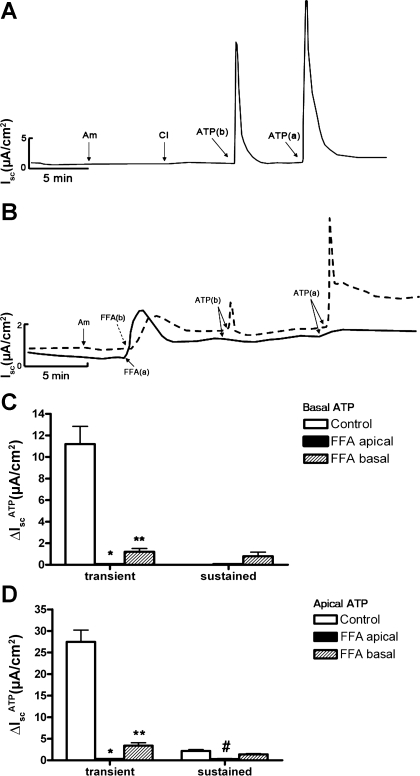
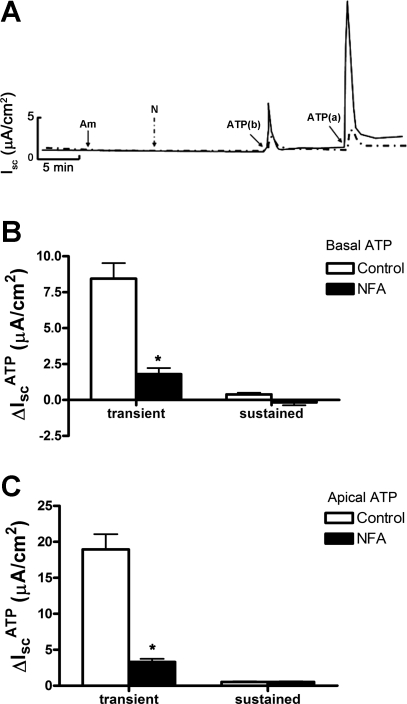
Since amiloride was added to the apical bath solution to inhibit ENaC-mediated Na+ absorption, the observed changes in IscATP likely involved a change in electrogenic anion secretion. To identify the ion channels involved in ATP-induced anion secretion, we pretreated cell sheets with a series of small molecular inhibitors. CFTR has been implicated as an ion channel that mediates apical Cl− secretion in mIMCD-K2 cells (18, 28, 38); however, apical addition of CFTR inhibitor-172, a selective inhibitor of CFTR, did not influence IscATP (Fig. 2A). Similarly, apical addition of DPAC (10−4 M, Sigma), an inhibitor of voltage-gated Cl− channels, did not influence IscATP (not shown). In contrast, addition of FFA (10−3 M), an inhibitor of CACC (13, 40), to the basal side of cell sheets significantly reduced the transient IscATP elicited by basal addition of ATP, and it diminished, but did not completely block, the transient and sustained IscATP elicited by apical addition of ATP (Fig. 2, B-D). Addition of FFA to the apical side of cell sheets completely blocked IscATP responses elicited by ATP addition to either side of cell sheets (Fig. 2, B-D). Similar results were observed with addition of niflumic acid (2 × 10−4 M), another inhibitor of CACC, to either side of cell sheets before ATP addition (Fig. 3).
Fig. 2.
Effects of cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor-172 and flufenamic acid (FFA) on ATP-induced Isc in mIMCD-K2 cells. A: representative trace of Isc in response to sequential addition of amiloride (Am; apical addition at 10−5 M), CFTR inhibitor-172 (CI; apical addition at 10−5 M), and basal or apical addition of ATP (10−5 M). Pretreatment with CFTR inhibitor-172 did not have a significant effect on ATP-induced Isc. B: superimposed representative traces of Isc responses to basal and apical addition of 10−5 M ATP following treatment with 10−3 M FFA added to either the basal (dashed trace) or the apical (solid trace) bath. Am, 10−5 M amiloride (apical addition); FFA(b), 10−3 M FFA added to the basal bath; FFA(a), 10−3 M FFA added to the apical bath; ATP(b), 10−5 M ATP added to the basal bath; ATP(a), 10−5 M ATP added to the apical bath. C: average Isc values in response to basal addition of ATP (ΔIscATP) to control cells (open bars), cells pretreated with FFA added to the apical side (closed bars), and cells pretreated with FFA added to the basal side (dashed bars). Values are represented as means ± SE (n = 11 filters). D: average Isc values in response to apical addition of ATP to control cells (open bars), cells pretreated with FFA added to the apical side (closed bars), and cells pretreated with FFA added to the basal side (dashed bars). Values are represented as means ± SE (n = 14 filters). *, **, #Values significantly different from control values.
Fig. 3.
Effect of niflumic acid (NFA) on ATP-induced Isc in mIMCD-K2 cells. A: superimposed representative traces of Isc responses to basal and apical addition of 10−5 M ATP following treatment with control (solid trace) or with NFA (dashed traces) added to cell sheets. Am, apical addition at 10−5 M amiloride; N, 2 × 10−4 M NFA (added to both sides); ATP(b) and ATP(a) indicate the addition of ATP added to basal and apical sides, respectively. B: average Isc values in response to basal addition of ATP (IscATP) in control cells (open bars) or in cells pretreated with NFA (closed bars). Isc responses are categorized as transient or subsequent sustained responses. Values are means ± SE (n = 12 filters). C: average Isc values in response to apical addition of ATP in control cells (open bars) or in cells pretreated with NFA (closed bars). Values are means ± SE (n = 12 filters). *Values significantly different from those in control cell sheets.
