Abstract
The renal extracellular 2′,3′-cAMP-adenosine and 3′,5′-cAMP-adenosine pathways (extracellular cAMPs→AMPs→adenosine) may contribute to renal adenosine production. Because mouse kidneys provide opportunities to investigate renal adenosine production in genetically modified kidneys, it is important to determine whether mouse kidneys express these cAMP-adenosine pathways. We administered (renal artery) 2′,3′-cAMP and 3′,5′-cAMP to isolated, perfused mouse kidneys and measured renal venous secretion rates of 2′,3′-cAMP, 3′,5′-cAMP, 2′-AMP, 3′-AMP, 5′-AMP, adenosine, and inosine. Arterial infusions of 2′,3′-cAMP increased (P < 0.0001) the mean venous secretion of 2′-AMP (390-fold), 3′-AMP (497-fold), adenosine (18-fold), and inosine (adenosine metabolite; 7-fold), but they did not alter 5′-AMP secretion. Infusions of 3′,5′-cAMP did not affect venous secretion of 2′-AMP or 3′-AMP, but they increased (P < 0.0001) secretion of 5′-AMP (5-fold), adenosine (17-fold), and inosine (6-fold). Energy depletion (metabolic inhibitors) increased the secretion of 2′,3′-cAMP (8-fold, P = 0.0081), 2′-AMP (4-fold, P = 0.0028), 3′-AMP (4-fold, P = 0.0270), 5′-AMP (3-fold, P = 0.0662), adenosine (2-fold, P = 0.0317), and inosine (7-fold, P = 0.0071), but it did not increase 3′,5′-cAMP secretion. The 2′,3′-cAMP-adenosine pathway was quantitatively similar in CD73 −/− vs. +/+ kidneys. However, 3′,5′-cAMP induced a 6.7-fold greater increase in 5′-AMP, an attenuated increase (61% reduction) in inosine and a similar increase in adenosine in CD73 −/− vs. CD73 +/+ kidneys. In mouse kidneys, 1) 2′,3′-cAMP and 3′,5′-cAMP are metabolized to their corresponding AMPs, which are subsequently metabolized to adenosine; 2) energy depletion activates the 2′,3′-cAMP-adenosine, but not the 3′,5′-cAMP-adenosine, pathway; and 3) although CD73 is involved in the 3′,5′-AMP-adenosine pathway, alternative pathways of 5′-AMP metabolism and reduced metabolism of adenosine to inosine compensate for life-long deficiency of CD73.
Keywords: 2′,3′-cAMP; 3′,5′-cAMP; 2′-AMP; 3′-AMP; 5′-AMP
adenosine is an autocrine/paracrine factor that is involved in the regulation of kidney function and structure (36); therefore, it is important to elucidate the mechanisms determining renal adenosine production. In this regard, there are multiple biochemical pathways that are responsible for adenosine biosynthesis in all organ systems including the kidneys.
One biochemical mechanism of adenosine production is the “extracellular 3′,5′-cAMP-adenosine pathway” that involves receptor-mediated intracellular production of 3′,5′-cAMP, active transport of 3′,5′-cAMP from the intracellular compartment to the cell surface, extracellular metabolism of 3′,5′-cAMP to 5′-AMP, and extracellular conversion of 5′-AMP to adenosine (3, 6–12). Studies in isolated, perfused rat kidneys, as well as in various rat kidney cells in culture, support the existence of a renal extracellular 3′,5′-cAMP-adenosine pathway (13–16, 19, 20, 27, 28).
Recent studies in isolated, perfused rat kidneys using high-performance liquid chromatograph tandem mass spectrometry (LC-MS/MS) identify a positional isomer of 3′,5′-cAMP, namely 2′,3′-cAMP (34). Moreover, subsequent investigations reveal that in addition to the renal extracellular 3′,5′-cAMP-adenosine pathway, there exists a renal “extracellular 2′,3′-cAMP-adenosine pathway” that involves intracellular production of 2′,3′-cAMP from mRNA, transport of 2′,3′-cAMP from the intracellular compartment to the cell surface, extracellular metabolism of 2′,3′-cAMP to 2′-AMP/3′-AMP, and extracellular conversion of 2′-AMP/3′-AMP to adenosine. Studies in isolated, perfused rat kidneys, as well as in various rat kidney cells in culture, support the existence of a renal extracellular 2′,3′-cAMP-adenosine pathway (17, 18, 34).
Both the extracellular 2′,3′-cAMP-adenosine pathway and the extracellular 3′,5′-cAMP-adenosine pathway exert biological effects. For example, in cultured rat preglomerular vascular smooth muscle cells and glomerular mesangial cells, extracellular 3′,5′-cAMP and 2′,3′-cAMP inhibit cell proliferation, in part via production of adenosine (17). Moreover, recent studies by Azarashvili et al. (1) demonstrate biological activity of 2′,3′-cAMP. These findings underscore the need to better clarify the enzymes involved in both of these cAMP-adenosine pathways.
An every growing repertoire of genetically altered mice are becoming available, many null with respect to enzymes involved in adenosine production. This resource affords the opportunity to investigate the involvement of specific enzymes in the metabolism of 3′,5′-cAMP and 2′,3′-cAMP to corresponding AMPs and adenosine. However, to date there is no information regarding whether mouse kidneys can express either the extracellular 3′,5′-cAMP-adenosine pathway or the extracellular 2′,3′-cAMP-adenosine pathway. The goal of the present study was to determine whether these cAMP-adenosine pathways exist in the mouse kidney and to examine the role of CD73 (ecto-5′-nucleotidase) in these pathways by employing kidneys obtained from CD73 knockout mice.
METHODS
Animals.
Male C57BL/6 mice were obtained from Taconic Farms (Germantown, NY) and CD73 −/− mice (2) (C57BL/6 × J129 background) and wild-type litter mates (CD73 +/+ mice) were bred and genotyped (2) at the University of Pittsburgh. Mice used for experiments were ∼10–12 wk of age. Animals were housed at the University of Pittsburgh Animal Facility and fed Pro Lab RHM 3000 rodent diet (PMI Feeds, St. Louis, MO). The Institutional Animal Care and Use Committee approved all procedures.
