Abstract
Extracellular nucleotides (e.g., ATP) activate ionotropic P2X and metabotropic P2Y receptors in the plasma membrane to regulate and maintain cell function and integrity. This includes the renal tubular and collecting duct system, where the locally released nucleotides act in a paracrine and autocrine way to regulate transport of electrolytes and water and maintain cell volume. A prominent role has been assigned to Gq-coupled P2Y2 receptors, which are typically activated by both ATP and UTP. Studies in gene knockout mice revealed an antihypertensive activity of P2Y2 receptors that is linked to vasodilation and an inhibitory influence on renal salt reabsorption. Flow induces apical ATP release in the thick ascending limb, and first evidence indicates an inhibitory influence of P2Y2 receptor tone on the expression and activity of the Na-K-2Cl cotransporter NKCC2 in this segment. The apical ATP/UTP/P2Y2 receptor system in the connecting tubule/cortical collecting duct mediates the inhibitory effect of dietary salt on the open probability of the epithelial sodium channel ENaC and inhibits ENaC activity during aldosterone escape. Connexin 30 has been implicated in the luminal release of the ATP involved in the regulation of ENaC. An increase in collecting duct cell volume in response to manipulating water homeostasis increases ATP release. The subsequent activation of P2Y2 receptors inhibits vasopressin-induced cAMP formation and water reabsorption, which facilitates water excretion and stabilizes cell volume. Thus recent studies have established the ATP/UTP/P2Y2 receptor system as a relevant regulator of renal salt and water homeostasis and blood pressure regulation. The pathophysiological relevance and therapeutic potential remains to be determined, but dual effects of P2Y2 receptor activation on both the vasculature and renal salt reabsorption implicate these receptors as potential therapeutic targets in hypertension.
Keywords: kidney, ENaC, open probability, aldosterone, aldosterone escape, connexin, vasopressin, aquaporin, collecting duct, volume regulation
the kidneys adapt renal excretion to the body's needs to maintain fluid and electrolyte homeostasis. In addition to systemic neurohumoral control, primary local mechanisms are important for renal function and integrity. Extracellular ATP and other nucleotides constitute such local intrarenal mechanisms that regulate physiological and pathophysiological functions by acting in a paracrine and autocrine way on ionotropic P2X receptors and metabotropic G protein-coupled P2Y receptors in the cell membrane. Currently seven P2X (P2X1–7) and eight P2Y (P2Y1,2,4,6,11–14) receptors have been identified (1, 6, 19). Whereas many of these receptor subtypes contribute to the regulation of renal salt and water transport, recent studies have assigned a prominent role to the P2Y2 receptor subtype. P2Y2 receptors are Gq-coupled receptors that activate phospholipase C (PLC) and increase cytosolic concentrations of Ca2+ ([Ca2+]i). They have been implicated in many extrarenal functions (1, 6, 20), and their role in renal transport mechanisms is the focus of this review.
Defining the functional role of extracellular nucleotides and specific P2 receptor subtypes has been hampered by the lack of receptor subtype-specific antagonists. Moreover, ecto-nucleotidases interconvert and degrade nucleotides and generate agonists that act on other P2 receptor subtypes or activate adenosine receptors. In addition, the formation of receptor heteromultimers (e.g., between the adenosine A1 receptor and P2Y2 receptor) (58) contributes to a system of great complexity (1). Despite these limitations, first information on the potential P2Y receptor subtype involved can be obtained based on their differences in agonist preference. For example, in rodents only the P2Y2 and P2Y4 receptor subtypes are similarly activated by ATP and UTP (1, 6). The more recent generation of gene knockout animals has provided an invaluable tool to better define the function and relevance of P2Y2 receptors as outlined in detail.
Since P2Y2 receptors are typically activated by both ATP and UTP, this review discusses the renal ATP/UTP/P2Y2 receptor system. After a brief and more general introduction of the determinants of extracellular concentrations of ATP and UTP, we will discuss in more detail the unique roles of this system in the regulation of renal transport mechanisms and its relevance for salt, water, and blood pressure homeostasis. In particular, recent studies have established the contribution of the ATP/UTP/P2Y2 receptor system to the regulation of the open probability of the epithelial sodium channel ENaC in the aldosterone-sensitive distal nephron (ASDN) and the regulation of water transport in the collecting duct (CD). Additional studies implicated the gap junction protein connexin 30 (Cx30), in the luminal release of ATP in murine CD and in the regulation of ENaC. These issues will be the major focus. More and more insights are also gained into the role of P2Y2 receptors in the proximal tubule and loop of Henle, which are briefly discussed. The interested reader is also referred to recent reviews on the role of P2 receptors in renal epithelia (4, 67, 72, 87, 88).
Determinants of Extracellular ATP and UTP
Cytosolic ATP concentrations in most cell types exceed 5 mM, which allows the cells to release physiologically relevant amounts of ATP without being compromised. Schwiebert's group (78) showed that renal epithelial cultures and cell lines derived from specific nephron segments release ATP into both apical and basolateral media with a predominant release across the apical membrane. Renal epithelial cells like other cells release ATP in response to multiple stimuli including increases in cell volume and flow rate (for a review, see Refs. 67, 78, and 88). Under physiological conditions, at least three mechanisms contribute to the regulated release of nucleotides: exocytosis of ATP-filled vesicles, ATP release by conductive ATP transport through “ATP release channels,” or by nonconductive, facilitated diffusion through an ATP transporter. In general, the molecular mechanisms involved and to what extent these mechanisms contribute to ATP release in renal epithelia are unclear (67, 78, 88). New studies indicate a specific role for Cx30, as discussed in detail below.
Vekaria et al. (92) directly measured intraluminal ATP concentrations in micropuncture experiments in renal proximal and distal tubules of anesthetized rats. They showed that proximal tubules release ATP into the lumen with ATP concentrations in the proximal tubular fluid in the range of 100- 300 nmol/l, whereas concentrations in the early distal tubule were ∼30 nmol/l and thus significantly lower (92). Nishiyama et al. (59) used a microdialysis method and reported ATP concentrations in the interstitial fluid of rat renal cortex of ∼7 nmol/l, which would be consistent with a proposed greater apical ATP release in renal epithelia (78). Nanomolar concentrations of extracellular UTP have also been detected in the medium bathing a range of different cell types (45). More recent data on ATP and UTP concentrations in urine are discussed below. In general, measurements of nucleotides in the bulk phase are thought to underestimate the true values at the cell membrane and receptor level by at least 20-fold such that the concentrations near the membrane receptors are well within the range of receptor affinities (1, 34).
Ecto-nucleotidases present in cell membranes degrade nucleotides, thereby modulating the ligand availability at nucleotide and nucleoside receptors (for a review, see Ref. 105). The expression pattern of these enzymes along the nephron and CD system may provide some clues as to their function (38, 88, 91), but little experimental data using specific inhibitors are available. Considering the current knowledge, luminal ATP released from the proximal convoluted tubule may be rather stable whereas the straight part of the proximal tubule, the thin ascending limb (tAL), and the thick ascending limb (TAL) have significant capacities to break down ATP and lower its luminal availability (88), which would be consistent with the micropuncture data by Vekaria et al. downstream (92). As a consequence, the luminal nucleotide milieu of the downstream ASDN may be functionally isolated, which, together with a low luminal expression of ecto-nucleotidases in cortical and outer medullary collecting ducts (CCD, OMCD, respectively) (88) may result in rather stable luminal nucleotides and allow for regulated and nucleotide-mediated autocrine and paracrine regulation of transport mechanisms as discussed below.
P2Y2 Receptors in Proximal Tubule and Loop of Henle
Expression of P2Y2 receptors in proximal tubule.
