Abstract
Endogenous cardiotonic steroids, through their interaction with the ouabain-binding site of the Na-K-ATPase α-subunit, have been implicated in a variety of cardiovascular disease states including hypertension. We have previously shown that ACTH-induced hypertension is abolished in mutant mice expressing ouabain-resistant α1- and α2-subunits. To further evaluate hypertension resistance in these mutant mice, we examined blood pressure changes in a modified model of 2-kidney, 1-clip (2K1C) renovascular hypertension. To reliably generate 2K1C hypertension, we used polyvinyl tubing (inner diameter: ∼0.27 mm) to accurately gauge the degree of renal artery stenosis. Using this method, virtually all of the clipped mice became hypertensive and there was no incidence of apparent renal ischemia. By telemetry, in response to renal artery clipping, blood pressure in wild-type mice (α1 ouabain-resistant, α2 ouabain-sensitive) increased from 97 ± 3 to 136 ± 7 mmHg. In α1-resistant, α2-resistant mice, pressure increased from 93 ± 2 to 123 ± 4 mmHg, and in α1-sensitive, α2-resistant mice, blood pressure increased from 95 ± 2 to 139 ± 5 mmHg. Blood pressure changes were equivalent in all three groups. In sham mice, blood pressure did not change (96 ± 1 to 95 ± 2 mmHg). Renin mRNA expression was dramatically elevated in the left vs. the right kidney, and plasma renin concentration was elevated similarly in all genotypes. These data indicate that sensitivity of the α1- or α2-Na-K-ATPase binding site to cardiotonic steroids is not a prerequisite for the development of 2K1C renovascular hypertension. In addition, use of a polyurethane cuff to constrict the renal artery provides a reliable method for producing 2K1C hypertension in mice.
Keywords: cardiotonic steroids, NKA, telemetry, blood pressure
endogenous cardiotonic steroids have been implicated in the pathogenesis of a number of cardiovascular disease states including human heart failure and hypertension (1). Elevated levels of these compounds have been reported in essential hypertension, renovascular hypertension, and preeclampsia (12, 14, 27). Commonly, these compounds are thought to bind to and inhibit the α-subunit of the Na-K-ATPase (α-NKA), of which there are four known isoforms (designated α1–4). The subsequent elevation in intracellular Na+ reduces Na+/Ca2+ exchanger activity, resulting in an increase in free intracellular Ca2+ concentration that is responsible for the positive inotropic effects in cardiac and smooth muscle. More recently, it has been proposed that these compounds can initiate specific intracellular signaling cascades that produce various physiological responses (1). In either case, high-affinity binding of the steroid to the α-NKA subunit is required to elicit a biological effect. Thus recent development of mutant mice with altered sensitivity of the α1- and α2-NKA isoforms to cardiotonic steroids has presented the opportunity to examine the specific role of these subunits in physiology and pathophysiology.
Accordingly, since previous studies had reported elevated levels of circulating ouabain-like compounds during ACTH-induced hypertension (39), we examined ACTH hypertension in our α-NKA mutant mice and found that blood pressure increases were markedly blunted in mutant mice expressing “ouabain-resistant” α1- and α2-NKA subunits, compared with mice in which either subunit is “ouabain sensitive” (10, 24). These findings confirmed that this form of stress-related hypertension is dependent upon the interaction between endogenous cardiotonic steroids and the α1- and/or α2- subunit. By contrast, it has been argued that the Na-K-ATPase plays a role in salt-dependent, but not salt-independent hypertension (16). We recently reported, however, that DOCA-salt hypertension persists in these α1/α2 ouabain-resistant mice (23). We therefore sought, in the present study, to examine the effects of altered ouabain sensitivity in a two-kidney, 1-clip (2K1C) model of high-renin, salt-independent, renovascular hypertension. Based on previous observations, we hypothesized that blood pressure increases would not differ between ouabain-sensitive and ouabain-resistant mice.
To perform these experiments successfully, it was necessary to develop a reliable and consistent model of 2K1C hypertension in the mouse, and we postulated that the use of a polyurethane cuff instead of a metal clip placed on the renal artery would provide this needed consistency. We provide data to validate this modified surgical approach that reliably and rapidly increased blood pressure in mice.
METHODS
Animal model.
Experiments were carried out with mutant mice expressing NKA isoforms with altered ouabain sensitivities: α1-resistant/α2-sensitive (α1R/R2S/S, wild-type), α1-resistant/α2-resistant (α1R/Rα2R/R, single-mutant), and α1-sensitive/α2-resistant (α1S/Sα2R/R, double-mutant). Alterations in ouabain sensitivity were produced by targeted mutations that produced amino acid substitutions at position 111 and 122 in the first extracellular loop of the α-subunit, as previously described (8, 9). Animals were obtained from two established colonies at the University of Cincinnati, which were both maintained on a mixed 129SvJ and Black Swiss background. The colony of single-mutant α1R/Rα2R/R mice were maintained by mating heterozygous male and female animals (α1R/Rα2S/R × α1R/Rα2S/R) (8). The colony of double-mutant (α1S/Sα2R/R) mice were maintained by mating homozygous double-mutant animals. Breeding pairs were periodically backcrossed to a parallel subcolony of wild-type mice to sustain a consistent genetic background between mutant and wild-type mice. Wild-type animals were obtained from both colonies and show no differences in any observed measurements. Genotypes were determined by PCR analysis of DNA from tail biopsies, as described. All experiments were approved by the University of Cincinnati Institutional Animal Care and Use Committee in accordance with established guidelines.
