Abstract
The H+-K+-ATPase α-subunit (HKα2) participates importantly in systemic acid-base homeostasis and defends against metabolic acidosis. We have previously shown that HKα2 plasma membrane expression is regulated by PKA (Codina J, Liu J, Bleyer AJ, Penn RB, DuBose TD Jr. J Am Soc Nephrol 17: 1833–1840, 2006) and in a separate study demonstrated that genetic ablation of the proton-sensing Gs-coupled receptor GPR4 results in spontaneous metabolic acidosis (Sun X, Yang LV, Tiegs BC, Arend LJ, McGraw DW, Penn RB, Petrovic S. J Am Soc Nephrol 21: 1745–1755, 2010). In the present study, we investigated the ability of chronic acidosis and GPR4 to regulate HKα2 expression in HEK-293 cells. Chronic acidosis was modeled in vitro by using multiple methods: reducing media pH by adjusting bicarbonate concentration, adding HCl, or by increasing the ambient concentration of CO2. PKA activity and HKα2 protein were monitored by immunoblot analysis, and HKα2 mRNA, by real-time PCR. Chronic acidosis did not alter the expression of HKα2 mRNA; however, PKA activity and HKα2 protein abundance increased when media pH decreased from 7.4 to 6.8. Furthermore, this increase was independent of the method used to create chronic acidosis. Heterologous expression of GPR4 was sufficient to increase both basal and acid-stimulated PKA activity and similarly increase basal and acid-stimulated HKα2 expression. Collectively, these results suggest that chronic acidosis and GPR4 increase HKα2 protein by increasing PKA activity without altering HKα2 mRNA abundance, implicating a regulatory role of pH-activated GPR4 in homeostatic regulation of HKα2 and acid-base balance.
Keywords: GPR4, kidney, metabolic acidosis, PKA
the kidney plays an essential role in acid-base balance and defends the body against chronic metabolic acidosis (CMA), a condition that commonly complicates chronic kidney disease (CKD) and has adverse effects on bone, nutrition, and metabolism. Beyond its manifestation in CKD, CMA impacts the pathology of numerous other organ systems and diseases including cardiac function and heart disease as well as osteopenia, sarcopenia, and stunting of growth and development (34). Therefore, improved understanding of the specific mechanisms by which the kidney responds to changes in systemic pH has potentially far-reaching clinical consequences. While many aspects of the adaptive response by the kidney to changes in pH and acid load are appreciated, our understanding of the afferent mechanisms that sense changes in pH, and link to efferent mechanisms that mediate the cellular response to acidosis, remains incomplete.
The preeminent role of the kidney in the regulation of acid-base homeostasis is in upregulating net acid excretion and bicarbonate reabsorption in response to metabolic acidosis (34). The cAMP-protein kinase A (PKA) signaling pathway has emerged as a critical regulator of cellular localization and function of H+ transporters. Recent evidence from our group and others suggests a critical role for PKA in the maturation and function of the H+-K+-ATPase α-subunit (HKα2) and H+-ATPase, a mechanism that involves phosphorylation at key serine residues (3, 11, 32).
Recently, a small number of G protein-coupled receptors (GPCRs) have been identified whose activation is stimulated by a reduction in extracellular pH, prompting the designation “proton-sensing” receptors. One of these putative proton-sensing GPCRs, GPR4 (39), is an upstream activator of cAMP-PKA signaling. Preliminary studies in our laboratory implicate GPR4 in the upregulation of proton transporters in the collecting duct of the kidney. Together with existing literature data on the activation of other proton-translocating ATPases, these findings indicate the presence of an underlying signaling network involved in coordinating the renal adaptive response to acidosis.
We recently reported that deletion of GPR4 in vivo was associated with a reduction in net acid excretion and a relatively alkaline urine pH, in parallel with a spontaneous nongap metabolic acidosis (42), thus providing evidence for an acidification defect in this model. Moreover, some of the features of the renal acidification defect in GPR4−/− resemble that reported for mice with deletion of one of the genes which encode for the rate-limiting bicarbonate transporter AE1 in the kidney collecting duct (36).
HKα2 mRNA and protein abundance has been demonstrated to increase in response to chronic hypokalemia in renal cortex and medulla, as well as in mouse outer medullary collecting duct (OMCD) and inner medullary collecting duct (IMCD) cells in culture (30). Additionally, HKα2 appears to be upregulated in rodent kidney in response to a number of other stimuli, including metabolic acidosis (37, 43, 44), respiratory acidosis (16), chronic metabolic alkalosis (with hypokalemia) (15), development of the newborn (9, 12), and potassium depletion (1, 7, 20). Expression and functionality of proton transporters in different experimental models, including HKα2, have been attributed to both transcriptional and posttranscriptional events (2, 6, 11, 14, 24, 45). In this context, Zhou et al. (46) demonstrated that increased CO2 stimulates Sch-28080-sensitive K+/Rb+ uptake mediated by H+-K+-ATPase in the cortical collecting duct of potassium-restricted rabbits. Because the experiment was performed in isolated tubules, the investigators concluded that acidosis induced by CO2 treatment appears to regulate, at least in part, insertion of the enzyme into the apical plasma membrane by exocytosis. Our laboratory (11) demonstrated that PKA-mediated phosphorylation of HKα2 at S955 promoted protein stability. Mutation of S955 to alanine decreased protein expression and 86Rb+ uptake, whereas mutation of S955 to aspartic acid augmented HKα2 protein expression and 86Rb+ uptake. Studies by Ludwig et al. (27) and Radu et al. (35) demonstrated that GPR4 expressed in HEK-293 cells induces accumulation of intracellular cAMP when the cells are challenged with a low extracellular pH. As a cAMP-generating/PKA-activating receptor, GPR4, therefore, has the potential to upregulate transporters in the collecting duct as an adaptive mechanism in response to acidosis.