Autoradiographical studies by Chan et al. (10) in 1998 provided evidence for binding of the stable ATP analog adenosine 5′-O-[3-thiotriphoshate] (ATPγS) to the basolateral membrane of the rat proximal tubule, which was competitively inhibited by UTP, suggesting the presence of P2Y2-like receptors, i.e., P2Y2 and/or P2Y4, which in rodents both bind and respond to ATP and UTP at similar concentrations. In accordance, addition of ATP or UTP to the bath of proximal convoluted tubules (including S1 segments) of isolated rat proximal tubules increased [Ca2+]i dose dependently (3, 9). Subsequent studies demonstrated the presence of mRNA for P2Y2 receptors in rat proximal convoluted and straight tubule (3, 37) (Table 1).
Table 1.
Expression of P2Y2 receptors along the native tubule and collecting duct system
| Proximal Tubule | Loop of Henle | Collecting Duct |
|---|---|---|
| mRNA: PCT (3, 37); PST (37) | IM: mTAL (intra), cTAL (intra) (86); mRNA: tDL, tAL (3, 37), mTAL (3, 37), cTAL (37) | IM: CCD (PC and IC, PC: intra) (97); OMCD (PC and IC, PC: intra-A/B) (97); MCD IC (86), IMCD (A>B) (37); IMCD (PC and IC, PC: B>A) (97); WB: IMCD (37); mRNA: CD (97); CCD (37), OMCD (3; 37), IMCD (37) |
A, apical; B, basolateral; CCD, cortical collecting duct; CD, collecting duct; cTAL, cortical thick ascending limb; IC, intercalated cells; IM, immunostaining; IMCD, inner medullary collecting duct; (intra), intracellular; OMCD, outer medullary collecting duct; PC, principal cell; PCT, proximal convoluted tubule; PST, proximal straight tubule; tAL, thin ascending limb; mTAL; thick ascending limb; tDL, thin descending limb; WB, Western blotting. Reference numbers appear in parentheses. All studies were performed in rat kidney unless otherwise stated. Modified from Ref. 88.
Activation of P2Y2-like receptors stimulates gluconeogenesis in proximal tubules in vitro.
Cha et al. (8) showed in freshly prepared rat renal cortical tubule suspensions that ATP increases [Ca2+]i and stimulates gluconeogenesis via P2Y receptor activation. Studies by Mo and Fisher (56) in isolated rat proximal tubules revealed that also UTP and the stable analog UTPγS stimulated gluconeogenesis, indicating a P2Y2-like receptor, and that these responses were dependent on PLC activation and an increase in [Ca2+]i. Based on additional pharmacological maneuvers the authors concluded that P2Y2 receptors rather than P2Y4 receptors were involved in the stimulation of gluconeogenesis (56), but more direct evidence is needed. Thus very little is known about the physiological and/or pathophysiological relevance of P2Y2 receptors in proximal tubule, even though the luminal ATP concentrations have been suggested to be highest at this site as discussed above.
P2Y2 receptors in thin limbs of Henle's loop.
Both thin descending limbs (tDL) and tAL of Henle's loop of the rat express mRNA for the P2Y2 receptor (3) (Table 1). Bailey et al. (3) measured changes in [Ca2+]i in freshly isolated rat tDL and tAL in response to basolateral application of receptor agonists and found that for both segments ATP, UTP, and ATPγS were equipotent, consistent with the presence of P2Y2-like receptors (3). The functional relevance of P2Y2 receptors in the thin limbs of Henle is unknown.
P2Y2 receptors in the TAL of Henle's loop.
Autoradiographical studies in rats indicated ATPγS binding sites in the basolateral membrane of the TAL, which were competitively inhibited by UTP, suggesting the presence of P2Y2-like receptors (2, 10). The presence of P2Y2 receptor mRNA (3, 37) and protein (predominantly intracellular) (86) was subsequently demonstrated in medullary and cortical TAL in the rat (Table 1).
Paulais et al. (62) showed in 1995 that ATP and UTP in the superfusate were equally effective to transiently increase [Ca2+]i in mouse cortical TAL (EC50 40 μM; sensitive to suramin) while ADP had little or no effect, consistent with a P2Y2-like receptor. Jensen et al. (33) measured changes in [Ca2+]i in perfused mouse mTAL and established a functional role of basolateral P2Y2 receptors by studying P2Y2 receptor knockout mice (P2Y2−/−). In addition, they found that luminal application of UTP or ATP increased [Ca2+]i in wild-type (WT) mice but never in P2Y2−/− mice. Other nucleotides such as UDP or ADP were without effect, suggesting that the P2Y2 receptor is the dominant P2 receptor expressed on the luminal membrane of mouse medullary TAL (33). The study also showed that luminal P2Y2 receptors promoted larger [Ca2+]i elevations compared with basolateral receptors and provided evidence that P2Y2 receptor activation increases [Ca2+]i via release of Ca2+ from internal stores and also activation of store-operated Ca2+ entry (33).
Flow-dependent ATP release in the TAL and inhibition of NKCC2 by P2Y2 receptor activation.
Increasing the perfusion pressure and thereby the luminal flow rate in isolated, perfused mouse TAL induces an apical and basolateral release of ATP, which by activation of apical and basolateral P2Y2 receptors triggers increases in [Ca2+]i (33). First evidence for an inhibitory influence of the P2Y2 receptor tone on NaCl reabsorption in TAL was provided by Rieg et al. (69), who showed that P2Y2−/− mice have a greater expression of NKCC2 and a greater furosemide-induced natriuresis compared with WT mice. Zhang et al. (102) confirmed greater medullary and cortical expression of NKCC2 in the kidney of P2Y2−/− mice. This effect of the ATP/UTP/P2Y2 receptor system may serve to inhibit and limit NaCl reabsorption in the TAL during an increase in flow rate and NaCl delivery, which may support and interact with the adenosine system in the metabolic control of TAL function (90).
P2Y2 Receptors in the Distal Nephron and CD
Autoradiographical studies revealed ATPγS binding sites in the basolateral membrane of rat CD segments, which were competitively inhibited by UTP, suggesting the presence of P2Y2-like receptors (10). Subsequent studies demonstrated the presence of mRNA for P2Y2 receptors in rat CCD (37) (Table 1). Wildman et al. (97) reported the protein expression of P2Y2 receptors in segments of rat CD and confirmed the mRNA expression of these receptors in microdissected CD (Table 1). The proposed localization of P2Y2 receptors to both apical and basolateral membranes of OMCD is consistent with the expression pattern found in other epithelia (30, 77).
Functional evidence for P2Y2-like receptors in the CCD.
Rouse et al. (75) reported in 1994 that ATP (100 nM and greater added to the bath) as well as UTP activate PLC and increase [Ca2+]i in the isolated, perfused rabbit CCD (75). Deetjen et al. (15) confirmed that basolateral ATP (EC50 of 34 μM) increases [Ca2+]i in the isolated, perfused rabbit CCD and went on to demonstrate that the isolated, perfused CCD of the mouse responded to both basolateral as well as luminal ATP and UTP (EC50 10–23 μM) with increases in [Ca2+]i while luminal ADP had no effect. Functional evidence for apical P2Y2-like receptors in the CCD was confirmed in the rabbit (99). Together, these studies provided functional evidence for the expression of both basolateral and luminal P2Y2-like receptors in principal cells of the CCD.
Inhibition of ENaC open probability by activation of P2Y2 receptors.
Regulation of the epithelial sodium channel ENaC plays a primary role in adapting renal reabsorption of sodium in physiological and pathophysiological states. Experiments by Koster et al. (43) in 1996 in cultured rabbit connecting tubule (CNT) and CCD cells provided first evidence for an inhibitory effect of ATP on sodium transport via ENaC, an effect that appeared to be independent of changes in [Ca2+]i. Short-circuit current measurements in M-1 mouse CCD cells revealed that extracellular ATP inhibits amiloride-sensitive Na+ absorption through activation of P2Y2-like receptors located in the apical and basolateral membrane (13). Follow-up studies in M-1 cells confirmed that the inhibitory effect of ATP on ENaC was independent of changes in [Ca2+]i (85). In 2002, Lehrmann et al. (47) showed in isolated, perfused CCD of mice kept on a low-salt diet that application of both ATP or UTP (100 μM) reduces amiloride-sensitive short-circuit currents by 30–50% when applied to the lumen and by 20% when applied from the basolateral surface, indicating that both luminal and basolateral activation of P2Y2-like receptors can inhibit ENaC-mediated Na+ reabsorption in that segment. Again, the inhibition occurred independently of an increase in [Ca2+]i. Ma et al. (51) reported in the same year that the P2Y receptor agonist ATPγS reduces the open probability (Po) of apical ENaC in the A6 cell line, an amphibian cell line that can form a polarized and high-resistance epithelium.