Isolated, perfused mouse kidney.
Mice were anesthetized with Inactin (100 mg/kg ip), the right ureter was ligated near the bladder, and the bladder was cannulated with PE-50 tubing to allow urine to drain from the left kidney. The aorta and vena cava were cleared above and below the left renal artery, and the distal aorta and vena cava were cannulated with PE-10 and PE-50 tubing, respectively, and these cannulas were advanced as near as possible to the origins of the left renal artery and vein. Tyrode's solution was perfused through the PE-10 tubing during this procedure to maintain renal perfusion during surgical procurement of the left kidney. All vessels branching from the aorta and vena cava near the left renal artery and renal vein were ligated, and the aorta and vena cava just proximal to the left renal artery and vein were ligated. The left kidney was transferred to a Hugo Sachs Elektronik-Harvard Apparatus GmbH (March-Hugstetten, Germany) kidney perfusion system. This system included the following components: model UP 100 Universal Perfusion System, model ISM 834 Channel Reglo Digital Roller Pump, a glass double-walled perfusate reservoir, a R 120144 glass oxygenator, mechanical integration of the oxygenator with the Universal Perfusion System UP 100, a Windkessel for absorption of pulsations, an inline holder for disc particle filters (80 μm), a temperature-controlled Plexiglas kidney chamber integrated with the UP 100, and a thermostatic circulator. The Plexiglas chamber contained a heat exchanger to maintain the temperature of the perfusate at 37°C at the point of entry into the tissue, and it also contained a device to extract bubbles from the perfusate just before the perfusate entered the kidney. The Tyrode's solution [137 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 1.1 mM MgCl2, 12 mM NaHCO3, 0.42 mM NaH2PO4, 5.6 mM D(+)-glucose, pH, 7.4, osmolality 295 mosmol/kgH2O] was maintained at 37°C in the double-walled perfusate reservoir, was bubbled with 95% oxygen-5% carbon dioxide, and was pumped by the roller pump: through a glass oxygenator (95% oxygen-5% carbon dioxide), through an inline particle filter, through an inline Windkessel, through a heat exchanger, through an inline bubble remover, and finally through the kidney. Kidneys were perfused (single-pass mode) at a constant flow (1.5 ml/min; approximates the normal renal blood flow for the mouse kidney) (30). Perfusion pressure was monitored with a Statham pressure transducer (model P23ID; Statham Division, Gould, Oxnard, CA) and recorded on a Grass model 79D polygraph (Grass Instruments, Quincy, MA). Perfusate exiting the renal vein was collected on ice and immediately frozen at −40°C for later analysis of purines by high-performance LC-MS/MS.
Experimental design.
Mouse kidneys were isolated and perfused as described above, and after a 2-h rest period (to allow endogenous purine production by the kidney to decline to a low and stable basal level), renal venous perfusate was collected for 1 min (1st basal sample). Next, either 3′,5′-cAMP or 2′,3′-cAMP was infused into the renal artery to achieve a final concentration in the renal arterial perfusate of 30 μM, and during the 5-min cAMP infusion, another 1-min renal venous perfusate sample was collected from 4 to 5 min into the infusion of the cAMP. After a 10-min washout period, a second 1-min basal sample of renal venous perfusate was collected, and the alternative cAMP was infused for 5 min with sampling of the renal venous perfusate from 4 to 5 min into the infusion. Whether a given cAMP was given first or second was chosen at random in this cross-over experimental design. To test the effects of energy depletion on the renal venous secretion of endogenous 2′,3′-cAMP, 3′,5′-cAMP, 2′-AMP, 3′-AMP, 5′-AMP, adenosine, and inosine, kidneys were isolated and perfused as described above, but after the rest period they were treated with either vehicle or a combination of metabolic inhibitors: iodoacetate (30 μM; inhibits glycolsis) (23–25) plus 2,4-dinitrophenol (30 μM; inhibits oxidative phosphorylation) (5, 21, 22). Renal venous samples were collected for 1 min before and 20 min into the treatment with the metabolic inhibitors. Renal perfusion pressures were ∼41 mmHg and were not affected by any of the treatments. The low-basal perfusion pressure reflects denervation and removal of the kidney from influences of circulating vasoconstrictors and is approximately what is obtained in the isolated, perfused rat kidney perfused at physiological flow rates with Tyrode's solution (29).
High-performance LC-MS/MS.
2′,3′-cAMP, 2′-AMP, 3′-AMP, 3′,5′-cAMP, 5′-AMP, adenosine, and inosine were from Sigma (St. Louis, MO), and 13C10-adenosine was from Medical Isotopes (Pelham, NH). Samples were injected onto an Agilent Zorbax eclipse XDB-C-18 column (3.5-μm beads; 2.1 × 100 mm) and quantified using a triple quadrupole mass spectrometer (TSQ Quantum-Ultra, ThermoFisher Scientific, San Jose, CA) operating in the selected reaction monitoring mode with a heated electrospray ionization source. The mobile phase, which was delivered by an ultra pressure liquid chromatographic system (Accela, ThermoFisher Scientific) at 300 μl/min, was a linear gradient of buffer A (0.1% formic acid in water) and buffer B (0.1% formic acid in methanol). The gradient (A/B) was 0 to 2 min, 98.5%/1.5%; 2 to 4 min, to 98%/2%; 5 to 6 min, to 92%/8%; 7 to 8 min, to 85%/15%; 9 to 11.5 min, to 98.5%/1.5%. Sample tray temperature was set at 4°C and the column temperature was kept at 20°C. For maximum sensitivities, 5′-AMP and adenosine were used for optimization of parameters of the ion source. Two separate tune files were used in the process of determination; 0 to 4.5 min, tune file 1 for the monitoring of 5′-AMP, 3′-AMP, and 2′,3′-cAMP; 4.5 to 11.5 min, tune file 2 for monitoring 2′-AMP, 3′,5′-cAMP, adenosine, internal standard, and inosine. The following parameters were the same in the TSQ tune files 1 and 2: ion spray voltage 3.8 kV, ion transfer tube temperature 270°C, source vaporization temperature 220°C, Q2 CID gas argon at 1.5 mTorr, sheath gas nitrogen at 50 psi, auxillary gas nitrogen at 40 psi, Q1/Q3 width 0.7/0.7 u full-width half-maximum, source CID off, scan width 0.5 u, scan time 0.05 s. The tube lens offset was 131 V for tune file 1 and 123 V for tune file 2. Five mass transitions were monitored: 330→136 for 2′,3′-cAMP and 3′,5′-cAMP with a collision energy of 28 V; 348→136 for 5′-AMP, 3′-AMP, and 2′-AMP with a collision energy of 21 V; 268→136 for adenosine with a collision energy of 19 V; 278→141 for 13C10-adenosine as internal standard with a collision energy of 19 V; 269→137 for inosine with a collision energy of 20 V.
