Abstract
We recently reported that necrotic renal proximal epithelial cells (RPTC) stimulate the expression of P2X7 receptor in renal fibroblasts and that P2X7 receptor mediates deleterious epithelial-fibroblast cross talk. The present study was carried out to investigate the signaling mechanism of necrotic RPTC-induced P2X7 expression in cultured renal interstitial fibroblasts (NRK-49F). Exposure of NRK-49F to necrotic RPTC supernatant (RPTC-Sup) induced a time- and dose-dependent phosphorylation of several signaling pathways including extracellular signal-regulated kinases (ERK1/2), p38, c-Jun N-terminal kinases (JNKs), and AKT in NRK-49F. Pharmacological inhibition of ERK1/2, but not p38, JNK, and AKT pathways, blocked RPTC-Sup-induced P2X7 expression and renal interstitial fibroblast death. Knockdown of ERK1/2 or MEK1, a direct upstream activator of ERK1/2, also reduced RPTC-Sup-induced P2X7 expression and cell death of renal fibroblasts. Conversely, overexpression of MEK1 enhanced these responses. Upon necrotic RPTC exposure, phosphorylation of Elk1, a transcriptional factor targeted by ERK1/2, was increased in NRK-49F, and knockdown of Elk1 by siRNA remarkably reduced RPTC-Sup-induced P2X7 expression as well as renal fibroblast death. Furthermore, silencing of MEK1 inhibited Elk1 phosphorylation in response to necrotic RPTC, whereas overexpression of MEK1 increased Elk1 phosphorylation. Taken together, these data reveal that necrotic RPTC induces P2X7 expression in renal fibroblasts through activation of the MEK1-ERK1/2-Elk1 signaling pathway.
Keywords: renal fibroblast, ERK1/2, Elk1
extracellular atp-sensitive receptors (purinergic receptors; P2 receptors) play a wide range of physiological role in kidney tissue. Most of the P2 receptors are normally expressed in kidney and involved in physiological events. But some of the P2 receptors are not expressed under normal condition, and their expression is increased during renal pathological conditions (15). P2X7 is such a receptor, which is barely expressed or not detectable in normal kidney but it is highly expressed in some disease models or human diseases (15, 17, 18). For example, the P2X7 expression is upregulated in rat model of glomerulonephritis and in patients with proliferative and membranous lupus glomerulonephritis (14, 16). Moreover, P2X7 is also expressed in diabetic and hypertensive rat kidney (18). Although the functional role of P2X7 in most of those diseases is not clear, a recent study showed that genetic or pharmacological blockade of P2X7 attenuates renal injury in experimental glomerulonephritis (14, 16), suggesting that the P2X7 receptor is a potential therapeutic target. As such, understanding the mechanism that regulates P2X7 receptor expression will aid in development of new therapeutic approach to treat kidney diseases.
Currently, very little is known about the signaling mechanisms underlying the expression of P2X7 induced under various pathological conditions. However, it is well-known that gene expression is regulated by different signaling pathways. Mitogen-activated protein kinases (MAPK) and the phosphatidylinositide 3,4,5-trisphosphate kinase (PI3K)/Akt signal transduction pathway are known to play an important role in driving gene expression in response to a variety of stimuli including growth factors, proinflammatory cytokines, and some stresses. MAPK pathways are composed of extracellular signal-regulated kinase (ERK)1/2, Jun-NH2-terminal kinase (JNK), and p38 pathways. Upon activation, these kinases are phosphoryated and then translocated to nuclei where they induce activation of various transcription factors including Elk1, Jun, and fos. Numerous studies showed that MAPKs are critical players in pathophysiology of renal damage and their activation/expression increased during several renal diseases (3, 7).
Elk1 is a transcriptional factor that can be transactivated by all three MAPK pathways and it plays a pivotal role in immediate early gene induction by various extracellular stimuli. Phosphorylation of Elk1 at serine 383 is crucial for its transcriptional activity (21). Mounting evidences indicate that activation of ERK1/2 and Elk1 can result in both apoptotic and necrotic form of cell death under certain pathological conditions including renal ischemia-reperfusion (11–13, 19, 20, 24). It has also been reported that some cytokines (i.e., interleukin-1β, tumor necrosis factor-α, and interferon-γ) induce expression of P2X7 and can also activate ERK1/2 and other MAPK pathways. Bioinformatics analysis of transcription enhancing region of P2X7 gene reveals that it contains putative binding sites for Elk1 (22), suggesting that there is a possible link between P2X7 expression and activation of MAPK-Elk1 pathways. This hypothesis has not been tested yet.
Recently, we showed that direct exposure of rat renal interstitial fibroblasts (NRK-49F) to cell supernatant from necrotic renal proximal tubular cells (RPTC) induces P2X7 expression (10), which is required for death of renal fibroblasts. However, it remains unknown about how P2X7 expression is upregulated in renal fibroblasts upon exposure of renal fibroblasts to necrotic RPTC supernatant. The present study was carried out to extend our previous findings and to delineate the signaling pathway leading to P2X7 expression under this condition. Using various pharmacological and molecular approaches, we identify that activation of the ERK/Elk1 pathway provides the signal necessary for necrotic RPTC-induced expression of P2X7 and death of renal fibroblasts.
MATERIALS AND METHODS
Chemicals and antibodies.