The first in vivo evidence for an inhibitory effect of luminal ATP on Na+ reabsorption in the CD was provided by Shirley et al. (79) in 2005 in microperfusion experiments in rats maintained on a low-Na+ diet. Subsequent experiments with more selective agonists, however, failed to identify in vivo the receptor responsible for this inhibitory effect.
Rieg et al. (69) observed in P2Y2−/− mice that despite having lower renal expression of the α-subunit of ENaC, amiloride-sensitive Na+ reabsorption was not significantly affected in these mice compared with WT mice. These in vivo findings would be consistent with the concept that lack of P2Y2 receptor activation results in greater ENaC Po, while the observed compensatory decreases in circulating aldosterone concentrations and ENaC expression serve to normalize net ENaC activity. Moreover, smaller increases in plasma aldosterone were required to adapt renal Na+ excretion to restricted intake in P2Y2−/− mice, suggesting a greater aldosterone sensitivity and facilitation of renal Na+ retention (69). This again could be explained by a greater ENaC Po in the absence of P2Y2 receptors, since in this situation a smaller aldosterone-induced increase in ENaC number in the apical membrane would be sufficient to induce a similar increase in total ENaC activity compared with WT mice.
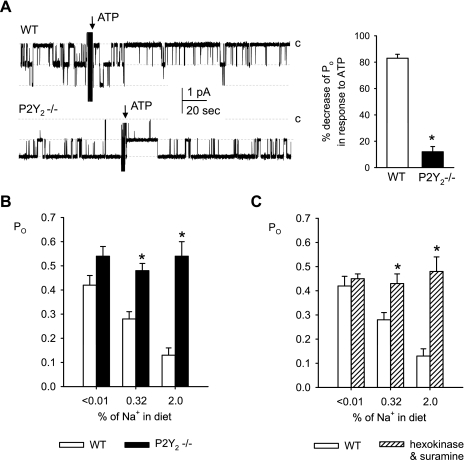
To further clarify this point, Pochynyuk et al. (64, 65) performed patch-clamp studies in freshly isolated split-open CNTs and CCDs to show that ATP decreases ENaC Po and activity in both mouse and rat principal cells. UTP mimicked the effects of ATP and the use of P2Y2−/− mice revealed that the decrease in ENaC activity was primarily mediated via apical P2Y2 receptors (Fig. 1). Concentration-response curves for the inhibitory effect of ATP and UTP on ENaC activity yielded IC50 values of 33 ± 2 and 49 ± 2 nM, respectively (81). Moreover, first evidence was presented for a tonic regulation of ENaC in the CD via locally released ATP inasmuch as scavenging endogenous ATP and inhibiting P2Y receptors rapidly increase ENaC Po and activity in WT mice, and ENaC resting Po was greater in CNT/CCD from P2Y2R−/− mice (64). These findings demonstrated that locally released ATP acts in an autocrine/paracrine manner via P2Y2 receptors to tonically regulate ENaC Po in the mammalian CD.
Fig. 1.
P2Y2 receptor activation determines the inhibitory effect of ATP as well as of dietary salt on epithelial sodium channel (ENaC) open probability (Po). A: representative continuous current traces from cell-attached patches on principal cells (containing ≥2 ENaC's) before and after application of ATP (100 μM) in connecting tubules (CNTs)/cortical collecting ducts (CCDs) harvested from wild-type (WT) and P2Y2 receptor knockout (P2Y2−/−) mice fed a regular 0.32% Na+ diet. Patches clamped to −Vp = −60 mV, and inward Li+ currents through ENaC are downward. Dashed lines indicate the respective current levels, with c denoting the closed state. Summary graphs of ENaC Po changes in response to ATP are shown on the right. *P < 0.05 vs. WT. B: summary graphs showing that suppression of ENaC Po by dietary NaCl intake is absent in CNT/CCD of P2Y2−/− mice. Patch-clamp studies were performed after 1 wk on a given diet. *P < 0.05 vs. WT. C: summary graph showing that inhibition of local ATP signaling [hexokinase (+glucose) to degrade local ATP plus suramin to prevent P2 receptor activation] prevents regulation of ENaC Po in CNT/CCD by dietary NaCl intake in cell-attached patches from WT mice. *P < 0.05 vs. WT control. Based on and modified from Ref. 65.
Inhibition of ENaC Po by P2Y2 receptors involves activation of PLC and hydrolysis of anionic phospholipids.
Ma et al. (51) showed that the P2Y receptor agonist ATPγS reduces the Po of apical ENaC in the A6 cell line by activation of PLC (51). They further showed in A6 cells that the regulation of ENaC by PLC-coupled receptors involves hydrolysis of the anionic phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2), and that the effects of PIP2 are independent of ENaC trafficking (52). Pochynyuk et al. (64) used total internal reflection fluorescence (TIRF) microscopy and a PIP2 reporter in mpkCCDc14 principal cells to show that ATP rapidly decreases plasma membrane PIP2 levels and ENaC Po and that this occurs with similar time courses. Additional studies showed that direct activation of PLC mimicked ATP action on ENaC in isolated CNT/CCD. Moreover, direct PLC stimulation decreased ENaC activity in CNT/CCD from P2Y2−/− mice, indicating intact signaling pathways downstream of the P2Y2 receptor (64). Wildman et al. (97) used pharmacological tools and whole-cell patch clamp of principal cells of split-open CDs from sodium-restricted rats and proposed that activation of P2 receptors (most likely the P2Y2 and/or P2Y4 subtype) via activation of PLC inhibited ENaC activity. Experiments by Kunzelmann et al. (44) in Xenopus laevis oocytes and M-1 mouse CCD cells provided further evidence for the following mechanism for the regulation of ENaC by P2Y2-like receptors, which in a similar form has been proposed by Ma and Eaton (50) (Fig. 2): under resting conditions, the inner leaflet of the lipid bilayer contains a high concentration of PIP2, the latter binding the N terminus of the β-subunit of ENaC, thereby maintaining the ENaC channel open. Stimulation of P2Y2-like receptors activates PLC, which hydrolyzes and lowers the concentration of PIP2 with resultant decreases in PIP2 binding to the N terminus of β-ENaC. The latter lowers ENaC activity by lowering the Po. A role for the cytosolic portion of the N terminus of β-ENaC for PIP2 regulation is consistent with previous studies by Yue et al. (100).
Fig. 2.
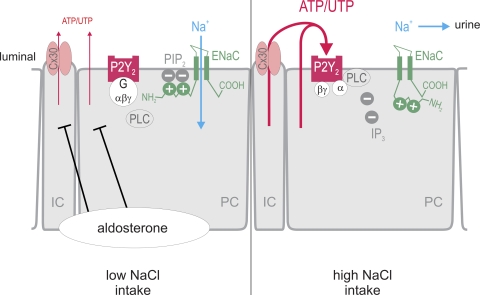
Inhibition of ENaC Po by dietary salt is mediated by the ATP/UTP/P2Y2 receptor system in the aldosterone-sensitive distal nephron. Low dietary salt intake increases aldosterone levels and suppresses the luminal release of ATP and UTP. As a consequence, apical P2Y2 receptors on principal cells (PC) are not activated, and the inner leaflet of the lipid bilayer contains a high concentration of negatively charged phosphatidylinositol 4,5-bisphosphate (PIP2), which binds to positively charged regions of the N terminus of the β-subunit of ENaC, thereby maintaining the ENaC channel open to reabsorb Na+. High dietary salt intake increases the release of ATP and UTP into the lumen, at least in part mediated by connexin 30 (Cx30) in intercalated cells (IC). The nucleotides activate P2Y2 receptors and, thereby, phospholipase C (PLC), which hydrolyzes and lowers the concentration of PIP2. This induces a conformation change in the N terminus of β-ENaC, lowers ENaC Po and activity, and increases Na+ excretion. Modified from Ref. 65. See text for details. IP3, inositol trisphosphate.