Statistical analyses.
Statistical compares were performed on a priori contrasts with nonparametric tests, i.e., Mann-Whitney U-test for two-sample hypothesis testing and Wilcoxon Signed-Rank test for one-sample hypothesis testing. The criterion of significance was P < 0.05. One sample was an extreme outlier and was rejected from the data set after applying Dixon's Q test (P < 0.001) (4).
RESULTS
Metabolism of cAMPs by the isolated, perfused mouse kidney.
As shown in Fig. 1 infusions of 2′,3′-cAMP increased the renal venous secretion of 2′,3′-cAMP (P < 0.0001), but not 3′,5′-cAMP; in contrast, infusions of 3′,5′-cAMP increased the renal venous secretion of 3′,5′-cAMP (P < 0.0001), but not 2′,3′-cAMP. Dividing the concentration of cAMPs in the renal venous perfusate by the concentrations in the renal arterial perfusate revealed that ∼95 ± 1% of arterial 2′,3′-cAMP was removed from the venous compartment during a single pass through the mouse kidney, whereas ∼88 ± 1% of 3′,5′-cAMP was extracted (P < 0.0001 vs. 2′,3′-cAMP).
Fig. 1.
Bar graphs depict effects of intrarenal infusions of 2′,3′-cAMP and 3′,5′-cAMP on renal venous secretion of 2′,3′-cAMP (A) and 3′,5′-cAMP (B) in the isolated, perfused mouse kidney. aP < 0.0001 vs. respective basal value. Values are means ± SE.
Figure 2 illustrates the effects of intrarenal infusions of 2′,3′-cAMP and 3′,5′-cAMP on renal venous secretion of 2′-AMP, 3′-AMP, and 5′-AMP in the isolated, pefused mouse kidney. Basal levels of 2′-AMP before infusions of 3′,5′-cAMP and 2′,3′-cAMP were 7.2 ± 2.9 and 7.0 ± 4.2 pmol·min−1·g kidney−1, respectively; basal levels of 3′-AMP before infusions of 3′,5′-cAMP and 2′,3′-cAMP were 9.9 ± 1.2 and 7.3 ± 3.8 pmol·min−1·g kidney−1, respectively; and basal levels of 5′-AMP before infusions of 3′,5′-cAMP and 2′,3′-cAMP were 11.5 ± 3.5 and 16.5 ± 6.1 pmol·min−1·g kidney−1, respectively. Arterial infusions of 2′,3′-cAMP increased (P < 0.0001) the mean venous secretion of 2′-AMP (390-fold), 3′-AMP (497-fold), but they did not alter 5′-AMP secretion. In contrast, infusions of 3′,5′-cAMP did not affect venous secretion of either 2′-AMP or 3′-AMP, but they increased (P < 0.0001) secretion of 5′-AMP (5-fold).
Fig. 2.
Bar graphs illustrate effects of intrarenal infusions of 2′,3′-cAMP and 3′,5′-cAMP on renal venous secretion of 2′-AMP (A), 3′-AMP (B), and 5′-AMP (C) in the isolated, perfused mouse kidney. aP < 0.0001 vs. respective basal value. Values are means ± SE.
Basal levels of adenosine before infusions of 3′,5′-cAMP and 2′,3′-cAMP were 216 ± 35 and 237 ± 36 pmol·min−1·g kidney−1, respectively, and basal levels of inosine before infusions of 3′,5′-cAMP and 2′,3′-cAMP were 327 ± 82 and 233 ± 33 pmol·min−1·g kidney−1, respectively. As shown in Fig. 3, arterial infusions of 2′,3′-cAMP increased (P < 0.0001) the mean venous secretion of adenosine (18-fold) and its metabolite inosine (7-fold). Similarly, infusions of 3′,5′-cAMP increased (P < 0.0001) secretion of adenosine (17-fold) and inosine (6-fold).
Fig. 3.
Bar graphs show effects of intrarenal infusions of 2′,3′-cAMP and 3′,5′-cAMP on renal venous secretion of adenosine (A) and inosine (B) in the isolated, perfused mouse kidney. aP < 0.0001 vs. respective basal value. Values are means ± SE.
Release of purines from isolated, perfused mouse kidney by metabolic inhibitors.
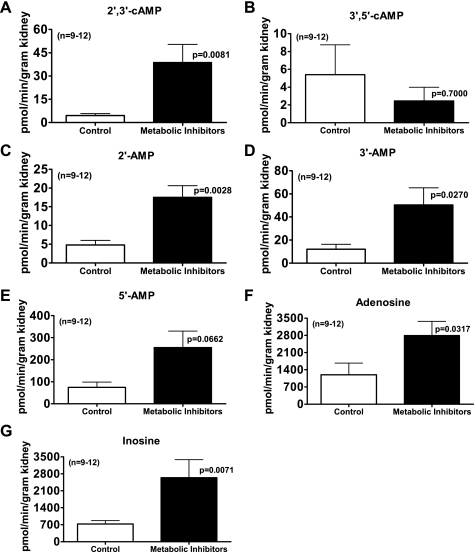
Figure 4 illustrates a typical LC-MS/MS chromatogram demonstrating unequivocal detection of endogenous 2′,3′-cAMP, 2′-AMP, 3′-AMP, 5′-AMP, adenosine, and inosine in the isolated, perfused mouse kidney during metabolic poisoning. As shown in Fig. 5, inhibiting energy production with the metabolic poisons iodoacetate plus 2,4-dinitrophenol increased the renal venous secretion of 2′,3′-cAMP by eightfold (P = 0.0081); in contrast, metabolic inhibition tended to suppress renal venous secretion of 3′,5′-cAMP (not statistically significant). In parallel with the increase in 2′,3′-cAMP, renal injury with metabolic inhibitors also increased the mean secretion of 2′-AMP by fourfold (P = 0.0028), 3′-AMP by fourfold (P = 0.0270), 5′-AMP by threefold (P = 0.0662), adenosine by twofold (P = 0.0317), and inosine by sevenfold (P = 0.0071).