2K1C model.
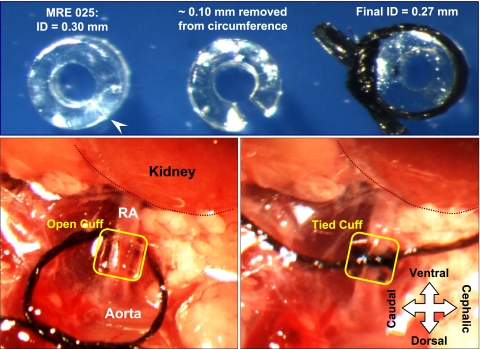
The Goldblatt model of 2K1C renovascular hypertension has been successfully reported in mice using the standard approach of clipping the renal artery with a silver clip, usually a 0.13-mm slit (6, 17, 38). However, we and others have found this approach to be unpredictable and inconsistent, as even small variations in slit dimensions result in insufficient constriction or renal ischemia. As an alternative, we used a small segment of polyurethane tubing, sliced lengthwise, to act as a cuff to produce a constriction around the left renal artery [MRE 025, Braintree Scientific; internal diameter (ID) = 0.30 mm; outside diameter (OD) = 0.63 mm; wall thickness, (WT) = 0.16 mm]. Since placement of a plastic cuff would result in constriction in two dimensions (constriction) rather than one (flattening), as with a metal clip, it was apparent that the appropriate cuff diameter would be substantially larger than 0.13. For reference, the OD of the renal artery in our mice typically ranges from 0.3 to 0.4 mm. Our initial attempts to test cuff size indicated that cuffs with an ID <0.2 mm resulted in blanching of the kidney and apparent cessation of renal blood flow. We therefore tested cuff diameters of 0.20, 0.24, and 0.27 mm in three separate pilot groups of NIH Swiss wild-type mice. As illustrated in Fig. 1, small segments of the circumference of the tubing could be removed so that cuffs with these calibers could easily be produced. The diameters noted above were achieved by removing ∼0.31, 0.19, and 0.10 mm of the circumference, respectively. In practice, the amount removed was estimated by recognizing that the wall thickness of the tubing was 0.16 mm, and then removing a segment that was relatively larger, smaller, or equivalent to that wall thickness; after cutting, the resulting cuff diameter was measured. For cuff placement, mice were anesthetized with isoflurane, placed on their right side, and the left renal artery was exposed and dissected free through a flank incision. The cuff was then placed around the renal artery and secured by gently tying with a 7-0 silk ligature to close the gap in the cuff. Sham mice underwent the same procedure, but the cuff was not placed.
Fig. 1.
Photographs showing the use of polyurethane tubing as a cuff to constrict the renal artery. Top, left: short length (∼0.6 mm) of MRE25 tubing [inner diameter (ID) 0.30 mm] that has been sliced lengthwise with a scalpel (at arrowhead); middle: a ∼0.1-mm wedge has been removed from the wall, so that when the tubing circumference is closed using a suture, the ID is ∼0.27 mm (right). The size of the wedge can be adjusted to finely tune the final ID. Bottom: cuff is shown after it is positioned around the left renal artery (RA; left) and after the suture is tied to constrict the artery (right). The position of the kidney has been outlined and labeled, and the orientation of the mouse, in a right lateral decubitus position, is indicated.
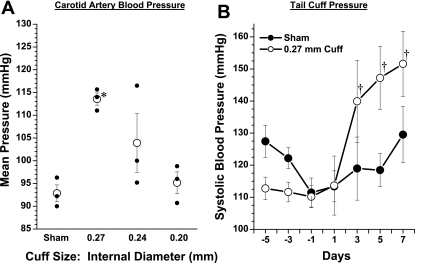
After 1 wk, animals were anesthetized with isoflurane for direct recording of blood pressure and analysis of right and left kidney renin mRNA (see below). In a subset of mice, systolic blood pressure was measured by tail-cuff before and after clipping (Visitech, BP2000, Apex, NC). We observed that blood pressure was significantly elevated in mice with 0.27-mm cuffs compared with sham, but unchanged in those with 0.20- or 0.24-mm cuffs (Fig. 2A). The left-to-right renin mRNA ratio was not different from 1 in the sham group and in the 0.24-mm group (0.88 ± 0.1 and 0.78 ± 0.03, respectively). In the 0.27-mm group, renin mRNA expression was significantly elevated in the left kidney, with a left-to right ratio of 3.0 ± 0.9. In the 0.20-mm group, left kidney renin expression was markedly suppressed, with a ratio of 0.18 ± 0.02. It was noted that the left kidneys from these mice appeared profoundly ischemic upon excision. In a subset of mice, we measured tail-cuff blood pressure in sham controls and in mice with 0.27-mm cuffs, and the results are shown in Fig. 2B. We found that within 48 h after cuff placement, systolic blood pressure increased significantly in the 0.27-mm 2K1C mice. We therefore chose a cuff size of ∼0.27 mm to perform all of the remaining studies.
Fig. 2.