Dietary salt inhibits Po of ENaC in CNT/CCD by enhancing apical P2Y2 receptor tone.
The above studies demonstrated that tonic apical release of ATP and/or UTP can activate P2Y2 receptors in CNT/CCD to inhibit the Po of ENaC. However, little was known about the regulation and physiological relevance of this system. In collaboration with the Stockand group, we showed in patch-clamp studies in freshly isolated mouse CNT/CCD that ENaC Po is negatively regulated by dietary salt (65, 89); i.e., an increase in dietary salt intake lowers ENaC Po consistent with a contribution to sodium homeostasis. Importantly, genetic deletion of P2Y2 receptors in mice did not affect the inhibitory effect of dietary salt on the number of ENaCs per patch (N) but completely prevented the dietary salt-induced lowering of ENaC Po (Fig. 1) (65). As a consequence, switching from control to a high-salt diet lowered ENaC activity (NPo) in WT and P2Y2−/− mice to 24 and 56% of control diet, respectively, such that ENaC activity was ∼150% greater in knockout vs. WT mice when given a high-salt diet (65). Similar effects on ENaC Po were observed with inhibition of apical ATP/P2Y receptor signaling (using suramin to block the receptors and hexokinase/glucose to remove ATP) in WT mice consistent with a dietary salt-dependent release of ATP/UTP from CNT/CCD that is preserved after harvesting these segments (Fig. 1). Much has been learned about the mechanisms that regulate ENaC trafficking (7), which determines the number of ENaCs in the apical membrane of the ASDN, as well as the control of ENaC Po by proteolytic cleavage (41). These new data show that dietary NaCl alters renal sodium reabsorption by regulating ENaC Po and that this regulation is mediated by the apical ATP/UTP/P2Y2 receptor system (65) (Fig. 2).
Consistent with the above concept, we observed (65) that a greater salt intake was associated with increased urinary-to-creatinine ratios of UTP as well as the ATP hydrolytic product, ADP, which may reflect greater ATP release in the ASDN and subsequent hydrolysis to ADP by ecto-ATPase activity (see below). Urinary ATP/creatinine ratios tended to be greatest in mice on a high-salt diet, but the values were more variable and lower than levels of UTP and ADP. The ratio of the sum of urinary ATP plus UTP to creatinine was significantly greater in mice on a high-salt diet (65). Previous studies reported a ratio of 1:3–5 for extracellular UTP/ATP both in resting and mechanically stimulated nonexcitable tissue, which closely reflects the relative intracellular abundance of these nucleotides. These previous studies also implied a potential common source and possibly a common mechanism for the release of both ATP and UTP (45, 46). Subsequent studies showed greater urinary ATP/creatinine ratios when dietary salt intake was increased (81). Thus dietary salt can increase the urinary concentrations of both P2Y2 receptor agonists, ATP and UTP. Even though ecto-ATPase activity and water reabsorption in downstream CDs and the bladder as a potential additional source of nucleotides (see below) are expected to affect luminal amounts of ATP and UTP, it is notable that the urinary concentrations of these two nucleotides were within the range of concentrations shown to alter ENaC activity in freshly isolated split-open CNT/CCD (81).
Relevance of ATP/UTP/P2Y2 receptor system for salt homeostasis and blood pressure regulation.
Figure 3 aims to illustrate the integrated renal and blood pressure phenotype of P2Y2−/− mice. Deleting P2Y2 receptors in mice caused a salt-resistant increase in blood pressure; i.e., an increase in salt intake did not augment the difference in blood pressure between WT and P2Y2−/− mice (69). However, whereas heart rate was not affected by salt intake in WT mice, heart rate was reduced and inversely related to salt intake in P2Y2−/− mice, indicating activation of baroreceptors. In addition, plasma aldosterone levels were consistently lower in P2Y2−/− mice (69). A dysfunction of arterial baroreceptors has been implicated in genetic forms of salt-sensitive hypertension in rats and humans (22), and thus the increase in blood pressure in P2Y2−/− mice may be salt resistant because the baroreceptor response to variations in salt intake is intact, allowing the effective increase in renal salt excretion by lowering aldosterone and the antinatriuretic tone of the sympathetic nervous system (69).
Fig. 3.
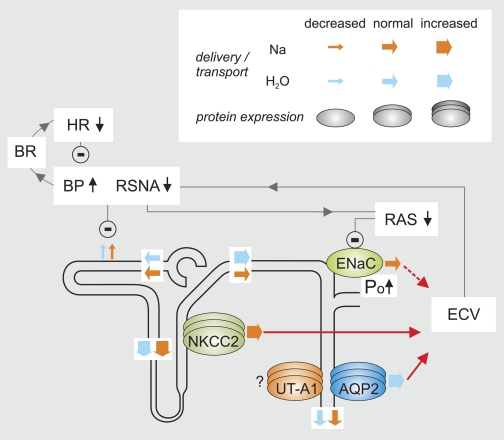
Integrated renal and blood pressure phenotype of P2Y2 receptor knockout mice. The renal expression and activity of Na-K-2Cl cotransporter (NKCC2) and aquaporin-2 (AQP2) are enhanced in P2Y2−/− mice, which increases NaCl and fluid reabsorption via these pathways. The increased ENaC Po also facilitates Na+ reabsorption. Impaired renal NaCl and fluid excretion increases the effective circulating volume (ECV), which enhances blood pressure (BP) and suppresses renal sympathetic nerve activity (RSNA) and the renin-angiotensin-aldosterone-system (RAS). Activation of baroreceptors (BR) reduces heart rate (HR) to lower BP. Suppression of aldosterone downregulates ENaC expression, which compensates for a greater Po and normalizes net Na+ reabsorption via ENaC. We speculate that lower RSNA and the greater BP via pressure natriuresis (54) inhibit proximal tubular reabsorption of NaCl and fluid. Since the glomerular filtration rate and thus the filtration of NaCl and fluid are normal in P2Y2−/− mice, this induces greater deliveries of NaCl and fluid out of the proximal tubule. Greater NaCl reabsorption via NKCC2 in water-impermeable TAL normalizes net NaCl delivery but causes the delivery of greater amounts of more hypotonic fluid to the early distal convoluted tubule. When the vasopressin system and thus the distal water permeability are suppressed, the latter facilitates free water clearance. Under basal conditions, however, the excess water is reabsorbed via increased AQP2 activity, such that net urinary NaCl and fluid excretion is normal in P2Y2−/− mice. The inhibitory influence of P2Y2 receptors on vascular tone is not illustrated. Modified from Ref. 69.
P2Y2−/− mice have increased blood pressure, but amiloride-sensitive renal sodium excretion is not significantly increased compared with WT mice on a regular NaCl diet (69), consistent with a similar net ENaC activity (NPo) in isolated CNT/CCD patches harvested in P2Y2−/− and WT animals on a normal-salt diet (65). The lower plasma aldosterone concentrations can explain the lower renal αENaC expression observed in P2Y2−/− mice and help normalize ENaC activity in the face of greater ENaC Po. Higher blood pressure in the presence of “normal” net ENaC activity, however, implies that P2Y2−/− mice have additional blood pressure-related defects, consistent with a proposed greater expression and activity of NKCC2 in the TAL (69).