Fig. 4.
Chromatogram demonstrating separation and detection of endogenous 2′,3′-cAMP, 3′,5′-cAMP, 2′-AMP, 3′-AMP, 5′-AMP, adenoisine, and inosine in the isolated, perfused mouse kidney.
Fig. 5.
Bar graphs summarize the effects of inhibiting energy production with the metabolic poisons iodoacetate plus 2,4-dinitrophenol (each at 30 μmol/l) on the renal venous secretion of 2′,3′-cAMP (A), 3′,5′-cAMP (B), 2′-AMP (C), 3′-AMP (D), 5′-AMP (E), adenosine (F), and inosine (G) in the isolated, perfused mouse kidney. Values represent means ± SE.
Effects of CD73 knockout on the 2′,3′-cAMP-adenosine and 3′,5′-cAMP-adenosine pathways.
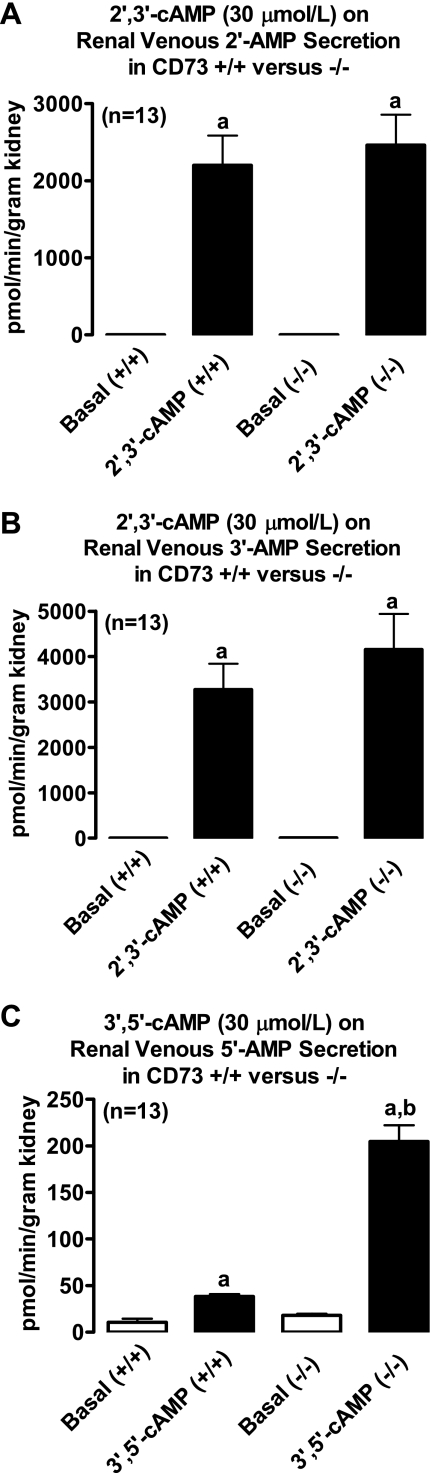
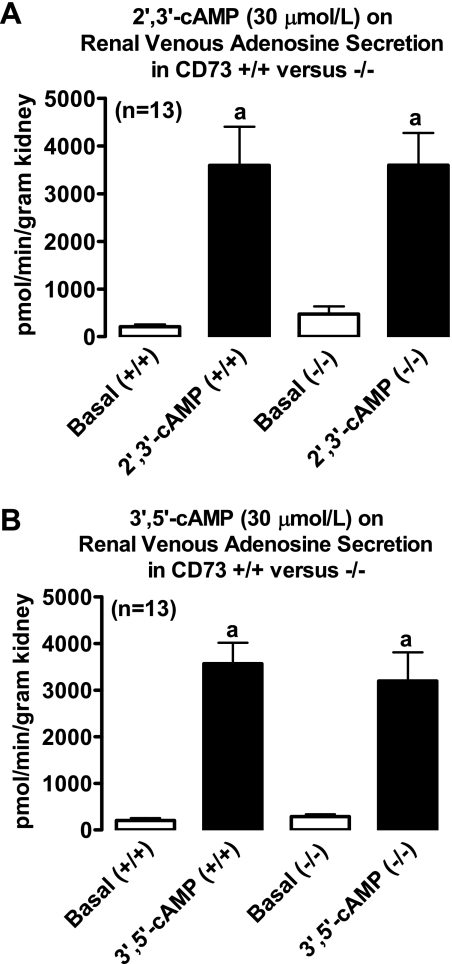
Intrarenal infusions of 2′,3′-cAMP significantly (P < 0.0001) and similarly increased renal venous secretion of 2′-AMP (Fig. 6), 3′-AMP (Fig. 6), adenosine (Fig. 7), and inosine (Fig. 8) in kidneys from CD73 +/+ vs. CD73 −/− mice. In contrast, the ability of 3′,5′-cAMP to increase renal venous secretion of 5′-AMP was 6.7-fold greater in CD73 −/− kidneys compared with CD73 +/+ kidneys (P < 0.0001; Fig. 6). Importantly, although 3′,5′-cAMP increased 5′-AMP levels much more in CD73 −/− kidneys compared with CD73 +/+ kidneys, this did not translate into an attenuated increase in adenosine. As shown in Fig. 7, 3′,5′-cAMP similarly increased renal venous adenosine secretion in CD73 +/+ vs. CD73 −/− kidneys. However, the 3′,5′-cAMP-induced increase in renal venous inosine secretion was significantly (P = 0.0016) attenuated in CD73 −/− kidneys (Fig. 8).
Fig. 6.