LY294002 was obtained from Biomol (Plymouth Meeting, PA). U0126, SB203580, and SP600125 were purchased from Calbiochem (San Diego, CA). The small interference RNA (siRNA) specific for rat P2X7, ERK1, ERK2, Elk1, and mitogen-activated ERK kinase 1 (MEK1) was purchased from Invitrogen (Carlsbad, CA). Antibodies to P2X7, p-Elk1, Elk1, and GAPDH were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). All other antibodies used in this study were purchased from Cell Signaling Technology (Danvers, MA). α-Tubulin and apyrase (Grade VII) and all other chemicals were purchased from Sigma (St. Louis, MO).
Cell culture.
Rat renal interstitial fibroblasts (NRK-49F) and immortalized mouse RPTC were used in this study. NRK-49F cells were obtained from American Type Culture Collection (Rockville, MD). Immortalized mouse RPTC were kindly provided by Dr. Elsa Bella-Reuss. This cell line has a brush border and a conserved epithelial morphology (5). Both of them were cultured in DMEM/F12 (Sigma) containing 5% FBS, penicillin, and streptomycin in an atmosphere of 5% CO2-95% air at 37°C. NRK-49F was confluent when used for various treatments. When necessary, various inhibitors were directly added to the culture and then incubated for desired time as indicated in the figure legends.
Preparation of necrotic RPTC cell lysate and treatment.
RPTC were harvested and washed twice with sterile PBS and then reconstituted to a cell number of 2 × 106/ml in complete culture media. For the induction of necrosis, RPTC cells were subjected to repetitive (5 cycles) freezing at −80°C and thawing at 37°C. Then, cell lysate was centrifuged at 15,000 rpm for 20 min to obtain necrotic cell supernatant (RPTC-Sup). For the experiments, 1/3 volume (330 μl/ml) of medium was removed and 330 μl of RPTC-Sup were added to the culture. When we replaced 330 μl/ml of medium with RPTC-Sup, the final concentration of cell lysate was equal to RPTC-Sup from 6.6 × 105 cells/ml. As a control, the same volume of complete medium (5 times freeze and thaw) was added.
Transfection of siRNA into cells.
siRNA oligonucleotides targeted specifically to rat P2X7, Elk1, MEK1, ERK1/2 were used in this experiment. siRNA (750 pmol) was transfected into NRK-49F (1 × 106 cells) using the Nucleofector Kit V and the Amaxa Nucleofector device according to the manufacturer's instructions (Gaithersburg, MD). In parallel, 750 pmol of scrambled siRNA were used to control for off-target changes in NRK-49F. After transfection, cells were cultured in DMEM/F-12 for 24 h before used for the experiments.
Transfection of adenoviral vector with MEK1.
NRK-49F cells were seeded to 60% confluence in complete medium on six-well culture plates. Infection of recombinant adenovirus contains constitutively active MEK1 (Ad-MEK1) was performed as described by Zhuang et al. (24). A recombinant adenovirus vector encoding β-galactosidase (Ad-LacZ) was used as a negative control. Briefly, cells were infected with Ad-LacZ or Ad-MEK1 at a multiplicity of infection of 100 pfu for 24 h at 37°C in a humidified 5% CO2 incubator. Afterward, the culture medium was changed and allowed to grow for an additional 24 h. Then, cells were treated with RPTC-Sup as described in the figure legends.
Determination of cell viability by the MTT assay.
Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After treatment, MTT was added (final concentration, 0.5 mg/ml) and incubated for 1 h. Tetrazolium released by the addition of DMSO and the optical density were determined with a spectrophotometer at 570-nm reader (Molecular Devices, Sunnyvale, CA).
Immunoblot analysis.
After various treatments, cells were washed once with ice-cold PBS and harvested in a cell lysis buffer. Proteins (20 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. After incubation with 5% skim milk for 1 h at room temperature, membranes were incubated with a primary antibody for overnight at 4°C and then incubated with appropriate horseradish peroxidase-conjugated secondary antibody for 1 h in room temperature. Bound antibodies were visualized by chemiluminescence detection.
Immunofluorescence staining.
NRK-49F cells were grown on glass coverslips placed inside 35-mm dish and treated with RPTC-Sup in the presence or absence of inhibitors. After various treatments, cells were washed twice with PBS and fixed with 4% paraformaldehyde for 20 min, then permeabilized with 0.1% (vol/vol) Triton X-100 and 0.1 mM glycine, and then blocked with PBS containing 5% fetal calf serum for 30 min in a moist chamber at room temperature. Cells were then treated with an antibody against P2X7 at room temperature for 1 h. After being washed with PBS, cells were incubated with a mixture of FITC-labeled goat anti-rabbit IgG antibody for 1 h at room temperature and then washed with PBS. Cells were also incubated with DAPI for 10 min for nuclear staining. Photographs of 10–15 random cortical fields (×400) from each sample were taken using a SPOT camera.
Densitometry.
The quantitative analysis of different proteins was carried out by using imageJ software provided by National Institutes of Health (NIH). The quantification is based on the intensity (density) of band, which is calculated by area and pixel value of the band. The quantification data are given as ratio between target protein and loading control (house keeping protein).
Statistical analysis.
Data are presented as means ± SD and were subjected to one-way ANOVA. Multiple means were compared using Tukey's test, and differences between two groups were determined by Student's t-test. P < 0.05 was considered statistically significant.
RESULTS
Necrotic RPTC supernatant induces activation of ERK1/2, AKT, p38, and JNK in cultured renal interstitial fibroblasts.