To test for a compensatory role of the suppressed aldosterone levels, we compared chronic effects of DOCA plus high salt intake in P2Y2−/− and WT mice. Elimination of differences in mineralocorticoid tone between genotypes unmasked the functional consequences of lacking an inhibitory tone on ENaC Po in P2Y2−/− mice, causing greater upregulation of net ENaC activity as well as NaCl sensitivity of blood pressure compared with WT mice (65). These effects in P2Y2−/− mice were associated with blunted DOCA-induced NaCl intake. DOCA is known to induce NaCl appetite or intake; i.e., when treated with DOCA and given the choice of water or NaCl solution, WT mice prefer to drink the NaCl solution. P2Y2−/− mice actually preferred tap water over the NaCl solution. Why knockout mice avoided the NaCl solution remained unclear, but the decrease in NaCl ingestion prevented an even greater increase in blood pressure (65).
The patch-clamp analysis of DOCA-treated mice further indicated that the lack of P2Y2 receptors blunts the dietary NaCl-induced suppression of the number of active ENaC per patch. This was not observed in the absence of DOCA, indicating that DOCA sensitizes active ENaC expression in the apical membrane to a P2Y2 receptor-mediated inhibitory influence. These studies implicate a potential influence of the ATP/UTP/P2Y2 receptor system on ENaC that goes beyond the regulation of Po (65). Moreover, these data provide strong evidence for a prominent role of P2Y2 receptor signaling in suppression of ENaC activity, when sodium transport is activated by high levels of mineralocorticoids in combination with high NaCl intake as observed in aldosterone escape (65, 81).
In addition to renal salt and fluid handling, P2Y2 receptors have also been implicated in ATP-evoked relaxation of the murine aorta (27). Moreover, P2Y2 receptors mediate the acute nitric oxide-independent blood pressure-lowering effect of the P2Y2/P2Y4 receptor agonist P1-(inosine 5′-)P4-(uridine 5′-)tetraphosphate, or Ip4U, indicating P2Y2 receptor-induced vascular responses to endothelium-derived hyperpolarizing factor (70). In addition to vasodilation, the latter studies also implicated the P2Y2 receptor in Ip4U-induced inhibition of renal sodium reabsorption, suggesting the potential utility of P2Y2 agonism in the treatment of hypertension (70). Based on the phenotype in knockout mice, we proposed that a blunting of P2Y2 receptor expression or activity is a new mechanism for salt-resistant arterial hypertension (69), which is part of the emerging role of P2 receptors in cardiovascular regulation and pathophysiology (20). Future studies will need to address the therapeutic potential and follow up on first genetic studies that associated polymorphisms in the gene for the P2Y2 receptor with essential hypertension in Japanese men (94) and with blood pressure in African Americans (23).
Cx30 mediates dietary salt-induced increases in luminal ATP in CNT/CCD.
The molecular mechanisms involved in the regulation of ATP/UTP concentrations at the apical membrane of the ASDN in general and following changes in salt intake are unclear but potentially may include changes in secretion and breakdown. Compared with further upstream nephron segments, the luminal expression of ecto-nucleotidases in the CCD and OMCD appears rather low and primarily localized to intercalated cells (for a review, see Ref. 88). As a consequence, ATP being released into the lumen by principal cells may be rather stable and able to stimulate ATP-sensitive P2 receptors to regulate transport mechanisms in an autocrine and paracrine way. Recent studies by Sipos et al. (80) suggested that the gap junction protein Cx30 may function as a hemichannel in the luminal release of ATP in the murine CD. They performed elegant studies in isolated split-open CDs to show that flow-induced ATP release is inhibited in Cx30 knockout (Cx30−/−) mice (80). Moreover, Cx30−/− mice have significantly lower urinary ATP/creatinine ratios with a control diet compared with WT mice. Importantly, also the bulk of the increase in urinary ATP observed in WT mice in response to high salt intake was prevented in Cx30−/− mice (81), supporting the concept that Cx30 expression, directly or indirectly, contributes to the regulation of renal epithelial ATP release by dietary salt. Mironova et al. (55) further tested this concept and performed patch-clamp studies in isolated split-open CNT/CCD. They found that ENaC activity and ENaC Po were still sensitive to changes in salt intake in Cx30−/− mice, but ENaC Po remained greater in the knockout mice on normal and high salt intake and total ENaC activity was greater with a high-salt diet vs. WT mice (55). These effects are likely to contribute to the observation that Cx30−/− mice show salt-sensitive increases in blood pressure that are prevented by the ENaC inhibitor benzamil as well as to the impaired pressure-induced natriuresis observed in these mice (80). Overall, these findings implicate Cx30 in local ATP release in the ASDN and that this contributes to the control of ENaC by dietary salt such that loss of this regulation affects sodium homeostasis and blood pressure (Fig. 2). Additional studies with DOCA/high salt revealed that Cx30 is required for normal DOCA escape of ENaC as indicated by significantly greater ENaC activity in Cx30−/− mice (55), similar to the above studies in P2Y2−/− mice. In contrast to Cx30−/− mice, mice lacking P2Y2 receptors have completely lost the downregulation of ENaC Po by dietary salt or the upregulation of ENaC Po by aldosterone. Further studies are needed to test whether this reflects other pathways of ATP release and whether Cx30 is also a determinant of UTP release. Other connexin isoforms have been proposed to be expressed in the distal nephron including Cx30.3 and Cx37, and it remains to be determined whether they are also involved in nucleotide release and/or formation of heteromers with Cx30 (29). Further studies are needed to clarify whether Cx30 is an actual conduit for ATP secretion or a regulator of nucleotide release.
Interestingly, McCulloch et al. (53) proposed that the renal expression of rabbit and murine Cx30 is mostly restricted to intercalated cells of the CNT/CCD, indicating a potential role of these cells in the regulation of luminal ATP release (Fig. 2). As noted above, also the luminal expression of ecto-ATPases as well as 5′-ectonucleotidase, which converts AMP to adenosine, although rather low, appears to be primarily localized to intercalated cells. The relevance of this constellation is unclear but could implicate a concerted regulation of the luminal availability of ATP/UTP by both secretion and local breakdown by these cells. In comparison, high expression of Cx30 was found throughout the luminal membrane continuously from the medullary TAL to the medullary CD system in the rat kidney, including principal cells, with the highest level in the distal convoluted tubule (53), indicating significant species differences.
What regulates release of ATP/UTP in CNT/CCD in response to salt intake or during aldosterone escape?
As noted above, Cx30 in intercalated cells potentially contributes to ATP/UTP release by dietary salt. Nonetheless, principal cells in the ASDN (31) as well as upstream cells in the proximal tubule (92) and the TAL (26, 33) are also potential sources of nucleotide release following an increase in salt intake. The prominent expression of ecto-ATPases in upstream tubular segments may limit their influence with regard to the delivery of ATP/UTP to the ASDN. Cell swelling is well established to induce tubular and CD ATP release, and the apical monocilia in the CD may sense the luminal flow rate to trigger the release of ATP (26, 33, 76, 80). Cell volume may determine luminal ATP release from CD cells in response to manipulating water homeostasis (see Ref. 72 and below), which may help to stabilize cell volume, but its role and specific usefulness in salt homeostasis is less clear. ENaC-mediated sodium uptake in principal cells is lower in response to a high-salt diet, which may reduce rather than increase cell volume. Whether a high-salt diet induces a sustained cell volume increase in intercalated cells to trigger ATP/UTP release via Cx30 remains to be determined. Notably, Cx30 has been implicated in flow-induced ATP release from the CD (80). Whether this is the primary stimulus relevant for the role of Cx30 in response to changes in salt intake is unclear. Changes in CD flow rate associated with a high salt intake can be modest, and not all changes in CD flow rate are associated with enhanced urinary ATP (Ref. 72 and see below). Alternatively or in addition, the local release and possibly breakdown/conversion of ATP/UTP in the ASDN may be directly or indirectly under the control of hormones involved in salt homeostasis. We observed that treatment of WT mice with DOCA increased the number of active ENaC and ENaC Po in isolated CNT/CCD; in comparison, DOCA did not alter ENaC Po in P2Y2−/− mice (65). This is consistent with the notion that mineralocorticoids increase ENaC Po by suppressing the inhibitory influence of the ATP/UTP/P2Y2 receptor system (Fig. 2). Previous studies have shown that mineralocorticoids enhance ENaC Po in A6 amphibian renal cells, a model for the mammalian distal nephron (16, 35, 48). Further studies are necessary to test these pathways/hypotheses. The mechanisms are likely different in mineralocorticoid escape (e.g., DOCA plus a high-salt diet), when ATP and/or UTP release may primarily be due to changes in cell volume (69, 72, 84, 93) as a consequence of DOCA-induced overactive sodium transport and/or increases in tubular flow rate.