The present study was designed to explore 1) whether HKα2 expression is regulated by chronic acidosis; 2) whether the regulation is posttranscriptional; and 3) the role of GPR4 in HKα2 protein expression during metabolic acidosis.
METHODS
Cell culture and transfection.
HEK-293 cells were grown at 37°C in DMEM media containing 10% newborn calf serum in the presence of 5% CO2 as described previously (10, 11). HEK-293 cells were transfected with rat HKα2 in pcDNA3.1(+)-Neo and rat NKβ1 in pcDNA3.1(+)-Zeo (8, 23) using the Lipofectamine PLUS method (10, 11) and selected with 250 μg/ml of G418 and 250 μg/ml zeocin. Individual colonies were isolated and tested for expression of HKα2 and NKβ1 by immunoblot analysis (7). Human GPR4 (pcDNA3GPR4) was transiently transfected into HEK-293 cells stably expressing HKα2/NKβ1.
Immunoblot analysis.
Immunoblot analysis was performed as described previously (7). Cell lysates were generated by scraping into 10 mM Tris·HCl, pH 8.0, 1 mM EDTA, 3 mM benzamidine, 1 mM PMSF, 1 μg/ml soybean trypsin inhibitor, and 1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate and incubating for 1 h at 4°C. Insoluble material was removed by centrifugation, the supernatant was retained, protein concentration was measured as described previously (26), and 75 μg of supernatant protein was subject to immunoblot analysis as described elsewhere (7). Equal loading of the lanes was verified by staining with Ponceau red or β-actin quantification. The bands were detected and quantified using an Odyssey Infrared Imaging System (Li-Cor) (11, 21).
Real-time PCR.
HEK-293 cells were scraped, collected by centrifugation, and washed with PBS at 4°C. RNA was isolated using RNAzol B (TelTest) as described previously (14). The total RNA concentration was quantified, converted to cDNA, and real-time PCR was performed using the Taqman system (Applied Biosystems) as described elsewhere (22). The cycle threshold (Ct) was calculated with MxPro software.
Induction of chronic acidosis by reduction of media HCO3− concentration.
Confluent HEK-293 cells stably expressing HKα2/NKβ1 or m-IMCD-3 cells were incubated in media containing decreasing concentrations of NaHCO3 (from 40 to 1.25 mM) for 24 h in a 5% CO2 incubator using serum-free DMEM media supplemented with 50 mM HEPES preadjusted to pH 7.5. Ionic strength of the media was corrected with NaCl. At the end of the experiment, the extracellular pH was measured with a pH meter and ranged from ∼7.4 (at 40 mM NaHCO3) to ∼6.8 (at 1.25 mM NaHCO3).
Reversibility of chronic acidosis on HKα2 protein expression.
To determine whether reversing CMA affects HKα protein expression, we performed the following experiment, in which the media of all cells was changed every 24 h. The first group of cells was maintained at extracellular pH ∼7.4 for 48 h. In the second group, the extracellular pH was maintained at 7.4 from 0 to 24 h and then decreased to extracellular pH 6.8 for 24 h (24–48 h) by manipulation of NaHCO3 concentration as described above. In the third group, cells were maintained at an extracellular pH of 6.8 from 0 to 24 h and then changed to extracellular pH 7.4 from 24 to 48 h. In the fourth group the cells were maintained at a constant extracellular pH of 6.8 for 48 h.
Model of chronic metabolic acidosis induced by addition of HCl.
Cells were incubated in serum-free DMEM media containing 25 mM NaHCO3 with increasing concentrations of HCl (from 0 to 10 mM) for 24 h in 5% CO2. Ionic strength of the media was corrected with NaCl. At the end of the experiment, the extracellular pH was measured with a pH meter and ranged from ∼7.4 (at 0 mM HCl) to ∼6.8 (at 10 mM HCl).
Model of respiratory acidosis.
Cells were incubated for 24 h in serum-free DMEM media containing 25 mM NaHCO3 in a CO2 incubator providing either 5 (control) or 10% (high) CO2.
Antibodies and other reagents.
Anti-HKα2 antibody was developed and characterized previously by our laboratory (7). Monoclonal p-VASP/VASP was purchased from Pharmigen, and forskolin from Fisher Scientific. Taqman probes to quantify HKα2 and GAPDH mRNA were purchased from Applied Biosystems. All other agents were from Sigma or previously identified sources (11). For data analysis, mean ± SE final pH values were calculated for both media pH and the corresponding measured outcome (e.g., HKα2 expression).
Statistical analyses.
All statistical analyses were performed using SigmaPlot 11.0. P values <0.05 were considered significant.
RESULTS
Chronic acidosis induced by reduction of media HCO3− concentration upregulates HKα2 protein abundance but not mRNA.