A and B depict effects of intrarenal infusions of 2′,3′-cAMP on renal venous secretion of 2′-AMP (A) and 3′-AMP (B) in isolated, perfused mouse kidneys obtained from CD73 wild-type mice (+/+) and CD73 null mice (−/−). C indicates effects of intrarenal infusions of 3′,5′-cAMP on renal venous secretion of 5′-AMP in isolated, perfused mouse kidneys obtained from +/+ and −/− mice. aP < 0.0001 vs. respective basal value. bP < 0.0001 vs. corresponding value in +/+ group. Values are means ± SE.
Fig. 7.
Bar graphs depict effects of intrarenal infusions of 2′,3′-cAMP (A) and 3′,5′-cAMP (B) on renal venous secretion of adenosine in isolated, perfused mouse kidneys obtained from CD73 wild-type mice (+/+) and CD73 null mice (−/−). aP < 0.0001 vs. respective basal value. Values are means ± SE.
Fig. 8.
Bar graphs depict effects of intrarenal infusions of 2′,3′-cAMP (A) and 3′,5′-cAMP (B) on renal venous secretion of inosine in isolated, perfused mouse kidneys obtained from CD73 wild-type mice (+/+) and CD73 null mice (−/−). aP < 0.0001 vs. respective basal value. bP < 0.002 vs. corresponding value in +/+ group. Values are means ± SE.
DISCUSSION
The present study shows that vascular delivery of 2′,3′-cAMP causes a phenomenal increase in renal venous secretion of both 2′-AMP and 3′-AMP, without affecting renal venous secretion of 5′-AMP. Because 2′,3′-cAMP is highly hydrophilic and would not readily diffuse into cells, most of the renal metabolism is likely extracellular conversion of 2′,3′-cAMP to downstream metabolites. These results imply that a major route of metabolism of extracellular 2′,3′-cAMP by the mouse kidney is by hydrolysis to 2′-AMP via an ecto-2′,3′-cAMP-3′-phosphodiesterase and to 3′-AMP via an ecto-2′,3′-cAMP-2′-phosphodiesterase. The lack of an increase in renal venous 5′-AMP by administration of 2′,3′-cAMP is expected since breaking either the 2′ or 3′ phosphoester linkage would not produce 5′-AMP. Associated with the extraction of 2′,3′-cAMP and appearance of 2′-AMP and 3′-AMP is a multifold increase in the renal venous secretion of both adenosine and its immediate metabolite inosine. These results strongly imply the presence of ecto-2′-nucleotidases and ecto-3′-nucleotidases that can metabolize 2′-AMP and 3′-AMP, respectively, to adenosine. Thus, as previously reported for the rat kidney (18), the mouse kidney supports a very robust extracellular 2′,3′-cAMP-adenosine pathway (extracellular 2′,3′-cAMP → 2′-AMP/3′-AMP → adenosine).
As with 2′,3′-cAMP, vascular 3′,5′-cAMP is also metabolized by the mouse kidney. In this regard, administration of 3′,5′-cAMP into the renal artery of the isolated, perfused mouse kidney causes a large increase in renal venous secretion of 5′-AMP, without affecting renal venous secretion of either 2′-AMP or 3′-AMP. As with 2′,3′-cAMP, 3′,5′-cAMP is highly hydrophilic and would not readily diffuse into cells, so most of the metabolism is likely extracellular conversion of 3′,5′-cAMP to downstream metabolites. These results imply that a major route of metabolism of extracellular 3′,5′-cAMP by the mouse kidney is by hydrolysis to 5′-AMP via an ecto-3′,5′-cAMP-3′-phosphodiesterase. The lack of an increase in renal venous 2′-AMP by administration of 3′,5′-cAMP is expected since breaking either the 3′ or 5′ phosphoester linkage would not produce 2′-AMP. Although in theory 3′,5′-cAMP could be metabolized to 3′-AMP, the kidney evidently does not express an ecto-3′,5′-cAMP-2′-phosphodiesterase that metabolizes 3′,5′-cAMP to 3′-AMP. Thus, any 3′-AMP formation by the kidney is likely from metabolism of 2′,3′-cAMP and not from metabolism of 3′,5′-cAMP. Similar to 2′,3′-cAMP, associated with the extraction of 3′,5′-cAMP and appearance of 5′-AMP is a multifold increase in the renal venous secretion of both adenosine and inosine. These results imply the presence of ecto-5′-nucleotidases that metabolize 5′-AMP to adenosine. Thus, as with the rat kidney (15, 19, 27, 28), the mouse kidney also supports a robust extracellular 3′,5′-cAMP-adenosine pathway (extracellular 3′,5′-cAMP → 5′-AMP → adenosine).
Importantly, nearly all 2′,3′-cAMP is removed from the vascular compartment in a single pass through the mouse kidney, and a high, but somewhat lesser, percentage of 3′,5′-cAMP in the vascular compartment is also extracted by the mouse kidney in a single pass. No doubt, robust vascular metabolism of these cAMPs contributes importantly to their high renal clearance. Nonetheless, 3′,5′-cAMP is filtered by the glomerulus and is metabolized in the tubular compartment (15), so urinary excretion of unchanged cAMPs and metabolism of cAMPs in the tubular compartment likely also contribute to the high degree of clearance of cAMPs by the kidney. Because the urine flow rate in a mouse kidney is only 0.7 μl/min (38) and we infused the cAMPs for 5 min only, we did not attempt to quantify the cAMPs and their metabolites in the minute volumes of urine.
The existence and role of 3′,5′-cAMP in cells and tissues are, of course, undisputed. However, the possibility that intact cells and tissues produce 2′,3′-cAMP is a very new concept. While studying the release of 3′,5′-cAMP from isolated, perfused rat kidneys, we observed a chromatographic peak that was due to an endogenous substance that was not 3′,5′-cAMP, yet mass spectrometry revealed that the unknown peak had the same parent ion as 3′,5′-cAMP and fragmented to the same daughter ion as 3′,5′-cAMP (34). Subsequently, we identified the substance as a positional isomer of 3′,5′-cAMP, namely 2′,3′-cAMP, and provided support for the notion that 2′,3′-cAMP derives from mRNA breakdown triggered by energy depletion (34). Very recently, Pabst et al. (31) using mass spectrometry (LC-ESI-MS with Q-TOF) independently confirm the biological existence of 2′,3′-cAMP, and recent studies by Rao et al. (33) reveal at least six different enzymes that hydrolyze 2′,3′-cAMP to 3′-AMP. Although the existence of 2′,3′-cAMP-3′-phosphodiesterase (CNPase; metabolizes 2′,3′-cAMP to 2′-AMP) has been recognized for decades, its role in physiology has been enigmatic (35, 37).