Role for P2Y2-like receptors in inhibition of K+ secretion in ASDN.
Patch-clamp studies of the apical membrane in split-open mouse CCD revealed that both ATP and UTP inhibited the small-conductance K+ channel of principal cells (49), thus implicating a P2Y2-like receptor in the regulation of renal K+ secretion. This effect may involve an increase in protein kinase G-sensitive phosphatase activity, which increases channel dephosphorylation (49). In contrast, in vitro studies have linked P2Y2 receptor activation to an increased activity of big-conductance Ca2+-activated K+ (BK) channels (28), which also contribute to basal renal K+ secretion (73). The BK channel is of particular interest since an increase in tubular flow cannot only increase ATP release and P2Y2 receptor activation (33) but stimulates K+ secretion via the BK channel in the ASDN, which may involve P2Y2 receptor-induced increases in [Ca2+]i, known to activate BK channels (63, 73, 98). Moreover, flow-induced ATP release in CD principal cells involves the primary monocilium (31), and studies in Madin-Darby canine kidney cells showed that the increase in [Ca2+]i associated with bending of the primary cilium activates the BK channels (66).
Mice lacking P2Y2 receptors have normal total renal excretion of K+, despite lower plasma concentrations of both K+ and aldosterone, which are primary stimulators of renal K+ excretion (69). This provided indirect evidence for a facilitated renal K+ excretion in P2Y2−/− mice on a standard-K+ diet. Since net ENaC activity seems normal in P2Y2−/− mice, greater ENaC activity is unlikely to explain this effect. However, it would be consistent with the proposed inhibitory influence of P2Y2-like receptors on the small-conductance K+ channel in principal cells discussed above. Facilitation of urinary K+ excretion in P2Y2−/− vs. WT mice dissipated in response to a high-K+ diet (69). Further studies are needed to test whether this may reflect increasing contributions of P2Y2 receptor-stimulated BK channels under these conditions.
P2Y2 Receptors and Water Transport in the CD
The antidiuretic hormone AVP is the primary regulator of water reabsorption in the renal CD and critically involved in the regulation of water balance and maintenance of plasma osmolality. AVP acts on the CD through the Gs protein-coupled vasopressin V2 receptor (V2R) to stimulate adenylyl cyclase (AC) and thus the synthesis of cAMP (71). cAMP activates PKA, which phosphorylates the water channel aquaporin-2 (AQP2), thereby resulting in apical plasma membrane insertion/retrieval of AQP2 (57) (Fig. 4). Importantly, AVP-mediated effects on water reabsorption are modulated by local factors. Local mechanisms may fine tune the effects induced by AVP to bodily needs and serve to more rapidly adapt CD water transport until changes in AVP concentrations (plasma half-life of 10–35 min) (14) can take over. These effects may also protect CD cells from excessive changes in cell volume as a consequence of rapid changes in extracellular osmolality. A perturbation in CD cell volume may release local factors and thus serve as a sensor of changes in plasma osmolality to accelerate AVP responses as well as to maintain cell volume and integrity. As illustrated in Fig. 4, local AVP-counterregulatory factors released from CD principal cells include endothelin-1 (ET-1) (42) and PGE2 (5). In the following, we outline the evidence that the CD system also releases nucleotides like ATP/UTP in response to changes in cell volume and that these nucleotides act locally, including the activation of P2Y2 receptors, to modulate water transport (40, 67, 72, 88).
Fig. 4.
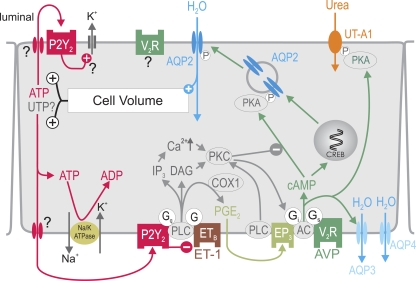
Proposed model for cell volume-dependent ATP release and water transport inhibition by P2Y2 receptor activation in inner medullary collecting duct (IMCD) cells. AVP activates the vasopressin V2 receptor (V2R) to stimulate AQP2-mediated water entry, which increases cell volume. The latter increases basolateral and apical release of ATP (and potentially UTP?) by an unknown mechanism. P2Y2 receptor activation inhibits water reabsorption via multiple signaling pathways and stimulates volume-regulatory K+ channels as shown in other cell types. These effects help to normalize cell volume and accelerate the excretion of free water in response to water loading before circulating AVP levels fall. In the latter situation, the relative hypotonic extracellular fluid increases cell volume. AC, adenylyl cyclase; COX1, cyclooxygenase 1; CREB, cAMP-responsive element-binding protein; DAG, diacylglycerol; EP3, PGE2 E3 receptor; ET-1, endothelin 1; ETB, endothelin B receptor; Gi, inhibitory G protein; Gq, Gs, stimulatory G protein; P, phosphorylation; UT-A1, urea transporter A1. Modified from Refs. 72 and 88.
P2Y2 receptor expression in the medullary CD and inhibition of AVP-induced water permeability by activation of P2Y2-like receptors.
P2Y2 receptor mRNA and protein are present in the inner medullary collecting duct (IMCD) of the rat (37). Protein expression of P2Y2 receptors has been localized in intercalated cells of the medullary CD (86) as well as in both apical and basolateral membranes of principal cells of the IMCD (37, 97) (Table 1). Whereas mRNA expression was not changed with acute water loading (70a) or hydration for 48 h (39), protein expression of the P2Y2 receptor was enhanced in the rat inner medulla by hydration for 48 h (39). In contrast, infusion of the V2R agonist dDAVP for 5 or 6 days decreased P2Y2 receptor protein abundance by ∼60% in the rat inner medulla (83).
Several studies in the rabbit and rat CCD, OMCD, and IMCD used the application of ATP and/or UTP to provide evidence for a P2Y2-like receptor that activates a PLC/Ca2+/PKC signaling pathway, thereby inhibiting cAMP formation and AVP-stimulated osmotic water permeability (9, 17, 18, 36, 75) (Fig. 4). Studies in mpkCCD cells indicated that P2Y2 receptors are translocated to the basolateral membrane in response to AVP and mediate the downregulation of AQP2-mediated water transport in response to basolateral ATP (96). Experiments in isolated IMCD suggested that ATP, by activation of P2Y2-like receptors, can stimulate the release of PGE2 by activation of cyclooxygenase 1 (17, 82, 95). Notably, urinary PGE2 and ATPγS-induced stimulation of PGE2 release in freshly isolated IMCD preparations were greater in hydrated rats compared with control rats, whereas the response to ATPγS was blunted in IMCD of dehydrated rats (82). Based on the upregulation of P2Y2 receptors and ATP-induced PGE2 release in IMCD of hydrated rats, Kishore and colleagues (39) proposed that an AVP-independent regulation of IMCD function by P2Y2 receptors may serve to facilitate renal free water excretion.
Lack of P2Y2 receptors affects expression of water channels and urea transporters in the kidney.
We observed that the expression of AQP2 in the renal medulla is increased in P2Y2−/− mice compared with WT mice, with free access to food and water, despite similar urinary AVP concentrations (69). Zhang et al. (104) confirmed these findings in mice fed a gel diet along with an increased expression of urea transporters UT-A1 (Figs. 3 and 4) and UT-A2 (in CD and thin descending limb of Henle's loop, respectively). Inner medullary protein expression of AQP2, AQP3, and UT-A1 were also greater in P2Y2−/− compared with WT mice during chronic activation of V2R (dDAVP given by osmotic minipumps for 5 days) (104). These findings indicate an inhibitory influence of P2Y2 receptors on the protein expression of these water channels and urea transporters.