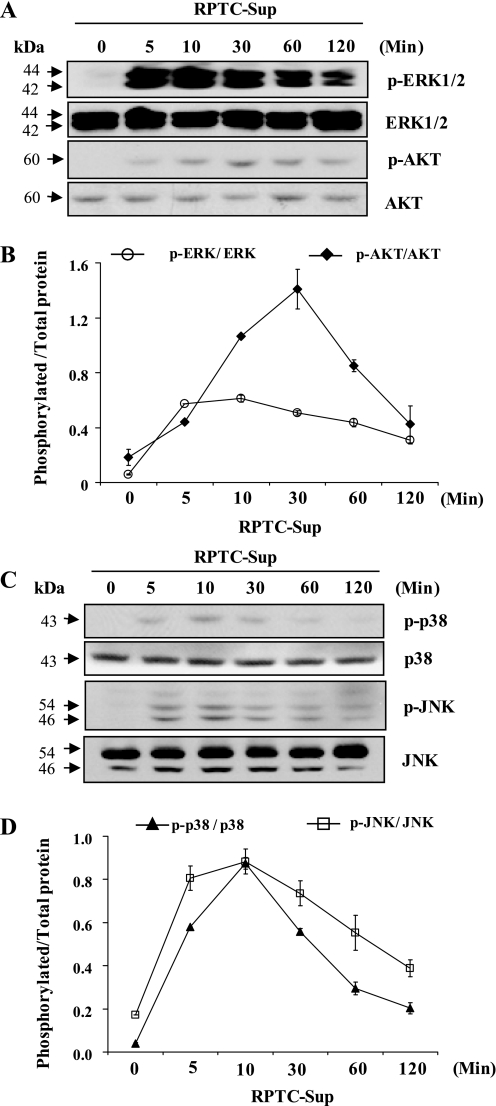
We recently reported that exposure of renal fibroblasts to necrotic RPTC induces the expression of P2X7 and it is responsible for necrotic RPTC-induced death of renal fibroblasts (10). To investigate the signaling pathway that regulates P2X7 expression, we first evaluated whether necrotic RPTC induces activation of various stress-responsive signaling molecules like p38, JNK, ERK1/2, and AKT. As shown in Fig. 1, RPTC-Sup exposure induced phosphorylation of AKT, ERK1/2, p38, and JNK, which was increased within 5 min and attained their maximum at various time points. The level of AKT phosphorylation reached maximum at 30 min (Fig. 1, A and B) and all other molecules reached their peak at 10 min after necrotic RPTC-Sup exposure (Fig. 1, A–D). Phosphorylation of these molecules was persistent for at least 120 min. In control cells (treated with 330 μl of freeze-thaw normal medium/ml), a very low level of phosphorylation of p38, JNK, ERK1/2, and AKT was observed. Total protein levels of these signaling molecules were not changed. These results indicate that necrotic RPTC induces activation of ERK1/2, AKT, JNK, p38 signaling pathways in renal fibroblasts.
Fig. 1.
Necrotic renal proximal epithelial cells (RPTC) induce phosphoryation of extracellular signal-regulated kinases (ERK1/2), AKT, p38, and c-Jun N-terminal kinases (JNK) in NRK-49F. NRK-49F cells were exposed to necrotic RPTC supernatant for the indicated time (0–120 min). Cell lysates were prepared and subjected to immunoblot analysis with antibodies for p-ERK1/2, ERK1/2, p-AKT, AKT, p-p38, p38, p-JNK, or JNK (A and C). Representative immunoblots from 3 experiments are shown. The phosphorylated and total level of ERK1/2, AKT, p38, and JNK were quantified by densitometry and phosphorylated protein levels were normalized to total protein levels (B and D). Values are means ± SD of 3 independent experiments.
Activation of ERK1/2 mediates P2X7 expression and cell death in renal fibroblasts after exposure of RPTC supernatant.
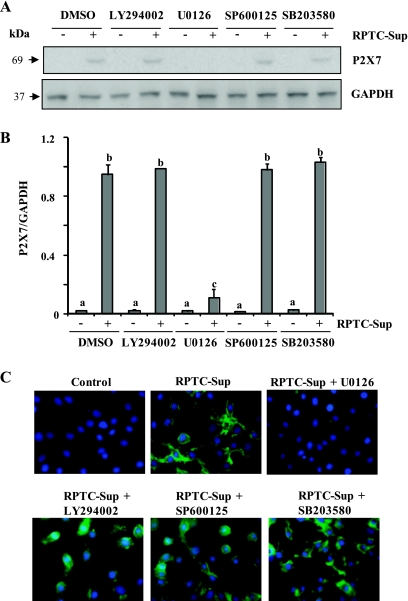
Next, we assessed whether inhibition of these molecules can affect necrotic RPTC-induced expression of P2X7 and cell death. For this study, the specific chemical inhibitors SB203580 (for p38), SP600125 (for JNK), LY294002 (for PI3K/AKT), and U0126 (for MEK 1-ERK1/2) were used. As shown in Fig. 2, A and B, inhibition of AKT, p38, or JNK did not influence the expression of P2X7. Interestingly, inhibition of ERK pathway with U0126 completely blocked P2X7 expression (Fig. 2, A and B). These inhibitors specifically inhibit each of those kinases (see Fig. 3, B and D).
Fig. 2.
Effect of ERK1/2, AKT, p38, and JNK pathway inhibitors on necrotic RPTC-induced P2X7 expression in NRK-49F. NRK-49F were pretreated with LY294002 (20 μM), U0126 (20 μM), SP600125 (10 μM), or SB 203580 (10 μM) for 1 h and then incubated with necrotic RPTC supernatant for 24 h. A: cell lysates were prepared and subjected to immunoblot analysis with specific antibodies against P2X7 or GADPH. B: levels of P2X7 receptor were quantified by densitometry and normalized with GADPH. Data are represented as means ± SD. Means with different superscript letters are significantly different from one another (P < 0.05). C: photomicrographs illustrating P2X7 (green) and nuclear staining (blue) of NRK-49F after various treatments.