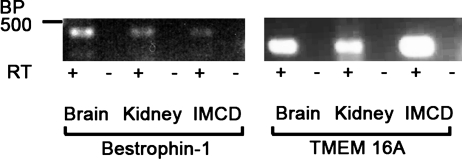
To begin the process of determining the molecular identities of CACC expressed in mIMCD-K2 cells, we performed RT-PCR to evaluate mRNA expression of bestrophin-1 and TMEM16A, which have been implicated as candidates responsible for CACC currents (19). We used a PCR-based approach because we anticipated that expression of these genes in mIMCD-K2 cells would be low. Mouse brain and kidney cDNA were run in parallel as positive controls. We detected both bestrophin-1 and TMEM16A mRNA expression in mouse brain, kidney, and mIMCD-K2 cells (Fig. 4), indicating that mIMCD-K2 cells express two gene products that may mediate CACC activity.
Fig. 4.
Expression of bestrophin-1 and TMEM16A mRNA in mIMCD-K2 cells. Photograph of gel showing PCR amplification products in mouse brain and kidney tissue (positive controls) and mIMCD-K2 cells (IMCD) after reverse transcription of mouse bestrophin-1 and TMEM16A mRNA. Samples containing reverse transcriptase (RT; +) or negative controls lacking RT (−) are included for each tissue/primer combination. BP 500 indicates a marker of 500 base pairs.
To evaluate the ion transporters and channels that are required for basolateral Cl− uptake during the ATP response, we pretreated mIMCD-K2 cells with bumetanide (Na+-K+-2Cl− cotransporter inhibitor), DIDS (Cl−/HCO3− exchanger and broad spectrum Cl− channel inhibitor), acetazolamide (carbonic anyhydrase inhibitor), and BaCl2 (broad spectrum K+ channel inhibitor) before ATP addition. Basal application of bumetanide, DIDS, and acetazolamide did not inhibit Cl− secretion (not shown), indicating that bumetanide-sensitive Na+-K+-2Cl− cotransporters and Cl−/HCO3− exchangers are not likely to be involved in ATP-induced basolateral Cl− uptake in mIMCD-K2 cells. However, basal application of BaCl2 (5 × 10−3 M) significantly inhibited IscATP elicited by either apical or basal addition of ATP (Fig. 5), suggesting that a basolateral K+ channel is responsible for recycling K+ to the extracellular compartment to promote Cl− secretion and uptake during the ATP response.
Fig. 5.
Basal addition of BaCl2 to mIMCD-K2 cells inhibits ATP-induced Isc. A: superimposed representative traces of Isc responses to apical and basal addition of 10−5 M ATP following treatment with control (solid trace) or 5 × 10−3 M BaCl2 (dashed trace). Am, 10−5 M amiloride (apical addition); Ba2+, 5 × 10−3 M BaCl2 (basal addition); ATP(a) and ATP(b) indicate 10−5 M ATP added to the apical and basal baths, respectively. B: average Isc values in response to apical addition of ATP (ΔIscATP) in control cells (open bars) or in cells pretreated with BaCl2 to the basal side (closed bars). Isc responses are categorized as transient or subsequent sustained responses. Values are means ± SE (n = 12 filters). C: average Isc values in response to basal addition of ATP in control filters (open bars) or in filters pretreated with BaCl2 to the basal side (closed bars). Values are means ± SE (n = 12 filters). *Values significantly different from those in control cell sheets.
ATP does not directly influence paracellular Na+ transport.
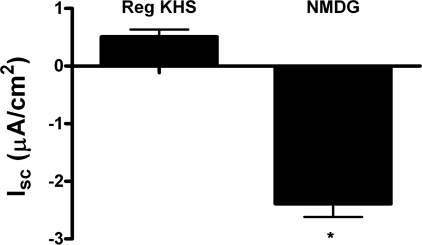
For transcellular Cl− secretion to persist, there must also be a parallel pathway for Na+ secretion to offset the electrochemical gradient generated by Cl− secretion. Under conditions where ENaC is downregulated, ATP-induced Cl− secretion may drive paracellular Na+ secretion. To establish the presence of paracellular Na+ transport, we performed Na+ substitution experiments, in which some of the Na+ was replaced with NMDG+ in the apical bath solution so that the Na+ concentration in the basal bath was 6.6-fold greater than that in the apical bath. Figure 6 shows that, in the presence of amiloride, Isc was significantly lower in mIMCD-K2 cells bathed in asymmetric solutions (Isc 0.51 ± 0.23 μA/cm2 for control apical bath solution compared with Isc −2.39 ± 0.23 μA/cm2 for NMDG-containing apical bath solution), suggesting that Na+ can be secreted through a paracellular pathway to decrease net Isc. We next repeated these Na+ substitution experiments in the presence of amiloride and FFA, to block transcellular NaCl transport, and then stimulated mIMCD-K2 cells with apical or basal ATP. Under these conditions, we observed no significant changes in Isc or Rte in response to apical (ΔIsc = 0.26 ± 0.16 μA/cm2; ΔRte = −0.21 ± 20 Ω·cm2) or basal (ΔIsc = −1.06 ± 0.17 μA/cm2; ΔRte = −38 ± 31 Ω·cm2) addition of ATP, indicating that ATP does not directly influence paracellular Na+ transport.