Enhanced AVP-dependent cAMP formation and water transport in P2Y2−/− mice.
Consistent with an inhibitory influence of P2Y2 receptors on AVP-induced cAMP formation, we found greater basal urinary cAMP excretion in P2Y2−/− than WT mice despite similar urinary AVP levels. Moreover, studies in freshly isolated IMCD showed that the more stable ATP analog, ATPγS, inhibited the stimulation of cAMP formation by the V2R agonist dDAVP in WT but not in P2Y2−/− mice (69). Furthermore, acute V2R blockade elicited a significantly greater diuresis and electrolyte free water clearance (Cle-H2O) in P2Y2−/− compared with WT mice and reduced urinary cAMP excretion to similar levels (69), demonstrating the greater V2R-mediated renal cAMP formation and water reabsorption in P2Y2−/− mice (Figs. 3 and 4). The fact that basal urinary excretion of fluid and Cle-H2O were not different between P2Y2−/− and WT mice is consistent with their similar fluid intake and was proposed to be the consequence of a greater delivery of a more hypotonic fluid to the distal nephron segments in P2Y2−/− mice, which relates to the integrated renal and blood pressure phenotype of these mice (Fig. 3) (69, 72, 88). Under basal conditions, the hyperactivity of the V2R-AQP2 system reabsorbs the excess water delivery, resulting in normal net fluid excretion in P2Y2−/− mice. Together, the data are consistent with the concept that P2Y2 receptor activation inhibits the AVP-induced cAMP formation and water transport in the CD (69).
How does a lack of P2Y2 receptors affect the response to acute water loading?
Acute oral water loading revealed a similar increase in Cle-H2O in P2Y2−/− and WT mice, even though urinary vasopressin and cAMP excretion remained significantly higher in knockout mice, and this was associated with significantly greater increases in urinary PGE2 compared with WT mice (69). Similar differences between genotypes were observed in preliminary studies with chronic water loading (0.6 M glucose in drinking water for 7 days) (68). A similar increase in Cle-H2O despite a lesser suppression of the vasopressin/cAMP axis indicates a facilitated rather than attenuated ability to enhance free water excretion in P2Y2−/− mice. This may be explained by a greater delivery of a more hypotonic fluid to the distal tubule (Fig. 3) as well as the greater PGE2 release in mice lacking P2Y2 receptors. The latter response was unexpected based on the concept that P2Y2 receptor activation increases the release of PGE2 in the CD (see above). Greater urinary PGE2 excretion has also been described in P2Y2−/− vs. WT mice in response to a high-salt diet (102). The greater PGE2 release in P2Y2−/− mice implied a role for other P2 receptors in ATP-induced PGE2 release, and/or the lack of P2Y2 receptors enhanced the release of PGE2 by other mechanisms. The latter may include a proposed inhibitory effect of P2Y2-like receptors on ET-1 mRNA, release, and effects in IMCD (9, 32) (Fig. 4). Whether ATP-induced inhibition of the endothelin system is physiological relevant and results in greater activation of this system in response to water loading in P2Y2−/− mice, which could enhance ETB-mediated PGE2 formation and free water excretion (25, 42), remains to be determined.
Treatment of WT mice with indomethacin for 30 min before an acute oral water load almost completely inhibited urinary PGE2 excretion and blunted the urine flow response in the 2-h experimental period (72). Remarkably, treatment of P2Y2−/− mice with indomethacin before water loading reduced urinary PGE2 excretion to the levels observed in WT mice without indomethacin treatment, and the responses in urinary flow rate and Cle-H2O were unaffected compared with untreated P2Y2−/− mice. Whether this is due to incomplete cyclooxygenase inhibition in P2Y2−/− or involves indomethacin-insensitive PGE2 formation, needs to be determined, but stresses potential differences in the quantitative or qualitative role of PGE2 in P2Y2−/− mice.
Cell volume-dependent and AVP-induced nucleotide release in CD cells.
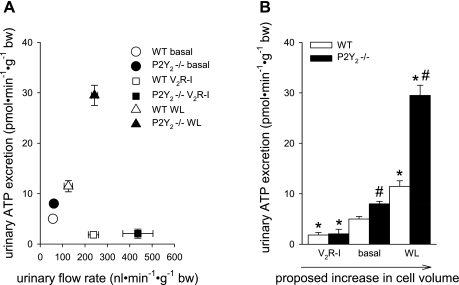
IMCD cells respond to AVP stimulation with an increase in cell volume (24), which is a known stimulus for ATP release (see above). Measuring urinary ATP excretion while manipulating water homeostasis in P2Y2−/− and WT mice provided indirect evidence for a cell volume-dependent ATP release in CD in vivo (69) (Fig. 5). We observed that urinary ATP excretion was similar in P2Y2−/− compared with WT mice during inhibition of water transport by V2R blockade but modestly greater under basal conditions and much greater in response to acute water loading in P2Y2−/− compared with WT mice as shown in Fig. 5. Moreover, V2R blockade and acute water loading both increased urinary flow rate and, therefore, probably exposed the urothelial cells of the lower urinary tract (including the bladder) to similar distension and luminal hypotonicity. However, the two maneuvers had opposite effects on urinary ATP excretion, which indicates that urinary flow rate alone is not a good predictor of urinary ATP excretion and argues against the notion that the exposure of the urothelial cells of the lower urinary tract to distension and hypotonicity is a dominant determinant of urinary ATP excretion following V2R blockade and water loading. Based on these data, we further propose that acute water loading increases ATP release in CD, which is secondary to increases in CD cell volume due to a reduction in extracellular tonicity. The released ATP activates P2Y2 receptors to inhibit CD water uptake and thereby limits the increase in cell volume and the stimulus for ATP release. This feedback mechanism on cell volume is missing in P2Y2−/− mice, and therefore they release more ATP than WT mice following acute water loading. In contrast, acute blockade of V2R reduces CD cell volume (which may include blockade of apical water entry and/or basolateral NaCl uptake) (11), thus offsetting the basal, AVP-, and cell volume-induced ATP release, thereby eliminating differences in ATP release between P2Y2−/− and WT mice (Figs. 4 and 5). In accordance, Odgaard et al. (60) proposed AVP-induced ATP/UTP release in isolated, perfused mouse CCD using a biosensor cell approach with stable transfection of the human P2Y2. The proposed concept would be consistent with an autocrine/paracrine signaling role of ATP release and P2Y/P2Y2 receptor activation in volume regulation, as suggested for other epithelia and cells (12, 21, 61, 74, 93), but needs to be confirmed with more direct assessment of cell volume regulation in mice lacking P2Y2 receptors.
Fig. 5.
Indirect evidence for a positive relationship between collecting duct cell volume, manipulated by different maneuvers in WT and P2Y2−/− mice, and urinary ATP excretion. A: acute vasopressin V2 receptor inhibition (V2R-I) and acute oral water loading (WL) both increased urinary flow rate but induced opposite effects on urinary ATP excretion. Urinary flow rate is not a good predictor of urinary ATP excretion. For further details see the text. B: we propose that V2R-I reduces CD water uptake and thus CD cell volume. In contrast, WL increases CD cell volume due to a reduction in extracellular tonicity. We propose that feedback regulation of cell volume via cell volume-induced ATP release is absent/reduced in P2Y2−/− mice, resulting in greater ATP release. This influence is minimized by reducing water uptake by V2R-I and maximized by WL-induced cell swelling. *P < 0.05 vs. basal. #P < 0.05 vs. WT. Based on and modified from Refs. 69 and 72.
P2Y2 receptors in renal pathophysiology.