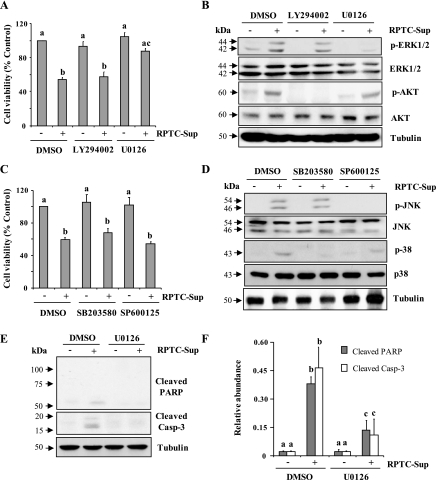
Fig. 3.
Effect of inhibition of ERK1/2, AKT, p38, and JNK on necrotic RPTC-induced renal fibroblast cell death. NRK-49F cells were pretreated with LY294002 (20 μM), U0126 (20 μM), SP600125 (10 μM), or SB 203580 (10 μM) for 1 h and then incubated with necrotic RPTC supernatant for 24 h (A, C, E) or 30 min (B and D). Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (A and B). Values are means ± SD of 3 independent experiments conducted in triplicates and expressed as the percentage of control. Bars with different letters (a–c) are significantly different from one another (P < 0.05). B and D: cells were harvested and cell lysates were subjected to immunoblot analysis with specific antibodies against p-ERK1/2, ERK1/2, p- AKT, AKT, p-p38, p38, p-JNK, JNK, or α-tubulin. E: NRK-49F cells were treated with necrotic RPTC supernatant for 24 h in the presence or absence of U0126 (20 μM) and then cell lysates were subjected to immunoblot analysis for active poly (adenosine diphosphate-ribose) polymerase (PARP), active caspase-3, or α-tubulin. The level of cleaved PARP and cleaved caspase-3 was quantified by densitometry analysis and normalized with α-tubulin (F). Data are represented as means ± SD. Bars with different superscript letters (a–c) are significantly different from one another (P < 0.05). Representative immunoblots from 3 experiments are shown.
To confirm the above results, we did immunofluorescent staining using P2X7-specific antibody. P2X7 was abundantly expressed in renal fibroblasts after incubation with necrotic RPTC-Sup for 24 h and primarily localized in plasma membrane. P2X7 expression was abolished in cells treated with U0126. In contrast, inhibition of AKT, p38, or JNK pathway did not influence P2X7 expression (Fig. 2C).
Given that P2X7 expression is required for death of renal fibroblasts after exposure to RPTC supernatant, we further examined whether ERK1/2 activation contributes to renal fibroblast death. As shown in Fig. 3A, treatment of renal fibroblasts with U0126 showed a remarkable protection against RPTC-Sup-induced cell death. Necrotic RPTC-Sup-induced renal fibroblasts death is characterized by cleavage of poly (adenosine diphosphate-ribose) polymerase (PARP) into to a 55-kDa fragment, and cleavage of caspase 3 to small fragments of 17 and 19 kDa, hallmarks of necrotic and apoptotic cell death, respectively (10). U0126 can completely block cleavage of both PARP and casapse-3, which were reduced up to threefold compared with RPTC-Sup alone treated cells (Fig. 3, E and F). In contrast, inhibition of AKT, p38, and JNK pathways did influence cell death induced by necrotic RPTC-Sup (Fig. 3, A and C). Figure 3, B and D, shows that concentration of inhibitor used in this study specifically blocked RPTC-Sup-induced activation of their corresponding protein kinases without alternating their total levels. These results suggest that ERK1/2 plays an important role in P2X7 expression and cell death induced by necrotic RPTC.
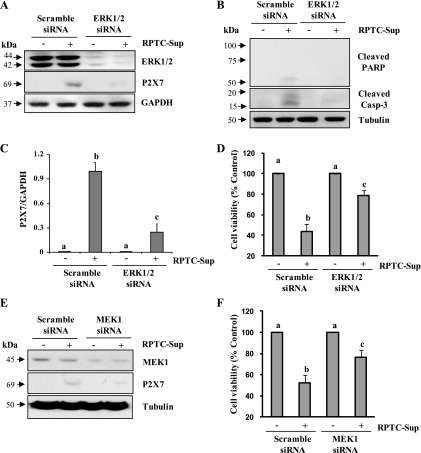
Knockdown of ERK1/2 and MEK1 attenuates necrotic RPTC-induced P2X7 expression and death of renal fibroblasts.
To validate the role of ERK1/2 in P2X7 expression and death of renal fibroblasts, we transfected renal fibroblast cells with rat-specific siRNA for ERK 1 and 2 and then cells were treated with necrotic RPTC. Figure 4A shows that knockdown of ERK 1/2 was successful and total ERK 1/2 expression was reduced more than 75%. Downregulation of ERK1/2 remarkably reduced RPTC-Sup-induced P2X7 expression and also protected against cell death in renal fibroblasts compared with P2X7 expression and cell death in scrambled siRNA-transfected cells treated with RPTC-Sup (Fig. 4, A–D). Consistent with inhibition of cleavage of PARP and caspase-3 by U0126, treatment with ERK1/2 siRNA also effectively reduced RPTC-Sup-induced cleavage of these two proteins (Fig. 4B). We further examined the effect of knockdown of MEK1 on RPTC-Sup-induced P2X7 expression and cell death. Figure 4, E and F, shows that necrotic RPTC induced P2X7 expression and cell death was significantly reduced in cells transfected with siRNA for MEK1. Together, these data reveal that ERK1/2 is a mediator of P2X7 expression and renal fibroblast cell death induced by necrotic RPTC.