Fig. 6.
Effect of substitution of Na+ with NMDG+ in the apical bath solution on basal Isc in mIMCD-K2 cells. NaCl was replaced with NMDG-Cl in the apical bath solution so that a basal-to-apical Na+ concentration gradient of 6.6:1 was established. The concentrations of all other ions in the apical and basal bath solutions were kept constant. Isc measurements were made after accounting for tight junction potentials created by the asymmetric bath solutions and after addition of 10−5 M amiloride to the apical bath. Average Isc values in cell sheets in control apical bath solution (Reg KHS) and in bath solution with Na+ substitution (NMDG). Values are represented as means ± SE (n = 12 filters). *Value significantly different from control value.
P2Y1 and P2Y2 receptors are expressed in mIMCD-K2 cells.
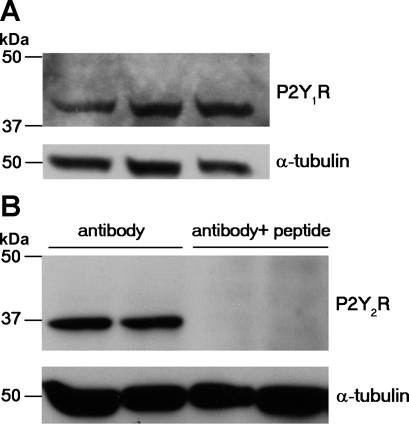
Although mIMCD-K2 cells are known to express P2Y1 and P2Y2 receptor mRNA (22), P2Y1 and P2Y2 receptor protein expression has not been demonstrated. Western blotting of mIMCD-K2 cell lysates with a previously characterized P2Y1 antibody (34) detected an immunoreactive band of 42 kDa, which matched the predicted size of mouse P2Y1 receptor protein (Fig. 7A). Western blotting of mIMCD-K2 cell lysates with an anti-P2Y2 receptor antibody showed an immunoreactive band of ∼36 kDa, which is slightly lower than the predicted molecular weight of 42 kDa. Anti-P2Y2 receptor antibody preincubated with blocking peptide, however, did not recognize this 36-kDa band, suggesting that this band represents P2Y2-immunoreactive protein (Fig. 7B).
Fig. 7.
P2Y1 and P2Y2 receptor protein expression by Western immunoblot in mIMCD-K2 cells. A: representative immunoblot showing whole cell lysates from 3 different passages of mIMCD-K2 cells probed with an anti-P2Y1 receptor (P2Y1R) antibody. Blots were also probed with an anti-α-tubulin antibody to assess protein loading. B: representative immunoblot showing whole cell lysates from 2 different passages incubated with either P2Y2 receptor (P2Y2R) antibody or P2Y2 receptor antibody preadsorbed with immunizing peptide.
P2Y1 and P2Y2 receptor activation stimulates Cl− secretion.
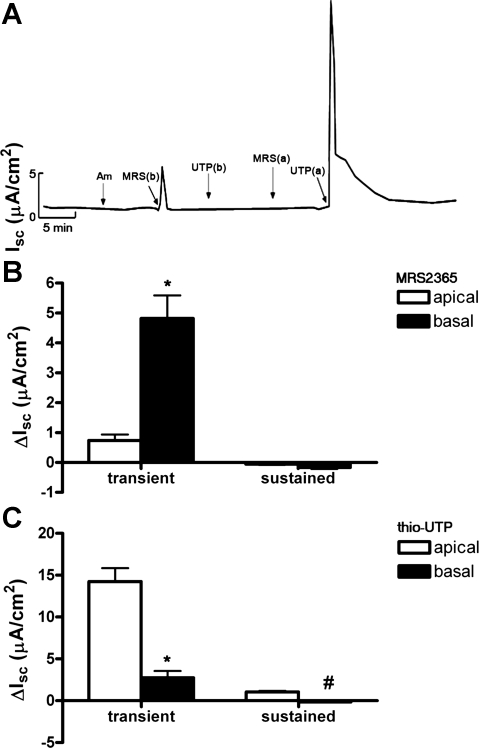
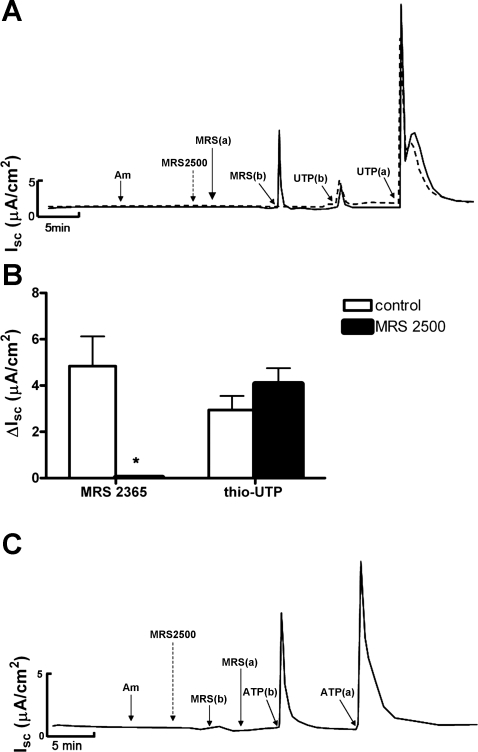
Since mIMCD-K2 cells express P2Y1 and P2Y2 receptors, we reasoned that ATP would stimulate Cl− secretion through activation of these receptors. To verify P2Y1 and P2Y2 receptor activation is responsible for IscATP, we used specific agonists, MRS2365 and thio-UTP, to activate P2Y1 and P2Y2 receptors, respectively. Basal addition of MRS2365 (10−6 M) to cell sheets caused a transient increase in Isc (4.8 ± 0.77 μA/cm2) without a subsequent sustained change in Isc (Fig. 8). Apical addition of MRS2365 induced a small, transient increase in Isc (0.7 ± 0.2 μA/cm2; Fig. 8).