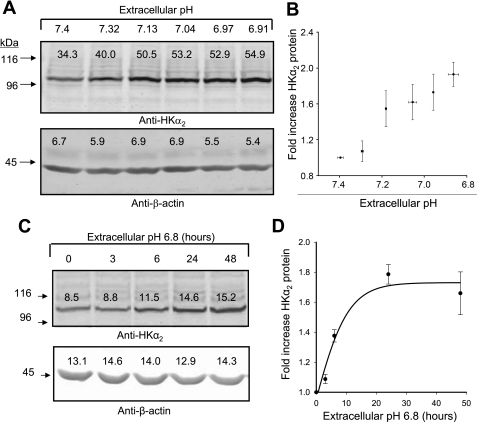
To test the regulatory effects of extracellular pH, PKA, and GPR4 on HKα2 expression, we employed a model system of HEK-293 cells with stable expression of rat HKα2 (13) [encoded in pcDNA3.1(+)-neo] and rat NKβ1 (25) [encoded in pcDNA3.1(+)-zeo] as described previously (11). Cells grown to confluence were exposed for 24 h to a decrease in extracellular pH by reducing media NaHCO3 concentration. A representative blot (Fig. 1A) and mean data (Fig. 1B) demonstrate that modest reductions in extracellular pH are sufficient to increase HKα2 expression in cells stably expressing the protein, and expression increases with progressive reduction of media pH. Although transcriptional regulation of recombinant HKα2 under a CMV promoter is unlikely here, we confirmed that HKα2 mRNA abundance was unaltered by chronic acidosis as assessed by real-time PCR. Mean Ct values for HKα2 were not significantly different between cells treated 24 h with media pH ∼7.4 [final media pH 7.39 ± 0.10, Ct (HKα2) = 25.64 ± 1.39; Ct (GAPDH) = 22.63 ± 0.37] vs. media pH ∼6.9 [pH 6.79 ± 0.19 Ct (HKα2) = 25.68 ± 1.45; Ct (GAPDH) = 22.23 ± 0.77]. Similar expression of HKα2 mRNA in both groups of cells was confirmed by Northern blot analysis (data not shown). In Fig. 1C, cells were incubated in media pH 6.8 for 0, 3, 6, 12, or 24 h, and lysates were harvested for immunoblotting HKα2 and β-actin. Figure 1D depicts the graph of means ± SE data from three independent experiments.
Fig. 1.
Chronic acidosis induced by reduction in media bicarbonate concentration increases the H+-K+-ATPase α-subunit (HKα2) protein, but not time-dependent mRNA abundance. HEK-293 cells stably expressing HKα2/NKβ1 as described previously, were exposed for up to 24 h to reduced extracellular pH by reducing media NaHCO3 concentration. Cell lysates were subsequently harvested and subjected to immunoblot analysis of HKα2 and β-actin (used to verify similar loading) as described in methods. Bands from immunoblots were detected and quantified using the Odyssey Infrared Imaging System (Li-Cor). A: representative blot in which media pH was progressively reduced by reducing media HCO3− concentration at the beginning of the experiment; lysates were harvested after 24 h. B: fold-change in HKα2 abundance relative to the value determined at pH 7.4 were calculated, and data were plotted as a function of media pH (media pH was measured at time of lysate harvest, means ± SE values were calculated, and 6 points, with X and Y error bars, were plotted); n = 23. C: immunoblots for HKα2 and β-actin of lysates harvested from cells maintained in media pH 6.8 for different durations. D: values are means ± SE of HKα2 from 3 different experiments.
Upregulation of HKα2 by chronic acidosis is reversible.
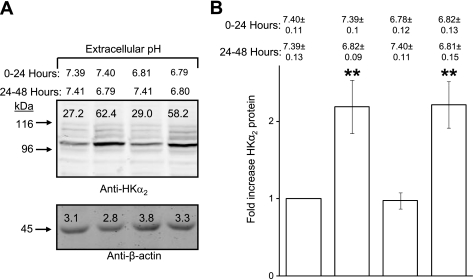
To test the reversibility of the upregulation of HKα2 promoted by chronic acidosis, cells were treated for 24 h in media pH 6.8, then washed and refed media pH 7.4 for another 24 h. Figure 2A depicts a representative blot demonstrating that induced HKα2 levels return to baseline if returned to media pH 7.4, yet remain elevated if media pH is sustained at pH 6.8. Figure 2B depicts the means ± SE values from three independent studies in which the fold-increase in HKα2 is calculated for each condition.
Fig. 2.
Acidosis-induced increase in HKα2 protein is reversible. A: cells were maintained in media pH ∼7.4 or ∼6.8 as indicated for 24 h, and then media was removed and replaced by media pH ∼7.4 or ∼6.8. Indicated pH values represent those measured for media after either the first (0–24 h) or second (24–48 h) 24-h treatment period. Cell lysates were harvested after each treatment period and subjected to immunoblot analysis of HKα2 and β-actin as described in methods. B: values are means ± SE from 3 independent experiments. **P < 0.01.
Upregulation of HKα2 protein is a robust phenomenon, induced by multiple means of decreasing extracellular pH.