As previously published, in the rat kidney, energy depletion stimulates the extracellular 2′,3′-cAMP-adenosine pathway (18, 34). In this regard, energy depletion causes breakdown of mRNA with the production of 2′,3′-cAMP from the processing of mRNA by RNAses. Intracellular 2′,3′-cAMP is then transported from the intracellular compartment to the cell surface where it is metabolized to 2′-AMP and 3′-AMP; with subsequent metabolism of the extracellular AMPs to adenosine. Given the novelty of the concept that energy depletion stimulates the extracellular 2′,3′-cAMP-adenosine pathway, it is important to test whether this occurs in the mouse kidney.
Importantly, as shown in Fig. 4, LC-MS/MS reveals unambiguously that 2′,3′-cAMP, 2′-AMP, and 3′-AMP are produced by the mouse kidney. Moreover, the present studies show that energy depletion with metabolic poisons (iodoacetate plus 2,4-dinitrophenol) causes a multifold increase in the renal venous secretion rate of not only 2′,3′-cAMP, but also 2′-AMP, 3′-AMP, adenosine, and inosine. Thus, the mouse kidney, like the rat kidney, expresses an endogenous extracellular 2′,3′-cAMP-adenosine pathway. As with the rat kidney (18, 34), energy depletion tends to inhibit, not stimulate, the renal venous secretion of 3′,5′-cAMP, so the increase in 5′-AMP induced by energy depletion is not due to production of 3′,5′-cAMP, but rather most likely is due to metabolism of ATP and ADP to 5′-AMP. The discovery that energy depletion activates the 2′,3′-cAMP-adenosine pathway means that the increase in adenosine produced by energy depletion can no longer be attributed solely to metabolism of high-energy adenine nucleotides (ATP and ADP) to 5′-AMP. The results of the present study in mouse kidneys and our previous results in rat kidneys suggest that energy depletion increases extracellular adenosine levels not only by metabolism of 5′-AMP to adenosine, but also via metabolism of 2′-AMP and 3′-AMP to adenosine.
CD73 is an ecto-enzyme that metabolizes extracellular 5′-AMP to adenosine (39). The present study demonstrates that there is no detectable quantitative difference in the metabolism of 2′,3′-cAMP to 2′-AMP, 3′-AMP, adenosine, or inosine in CD73 −/− vs. CD73 +/+ kidneys, indicating no involvement of this ecto-enzyme in the extracellular 2′,3′-cAMP-adenosine pathway. In contrast, our results do indicate the participation of CD73 in the extracellular 3′,5′-cAMP-adenosine pathway. In this regard, administration of 3′,5′-cAMP to CD73 −/− kidneys causes a much greater accumulation of 5′-AMP in the renal venous perfusate in CD73 −/− compared with CD73 +/+ kidneys. This result has two implications. One implication is that the activity of CD73, by metabolizing extracellular 5′-AMP to adenosine, masks the magnitude of the flux of extracellular 3′,5′-cAMP to extracellular 5′-AMP. Thus, an important conclusion from the present study is that extracellular 3′,5′-cAMP is metabolized to 5′-AMP by an ecto-phosphodiesterase much more readily than previously envisioned. A second implication is that because knockout of CD73 causes a large accumulation of 5′-AMP behind the metabolic block, any differential phenotype between CD73 −/− and +/+ mice could well be due to the accumulation and biological effects of 5′-AMP. Indeed, a recent report by Rajakumar and co-workers (32) supports the concept that inhibition of CD73 may be renoprotective by increasing renal levels of 5′-AMP.
Interestingly, despite the obvious role of CD73 in metabolizing 5′-AMP to adenosine as evidence by the remarkable accumulation of 5′-AMP, the production of adenosine from 3′,5′-cAMP is unimpaired in CD73 −/− kidneys. This is an important observation because it implies that CD73 knockout mice are not necessarily adenosine deficient. Most likely the lack of effect of CD73 knockdown on adenosine levels is due to two mechanisms. First, as 5′-AMP accumulates in CD73 −/−, it is likely metabolized to adenosine by alternative low-affinity/high-capacity ecto-nucleotidases that provide “shunt” pathways. A second mechanism may involve adenosine deaminase. Although 3′,5′-cAMP increases adenosine similarly in CD73 −/− vs. +/+ kidneys, the increase in inosine is without a doubt reduced in CD73 −/− kidneys. This finding supports the conclusion that deficiency in CD73 in some manner reduces adenosine deaminase activity, a mechanism that also would preserve extracellular adenosine levels in the face of reduced CD73 activity. Whether these compensatory pathways are engaged only after life-long knockout of CD73 in CD73 −/− mice is unknown. However, previous studies with pharmacological inhibition of CD73 demonstrate that in the rat kidney acute blockade of CD73 limits the conversion of 3′,5′-cAMP to adenosine (28), a finding supporting the concept that the compensatory pathways observed in CD73 −/− mice are the result of chronic, not acute, CD73 deficiency.