Fig. 8.
Effects of the P2Y1 receptor agonist MRS2365 and the P2Y2 receptor agonist thio-UTP on Isc in mIMCD-K2 cells. A: representative trace of Isc in response to sequential addition of the following: Am, 10−5 M amiloride (apical addition); MRS(b) and UTP(b), basal addition of MRS2365 (10−6 M) and thio-UTP (10−6 M), respectively; MRS(a) and UTP(a), apical addition of MRS2365 and thio-UTP, respectively. B: average Isc values after addition of MRS2365 (ΔIsc) to the apical side (open bars) or the basal side (closed bars) of cell sheets. Isc responses are categorized as transient or subsequent sustained responses. Values are represented as means ± SE (n = 14 filters). C: average Isc values after addition of thio-UTP to the apical side (open bars) or the basal side (closed bars). Values are means ± SE (n = 14 filters). *, #Values that are significantly different from those induced by the same agonist added to the opposite side.
Basal addition of the specific P2Y2 receptor agonist thio-UTP (10−6 M) to cell sheets induced a small, transient increase in Isc (2.7 ± 0.8 μA/cm2), which is not apparent in the representative trace in Fig. 8A. Similar to the effect of apical addition of ATP on Isc, apical addition of thio-UTP induced a large, transient increase in Isc (14.24 ± 1.6 μA/cm2) that transitioned to a sustained Isc (1.05 ± 0.12 μA/cm2; Fig. 8).
To further confirm the involvement of P2Y1 receptors in IscATP, we incubated cell sheets with the P2Y1 receptor antagonist MRS2500 before the addition of MRS2365. Pretreatment of both sides of cell sheets with MRS2500 (10−6 M) completely blocked the stimulatory effect of basal addition of P2Y1 receptor agonist MRS2365 on Isc (Fig. 9), confirming that P2Y1 receptor activation by MRS2365 at the basal side of cell sheets enhances Isc. In contrast, pretreatment of cell sheets with MRS2500 did not influence the stimulatory effects of basal or apical addition of P2Y2 receptor agonist thio-UTP on Isc (Fig. 9). Pretreatment with MRS2500 also did not have any effect on the Isc response to basal ATP addition (Fig. 9C), indicating that P2Y2-mediated stimulation of IscATP can occur despite P2Y1 receptor blockade.
Fig. 9.
Effects of the P2Y1 receptor antagonist MRS2500 on MRS2365-, thio-UTP-, or ATP-induced Isc in mIMCD-K2 cells. A: superimposed representative traces of Isc responses to the addition of the P2Y1 agonist MRS2365 or the P2Y2 agonist thio-UTP following treatment with control (solid trace) or with 10−6 M P2Y1 receptor antagonist MRS2500 (dashed trace) added to both sides of cell sheets. Am, 10−5 M amiloride; MRS2500, 10−6 M MRS2500; MRS(a) and MRS(b) indicate addition of 10−6 M P2Y1 agonist MRS2365 to the apical and basal sides, respectively. UTP(b) and UTP(a) indicate the addition of 10−6 M P2Y2 agonist thio-UTP to the basal and apical sides, respectively. B: average Isc values in the transient response to basal addition of either MRS2365 (ΔIsc) or thio-UTP in control cells (open bars) or in cells pretreated with MRS2500 (closed bars). Values are means ± SE (n = 9 filters). *Value significantly different from control value. C: representative trace of Isc showing that pretreatment of both sides of cell sheets with the P2Y1 antagonist MRS2500 (10−6 M) blocked the stimulatory effects of the P2Y1 agonist MRS2365 (10−6 M) added to the basal side of cell sheets [MRS(b)]. MRS(a) indicates MRS2365 (10−6 M) added to the apical side. Pretreatment of MRS2500 did not affect the stimulatory effects of ATP on Isc when added to either the basal [ATP(b)] or apical [ATP(a)] sides of cell sheets (n = 6 filters).
ATP signals through phospholipase C and intracellular calcium.