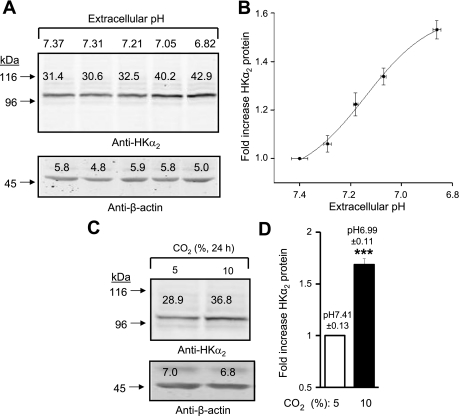
To further explore the effect of extracellular pH on HKα2 expression, serum-free media (containing 25 mM NaHCO3) pH was reduced by sequentially adding increasing concentrations of HCl (0–10 mM) for 24 h in a 5% CO2 incubator. The results of a representative experiment are displayed in Fig. 3A, and means ± SE values for the fold-increase in HKα2 abundance from three independent studies are displayed in Fig. 3B. In the experiments displayed in Fig. 3C, cells were grown for 24 h in serum free-media under conditions of 5 or 10% CO2 to produce the indicated media pH of 7.4 and 7.0. In Fig. 3D, means ± SE values from three independent studies are depicted. Results demonstrate that these two approaches for imposing chronic acidosis also increase expression of HKα2 protein, similar to the effect of titrating media bicarbonate (Fig. 1).
Fig. 3.
Different methods of media pH reduction promote increased HKα2 protein abundance. A: media pH was adjusted as indicated by addition of HCl. B: experiment was repeated 3 times, and fold-increase in HKα2 vs. extracellular pH is shown. C: cells were cultured in incubators set for 5 or 10% CO2 to achieve a media pH of 7.4 or 7.0, respectively. D: values are means ± SE for fold-increase in HKα2 at 10 vs. 5% CO2 from 3 independent experiments. ***P < 0.001.
Inhibition of carbonic anhydrase during respiratory acidosis does not affect abundance of HKα2.
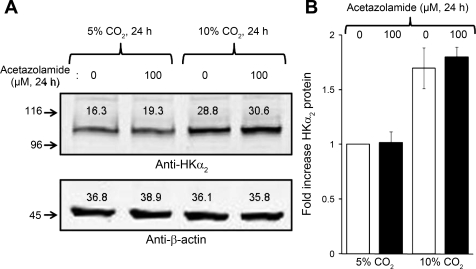
It has been suggested that soluble adenylyl cyclase (sAC)-regulated cAMP signaling may constitute a general sensing mechanism for regulating H+-ATPase-mediated proton transport (31) and is inhibited by acetazolamide (19). As applied in the current study, reductions in media pH caused by either reduction of bicarbonate or addition of HCl would decrease rather than increase cellular bicarbonate levels. Since sAC appears to respond to an increase in intracellular HCO3− concentration, we therefore tested the effect of acetazolamide on the observed induction of HKα2 by increasing the ambient concentration of CO2 in the incubator with and without inhibition of carbonic anhydrase. As shown in Fig. 4, A and B, while an increase in CO2 concentration in the incubator clearly increased the abundance of HKα2, application of 100 μM acetazolamide had no effect on the induction of HKα2 by CO2.
Fig. 4.
Inhibition of carbonic anhydrase does not affect upregulation of HKα2 during increase in CO2. Cells were subjected to 24-h treatment in either 5 or 10% CO2 in the presence of vehicle or 100 μM acetazolamide. A: representative immunoblot of HKα2 and β-actin expression. B: values are means ± SE from 4 independent experiments. **P < 0.01.
Stimulators of PKA upregulate HKα2 protein, and decreasing extracellular pH increases PKA activity.
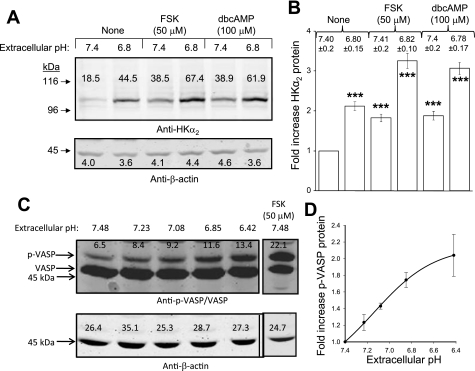
Despite the apparent lack of a mechanistic role for soluble adenylyl cyclase in mediating the observed upregulation of HKα2 in the current studies, the potential role of PKA in this phenomenon was further examined. Consistent with previous studies from our laboratory (11), 24 h of treatment with activators of intracellular PKA activity forskolin (FSK) or dibutyryl-cAMP (db-cAMP) increased HKα2 protein expression in stably transfected cells. A representative experiment is displayed in Fig. 5A. Figure 5B depicts means ± SE values (n = 3) for the fold-increase in HKα2 induced by the indicated agent/pH. Decreasing media pH was shown to rapidly increase intracellular PKA activity (demonstrated by the increase in the 50-kDa band of PKA substrate VASP) (5, 21) (Fig. 5, C and D), although phospho-VASP levels induced by reduced extracellular pH were not as great as those induced by FSK.
Fig. 5.
Stimulators of protein kinase A (PKA) upregulate HKα2 protein; decreasing extracellular pH increases PKA activity. A: 24-h treatment of cells with PKA-activating agents forskolin (FSK) or dibutyryl-cAMP (db-cAMP) increases HKα2 protein expression in stably transfected cells. B: values are means ± SE derived from 4 independent experiments. C: stimulation of cells for 5 min with media of progressively lower pH causes a rapid increase in intracellular PKA activity, indicated by the increased expression of the 50-kDa band of the PKA substrate VASP. D: values are means ± SE for fold-increase in phospho-VASP levels from 3 independent experiments.
GPR4 expression increases basal and acid-induced PKA activity, HKα2 expression.