Fig. 4.
Effect of downregulation of ERK1/2 and MEK1 on necrotic RPTC-induced P2X7 expression and cell death. NRK-49F cells were transfected with siRNA targeting ERK1 and ERK2 or scrambled siRNA and 24 h after transfection, cells were treated with necrotic RPTC for 24 h (A–D). Cell lysates were prepared and subjected to immunoblot analysis with antibodies for ERK1/2, GAPDH, P2X7, cleaved PARP, cleaved caspase-3, or α-tubulin (A and B). The expression of P2X7 was quantified by densitometry and normalized to α-tubulin level (C). Bars with different letters (a–d) are significantly different from one another (P < 0.05). NRK-49F cells were transfected with scrambled siRNA or MEK1-specific siRNA and treated with necrotic RPTC for 24 h. Then, cells were harvested and subjected to immunoblot analysis for MEK1, P2X7, or α-tubulin (E). Cell viability was determined by the MTT assay (D and F). Values are means ± SD of 3 independent experiments conducted in triplicates and expressed as the percentage of control. Representative immunoblots from 3 experiments are shown.
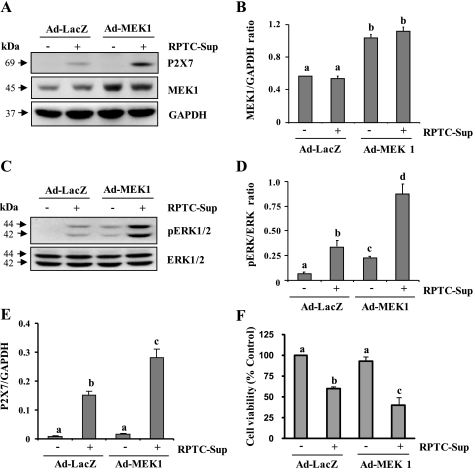
Overexpression of MEK1 enhances P2X7 expression and cell death.
To further confirm the role of ERK1/2 in P2X7 expression, we evaluated the effect of overexpression of MEK1 on necrotic RPTC-induced expression of P2X7 in NRK-49F. For this study, constitutively active MEK1 was overexpressed in NRK-49F cells by using recombinant adenoviral vectors (Ad-MEK1). Upon exposure of renal fibroblasts overexpressing MEK1 to RPTC-Sup, a significant increase of P2X7 expression was observed compared with their expression in RPTC-Sup-treated control NRK-49F (Ad-LacZ-infected cells; Fig. 5, A and E). As shown in Fig. 5, A and B, MEK1 expression level was increased up to twofold in Ad-MEK1-infected cells compared with Ad-LacZ. In addition, RPTC-Sup-induced phosphorylation of ERK1/2 was significantly enhanced in cells overexpressing MEK1 (Fig. 5, C and D). The cell viability assay showed that necrotic RPTC-induced cell death was also significantly enhanced in NRK-49F overexpressing Ad-MEK1 (Fig. 5F). Hence, our results confirm that ERK1/2 is a major pathway for P2X7 expression in renal fibroblasts.
Fig. 5.
Effect of overexpression of wild-type MEK1 on P2X7 expression. NRK-49F cells were infected with Ad-Lacz or Ad-wild-type MEK1 as described in materials and methods and then treated with necrotic RPTC for 24 h (A and F) or 30 min (C). After treatment, cells were harvested and subjected to immunoblot analysis for MEK1, P2X7, p-ERK1/2, ERK1/2, or GAPDH (A and C). The levels of MEK1 (B) and P2X7 (E) were quantified by densitometry and normalized to GAPDH level. The levels of p-ERK1/2 and ERK1/2 were quantified by densitometry and the ratio between them was calculated (D). Bars with different letters (a–c) are significantly different from one another (P < 0.05). Cell viability was determined by the MTT assay (F). Values are means ± SD of 3 independent experiments conducted in triplicates and expressed as the percentage of control. Representative immunoblots from 3 experiments are shown.
Activation of Elk1 is necessary for P2X7 expression.
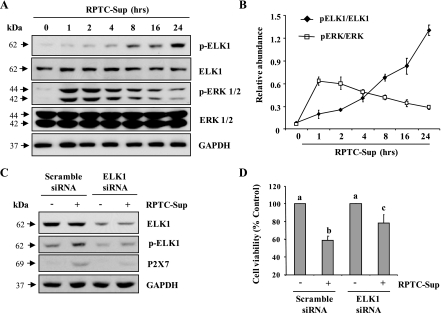
Recently, Zhou et al. (22) described that P2X7 receptor gene possesses binding sites for transcriptional factor, Elk1. Since this transcriptional factor can be activated by ERK1/2 and is associated with regulation of cell death in different cell types, we further examined the phosphorylation level of Elk1 in RPTC-Sup-treated NRK-49F cells. The basal level of phosphorylated Elk1 at Serine 383 was detected in cultured renal fibroblasts, and necrotic RPTC-Sup induced an increase in Elk1 phosphorylation in a time-dependent manner with the maximum induction at 24 h (Fig. 6, A and B). In addition, the level of p-ERK 1/2 was significantly increased in early time points and it gradually decreased in later time points. Although ERK 1/2 phosphorylation decreased after 2 h, its level was still high (∼3-fold more than control) after 24 h of RPTC-Sup exposure. But total ERK 1/2 or Elk1 level did not alter through out the time points (Fig. 6, A and B).