Data from pilot experiments to evaluate different cuff sizes. A: mean arterial pressure measured from a carotid catheter under isoflurane anesthesia in mice fitted with various sizes of renal artery cuff vs. sham after 1 wk. B: tail-cuff pressures measured every other day in sham-operated mice and mice fitted with a 0.27-mm renal artery cuff. *P < 0.05 compared with sham. †P < 0.05 compared with day −1.
Telemetric measurement of blood pressure.
In α1R/R2S/S, α1R/Rα2R/R, and α1S/Sα2R/R mice, continuous blood pressure recordings were made as previously described using the TA11PA-C10 pressure transmitter (Data Sciences International, St. Paul, MN)(24). Transmitters were implanted under isoflurane anesthesia using carotid artery cannulation and subcutaneous transmitter placement. Mice were synchronized to a 14:10 h light-dark schedule with lights on at 6:00 A.M. Following telemeter implantation, mice were allowed to recover for 5–7 days before collection of baseline data for an additional 3–5 days. Renal artery cuffs with 0.27-mm ID (based on preliminary results) were then placed as described. Blood pressure was monitored continuously between 10:00 AM and 4:00 PM daily for at least 6 days using the PowerLab system and Chart software (ADInstruments, Colorado Springs, CO). Experiments on α1R/Rα2S/S and α1R/Rα2R/R mice were conducted first, and these mice were euthanized at 6–7 days to correlate renal renin expression with changes in blood pressure. Experiments in α1S/Sα2R/R mice were performed at a later time, and these mice were euthanized at 4 wk to examine the long-term time course of blood pressure changes in response to renal artery stenosis. Three mice of each genotype underwent sham surgery, and data were pooled.
Evaluation of kidney renin mRNA and plasma renin concentration.
Kidney renin mRNA was measured by real-time PCR. At the time of death, total RNA was isolated from left and right kidneys using Tri-reagent (TR118; Molecular Research Center). RNA concentration was quantified by UV spectrophotometry, and samples were stored at −80°C. cDNA was synthesized using oligo dT primers in the SuperScript III first-strand synthesis system (18080–051; Invitrogen). Real-time PCR analysis was carried out using the iQ SYBR Green system (170–8882; Bio-Rad Laboratories) and the Opticon2 DNA Engine (MJ Research). Levels of GAPDH and renin were determined using primers from PrimerBank (GAPDH: PrimerBank ID 6679937a3; renin: Primer Bank ID 13676837a1) (31, 34) and Opticon Monitor Analysis Software (Ver. 2.02). All reactions were run in triplicate, and renin levels were normalized to GAPDH levels.
For measurements of plasma renin concentration (PRC), blood samples were obtained from conscious mice at 1 wk of 2K1C by tail-cut using EDTA as an anticoagulant and assayed as previously described (26). From each sample, 2 μl of plasma was added to an incubation cocktail containing 158 μl of modified phosphate incubation buffer (0.1 M phosphate buffer, 0.06 M disodium EDTA, 0.2% gelatin, pH 6.5), 2 μl of PMSF, and 138 μl substrate (containing 250 ng angiotensinogen, obtained from 48-h nephrectomized rats). This sample was incubated at 37°C for 90 min, boiled for 10 min to stop the reaction, centrifuged for 10 min at 2,500 g, and the supernatant was removed and frozen for later analysis (typically in <48 h). ANG I generation was analyzed by radioimmunoassay using a Gamma Coat PRA kit (DiaSorin, Stillwater, MN) according to the manufacturer's instructions. The assay was run with buffer blanks and internal quality controls.
Statistical analysis.
Data were analyzed using a one-way or mixed two-way ANOVA, with repeated measures on the second factor. Post hoc analysis for pairwise comparisons was performed using the Holmes-Sidak test (SigmaPlot). Summary data are presented as the means ± SE.
RESULTS
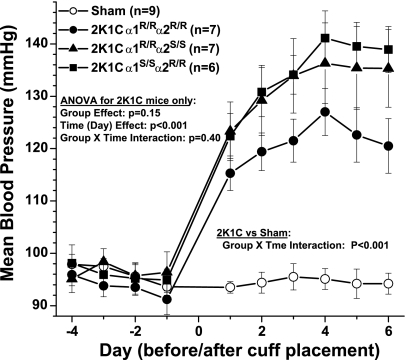
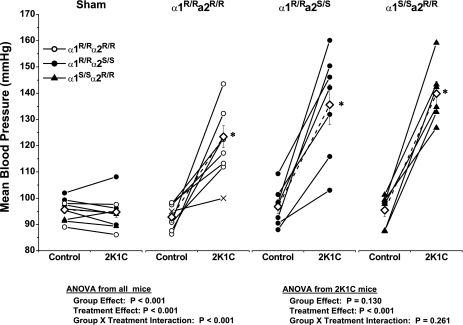
A total of 32 mice were prepared for telemetry. Of these, one mouse died perioperatively and one mouse did not sustain a patent catheter beyond 5 days. The remainder were included in the analysis. As shown in Fig. 3, blood pressure increased significantly in all three genotype groups of mice within 1 day of cuff placement and continued to rise gradually over the next 3 days. Blood pressure did not change in sham mice. Compared with α1R/Rα2S/S and α1S/Sα2R/R, blood pressure in the α1R/Rα2R/R mice was ∼10 mmHg lower in the days following cuff placement, but this difference was not significant. To examine the reliability and consistency of hypertension development in the 2K1C mice, blood pressure plateau values on days 4–6 were averaged and compared with the baseline average from day −3 to −1 in each mouse. Nine mice received sham surgery, and 21 mice were implanted with renal artery cuffs. Data from each mouse are shown in Fig. 4, along with group averages. Blood pressure increased by at least 15 mmHg in 18 of 20 mice studied. In one α1S/Sα2R/R mouse, the increase was 12.5 mmHg. In one α1R/Rα2R/R mouse, there was only a 5-mmHg increase in blood pressure, but it was discovered at necropsy that the cuff had slipped off the artery at some point after implantation. Data from this mouse, denoted by ×-symbols in Fig. 4 (second panel from the left), were omitted from the statistical analysis. None of the 20 clipped kidneys exhibited overt signs of ischemic damage at necropsy. Statistical analysis of plateau levels (days 4–6) indicated that the increase in blood pressure was not significantly different between the three genotypes.