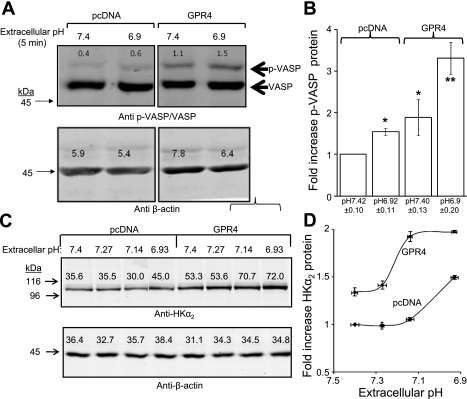
We previously demonstrated the mice lacking GPR4 exhibit spontaneous metabolic acidosis, and GPR4 heterologously expressed in HEK-293 cells stimulates cAMP generation in response to a reduction in extracellular pH (42). Accordingly, GPR4 may contribute to homeostatic acid-base regulation by upregulating the expression of (PKA-regulated) transporters in response to acid load. We therefore tested the capacity of GPR4 to activate PKA and promote HKα2 upregulation in HEK-293 cells stably expressing HKα2. Figure 6A is a representative immunoblot demonstrating that GPR4 overexpression in these cells was sufficient to increase basal PKA as well as acid-stimulated PKA activity; means ± SE values from three independent experiments are displayed in Fig. 6B. Moreover, HKα2 expression in GPR4-expressing cells was higher than in vector-transfected control cells when cultured in media pH 7.4. A progressive reduction in media pH to 6.8 further augmented HKα2 relative to that observed in vector-transfected control cells (Fig. 6, C and D).
Fig. 6.
G protein proton-sensing receptor GPR4 expression increases basal and acid-induced PKA activity, HKα2 expression. A: transient expression of GPR4 in HEK-293 cells increases both basal and acid-stimulated PKA activity. B: values are means ± SE depicting effect of GPR4 expression and reduced media pH on fold-expression of phospho-VASP levels (normalized to corresponding β-actin values); n = 4. C: progressive reduction in media pH to 6.9 further augments HKα2 relative to that observed in vector-transfected control cells. D: values are means ± SE from 3 independent experiments. *P < 0.05. **P < 0.01.
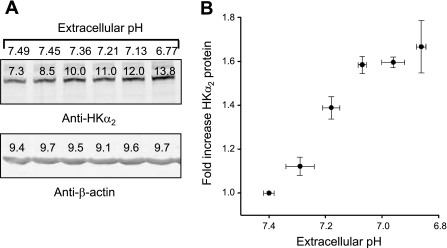
Regulation of endogenous HKα2 expression by extracellular pH.
The m-IMCD-3 cell line is derived from murine IMCD cells and can serve as a system for analysis of regulation of endogenous HKα2 expression. We previously demonstrated a high level of expression of GPR4 mRNA in m-IMCD-3 cells as well as in tissue from the mouse IMCD (41). We therefore grew m-IMCD-3 cells to near confluency, rendered them quiescent by incubation in serum-free media for 24 h, then reduced media pH by manipulation of bicarbonate concentration and cultured cells for another 24 h. Figure 7A shows a representative immunoblot that demonstrates that progressive reduction of extracellular pH from ∼pH 7.5 to ∼pH 6.8 increased HKα2 expression. Means ± SE values from three independent experiments are displayed in Fig. 7B. As was the case in experiments examining regulation of (recombinant) HKα2, this increase in protein expression was not associated with an increase in mRNA levels (HKα2 mRNA increased 0.2+0.2-fold in m-IMCD-3 cells subject to reduced extracellular pH; n = 5).
Fig. 7.
Regulation of endogenous HKα2 expression in m-IMCD-3 cells by extracellular pH. m-IMCD-3 cells were grown to near confluency, arrested 24 h in serum-free media, then subject to reduced extracellular pH by reduction of media bicarbonate concentration for another 24 h. A: representative immunoblot analysis of pH effect on HKα2 expression. B: values are means ± SE of data derived from 3 independent experiments.
DISCUSSION
The present study reports the novel finding that a reduction in extracellular pH increases HKα2 expression, via a posttranscriptional mechanism. The capacity of the proton-sensing GPR4 receptor to mediate such pH-dependent regulation is further demonstrated.
Importantly, we demonstrate that pH-dependent regulation of HKα2 is a robust phenomenon that is effected by multiple means when a heterologous expression system for HKα2 is used. Moreover, reducing extracellular pH also increases endogenous HKα2 expression in the murine collecting duct cell line m-IMCD-3. Three different methods to reduce extracellular pH caused an increase in HKα2 protein expression: 1) reducing HCO3− concentration of the media (Fig. 1); 2) adding HCl to the media (Fig. 3, A and B); or 3) increasing the concentration of CO2 in the incubator (Fig. 3, C and D) in HEK-293 cells stably transfected with HKα2 plus NKβ1. The effect is reversible since, after termination of acidosis, HKα2 protein abundance returns to basal levels (Fig. 2). The changes in HKα2 protein occur without change in the expression of HKα2 mRNA.