Fig. 6.
Effect of inhibition of Elk1 on necrotic RPTC-induced P2X7 expression and cell death. NRK-49F were treated with RPTC-Sup supernatant for the indicated time (0–24 h) and cell lysates were subjected to immunoblot analysis for p-Elk1, Elk1, p-ERK1/2, ERK1/2, or GAPDH (A). The phosphorylated and total levels of p-Elk1, Elk1, p-ERK1/2, and ERK 1/2 were quantified by densitometry and phosphorylated protein levels were normalized to total protein levels (B). Cultured NRK-49F cells were transfected with scrambled siRNA or siRNA specific for Elk1. At 24 h after posttransfection, cells were treated with necrotic RPTC for 24 h and cell lysates were subjected to immunoblot analysis for Elk1, pElk1, P2X7, or GAPDH (C). The cell viability was assessed by MTT (D). Values are means ± SD of 3 independent experiments conducted in triplicates and expressed as the percentage of control.
To determine the role of Elk1 in P2X7 expression and renal fibroblast death, Elk1 was silenced with siRNA and the expression of P2X7 and renal fibroblast viability after treatment with necrotic RPTC-Sup was examined. As shown in Fig. 6C, RPTC-Sup-induced expression of P2X7 was significantly reduced in Elk1siRNA-transfected fibroblast cells relevant to that in control. Similarly, the cell viability of renal fibroblasts was also significantly increased upon knock down of Elk1 (Fig. 6D). These data suggest that Elk1 plays a critical role in transducing activation of ERK pathway-mediated P2X7 expression, which results in renal fibroblast cell death.
ERK pathway mediates activation of Elk1.
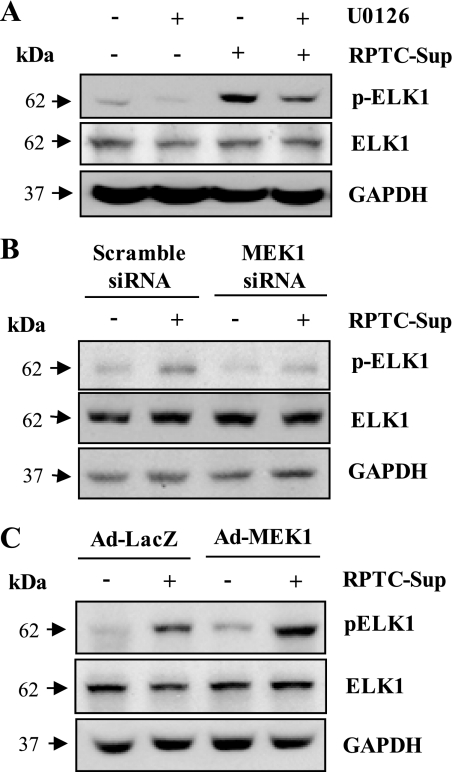
If ERK1/2 mediates P2X7 expression through Elk1 activation, inhibition of ERK1/2 pathway should block RPTC-Sup-induced phosphorylation of Elk1. To test this hypothesis, we examined the influence of ERK1/2 inhibition on Elk1 phosphorylation by using chemical inhibitor (U0126), siRNA specific for MEK1, or adenovirus vector encoding wild-type MEK1. As expected, inhibition of MEK1 with U0126 or siRNA effectively blocked RPTC-Sup-induced Elk1 phosphorylation without any significant change in total Elk1 level (Fig. 7, A and B). In addition, the level of Elk1 phosphorylation was increased in NRK-49F cells overexpressing MEK1 and RPTC-Sup further enhanced phosphorylation of Elk1 (Fig. 7C). These results suggest that Elk1 activation is involved in upregulation of P2X7 expression and MEK1-ERK1/2 pathway is an important mediator of Elk1 activation (Fig. 8).
Fig. 7.
Effect of inhibition of MEK1 or overexpression of MEK1 on necrotic RPTC-induced Elk1 phosphorylation. Cultured NRK-49F cells were treated with U0126 (20 μM) for 1 h and then exposed to necrotic RPTC supernatant for 24 h (A). NRK-49F cells were transfected with siRNA specific for MEK1 (B) or infected with adenovirus encoding MEK1 (Ad-MEK1; C), and cells were treated with necrotic RPTC for 24 h. Cells were harvested and cell lysates were subjected to immunoblot analysis for pElk1, Elk1, or GAPDH. Representative immunoblots from 3 experiments are shown.
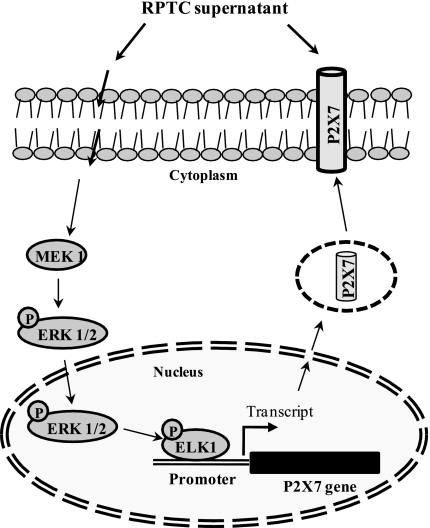
Fig. 8.
Scheme of ERK pathway-mediated P2X7 expression in renal fibroblasts. Exposure of renal necrotic RPTC induces activation of the MEK1/ERK pathway, which in turn activates Elk1, a nuclear transcriptional factor. Activated Elk1 binds to P2X7 gene and drives its expression thereby increasing P2X7 protein levels on cell surface and it induces cell death in renal fibroblasts.