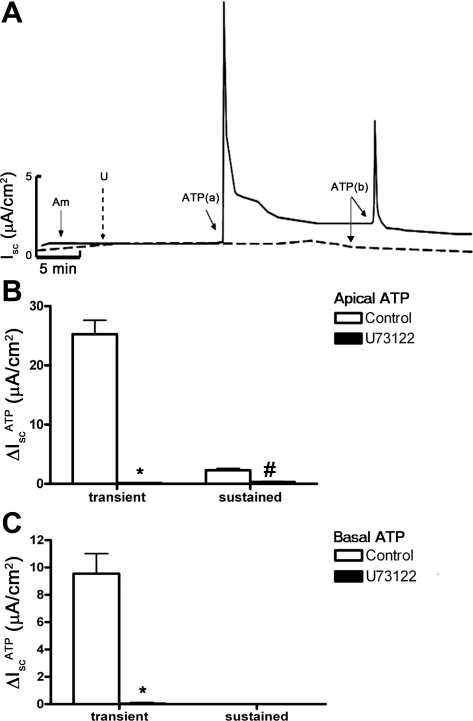
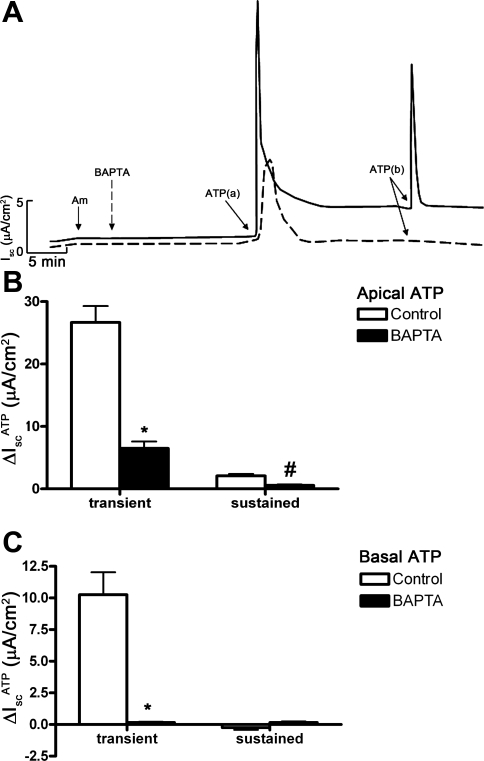
P2Y receptors are linked to G proteins that activate PLC enzyme activity (8, 9), which can lead to activation of downstream pathways involving PKC and intracellular Ca2+. To further characterize the signaling pathways involved in IscATP, we treated mIMCD-K2 cells with the PLC inhibitor U73122 before the addition of ATP. Pretreatment of cell sheets with U73122 (10−6 M) completely abolished the IscATP response elicited from either side of cell sheets (Fig. 10), confirming that ATP action on ion transport in mIMCD-K2 cells involves stimulation of PLC activity. We also used PKC inhibitors GF109203X (10−5 M) and Go6983 (2 × 10−5 M) to evaluate whether PKC is involved in IscATP, but these inhibitors did not alter IscATP responses (not shown). To confirm whether IscATP involves Ca2+ signaling pathways and CACC-mediated Cl− secretion, we used the Ca2+ chelator BAPTA-AM to decrease the intracellular Ca2+ concentration in mIMCD-K2 cells. Pretreatment of cell sheets with BAPTA-AM (5 × 10−5 M) significantly diminished IscATP responses elicited by either apical (transient response 6.48 ± 1.10, and sustained response 0.57 ± 0.13 μA/cm2) or basal (no response) ATP addition to cell sheets (Fig. 11).
Fig. 10.
Phospholipase C (PLC) inhibitor U73122 inhibits ATP-induced Isc in mIMCD-K2 cells. A: superimposed representative traces of Isc responses to apical and basal addition of 10−5 M ATP following treatment with control (solid trace) or 10−6 M U73122 (dashed trace). Am, 10−5 M amiloride (apical addition); U, U73122; ATP(a) and ATP(b) indicate 10−5 M ATP added to the apical and basal baths, respectively. B: average Isc values in response to apical addition of ATP (10−5 M) in control cells (open bars) or in cells pretreated with U73122 on both sides (closed bars). Isc responses are categorized as transient or subsequent sustained responses. Values are means ± SE (n = 14 filters). C: average Isc values in response to basal addition of ATP (10−5 M) in control filters (open bars) or in filters pretreated with U73122 (closed bars). Values are means ± SE (n = 14 filters). *, #Values significantly different from those in control cell sheets.
Fig. 11.
BAPTA-AM inhibits ATP-induced Isc in mIMCD-K2 cells. A: superimposed representative traces of Isc responses to apical and basal addition of 10−5 M ATP following treatment with control (solid trace) or BAPTA-AM (dashed trace). Am, 10−5 M amiloride (apical addition); BAPTA, 5 × 10−5 M BAPTA-AM (added to both sides); ATP(a) and ATP(b) indicate 10−5 M ATP added to the apical and basal baths, respectively. B: average Isc values in response to apical addition of ATP (ΔIscATP) in control filters (open bars) or in filters pretreated with BAPTA-AM (closed bars). Isc responses are categorized as transient or subsequent sustained responses. Values are means ± SE (n = 12 filters). C: average Isc values in response to basal addition of ATP in control filters (open bars) or in filters pretreated with BAPTA-AM (closed bars). Values are means ± SE (n = 12 filters). *, #Values significantly different from those in control cell sheets.