The observed upregulation of HKα2 is consistent with our previous study, which revealed that cAMP-generating agents increased plasma membrane expression of HKα2 via a PKA-dependent, posttranscriptional mechanism. We used multiple approaches to establish that PKA activation and phosphorylation of HKα2 promote its maturation and plasma membrane expression, without changes in HKα2 mRNA levels (11). Moreover, the rate of degradation was similar for (35S-methionine/cysteine-labeled) wild-type HKα2, HKα2 (S955/A) (an HKα2 mutant in which the critical PKA substrate Ser955 is mutated to an alanine), and the HKα2(S955/D) mutant (mutation to aspartate mimics phosphorylation), suggesting that the posttranslational mechanism involved PKA phosphorylation of HKα2 and affected the early stages of new protein synthesis, possibly minimizing the amount of nascent misfolded protein. Well-documented examples of other transport proteins and channels in the distal tubule and collecting duct, which are regulated by phosphorylation, include the water channel aquaporin (17) and several sodium transport proteins (28, 38). Accumulating evidence emphasizes a critical role for phosphorylation of the water channel aquaporin-2 for membrane trafficking and endosomal recycling, both as a feature of its physiological response to vasopressin as well as participation in the pathophysiological mechanism of human nephrogenic diabetes insipidus (4, 18). The activity of sodium transporters Na-K-2Cl cotransporter (in the thick ascending limb of Henle's loop) and Na-Cl cotransporter (in the distal tubule) are predominantly regulated through phosphorylation/dephosphorylation by specific kinases and phosphatases. Abnormalities in these transporters have been shown to cause inherited forms of blood pressure regulation and electrolyte abnormalities, Bartter's, Gitelman's, and Gordon's syndromes, respectively. Moreover, in addition to our observations with HKα2, as mentioned above (11), Pastor-Soler's laboratory (3) recently reported that V-ATPase subcellular localization, and therefore, its activity in intercalated cells, may be coupled to acid-base status via PKA, and metabolic status via AMPK.
In the present study, we note that reducing extracellular pH caused a modest, but consistent increase in intracellular PKA activity as evidenced by increased phosphorylation of the PKA substrate VASP. Although the mechanism(s) by which a decrease in extracellular pH stimulates intracellular PKA activity and HKα2 expression is unclear, recent studies have identified a subfamily of pH-sensitive GPCRs (reviewed in Ref. 27). Within this subfamily are two Gs-coupled receptors, T cell death-associated gene 8 (TDAG8) and GPR4, that stimulate cAMP accumulation when acutely exposed to a reduction in media pH. Real-time PCR suggests extremely low TDAG8 mRNA levels in murine whole kidney, whereas GPR4 mRNA levels are high (Ref. 41 and Sun X and Petrovic S, unpublished observations). We (41) recently demonstrated 1) a role for GPR4 in the homeostatic regulation of acid-base balance; 2) acid-stimulated cAMP accumulation in HEK-293 cells transfected to express GPR4; and 3) a reduction in acid-stimulated cAMP in OMCD cells in which GPR4 is knocked down by small interference RNA. To explore the capacity of GPR4 to mediate an increase in HKα2 expression in response to media acidification, we transfected our HEK-293 cells stably expressing HKα2. We observed that (over)-expression of GPR4 alone was sufficient to increase intracellular PKA activity and HKα2 expression. In addition, GPR4-expressing HEK-293 cells were more responsive to reductions in extracellular pH by increasing HKα2 expression.
Although our previous study demonstrated only minor production of intracellular cAMP in untransfected HEK-293 cells exposed to acid pH (41), the increase in phospho-VASP levels following reduction in extracellular pH strongly suggests intracellular PKA activation, perhaps mediated by a compartmentalized cAMP signal not readily detected in assays of whole-cell cAMP accumulation. Compartmentalized cAMP signaling is mediated via A-kinase anchoring proteins (AKAPs), which function as a scaffold for PKA as well as PKA substrates and regulatory molecules (40). What transduces the extracellular stimulus (reduction in media pH) into an increase in intracellular cAMP and PKA activation in HEK-293 cells is presently unclear. Although endogenous GPR4 or TDAG8 are possibilities, their proteins are not detectable with available antibodies (not shown) and their mRNA levels are also low (not shown), again suggesting either very low or absent expression. An alternative explanation is the existence of another “proton sensor” such as intracellular Pyk2, previously shown to mediate an increase in Na/H exchanger type 3 in response to acidosis (24). However, the mechanism of Pyk2 activation by a reduction in extracellular pH remains unclear and probably does not involve PKA (24).
The precise mechanism by which reduced extracellular pH causes an increase in the abundance of HKα2 remains to be established. Our findings with acetazolamide during high ambient CO2 suggest that changes in intracellular HCO3− (and therefore, sAC), may not be involved. We recognize, however, that this view is based on the assumption that acetazolamide, which was present throughout the incubation period, completely inhibited carbonic anhydrase and that the generation of HCO3− from the uncatalyzed reaction was minimal. Although a role for endogenous GPR4 in mediating the upregulation of HKα2 in HEK-293 by acid pH cannot be readily discerned, heterologously expressed GPR4 can be shown to mediate the same effect of extracellular pH in this system (Fig. 5B). In addition, m-IMCD-3 cells, which express GPR4 and increase intracellular cAMP in response to media acidification (Petrovic S, unpublished observations), exhibit a progressive increase in HKα2 expression as pH is reduced from ∼pH 7.5 to pH ∼6.8, which corresponds to the dynamic range of GPR4 activation. Thus GPR4 may function as a potential proton-sensing mechanism in collecting duct cells. That this potential pH sensor plays an important role in the defense against metabolic acidosis was suggested by the recent observation that GPR4-deficient mice develop spontaneous nongap metabolic acidosis in the face of defective net acid excretion. GPR4 knockout mice also excrete an inappropriately alkaline urine pH, when corrected for the degree of systemic acidosis, compared with wild-type mice.