DISCUSSION
In normal adult rat kidney, there is little or no expression of P2X7 receptor (15, 17, 18); however, increased expression has been observed in some experimental kidney diseases such as the glomeruli of diabetic, hypertensive and glomerulonephritis. P2X7 is also detected in cultured mesangial cells on exposure to TNF-α (6) and podocyte and renal tubular cells under chronic and inflammatory condition (18). However, the signaling mechanism(s) responsible for P2X7 expression remain elusive. We recently demonstrated that necrotic RPTC induces P2X7 expression, which is required for death of renal fibroblasts. The purpose of this study is to elucidate the signaling mechanism that mediates P2X7 expression and subsequent cell death in renal interstitial fibroblasts. Our data show that at least four pathways, namely, ERK1/2, Akt, p38, JNK, are activated upon exposure of renal fibroblasts to necrotic RPTC supernatant. However, inhibition of ERK1/2, but not other pathways, blocks the P2X7 expression. Furthermore, we demonstrate that inhibition of the ERK pathway protects against renal fibroblast death. Therefore, we suggest that activation of ERK pathway is a key mechanism for necrotic RPTC to induce P2X7 expression and cell death in renal fibroblasts.
To our knowledge, the ERK pathway is the first one that has been identified to regulate of P2X7 expression. This conclusion is supported by several observations. First, necrotic RPTC induces phosphorylation of ERK1/2 and its upstream activator, MEK1, in renal fibroblasts. Second, pharmacological inhibition of ERK1/2 pathway by U0126 blocked necrotic RPTC-induced P2X7 expression. Third, knockdown of either ERK1/2 or MEK1 attenuated P2X7 expression. Fourth and finally, overexpression of MEK1 increased expression of P2X7. As P2X7 expression is required for induction of renal fibroblast death, we also examined whether activation of the ERK pathway contributes to the death of renal fibroblasts after treatment with necrotic RPTC supernatant. Our data showed that inhibition of this pathway by either U0126 or siRNA specifically targeting ERK1/2 or MEK1 attenuated necrotic RPTC supernatant-induced death of renal fibroblasts, whereas overexpression of MEK increased death of this cell type. These data clearly indicate that activation of the MEK1/ERK1/2 pathway is required for necrotic RPTC to induce P2X7 expression as well as cell death in renal fibroblasts.
In general, ERK pathway plays an essential role in cell proliferation and survival but a growing body of literature indicates that activation of this pathway also contributes to cell death in some cell types and tissues under certain pathological conditions (9, 19, 24). We previously showed that activation of the ERK pathway precedes mitochondrial swelling and dysfunction by altering mitochondrial membrane permeability, which results in necrotic cell death in renal tubular cells (23). ERK1/2 activation has been also reported to contribute to the pathogenesis of acute kidney injury induced by cisplatin or ischemia-reperfusion (9, 19, 23, 24). Depending on the duration, magnitude, and its subcellular localization, ERK activation controls various cell responses (1). In studies related to ERK-mediated cell death, prolonged activation of ERK has been observed and ERK phosphorylation is maintained at least 6 h and maximum ∼72 h, which indicates that persistent activation of ERK is crucial in implication of cell death (1). In the current study, we also observed that ERK1/2 phosphorylation escalated within 5 min when exposed to necrotic RPTC supernatant and remained elevated for at least 24 h. Blockade of ERK1/2 activation inhibits both necrotic and apoptotic cell death markers as indicated by decreased levels of active PARP and caspase-3, suggesting the importance of ERK pathway in mediating death of renal fibroblasts under the pathological conditions, which is accompanied by necrotic tubular cell death and release of cellular contents.
Our data suggest that the MEK-ERK pathways regulate P2X7 expression through activation of Elk1. Elk1 is a transcriptional factor that can be phosphorylated by the MEK-ERK cascade. Bioinformatics analysis of transcription enhancing region of P2X7 gene reveals that it contains putative binding sites for Elk1 (22). As such, we assumed that Elk1 may play a role in transcriptional regulation of P2X7 expression. To test this hypothesis, we examined the effect of Elk1 knockdown on P2X7 expression in renal fibroblasts exposed to necrotic RPTC supernatant. Our data clearly show that knockdown of Elk1 by its specific siRNA significantly reduced P2X7 expression. Consistent with the prodeath action of P2X7, silencing of Elk1 also protects renal fibroblasts against necrotic RPTC-induced cell death. In addition, we found that knockdown of MEK reduced Elk1 phosphorylation, while overexpression of MEK1 increased Elk1 phosphorylation. These data strongly support the idea that Elk1 plays an essential role in transmitting ERK1/2 activation to P2X7 expression.
Currently, the initial events triggered by necrotic RPTC supernatant for the activation of ERK1/2 pathway in renal fibroblasts remain unclear. It has been documented that injured or dying epithelial cells can produce and secrete proinflammatory cytokines like IL-1β and TNF-α (2, 8). Donnahoo et al. (4) observed that renal tubular cells locally produce TNF-α following ischemia-reperfusion injury. In this context, necrotic RPTC might produce those cytokines and then activate their receptors in renal fibroblasts, leading to activation of ERK signaling pathway and P2X7 expression. Further studies are necessary to identify cell surface receptor that mediates activation of the MEK1-ERK pathway in response to necrotic RPTC supernatant.