We recently showed that adenosine stimulates Cl− secretion though CFTR in mIMCD-K2 cells by activating apical adenosine A2b receptors and signaling through cAMP/PKA (28). Since the magnitude of the sustained IscATP was similar to that of adenosine-stimulated Isc (Iscadenosine), we used a series of small molecule compounds to determine whether IscATP is distinct from Iscadenosine. Addition of PSB603 (adenosine A2b receptor inhibitor), DPCPX (broad spectrum adenosine receptor inhibitor), and H-89 (PKA inhibitor) had no effects on the transient or sustained IscATP elicited by ATP addition to cell sheets (not shown), indicating that adenosine receptor signaling pathways are not involved in the sustained IscATP response.
DISCUSSION
In this study, we used the mIMCD-K2 cell line to examine direct effects of extracellular nucleotides on NaCl transport across IMCD epithelia. Our data show that ATP stimulates CACC in mIMCD-K2 cells by activating both P2Y1 and P2Y2 receptor pathways, which are localized on different sides of polarized epithelia and signal through PLC and intracellular Ca2+. Our experiments further demonstrate that ATP stimulates Cl− secretion in the presence of amiloride, indicating that ATP plays an important role in regulating ion transport under conditions of minimal ENaC activity. While ATP stimulates Cl− secretion under these conditions, ATP does not directly stimulate paracellular Na+ secretion, but rather it secondarily drives paracellular Na+ transport by inducing transcellular Cl− secretion.
The molecular identity of the proteins responsible for CACC currents in mIMCD-K2 cells (4, 5), as well as in other cells and tissues (15), is currently unresolved. However, emerging studies implicated the bestrophin and TMEM16 family of proteins as candidates that recapitulate many of the properties of CACC currents (3, 6, 32, 42). Using a PCR-based approach, we detected bestrophin-1 and TMEM16A mRNA in mIMCD-K2 cells. It is not presently clear, however, whether bestrophin-1, TMEM16A, or both proteins might be responsible for CACC activity in these cells. The expression studies presented here should provide guidance for future studies regarding the molecular identity of the proteins responsible for ATP-regulated CACC currents in mIMCD-K2 cells.
McCoy et al. (22) originally demonstrated that extracellular nucleotides stimulate Cl− secretion in mIMCD-K2 cells and they suggested, based on mRNA expression studies, that P2Y1 and P2Y2 receptors regulate Cl− secretion. Using a panel of purinergic receptor agonists and antagonists, we confirmed this hypothesis and showed further that P2Y1 and P2Y2 receptors regulate Cl− secretion through activation of CACC. Although P2Y receptor activation stimulated CACC-mediated Cl− secretion from either side of IMCD epithelia, the Isc response profiles differed depending on which side was stimulated. Apical addition of ATP or UTP, but not the P2Y1 agonist MRS2365, caused transient increases in Isc and subsequent sustained increases in Isc, while basal addition of ATP, UTP, or MRS2365 induced isolated, transient increases in Isc. The mechanisms responsible for separate Isc response profiles that vary according to sidedness (apical vs. basal) rather than receptor subtype (P2Y1 vs. P2Y2) remain to be determined.
We also found that ATP-induced basolateral Cl− uptake was not sensitive to bumetanide, which has also been described in mIMCD-K2 cells as well as in primary cultures of the rat IMCD cells (16, 18). Additional studies will be required to more fully characterize the molecular mechanisms controlling basolateral Cl− uptake in mIMCD-K2 cells. Interestingly, the mechanisms involved in ATP-induced basolateral Cl− uptake appear to be distinct from those involved in cAMP or vasopressin-induced Cl− uptake via bumetanide-sensitive Na+-K+-2Cl− cotransporters and Cl−/HCO3− exchangers (18). The mechanisms involved in ATP-induced apical Cl− exit via CACC are also different from those involved in vasopressin-induced Cl− exit via CFTR (18, 38). Collectively, these findings suggest that a variety of hormones can regulate signal transduction and Cl− transport pathways in a context-specific manner in mIMCD-K2 cells.
To our knowledge, this is the first demonstration of P2Y1 receptor-mediated stimulation of Cl− secretion in a kidney model system. Previous studies described P2Y1 mRNA expression in mIMCD-K2 cells (22) and implicated P2Y1 receptors in Ca2+ signaling pathways in the outer medullary CD (7). In the few studies that delineated P2Y1 expression in the kidney, P2Y1 receptor expression has been localized only to basolateral surfaces (1). This finding is congruent with our data in mIMCD-K2 cells that activation of basal P2Y1 receptors stimulates Cl− secretion. Bailey et al. (1, 2) demonstrated that P2Y1 and P2Y2 receptors are present together in cells from microdissected rat CD and suggested that both receptors share a common pool of intracellular Ca2+. Our findings similarly suggest that both P2Y1 and P2Y2 receptor pathways signal through PLC to increase intracellular Ca2+ concentration in IMCD cells, thus activating CACC and increasing Cl− secretion. Given that ATP and ADP content in rat kidney tissue slices have been observed to increase under conditions of high-dietary salt intake (12), the presence of P2Y1 receptors on the basolateral surface of IMCD cells may constitute a potential mechanism by which ATP and by-products such as ADP stimulate Cl− secretion as an adaptive response to enhance urine NaCl excretion.