Fig. 3.
Telemetric blood pressure in 2-kidney, 1-clip (2K1C) hypertensive mice vs. sham 4 days before and 6 days following 2K1C surgery in Na-K-ATPase (NKA) mutant mice as indicated. Three mice from each genotype underwent sham surgery, and data were pooled (open symbols). ANOVA on 2K1C mice showed that the increase in pressure was equivalent in all groups (P = 0.40).
Fig. 4.
Mean arterial pressure values from individual sham and 2K1C mice. Individual values represent the average for the last 3 days before clipping and the average of days 4–6 after clipping. ◊, Group means; ×, values from a mouse in which the cuff had slipped off the artery, and these values are not included in the analysis. *P < 0.05 compared with respective control.
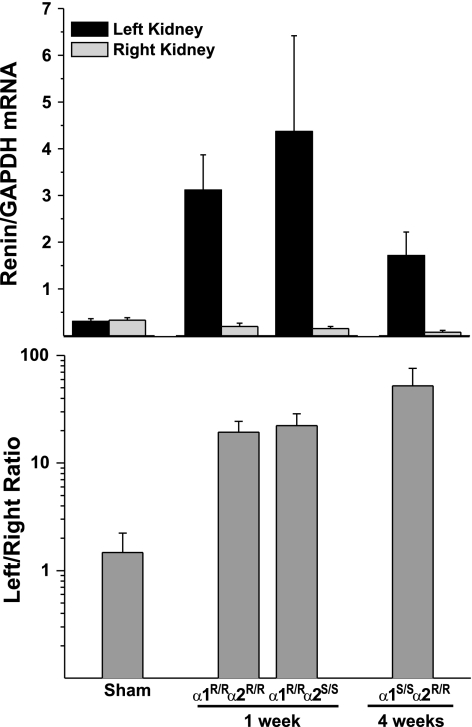
At termination of the experiments, both left and right kidneys were carefully dissected and weighed, and the cuff was removed intact for determination of in situ diameter. Cuffs were photographed with ligatures in place, and the lumen diameter was measured using NIH ImageJ. Left and right kidney weights and cuff diameters are given in Table 1. The cuff diameter for all 2K1C mice was 0.276 ± 0.003 mm and was not different between the three groups of clipped mice. Other morphometrical data are presented in Table 2. After 1 wk of 2K1C hypertension, both α1R/Rα2R/R and α1R/Rα2S/S mice showed modest, but significant decreases in left kidney weight and left/right kidney weight ratio. There was no evidence of cardiac hypertrophy after 1 wk. As shown in Fig. 5, at 1 wk of left renal artery clip, the relative expression of renal renin mRNA was markedly upregulated in the left kidney of both α1R/Rα2R/R and α1R/Rα2S/S mice, by 19- and 22-fold, respectively.
Table 1.
Cuff diameters at necropsy
| Internal Diameter, mm |
||
|---|---|---|
| Mean | Range | |
| All mice (n = 19) | 0.276 ± 0.003 | 0.251–0.293 |
| α1R/Rα2R/R (n = 7) | 0.269 ± 0.005 | 0.253–0.291 |
| α1R/Rα2S/S (n = 7) | 0.278 ± 0.006 | 0.251–0.291 |
| α1S/Sα2R/R (n = 5) | 0.281 ± 0.003 | 0.274–0.293 |
Table 2.
Morphometrical data at 1 or 4 wk
| Kidney Weight |
||||||
|---|---|---|---|---|---|---|
| Body Weight, g | Left, mg | Right, mg | Left/right ratio | Heart Weight, mg | Heart Wt/Body Wt | |
| 1 Wk | ||||||
| Sham | 36.6 ± 1.5 | 289 ± 21 | 278 ± 15 | 1.04 ± 0.04 | 156 ± 7 | 4.27 ± 0.10 |
| α1R/Rα2R/R | 36.9 ± 0.9 | 212 ± 16* | 282 ± 16 | 0.76 ± 0.07* | 165 ± 12 | 4.50 ± 0.37 |
| α1R/Rα2S/S | 34.1 ± 0.7 | 212 ± 18* | 301 ± 15 | 0.71 ± 0.05* | 173 ± 7 | 5.01 ± 0.20 |
| 4 Wk | ||||||
| α1S/Sα2R/R | 43.2 ± 0.6*† | 141 ± 28*† | 444 ± 15*† | 0.33 ± 0.08*† | 233 ± 12*† | 5.39 ± 0.22* |
Values are means ± SE.