What is the relative contribution of extracellular 3′,5′-cAMP vs. 2′,3′-cAMP vs. extracellular nucleotides (ATP and ADP) to the extracellular levels of adenosine in the kidney? Most likely the relative contributions of these pathways depend on the precise physiological/pathophysiological situation and renal biophase. Previous studies show that activation of β-adrenoceptors or direct activation of adenylyl cyclase stimulates (as expected) the 3′,5′-cAMP-adenosine pathway (8, 10, 27). In anesthetized rats, in vivo inhibition of the ecto-phosphodiesterase that metabolizes extracellular 3′,5′-cAMP to extracellular 5′-AMP reduces urinary adenosine by ∼50% (14) and renal interstitial adenosine by ∼50% (26). Thus, under these conditions, adenylyl cyclase appears to contribute to some compartments of extracellular adenosine in the kidney. Also, in anesthetized rats, intraportal glucagon increases plasma levels of 3′,5′-cAMP by 10-fold and this is associated with a 2-fold increase in renal interstitial levels of adenosine (assessed by microdialysis) and a 2-fold increase in the urinary excretion of adenosine (15). Therefore, during hormonal stimulation of adenylyl cyclase, the contribution of the 3′,5′-cAMP-adenosine pathway to renal adenosine is higher. Presumably, under physiological conditions in which adenylyl cyclase activity is low, the 3′,5′-cAMP-adenosine pathway would not contribute meaningfully to extracellular adenosine. Less is known regarding the potential for 2′,3′-cAMP to contribute to adenosine. As shown in the present study, energy depletion inhibits 3′,5′-cAMP production, so this pathway of adenosine formation does not contribute to adenosine production in response to energy depletion. On the other hand, energy depletion causes a multifold increase in 2′,3′-cAMP, 2′-AMP, 3′-AMP, and adenosine, suggesting that the 2′,3′-cAMP-adenosine pathway may importantly contribute to adenosine production under these conditions. However, 5′-AMP also increases so it is not possible to deduce precisely how much adenosine arises from 5′-AMP and how much arises from 2′-AMP and 3′-AMP. It is conceivable that initially 5′-AMP is the primary source of adenosine, with the relative contribution of 2′-AMP and 3′-AMP increasing as the ATP/ADP pool is depleted and mRNA breakdown proceeds.
Are extracellular levels of 3′,5′-cAMP and 2′,3′-cAMP sufficient to exert biological effects via adenosine? Nanomolar concentrations of 2′,3′-cAMP and 3′,5′-cAMP are capable of affecting cell proliferation (17), and stimulation of adenylyl cyclase can achieve nanomolar plasma levels of 3′,5′-cAMP (15). Also, based on pharmacological evidence, efflux of 3′,5′-cAMP from vascular smooth cells during stimulation of adenylyl cyclase is sufficient to inhibit cell proliferation via conversion of 3′,5′-cAMP to adenosine with activation of A2 receptors (10). It is likely that both endogenous circulating and locally produced 3′,5′-cAMP have biological effects via adenosine. The situation with 2′,3′-cAMP is more difficult to assess experimentally. In pilot studies, we examined the half-life of 2′,3′-cAMP, 2′-AMP, and 3′-AMP in blood and plasma and found that the half-life of these purines is exceedingly short (a few seconds) due to rapid conversion to adenosine in blood and plasma. Clearly, these purines are not circulating autocoids, and measurements of plasma levels of 2′,3′-cAMP, 2′-AMP, and 3′-AMP may be of little value. Whether energy depletion (or cell injury) raises 2′,3′-cAMP levels at the biophase of the cell membrane sufficiently to have biological effects via adenosine is presently unknown and must await development of selective methods of blocking this pathway.
In conclusion, the present study establishes that mouse kidneys convert 2′,3′-cAMP and 3′,5′-cAMP to their corresponding AMPs, which are subsequently metabolized to adenosine. In this regard, energy depletion engages the 2′,3′-cAMP-adenosine, but not the 3′,5′-cAMP-adenosine, pathway. This study also establishes that although CD73 is involved in the 3′,5′-cAMP-adenosine pathway, compensatory mechanisms (i.e., shunt pathways and modulation of adenosine metabolism pathways) yield CD73's participation nonessential, at least in the intact kidney with chronic CD73 deficiency.
GRANTS
This work was supported by National Institutes of Health Grants HL069846, DK068575, and DK079307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Jürgen B. Schnermann (National Institute of Diabetes and Digestive and Kidney Diseases) for the CD73 knockout mice.
REFERENCES
- 1. Azarashvili T, Krestinina O, Galvita A, Grachev D, Baburina Y, Stricker R, Evtodienko Y, Reiser G. Ca2+-dependent permeability transition regulation in rat brain mitochondria by 2′,3′-cyclic nucleotides and 2′,3′-cyclic nucleotide 3′-phosphodiesterase. Am J Physiol Cell Physiol 296: C1428–C1439, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest 114: 634–642, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiavegatti T, Costa VL, Jr, Araujo MS, Godinho RO. Skeletal muscle expresses the extracellular cyclic AMP-adenosine pathway. Br J Pharmacol 153: 1331–1340, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dean RB, Dixon WJ. Simplified statistics for small numbers of observations. Anal Chem 23: 636–638, 1951 [Google Scholar]
- 5. Desquiret V, Loiseau D, Jacques C, Douay O, Malthiery Y, Ritz P, Roussel D. Dinitrophenol-induced mitochondrial uncoupling in vivo triggers respiratory adaptation in HepG2 cells. Biochim Biophys Acta 1757: 21–30, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Dubey RK, Gillespie DG, Jackson EK. Cyclic AMP-adenosine pathway induces nitric oxide synthesis in aortic smooth muscle cells. Hypertension 31: 296–302, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Dubey RK, Gillespie DG, Mi Z, Jackson EK. Cardiac fibroblasts express the cAMP-adenosine pathway. Hypertension 36: 337–342, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Dubey RK, Gillespie DG, Mi Z, Jackson EK. Endogenous cyclic AMP-adenosine pathway regulates cardiac fibroblast growth. Hypertension 37: 1095–1100, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Dubey RK, Gillespie DG, Mi Z, Jackson EK. Extracellular 3′,5′-cyclic AMP-adenosine pathway inhibits glomerular mesangial cell growth. J Pharmacol Exp Ther 333: 808–815, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dubey RK, Gillespie DG, Mi Z, Rosselli M, Keller PJ, Jackson EK. Estradiol inhibits smooth muscle cell growth in part by activating the cAMP-adenosine pathway. Hypertension 35: 262–266, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Dubey RK, Mi Z, Gillespie DG, Jackson EK. Cyclic AMP-adenosine pathway inhibits vascular smooth muscle cell growth. Hypertension 28: 765–771, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Giron MC, Bin A, Brun P, Etteri S, Bolego C, Florio C, Gaion RM. Cyclic AMP in rat ileum: evidence for the presence of an extracellular cyclic AMP-adenosine pathway. Gastroenterology 134: 1116–1126, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Jackson EK, Mi Z. Preglomerular microcirculation expresses the cAMP-adenosine pathway. J Pharmacol Exp Ther 295: 23–28, 2000 [PubMed] [Google Scholar]