Although P2Y2 receptors are known to mediate the stimulatory effects of extracellular ATP on CACC-mediated Cl− secretion in cortical CD cells (10), the physiological role of Cl− secretion in the cortical CD is not clear. In fact, the only nephron segment that has been implicated as an in vivo site for Cl− secretion by the kidney is the IMCD. Wallace et al. (39) microperfused isolated rat IMCD segments and demonstrated fluid secretion that was coupled to active NaCl secretion, with the highest rate of fluid secretion in the initial segment of the IMCD, the same segment from which mIMCD-K2 cells are derived. Sonnenberg (35, 39) used retrograde microcatheterization to show that rat IMCD has the capacity to secrete up to 10% of the filtered load of NaCl when ECF volume is expanded. While the capacity for NaCl secretion by the IMCD may appear limited, changes in net tubular excretion in the IMCD as low as 0.055% of the filtered NaCl load can be sufficient to cause significant changes in ECF volume over time (14). Thus, in states of NaCl excess, Cl− secretion pathways in IMCD, including P2Y receptor pathways identified here, could play an important role in the daily regulation of urinary NaCl excretion.
Recently, we demonstrated that extracellular adenosine stimulates Cl− secretion through CFTR in mIMCD-K2 cells by activating apical A2b receptors and signaling through the cAMP/PKA pathway (28). Adenosine is derived from ATP through the action of ecto-nucleotidases, which are abundantly expressed at the apical membrane surface of kidney epithelial cells (37). The action of both extracellular nucleosides and nucleotides, signaling through separate but parallel Cl− channel pathways in the IMCD, may represent a membrane signaling network that can fine-tune the regulation of urinary NaCl excretion.
In conclusion, the integrated control by the kidney of NaCl retaining and excreting systems determines NaCl balance, ECF volume, and blood pressure. Under conditions of high-dietary salt intake in mice, urinary nucleotide concentration increases; we propose that extracellular nucleotides may activate both P2Y1 and P2Y2 receptor pathways in IMCD cells, stimulating both sustained and repeated bursts of Cl− secretion through CACC channels as an alternative adaptive mechanism for enhancing urinary NaCl excretion. The activation of these signaling pathways would become particularly important under states of ECF volume expansion, when levels of serum aldosterone are low and ENaC activity is suppressed. Defects in this pathway could contribute to the pathogenesis of salt-sensitive hypertension because inappropriate control of renal salt handling could result in expansion of ECF volume and elevated blood pressure.
GRANTS
This work was supported by grants from the National Institutes of Health (K08-DK-073487 to A. C. Pao and T32-DK07357-26A1 to P. P. Kathpalia), Amgen (2009 Amgen Nephrology Junior Faculty Research Support Program to A. C. Pao), Satellite Healthcare (2008 Norman S. Coplon Extramural Grant to A. C. Pao), the Forest Research Institute (2009 Emerging Leaders in Hypertension Program to P. P. Kathpalia), and Stanford University (Dean's Fellowship Award to S. V. Thomas).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We are grateful to Dr. Bruce Stanton for providing mIMCD-K2 cells for the study. We thank Drs. Carol A. Charlton and Vivek Bhalla for valuable discussions and helpful comments on the manuscript.
REFERENCES
- 1. Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Srai K, Burnstock G, Unwin RJ. Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int 58: 1893–1901, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Bailey MA, Shirley DG. Effects of extracellular nucleotides on renal tubular solute transport. Purinergic Signal 5: 473–480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barro Soria R, Spitzner M, Schreiber R, Kunzelmann K. Bestrophin-1 enables Ca2+-activated Cl− conductance in epithelia. J Biol Chem 284: 29405–29412, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boese SH, Aziz O, Simmons NL, Gray MA. Kinetics and regulation of a Ca2+-activated Cl− conductance in mouse renal inner medullary collecting duct cells. Am J Physiol Renal Physiol 286: F682–F692, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Boese SH, Glanville M, Aziz O, Gray MA, Simmons NL. Ca2+ and cAMP-activated Cl− conductances mediate Cl− secretion in a mouse renal inner medullary collecting duct cell line. J Physiol 523: 325–338, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Cha SH, Sekine T, Endou H. P2 purinoceptor localization along rat nephron and evidence suggesting existence of subtypes P2Y1 and P2Y2. Am J Physiol Renal Physiol 274: F1006–F1014, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Communi D, Janssens R, Suarez-Huerta N, Robaye B, Boeynaems JM. Advances in signalling by extracellular nucleotides. The role and transduction mechanisms of P2Y receptors. Cell Signal 12: 351–360, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal 3: re1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cuffe JE, Bielfeld-Ackermann A, Thomas J, Leipziger J, Korbmacher C. ATP stimulates Cl− secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells. J Physiol 524: 77–90, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis AJ, Forrest AS, Jepps TA, Valencik ML, Wiwchar M, Singer CA, Sones WR, Greenwood IA, Leblanc N. Expression profile and