Collectively, we demonstrate that the ERK1/2 pathway is responsible for consecutive stimulation of P2X7 expression and death of renal fibroblasts after being exposed to necrotic RPTC supernatant. In addition to renal fibroblasts, increased expression of P2X7 has been observed in several other cell types in the kidney under pathological conditions of animal models or in renal biopsy tissue from patients with lupus nephritis. Identification of ERK1/2 activation as a regulatory mechanism for P2X7 expression provides a basis to further explore the signaling events that control P2X7 expression in diseased condition.
GRANTS
This work was supported by a grant from the National Institutes of Health (DK-071997, DK-085065) to S. Zhuang.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J 277: 2–21, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Chang CH, Huang Y, Anderson R. Activation of vascular endothelial cells by IL-1alpha released by epithelial cells infected with respiratory syncytial virus. Cell Immunol 221: 37–41, 2003 [DOI] [PubMed] [Google Scholar]
- 3. De Borst MH, Wassef L, Kelly DJ, Van Goor H, Navis GJ. Mitogen-activated protein kinase signaling in the kidney: target for intervention? Signal Transduction 6: 32–53, 2006 [Google Scholar]
- 4. Donnahoo KK, Meng X, Ao L, Ayala A, Shames BD, Cain MP, Harken AH, Meldrum DR. Differential cellular immunolocalization of renal tumour necrosis factor-alpha production during ischaemia versus endotoxaemia. Immunology 102: 53–58, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ernest S, Bello-Reuss E. Expression and function of P-glycoprotein in a mouse kidney cell line. Am J Physiol Cell Physiol 269: C323–C333, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Harada H, Chan CM, Loesch A, Unwin R, Burnstock G. Induction of proliferation and apoptotic cell death via P2Y and P2X receptors, respectively, in rat glomerular mesangial cells. Kidney Int 57: 949–958, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Mehta A, Sekhon CP, Giri S, Orak JK, Singh AK. Attenuation of ischemia/reperfusion induced MAP kinases by N-acetyl cysteine, sodium nitroprusside and phosphoramidon. Mol Cell Biochem 240: 19–29, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Meldrum KK, Meldrum DR, Hile KL, Yerkes EB, Ayala A, Cain MP, Rink RC, Casale AJ, Kaefer MA. p38 MAPK mediates renal tubular cell TNF-α production and TNF-α-dependent apoptosis during simulated ischemia. Am J Physiol Cell Physiol 281: C563–C570, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Pavlovic D, Andersen NA, Mandrup-Poulsen T, Eizirik DL. Activation of extracellular signal-regulated kinase (ERK)1/2 contributes to cytokine-induced apoptosis in purified rat pancreatic beta-cells. Eur Cytokine Netw 11: 267–274, 2000 [PubMed] [Google Scholar]
- 10. Ponnusamy M, Ma L, Gong R, Pang M, Chin YE, Zhuang S. P2X7 receptors mediate deleterious renal epithelial-fibroblast cross talk. Am J Physiol Renal Physiol 300: F62–F70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma A, Callahan LM, Sul JY, Kim TK, Barrett L, Kim M, Powers JM, Federoff H, Eberwine J. A neurotoxic phosphoform of Elk-1 associates with inclusions from multiple neurodegenerative diseases. PLos One 5: e9002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stefanelli C, Tantini B, Fattori M, Stanic I, Pignatti C, Clo C, Guarnieri C, Caldarera CM, Mackintosh CA, Pegg AE, Flamigni F. Caspase activation in etoposide-treated fibroblasts is correlated to ERK phosphorylation and both events are blocked by polyamine depletion. FEBS Lett 527: 223–228, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Subramaniam S, Unsicker K. ERK and cell death: ERK1/2 in neuronal death. FEBS J 277: 22–29, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Taylor SR, Turner CM, Elliott JI, McDaid J, Hewitt R, Smith J, Pickering MC, Whitehouse DL, Cook HT, Burnstock G, Pusey CD, Unwin RJ, Tam FW. P2X7 deficiency attenuates renal injury in experimental glomerulonephritis. J Am Soc Nephrol 20: 1275–1281, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turner CM, Elliott JI, Tam FW. P2 receptors in renal pathophysiology. Purinergic Signal 5: 513–520, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turner CM, Tam FW, Lai PC, Tarzi RM, Burnstock G, Pusey CD, Cook HT, Unwin RJ. Increased expression of the pro-apoptotic ATP-sensitive P2X7 receptor in experimental and human glomerulonephritis. Nephrol Dial Transplant 22: 386–395, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs 175: 105–117, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Vonend O, Turner CM, Chan CM, Loesch A, Dell'Anna GC, Srai KS, Burnstock G, Unwin RJ. Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int 66: 157–166, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem 275: 39435–39443, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Wang X, Wang H, Xu L, Rozanski DJ, Sugawara T, Chan PH, Trzaskos JM, Feuerstein GZ. Significant neuroprotection against ischemic brain injury by inhibition of the MEK1 protein kinase in mice: exploration of potential mechanism associated with apoptosis. J Pharmacol Exp Ther 304: 172–178, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci 23: 213–216, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Zhou L, Luo L, Qi X, Li X, Gorodeski GI. Regulation of P2X7 gene transcription. Purinergic Signal 5: 409–426, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhuang S, Kinsey GR, Yan Y, Han J, Schnellmann RG. Extracellular signal-regulated kinase activation mediates mitochondrial dysfunction and necrosis induced by hydrogen peroxide in renal proximal tubular cells. J Pharmacol Exp Ther 325: 732–740, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol Renal Physiol 292: F440–F447, 2007 [DOI] [PubMed] [Google Scholar]