P < 0.05 compared with sham.
P < 0.05 compared with other two 2K1C groups.
Fig. 5.
Left and right kidney renin mRNA expression in sham and 2K1C mice. Top: relative expression of renin in each kidney, normalized to levels of GAPDH. Bottom: fold-expression as a ratio of left to right kidney renin expression. Sham, α1R/Rα2R/R, and α1R/Rα2S/S mice were euthanized at 1 wk; α1S/Sα2R/R mice were euthanized at 4 wk.
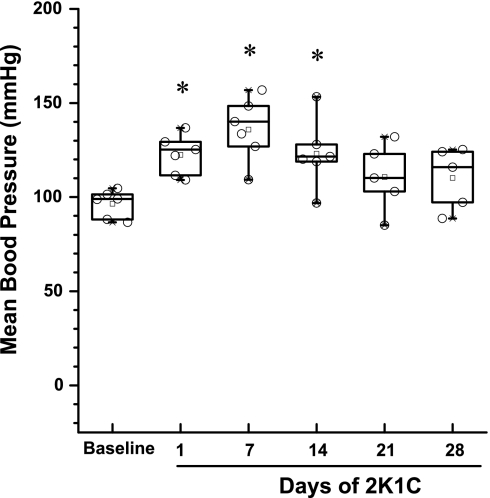
In the 2K1C α1S/Sα2R/R mice, we opted to extend the 2K1C hypertension period to 4 wk to determine whether blood pressure elevation was sustained for an extended period. Importantly, we observed in these mice that blood pressure was highest at 7 days and thereafter tended to decline to various degrees in each mouse, such that by day 21 the difference from baseline was not significant, as shown in Fig. 6. This decline in pressure was associated with a substantial reduction in left kidney weight and increase in right kidney weight by 4 wk, as shown in Table 2. There was also some indication of cardiac hypertrophy in these mice. Also, at 4 wk in α1S/Sα2R/R mice, the relative expression of renin mRNA in both the right and left kidneys was substantially reduced (Fig. 5), but the expression of renin mRNA in the left kidney remained markedly higher than in the right.
Fig. 6.
Distribution of blood pressure values in the α1S/Sα2R/R mice at baseline and during 4 wk of 2K1C hypertension. Blood pressure was elevated during the first 2 wk, but by week 3 values were not different from baseline. Boxes depict the 25th/75th percentiles and whiskers the 5th/95th percentile. *P < 0.05 compared with baseline.
PRC was measured in all groups of mice at 1 wk of 2K1C hypertension. Compared with the sham value of 0.30 ± 0.05 μg ANG I·ml−1·h−1 (n = 7), 2K1C mice had significantly elevated levels of PRC (P = 0.022): 1.79 ± 0.40 μg ANG I·ml−1·h−1 in α1R/Rα2R/R, (n = 5) 2.27 ± 1.02 μg ANG I·ml−1·h−1 in α1R/Rα2S/S, (n = 6), and 3.19 ± 0.80 μg ANG I·ml−1·h−1 in α1S/Sα2R/R mice (n = 6). PRC values between the three groups of 2K1C mice were not significantly different.
DISCUSSION
Role of NKA α-subunit and its endogenous ligands in renovascular hypertension.
We have previously reported that ACTH-induced hypertension is blunted in mutant mice expressing a ouabain-resistant α2-Na-K-ATPase subunit (10, 24). Our explanation of this finding was that this form of hypertension was dependent on elevated levels of an endogenous cardiotonic steroid ligand that interacts with the α2-subunit to produce this form of hypertension. It has been argued that these endogenous ouabain-like compounds contribute to other forms of hypertension as well, including essential hypertension (2, 15), preeclampsia (12, 33), and DOCA-salt hypertension (14, 19, 32). Although ACTH-induced hypertension is commonly regarded as a stress-related form of hypertension involving multiple regulatory pathways, the pathogenesis of this form of high blood pressure remains somewhat enigmatic, and there appears to be a substantial amount of species heterogeneity (37). It therefore became important to determine whether resistance to hypertension in the α1R/Rα2R/R animals was a general phenomenon, or alternatively, whether the resistance was peculiar to this form of stress-related hypertension. Accordingly, we recently reported that DOCA-salt hypertension persists in mice with a ouabain-resistant α2-subunit, which argues against a particular role for this isoform in salt-dependent hypertension (23).
The purpose of this study, therefore, was to explore the role of the α1- and α2-isoforms in a third well-characterized model of experimental hypertension, specifically the 2K1C model of renovascular hypertension. We demonstrated that in response to unilateral renal artery constriction, increases in blood pressure were statistically equivalent in ouabain-resistant mice (α1R/Rα2R/R) compared with mice with ouabain-sensitive α1- and α2-subunits (α1R/Rα2S/S and α1S/Sα2R/R). Based on the data shown in Figs. 3 and 4, it is tempting to consider the possibility that the blood pressure increase seen in the α1R/Rα2R/R mice might be blunted to some degree since the change in blood pressure at its plateau was ∼30 mmHg in the α1R/Rα2R/R mice vs. 39 and 44 mmHg in the α1R/Rα2S/S and α1S/Sα2R/R mice, respectively. We cannot rule out the possibility that a more extensive study with more mice might better define a subtle influence of ouabain sensitivity. Nevertheless, our data definitively demonstrate that the primary mechanisms that elevate blood pressure in 2K1C hypertension do not require α1 or α2 ouabain sensitivity. We can allow for the possibility, however, that endogenous cardiotonic steroids interacting with the α1- and/or α2-subunits might modestly augment the hypertensive response.