- 14. Jackson EK, Mi Z, Dubey RK. The extracellular cAMP-adenosine pathway significantly contributes to the in vivo production of adenosine. J Pharmacol Exp Ther 320: 117–123, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Jackson EK, Mi Z, Zacharia LC, Tofovic SP, Dubey RK. The pancreatohepatorenal cAMP-adenosine mechanism. J Pharmacol Exp Ther 321: 799–809, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Jackson EK, Raghvendra DK. The extracellular cyclic AMP-adenosine pathway in renal physiology. Annu Rev Physiol 66: 571–599, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Jackson EK, Ren J, Gillespie DG, Dubey RK. Extracellular 2′,3′-cyclic adenosine 5′-monophosphate is a potent inhibitor of preglomerular vascular smooth muscle cell and mesangial cell growth. Hypertension 56: 151–158 (PMC2892387), 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem 284: 33097–33106 (PMC2785151), 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jackson EK, Ren J, Zacharia LC, Mi Z. Characterization of renal ecto-phosphodiesterase. J Pharmacol Exp Ther 321: 810–815, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Jackson EK, Zacharia LC, Zhang M, Gillespie DG, Zhu C, Dubey RK. cAMP-adenosine pathway in the proximal tubule. J Pharmacol Exp Ther 317: 1219–1229, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Joel CD, Neaves WB, Rabb JM. Mitochondria of brown fat: oxidative phosphorylation sensitive to 2,4,-dinitrophenol. Biochem Biophys Res Commun 29: 490–495, 1967 [DOI] [PubMed] [Google Scholar]
- 22. Kaminsky YG, Kosenko EA. Different effects of 2,4-dinitrophenol on rat liver mitochondrial oxidation of various substrates: succinate and glutamate vs 3-hydroxybutyrate and glycerol 3-phosphate. Int J Biochem 19: 97–99, 1987 [DOI] [PubMed] [Google Scholar]
- 23. Konings AW. The influence of iodoacetate on the mechanism of nuclear glucose oxidation. Experientia 27: 253–254, 1971 [DOI] [PubMed] [Google Scholar]
- 24. McKee RW, Parks ME, Dickey A. Influence of iodoacetate on glycolytic intermediates and on respiration in Ehrlich-Lettre ascites carcinoma cells. Arch Biochem Biophys 124: 450–455, 1968 [DOI] [PubMed] [Google Scholar]
- 25. McKee RW, Wong W, Landman M. Effects of iodoacetate on glycolysis and respiration in Ehrlich-Lettre ascites carcinoma cells. Biochim Biophys Acta 105: 410–423, 1965 [DOI] [PubMed] [Google Scholar]
- 26. Mi Z, Herzer WA, Zhang Y, Jackson EK. 3-Isobutyl-1-methylxanthine decreases renal cortical interstitial levels of adenosine and inosine. Life Sci 54: 277–282, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Mi Z, Jackson EK. Evidence for an endogenous cAMP-adenosine pathway in the rat kidney. J Pharmacol Exp Ther 287: 926–930, 1998 [PubMed] [Google Scholar]
- 28. Mi Z, Jackson EK. Metabolism of exogenous cyclic AMP to adenosine in the rat kidney. J Pharmacol Exp Ther 273: 728–733, 1995 [PubMed] [Google Scholar]
- 29. Oellerich WF, Malik KU. Neuropeptide Y modulates the vascular response to periarterial nerve stimulation primarily by a postjunctional action in the isolated perfused rat kidney. J Pharmacol Exp Ther 266: 1321–1329, 1993 [PubMed] [Google Scholar]
- 30. Oppermann M, Hansen PB, Castrop H, Schnermann J. Vasodilatation of afferent arterioles and paradoxical increase of renal vascular resistance by furosemide in mice. Am J Physiol Renal Physiol 293: F279–F287, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Pabst M, Grass J, Fischl R, Leionard R, Jin C, Hinterkoirner G, Borth N, Altmann F. Nucleotide and nucleotide sugar analysis by liquid chromatography electrospray ionization-mass spectrometry on surface-conditioned porous graphitic carbon. Anal Chem 82: 9782–9788, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rajakumar SV, Lu B, Crikis S, Robson SC, d′Apice AJF, Cowan PJ, Dwyer KM. Deficiency or inhibition of CD73 protects in mild kidney ischemia-reperfusion injury. Transplantation 90: 1260–1264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rao F, Qi Y, Murugan E, Pasunooti S, Ji Q. 2′,3′-cAMP hydrolysis by metal-dependent phosphodiesterases containing DHH, EAL, and HD domains is nonspecific: implications for PDE screening. Biochem Biophys Res Commun 398: 500–505, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Ren J, Mi Z, Stewart NA, Jackson EK. Identification and quantification of 2′,3′-cAMP release by the kidney. J Pharmacol Exp Ther 328: 855–865 (PMC2646794), 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sprinkle TJ. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit Rev Neurobiol 4: 235–301, 1989 [PubMed] [Google Scholar]
- 36. Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 86: 901–940, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Vogel US, Thompson RJ. Molecular structure, localization, and possible functions of the myelin-associated enzyme 2′,3′-cyclic nucleotide 3′-phosphodiesterase. J Neurochem 50: 1667–1677, 1988 [DOI] [PubMed] [Google Scholar]
- 38. Yu Y, Stubbe J, Ibrahim S, Song WL, Smyth EM, Funk CD, Fitzgerald GA. Cyclooxygenase-2-dependent prostacyclin formation and blood pressure homeostasis: targeted exchange of cyclooxygenase isoforms in mice. Circ Res 106: 337–345, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zimmermann H. Ectonucleotidases in the nervous system. Novartis Found Symp 276: 113–128, 2006 [PubMed] [Google Scholar]