Although the present study established the ability of a reduction in extracellular pH to induce HKα2 in two different cell models (one derived from the collecting duct), additional studies are needed to clarify: 1) the relative contribution of GPR4 to this induction in relevant renal cells and tissues in which other putative proton-sensing mechanisms may participate; and 2) the specific role of PKA in mediating the pH- and GPR4-dependent induction. These latter studies will require an effective means of inhibiting PKA in primary cells/tissue or collecting duct cell lines, a challenging task given the poor transfection efficiency of such cells, and traditionally inherent problems in selectively and significantly inhibiting PKA in intact cell systems (21, 29, 33). Whereas the recent findings of Sun et al. (41, 42) implicate a physiological requirement for GPR4 in the renal control of acid-base balance, it would not be surprising that other mechanisms, acting through distinct pathways on various renal transporters, exist to facilitate the ability of the organism to defend against metabolic acidosis.
GRANTS
This work was supported, in part, by National Institutes of Health Grants DK 30603 (T. D. DuBose, Jr.), HL58506 (R. B. Penn), and CA136810 (C. Furdui) and American Heart Association Grant 083J3131 (S. Petrovic). T. S. Opyd and Z. B. Powell were supported by the Medical Student Research Program of Wake Forest University Health Sciences.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Amanda Goode for assistance with the composition of the manuscript.
REFERENCES
- 1. Ahn KY, Park KY, Kim KK, Kone BC. Chronic hypokalemia enhances expression of the H+-K+-ATPase α2-subunit gene in renal medulla. Am J Physiol Renal Fluid Electrolyte Physiol 271: F314–F321, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Alpern RJ, Preisig PA. Dietary acid, endothelins, and sleep. Trans Am Clin Climatol Assoc 115: 385–394, 2004 [PMC free article] [PubMed] [Google Scholar]
- 3. Alzamora R, Thali RF, Gong F, Smolak C, Li H, Baty CJ, Bertrand CA, Auchli Y, Brunisholz RA, Neumann D, Hallows KR, Pastor-Soler NM. PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells. J Biol Chem 285: 24676–24685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouley R, Lu HA, Nunes P, Da SN, McLaughlin M, Chen Y, Brown D. Calcitonin has a vasopressin-like effect on aquaporin-2 trafficking and urinary concentration. J Am Soc Nephrol 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem 269: 14509–14517, 1994 [PubMed] [Google Scholar]
- 6. Cheval L, Morla L, Elalouf JM, Doucet A. Kidney collecting duct acid-base “regulon.” Physiol Genomics 27: 271–281, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Codina J, Delmas-Mata JT, DuBose TD., Jr Expression of HKα2 protein is increased selectively in renal medulla by chronic hypokalemia. Am J Physiol Renal Physiol 275: F433–F440, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Codina J, Kone BC, Delmas-Mata JT, DuBose TD., Jr Functional expression of the colonic H+,K+-ATPase α-subunit. Pharmacologic properties and assembly with X+,K+-ATPase β-subunits. J Biol Chem 271: 29759–29763, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Codina J, Li J, DuBose TD., Jr Expression of a splice variant of the colonic H+,K+-ATPase in kidney of newborn rats. (Abstract). J Am Soc Nephrol 15: 72A, 2004 [Google Scholar]
- 10. Codina J, Li J, DuBose TD., Jr CD63 interacts with the carboxy terminus of the colonic H+-K+-ATPase to decrease plasma membrane localization and 86Rb+ uptake. Am J Physiol Cell Physiol 288: C1279–C1286, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Codina J, Liu J, Bleyer AJ, Penn RB, DuBose TD., Jr Phosphorylation of S955 at the protein kinase A consensus promotes maturation of the α-subunit of the colonic H+,K+-ATPase. J Am Soc Nephrol 17: 1833–1840, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Constantinescu A, Silver RB, Satlin LM. H+-K+-ATPase activity in PNA-binding intercalated cells of newborn rabbit cortical collecting duct. Am J Physiol Renal Physiol 272: F167–F177, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Crowson MS, Shull GE. Isolation and characterization of a cDNA encoding the putative distal colon H+,K+-ATPase. Similarity of deduced amino acid sequence to gastric H+,K+-ATPase and Na+,K+-ATPase and mRNA expression in distal colon, kidney, and uterus. J Biol Chem 267: 13740–13748, 1992 [PubMed] [Google Scholar]
- 14. DuBose TD, Jr, Codina J, Burges A, Pressley TA. Regulation of H+-K+-ATPase expression in kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F500–F507, 1995 [DOI] [PubMed] [Google Scholar]
- 15. DuBose TD, Jr, Good DW. Effects of chronic Cl depletion alkalosis on proximal tubule transport and renal production of ammonium. Am J Physiol Renal Fluid Electrolyte Physiol 269: F508–F514, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Eiam-Ong S, Laski ME, Kurtzman NA, Sabatini S. Effect of respiratory acidosis and respiratory alkalosis on renal transport enzymes. Am J Physiol Renal Fluid Electrolyte Physiol 267: F390–F399, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Eto K, Noda Y, Horikawa S, Uchida S, Sasaki S. Phosphorylation of aquaporin-2 regulates its water permeability. J Biol Chem 285: 40777–40784, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci USA 105: 3134–3139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, Pastor-Soler NM. Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol 298: F1162–F1169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guntupalli J, Onuigbo M, Wall S, Alpern RJ, DuBose TD., Jr Adaptation to low-K+ media increases H+-K+-ATPase but not H+-ATPase-mediated pHi recovery in OMCD1 cells. Am J Physiol Renal Physiol 273: F558–F571, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Guo M, Pascual RM, Wang S, Fontana MF, Valancius CA, Panettieri RA, Jr, Tilley SL, Penn RB. Cytokines regulate β2-adrenergic receptor responsiveness in airway smooth muscle via multiple PKA- and EP2 receptor-dependent mechanisms. Biochemistry 44: 13771–13782, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Kong KC, Gandhi U, Martin TJ, Anz CB, Yan H, Misior AM, Pascual RM, Deshpande DA, Penn RB. Endogenous Gs-coupled receptors in smooth muscle exhibit differential susceptibility to GRK2/3-mediated desensitization. Biochemistry 47: 9279–9288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li J, Codina J, Petroske E, Werle MJ, Willingham MC, DuBose TD., Jr The effect of beta-subunit assembly on function and localization of the colonic H+,K+-ATPase α-subunit. Kidney Int 66: 1068–1075, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Li S, Sato S, Yang X, Preisig PA, Alpern RJ. Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J Clin Invest 114: 1782–1789, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lingrel JB. Na-ATPase: isoform structure, function, and expression. J Bioenerg Biomembr 24: 263–270, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 27. Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature 425: 93–98, 2003 [DOI] [PubMed] [Google Scholar]
- 28. McEneaney V, Dooley R, Yusef YR, Keating N, Quinn U, Harvey BJ, Thomas W. Protein kinase D1 modulates aldosterone-induced ENaC activity in a renal cortical collecting duct cell line. Mol Cell Endocrinol 325: 8–17, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Nevo I, vidor-Reiss T, Levy R, Bayewitch M, Heldman E, Vogel Z. Regulation of adenylyl cyclase isozymes on acute and chronic activation of inhibitory receptors. Mol Pharmacol 54: 419–426, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Ono S, Guntupalli J, DuBose TD., Jr Role of H+-K+-ATPase in pHi regulation in inner medullary collecting duct cells in culture. Am J Physiol Renal Fluid Electrolyte Physiol 270: F852–F861, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Paunescu TG, Da SN, Russo LM, McKee M, Lu HA, Breton S, Brown D. Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol 294: F130–F138, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol 298: F643–F654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Penn RB, Parent JL, Pronin AN, Panettieri RA, Jr, Benovic JL. Pharmacological inhibition of protein kinases in intact cells: antagonism of beta adrenergic receptor ligand binding by H-89 reveals limitations of usefulness. J Pharmacol Exp Ther 288: 428–37, 1999 [PubMed] [Google Scholar]
- 34. Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, Wesson DE. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int 77: 617–623, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Radu CG, Nijagal A, McLaughlin J, Wang L, Witte ON. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc Natl Acad Sci USA 102: 1632–1637, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rothenberger F, Velic A, Stehberger PA, Kovacikova J, Wagner CA. Angiotensin II stimulates vacuolar H+-ATPase activity in renal acid-secretory intercalated cells from the outer medullary collecting duct. J Am Soc Nephrol 18: 2085–2093, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Sabatini S, Laski ME, Kurtzman NA. NEM-sensitive ATPase activity in rat nephron: effect of metabolic acidosis and alkalosis. Am J Physiol Renal Fluid Electrolyte Physiol 258: F297–F304, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Scheuer T. Regulation of sodium channel activity by phosphorylation. Semin Cell Dev Biol 22: 160–165, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seuwen K, Ludwig MG, Wolf RM. Receptors for protons or lipid messengers or both? J Recept Signal Transduct Res 26: 599–610, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Smith FD, Langeberg LK, Scott JD. The where's and when's of kinase anchoring. Trends Biochem Sci 31: 316–323, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Sun X, McGraw DW, Petrovic S. The proton-sensing receptor, GPR4, is a good candidate for an acid-sensor in the kidney (Abstract). J Am Soc Nephrol 17: 14A, 2006 [Google Scholar]
- 42. Sun X, Yang LV, Tiegs BC, Arend LJ, McGraw DW, Penn RB, Petrovic S. Deletion of the pH sensor GPR4 decreases renal acid excretion. J Am Soc Nephrol 21: 1745–1755, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wesson DE. Na+/H+ exchange and H+,K+-ATPase increase distal tubule acidification in chronic alkalosis. Kidney Int 53: 945–951, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Wingo CS, Smolka AJ. Function and structure of H+-K+-ATPase in the kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F1–F16, 1995 [DOI] [PubMed] [Google Scholar]
- 45. Yang X, Amemiya M, Peng Y, Moe OW, Preisig PA, Alpern RJ. Acid incubation causes exocytic insertion of NHE3 in OKP cells. Am J Physiol Cell Physiol 279: C410–C419, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Zhou X, Nakamura S, Xia SL, Wingo CS. Increased CO2 stimulates K+/Rb+ reabsorption mediated by H+-K+-ATPase in CCD of potassium-restricted rabbit. Am J Physiol Renal Physiol 281: F366–F373, 2001 [DOI] [PubMed] [Google Scholar]