Four α-isoforms of Na-K-ATPase, α1–α4, have been identified, and expression levels are tissue and species dependent (22). The α1-isoform is expressed abundantly in most tissues; the α2-isoform is detected in brain, heart, skeletal and vascular smooth muscle, and adipocytes; the α3-isoform is predominant in neurons and ovaries; the α4-isoform is exclusively expressed in sperm. In mice, it has been reported that in both cardiac muscle and vascular smooth muscle, the expression of the α1-isoform represents ∼70% of the total, and the α2 represents ∼30% of the total (29). Renal epithelial cells express almost exclusively the α1-isoform (11, 25). Since other α-isoforms (α3 or α4) have not been detected in these cardiovascular/renal cell types, our present data suggest that endogenous cardiotonic steroids acting on the heart, vasculature, or kidney do not play a substantial role in the pathogenesis of renovascular hypertension. On the other hand, since both α1R/Rα2S/S and α1R/Rα2R/R mice express a ouabain-sensitive α3-subunit, which is a dominant isoform in neurons, a potential role for the α3-Na-K-ATPase in 2K1C renovascular hypertension remains possible. Such a role for endogenous cardiotonic steroids acting in the central and peripheral nervous system to promote hypertension has been extensively investigated and is well supported (21).
Modified approach for 2K1C hypertension in mice.
In developing a useful model of 2K1C Goldblatt hypertension for the mouse, our original attempts using conventional silver clips, with slit dimensions ranging from 0.11 to 0.13 mm, resulted in low success rates. We found that in more than half of the mice, the degree of constriction was either too small, leading to insufficient blood flow restriction, or too great, leading to global renal ischemia. In either case, no hypertension developed. Since the result of this inconsistency would inevitably lead to artifactual selection of our treatment groups, and since this self-selection process could very likely be inconsistent between our genotypes, we found it necessary to seek a more reliable and consistent approach for generating 2K1C renovascular hypertension in our mice. We reasoned that application of a circumferential constriction, in the form of a polyurethane cuff would provide better control of the caliber of the constriction and blood flow restriction. We originally experimented with a variety of tubing types (polyethylene, Teflon, and polyurethane) with various diameters and wall thickness but determined that the MRE 025 polyurethane tubing (Braintree) offered the best advantages: it could be easily trimmed to the desired caliber, was highly biocompatible, and could be securely tied in place without compressing. Based on our preliminary experiments, we determined that a target cuff diameter of ∼0.27 mm produced the most consistent results. Actual cuff diameter ranged from 0.25 to 0.29 mm, based on postmortem analyses. Our results demonstrate the effectiveness of this approach. Of the 30 mice used in our telemetry experiments, 9 were designated for sham surgery, and 21 received renal artery cuffs. Of these 21 mice, all but one developed some meaningful degree of hypertension (i.e., >10 mmHg). In the mouse with no hypertension, it was discovered at necropsy that the polyethylene cuff had become free from the renal artery postoperatively and there was no constriction. Upon gross examination at the end of the experiment, there was no apparent indication of overt renal ischemia in any of the clipped kidneys.
Other investigators have used the silver clip (or stainless steel) approach in mice with various levels of success. One of the earliest and most thorough investigations in mice was reported by Wiesel et al. (38) in 1997. They evaluated stainless steel clips with openings ranging from 0.04 to 0.18 mm and found that the optimal opening size was 0.12 mm. They reported that openings of below 0.11 mm always resulted in infarction, and those larger than 0.12 mm did not produce hypertension. Despite the constraints of these tight tolerances (<0.02 mm), the incidence of infarction at 2–4 wk remained significant in both the 0.11-mm group (40–80%) and the 0.12-mm group (15%). By contrast, despite the fact that the diameter of our cuffs varied from 0.25 to 0.29 mm, our success rate for producing hypertension was nearly 100%, and the incidence of infarction was nil.
While Wiesel et al. (38) did not examine the time course of hypertension, other groups using the metal clip approach have reported that full development of hypertension requires often 7–14 days (4, 5, 20) whereas others have found that it occurs within 1–3 days (13, 28). In rats and dogs, the development of 2K1C hypertension has been found to be quite variable, and to some extent, investigator and technique dependent (3, 18, 36). Highlighting this variability, Sigmon et al. (30) showed in rats that placement of metal clips resulting in a wide range of flow restriction could produce increases in pressure that were quantitatively similar, but qualitatively different in terms of mechanism. Using our approach for 2K1C hypertension, we found that blood pressure increased markedly in the first 24 h following placement of the renal artery cuff and that blood pressure plateaued at its maximum value within 3–4 days (Fig. 3). This response was consistent in all of the mice analyzed. This model also demonstrated many of the other hallmarks of renovascular hypertension, including elevation of renin mRNA expression in the clipped kidney, sustained elevation of plasma renin concentration, and an increase in the ratio of left to right kidney weight.