protein translation of TMEM16A in murine smooth muscle. Am J Physiol Cell Physiol 299: C948–C959, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dobrowolski L, Walkowska A, Kompanowska-Jezierska E, Kuczeriszka M, Sadowski J. Effects of ATP on rat renal haemodynamics and excretion: role of sodium intake, nitric oxide and cytochrome P450. Acta Physiol (Oxf) 189: 77–85, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Fischer H, Illek B, Finkbeiner WE, Widdicombe JH. Basolateral Cl channels in primary airway epithelial cultures. Am J Physiol Lung Cell Mol Physiol 292: L1432–L1443, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Grantham JJ, Wallace DP. Return of the secretory kidney. Am J Physiol Renal Physiol 282: F1–F9, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol 67: 719–758, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Husted RF, Volk KA, Sigmund RD, Stokes JB. Anion secretion by the inner medullary collecting duct. Evidence for involvement of the cystic fibrosis transmembrane conductance regulator. J Clin Invest 95: 644–650, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med 64: 193–198, 1978 [DOI] [PubMed] [Google Scholar]
- 18. Kizer NL, Vandorpe D, Lewis B, Bunting B, Russell J, Stanton BA. Vasopressin and cAMP stimulate electrogenic chloride secretion in an IMCD cell line. Am J Physiol Renal Fluid Electrolyte Physiol 268: F854–F861, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Kunzelmann K, Kongsuphol P, Aldehni F, Tian Y, Ousingsawat J, Warth R, Schreiber R. Bestrophin and TMEM16-Ca2+ activated Cl− channels with different functions. Cell Calcium 46: 233–241, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest 110: 1651–1658, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Makhanova N, Hagaman J, Kim HS, Smithies O. Salt-sensitive blood pressure in mice with increased expression of aldosterone synthase. Hypertension 51: 134–140, 2008 [DOI] [PubMed] [Google Scholar]
- 22. McCoy DE, Taylor AL, Kudlow BA, Karlson K, Slattery MJ, Schwiebert LM, Schwiebert EM, Stanton BA. Nucleotides regulate NaCl transport in mIMCD-K2 cells via P2X and P2Y purinergic receptors. Am J Physiol Renal Physiol 277: F552–F559, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Murray RH, Luft FC, Bloch R, Weyman AE. Blood pressure responses to extremes of sodium intake in normal man. Proc Soc Exp Biol Med 159: 432–436, 1978 [DOI] [PubMed] [Google Scholar]
- 24. Park H, Oh SJ, Han KS, Woo DH, Mannaioni G, Traynelis SF, Lee CJ. Bestrophin-1 encodes for the Ca2+-activated anion channel in hippocampal astrocytes. J Neurosci 29: 13063–13073, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283: 36599–36607, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pochynyuk O, Rieg T, Bugaj V, Schroth J, Fridman A, Boss GR, Insel PA, Stockand JD, Vallon V. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2 receptor tone. FASEB J 24: 2056–2065, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajagopal M, Fischer H, Widdicombe JH. Hormonal and purinergic stimulation of bicarbonate secretion in oviducts of rhesus monkey. Am J Physiol Endocrinol Metab 295: E55–E62, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Rajagopal M, Pao AC. Adenosine activates a2b receptors and enhances chloride secretion in kidney inner medullary collecting duct cells. Hypertension 55: 1123–1128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Roos JC, Koomans HA, Dorhout Mees EJ, Delawi IM. Renal sodium handling in normal humans subjected to low, normal, and extremely high sodium supplies. Am J Physiol Renal Fluid Electrolyte Physiol 249: F941–F947, 1985 [DOI] [PubMed] [Google Scholar]
- 32. Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwiebert EM, Kishore BK. Extracellular nucleotide signaling along the renal epithelium. Am J Physiol Renal Physiol 280: F945–F963, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Song Z, Gomes DA, Stevens W. Role of purinergic P2Y1 receptors in regulation of vasopressin and oxytocin secretion. Am J Physiol Regul Integr Comp Physiol 297: R478–R484, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Sonnenberg H. Secretion of salt and water into the medullary collecting duct of Ringer-infused rats. Am J Physiol 228: 565–568, 1975 [DOI] [PubMed] [Google Scholar]
- 36. Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294: F10–F27, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 86: 901–940, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Vandorpe D, Kizer N, Ciampollilo F, Moyer B, Karlson K, Guggino WB, Stanton BA. CFTR mediates electrogenic chloride secretion in mouse inner medullary collecting duct (mIMCD-K2) cells. Am J Physiol Cell Physiol 269: C683–C689, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Wallace DP, Rome LA, Sullivan LP, Grantham JJ. cAMP-dependent fluid secretion in rat inner medullary collecting ducts. Am J Physiol Renal Physiol 280: F1019–F1029, 2001 [DOI] [PubMed] [Google Scholar]
- 40. White MM, Aylwin M. Niflumic and flufenamic acids are potent reversible blockers of Ca2+-activated Cl− channels in Xenopus oocytes. Mol Pharmacol 37: 720–724, 1990 [PubMed] [Google Scholar]
- 41. Xia SL, Wang L, Cash MN, Teng X, Schwalbe RA, Wingo CS. Extracellular ATP-induced calcium signaling in mIMCD-3 cells requires both P2X and P2Y purinoceptors. Am J Physiol Renal Physiol 287: F204–F214, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008 [DOI] [PubMed] [Google Scholar]