Experiments in the α1S/Sα2R/R mice were conducted after the other groups were completed, and in these mice we elected to follow the progression of hypertension over a 4-wk period, rather than euthanizing them at 1 wk. We discovered that the blood pressure peaked at 7 days and thereafter tended to decline. In two of the mice, blood pressure returned nearly to baseline (see Fig. 6), and we found that the kidneys of these mice had atrophied markedly. It appears that with increasing time, there was a progressive stenosis in these two mice that eventually led to complete renal ischemia, and consequent resolution of the hypertension. Similar declines have been observed with conventional renal artery clips in both rats and mice (13, 18), but sustained increases have also been reported for both species (4, 7, 17). It is possible that the progressive stenosis observed in the α1S/Sα2R/R mice is not a general phenomenon but may be peculiar to these mutant mice specifically. Indeed, we have recently reported that the α1S/Sα2R/R mice are more susceptible to pressure overload-induced myocardial fibrosis (35). It is not unreasonable to suppose that a similar phenomenon may occur in the renal vasculature in response to unilateral stenosis. In any case, the chronic phase of 2K1C hypertension in mice, using this method or the conventional metal clip approach, requires further investigation.
In summary, we have developed and validated a modified approach for 2K1C renovascular hypertension that reliably and consistently elevates blood pressure by 20–40 mmHg. The facile nature of this method should simplify the use of this model in the mouse and could be extended for use in rats. Using this model, our data indicate that the development of 2K1C hypertension in mice does not depend on the ouabain sensitivity of either the α1- or α2-Na-K-ATPase. These data imply that circulating cardiotonic steroids acting on the heart or vasculature do not participate in the pathogenesis of this form of hypertension.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants DK57552 (to J. N. Lorenz), HL 28573 and HL 28573 (to J. B. Lingrel), PPG 5PO1HL090550-02 (to W. H. Beierwaltes), and DK050594 (to G. E. Shull).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev 61: 9–38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blaustein MP. Endogenous ouabain: role in the pathogenesis of hypertension. Kidney Int 49: 1748–1753, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Burgelova M, Vanourkova Z, Thumova M, Dvorak P, Opocensky M, Kramer HJ, Zelizko M, Maly J, Bader M, Cervenka L. Impairment of the angiotensin-converting enzyme 2-angiotensin-(1–7)-Mas axis contributes to the acceleration of two-kidney, one-clip Goldblatt hypertension. J Hypertens 27: 1988–2000, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Cervenka L, Horacek V, Vaneckova I, Hubacek JA, Oliverio MI, Coffman TM, Navar LG. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension 40: 735–741, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Cervenka L, Vaneckova I, Huskova Z, Vanourkova Z, Erbanova M, Thumova M, Skaroupkova P, Opocensky M, Maly J, Chabova VC, Tesar V, Burgelova M, Viklicky O, Teplan V, Zelizko M, Kramer HJ, Navar LG. Pivotal role of angiotensin II receptor subtype 1A in the development of two-kidney, one-clip hypertension: study in angiotensin II receptor subtype 1A knockout mice. J Hypertens 26: 1379–1389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng J, Zhou W, Warner GM, Knudsen BE, Garovic VD, Gray CE, Lerman LO, Platt JL, Romero JC, Textor SC, Nath KA, Grande JP. Temporal analysis of signaling pathways activated in a murine model of two-kidney, one-clip hypertension. Am J Physiol Renal Physiol 297: F1055–F1068, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collis MG, Vanhoutte PM. Increased renal vascular reactivity to angiotensin II but not to nerve stimulation or exogenous norepinephrine in renal hypertensive rats. Circ Res 43: 544–552, 1978 [DOI] [PubMed] [Google Scholar]
- 8. Dostanic I, Lorenz JN, Schultz Jel J, Grupp IL, Neumann JC, Wani MA, Lingrel JB. The α2 isoform of Na,K-ATPase mediates ouabain-induced cardiac inotropy in mice. J Biol Chem 278: 53026–53034, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Dostanic I, Schultz Jel J, Lorenz JN, Lingrel JB. The α1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J Biol Chem 279: 54053–54061, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Dostanic-Larson I, Van Huysse JW, Lorenz JN, Lingrel JB. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc Natl Acad Sci USA 102: 15845–15850, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farman N. Na,K-pump expression and distribution in the nephron. Miner Electrolyte Metab 22: 272–278, 1996 [PubMed] [Google Scholar]
- 12. Graves SW, Lincoln K, Cook SL, Seely EW. Digitalis-like factor and digoxin-like immunoreactive factor in diabetic women with preeclampsia, transient hypertension of pregnancy, and normotensive pregnancy. Am J Hypertens 8: 5–11, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Griol-Charhbili V, Sabbah L, Colucci J, Vincent MP, Baudrie V, Laude D, Elghozi JL, Bruneval P, Picard N, Meneton P, Alhenc-Gelas F, Richer C. Tissue kallikrein deficiency and renovascular hypertension in the mouse. Am J Physiol Regul Integr Comp Physiol 296: R1385–R1391, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Hamlyn JM. Increased levels of a humoral digitalis-like factor in deoxycorticosterone acetate-induced hypertension in the pig. J Endocrinol 122: 409–420, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Hamlyn JM, Ringel R, Schaeffer J, Levinson PD, Hamilton BP, Kowarski AA, Blaustein MP. A circulating inhibitor of (Na+ + K+)ATPase associated with essential hypertension. Nature 300: 650–652, 1982 [DOI] [PubMed] [Google Scholar]
- 16. Iwamoto T, Kita S, Zhang J, Blaustein MP, Arai Y, Yoshida S, Wakimoto K, Komuro I, Katsuragi T. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med 10: 1193–1199, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Johns C, Gavras I, Handy DE, Salomao A, Gavras H. Models of experimental hypertension in mice. Hypertension 28: 1064–1069, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Kost CK, Jr, Li P, Pfeifer CA, Jackson EK. Telemetric blood pressure monitoring in benign 2-kidney, 1-clip renovascular hypertension: effect of chronic caffeine ingestion. J Pharmacol Exp Ther 270: 1063–1070, 1994 [PubMed] [Google Scholar]
- 19. Krep H, Price DA, Soszynski P, Tao QF, Graves SW, Hollenberg NK. Volume sensitive hypertension and the digoxin-like factor reversal by a fab directed against digoxin in DOCA-salt hypertensive rats. Am J Hypertens 8: 921–927, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Lazartigues E, Lawrence AJ, Lamb FS, Davisson RL. Renovascular hypertension in mice with brain-selective overexpression of AT1a receptors is buffered by increased nitric oxide production in the periphery. Circ Res 95: 523–531, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Leenen FHH. The central role of the brain aldosterone-“ouabain” pathway in salt-sensitive hypertension. Biochim Biophys Acta 1802: 1132–1139, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Lingrel JB. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu Rev Physiol 72: 395–412, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loreaux EL, Oshiro N, Lingrel JB, Lorenz JN. DOCA-salt hypertension persists in mice with ouabain-resistant α1 and α2 Na,K-ATPase (NKA) (Abstract). FASEB J 22: 1210A, 2008 [Google Scholar]
- 24. Lorenz JN, Loreaux EL, Dostanic-Larson I, Lasko V, Schnetzer JR, Paul RJ, Lingrel JB. ACTH-induced hypertension is dependent on the ouabain-binding site of the α2-Na+-K+-ATPase subunit. Am J Physiol Heart Circ Physiol 295: H273–H280, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lucking K, Nielsen JM, Pedersen PA, Jorgensen PL. Na-K-ATPase isoform (α3, α2, α1) abundance in rat kidney estimated by competitive RT-PCR and ouabain binding Am J Physiol Renal Fluid Electrolyte Physiol 271: F253–F260, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Lum C, Shesely EG, Potter DL, Beierwaltes WH. Cardiovascular and renal phenotype in mice with one or two renin genes. Hypertension 43: 79–86, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Manunta P, Stella P, Rivera R, Ciurlino D, Cusi D, Ferrandi M, Hamlyn JM, Bianchi G. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension 34: 450–456, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Salguero G, Akin E, Templin C, Kotlarz D, Doerries C, Landmesser U, Grote K, Schieffer B. Renovascular hypertension by two-kidney one-clip enhances endothelial progenitor cell mobilization in a p47phox-dependent manner. J Hypertens 26: 257–268, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Shelly DA, He S, Moseley A, Weber C, Stegemeyer M, Lynch RM, Lingrel J, Paul RJ. Na+ pump α2-isoform specifically couples to contractility in vascular smooth muscle: evidence from gene-targeted neonatal mice. Am J Physiol Cell Physiol 286: C813–C820, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Sigmon DH, Beierwaltes WH. Degree of renal artery stenosis alters nitric oxide regulation of renal hemodynamics. J Am Soc Nephrol 5: 1369–1377, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res 38: D792–D799, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Huysse JW. Endogenous brain Na pumps, brain ouabain-like substance and the α2 isoform in salt-dependent hypertension. Pathophysiology 14: 213–220, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Vu HV, Ianosi-Irimie MR, Pridjian CA, Whitbred JM, Durst JM, Bagrov AY, Fedorova OV, Pridjian G, Puschett JB. Involvement of marinobufagenin in a rat model of human preeclampsia. Am J Nephrol 25: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res 31: e154, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wansapura AN, Lasko VM, Lingrel JB, Lorenz JN. Mice expressing ouabain-sensitive α1-Na,K-ATPase have increased susceptibility to pressure overload-induced cardiac hypertrophy. Am J Physiol Heart Circ Physiol 300: H347–H355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watkins BE, Davis JO, Hanson RC, Lohmeier TE, Freeman RH. Incidence and pathophysiological changes in chronic two-kidney hypertension in the dog. Am J Physiol 231: 954–960, 1976 [DOI] [PubMed] [Google Scholar]
- 37. Whitworth JA, Zhang Y, Mangos G, Kelly JJ. Species variability in cardiovascular research: the example of adrenocorticotrophin-induced hypertension. Clin Exp Pharmacol Physiol 33: 887–891, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Wiesel P, Mazzolai L, Nussberger J, Pedrazzini T. Two-kidney, one clip and one-kidney, one clip hypertension in mice. Hypertension 29: 1025–1030, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Yamada K, Goto A, Omata M. Adrenocorticotropin-induced hypertension in rats: role of ouabain-like compound. Am J Hypertens 10: 403–408, 1997 [PubMed] [Google Scholar]