Studies by Zhang et al. (103) showed that lithium-induced nephrogenic diabetes insipidus (NDI) in rats is associated with reduced medullary CD mRNA expression of the P2Y2 receptor and that purinergic signaling in the medullary CD is sensitized to activation of a P2Y2-like receptor, i.e., P2Y2 or P2Y4, with regard to increased production of PGE2. Zhang et al. (101) used bilateral ureteral obstruction and release (BUO/R) as a model of postobstructive uropathy and polyuria and found a ∼3.5-fold greater inner medullary P2Y2 protein expression compared with sham rats. Moreover, both ATP and UTP induced greater PGE2 release from freshly isolated medullary CD of BUO/R rats compared with sham rats, indicating augmented signaling via a P2Y2-like receptor. Further studies in P2Y2−/− mice are needed to establish any functional relevance of the P2Y2 receptors in these models.
Summary and Perspectives
We are beginning to appreciate the physiological relevance of cellular nucleotide release and the multitude of cellular processes and functions that are being regulated by extracellular nucleotides acting on P2 receptors in the plasma membrane. This includes the kidney, where the regulation of ENaC by the apical ATP/UTP/P2Y2 receptor system in the CNT/CCD in response to changes in dietary salt is one rather well-understood example. This example also points to the fact that local nucleotide/P2 receptor systems are not only used to preserve cell volume and integrity but that nucleotide release can be regulated by stimuli that derive from the homeostasis of the whole organism. Moreover, the implication of Cx30 in the regulation of nucleotide release by dietary salt is an important step to better understand the molecular nature of renal epithelial nucleotide release pathways and may help to further define the signals and signaling cascades involved in their regulation. We have gained first insights into the renal localization of P2Y2 receptor expression and their function under basal condition but still know very little about how the expression is regulated and affected by physiological and pathophysiological conditions. Even less is known about the regulation along the tubular and CD system of the enzymes involved in nucleotide degradation and interconversion or the interactions between nucleotide and adenosine receptor-mediated signaling including the formation of receptor heteromultimers (e.g., for P2Y2 and adenosine A1 receptors) (1, 58). Dual effects of P2Y2 receptor activation on both the vasculature and renal salt reabsorption implicate these receptors as potential therapeutic targets in hypertension. To better define the functional role and therapeutic potential, specific and more stable P2Y2 receptor antagonists and agonists are very much needed.
GRANTS
The authors were supported by grants provided by the National Institutes of Health (R01HL094728, R01GM66232, R01DK56248, R01DK28602, P30DK079337 to V. Vallon), the American Heart Association (GRNT3440038 to V. Vallon, 10SDG2610034 to T. Rieg), a Carl W. Gottschalk Research Grant from the American Society of Nephrology (to T. Rieg), and the Department of Veterans Affairs (V. Vallon, T. Rieg).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII Update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailey MA, Hillman KA, Unwin RJ. P2 receptors in the kidney. J Auton Nerv Syst 81: 264–270, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Srai K, Burnstock G, Unwin RJ. Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int 58: 1893–1901, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bailey MA, Shirley DG. Effects of extracellular nucleotides on renal tubular solute transport. Purinergic Signal 5: 473–480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol 279: F12–F23, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Burnstock G. Purinergic signalling. Br J Pharmacol 147, Suppl 1: S172–S181, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butterworth MB, Edinger RS, Frizzell RA, Johnson JP. Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol 296: F10–F24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cha SH, Jung KY, Endou H. Effect of P2Y-purinoceptor stimulation on renal gluconeogenesis in rats. Biochem Biophys Res Commun 211: 454–461, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Cha SH, Sekine T, Endou H. P2 purinoceptor localization along rat nephron and evidence suggesting existence of subtypes P2Y1 and P2Y2. Am J Physiol Renal Physiol 274: F1006–F1014, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Chan CM, Unwin RJ, Burnstock G. Potential functional roles of extracellular ATP in kidney and urinary tract. Exp Nephrol 6: 200–207, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Chou CL, Yu MJ, Kassai EM, Morris RG, Hoffert JD, Wall SM, Knepper MA. Roles of basolateral solute uptake via NKCC1 and of myosin II in vasopressin-induced cell swelling in inner medullary collecting duct. Am J Physiol Renal Physiol 295: F192–F201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal 3: re1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuffe JE, Bielfeld-Ackermann A, Thomas J, Leipziger J, Korbmacher C. ATP stimulates Cl- secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells. J Physiol 524: 77–90, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Czaczkes JW, Kleeman CR, Koenig M. Physiological studies of antidiuretic hormone by its direct measurement in human plasma. J Clin Invest 43: 1625–1640, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deetjen P, Thomas J, Lehrmann H, Kim SJ, Leipziger J. The luminal P2Y receptor in the isolated perfused mouse cortical collecting duct. J Am Soc Nephrol 11: 1798–1806, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Duchatelle P, Ohara A, Ling BN, Kemendy AE, Kokko KE, Matsumoto PS, Eaton DC. Regulation of renal epithelial sodium channels. Mol Cell Biochem 114: 27–34, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Ecelbarger CA, Maeda Y, Gibson CC, Knepper MA. Extracellular ATP increases intracellular calcium in rat terminal collecting duct via a nucleotide receptor. Am J Physiol Renal Fluid Electrolyte Physiol 267: F998–F1006, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Edwards RM. Basolateral, but not apical, ATP inhibits vasopressin action in rat inner medullary collecting duct. Eur J Pharmacol 438: 179–181, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflügers Arch 452: 552–562, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal 4: 1–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feranchak AP, Fitz JG, Roman RM. Volume-sensitive purinergic signaling in human hepatocytes. J Hepatol 33: 174–182, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Folkow B, Ely D. Importance of the blood pressure-heart rate relationship. Blood Press 7: 133–138, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Fox ER, Young JH, Li Y, Dreisbach AW, Keating BJ, Musani SK, Liu K, Morrison AC, Ganesh S, Kutlar A, Ramachandran VS, Polak JF, Fabsitz RR, Dries DL, Farlow DN, Redline S, Adeyemo A, Hirschorn JN, Sun YV, Wyatt SB, Penman AD, Palmas W, Rotter JI, Townsend RR, Doumatey AP, Tayo BO, Mosley TH, Jr, Lyon HN, Kang SJ, Rotimi CN, Cooper RS, Franceschini N, Curb JD, Martin LW, Eaton CB, Kardia SL, Taylor HA, Caulfield MJ, Ehret GB, Johnson T, Chakravarti A, Zhu X, Levy D. Association of genetic variation with systolic and diastolic blood pressure among African Americans: the Candidate Gene Association Resource study. Hum Mol Genet 20: 2273–2284, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ganote CE, Grantham JJ, Moses HL, Burg MB, Orloff J. Ultrastructural studies of vasopressin effect on isolated perfused renal collecting tubules of the rabbit. J Cell Biol 36: 355–367, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ge Y, Ahn D, Stricklett PK, Hughes AK, Yanagisawa M, Verbalis JG, Kohan DE. Collecting duct-specific knockout of endothelin-1 alters vasopressin regulation of urine osmolality. Am J Physiol Renal Physiol 288: F912–F920, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Geyti CS, Odgaard E, Overgaard MT, Jensen ME, Leipziger J, Praetorius HA. Slow spontaneous [Ca2+]i oscillations reflect nucleotide release from renal epithelia. Pflügers Arch 455: 1105–1117, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Guns PJ, Van Assche T, Fransen P, Robaye B, Boeynaems JM, Bult H. Endothelium-dependent relaxation evoked by ATP and UTP in the aorta of P2Y2-deficient mice. Br J Pharmacol 147: 569–574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hafting T, Sand O. Purinergic activation of BK channels in clonal kidney cells (Vero cells). Acta Physiol Scand 170: 99–109, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol 298: R1143–R1155, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol 150: 1349–1360, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, Fintha A, Komlosi P, Liu W, Satlin LM, Bell PD, Yoder BK, Schwiebert EM. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal 4: 155–170, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hughes AK, Stricklett PK, Kishore BK, Kohan DE. Adenosine triphosphate inhibits endothelin-1 production by rat inner medullary collecting duct cells. Exp Biol Med (Maywood ) 231: 1006–1009, 2006 [PubMed] [Google Scholar]
- 33. Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]I increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062–2070, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem 278: 23331–23342, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Kemendy AE, Kleyman TR, Eaton DC. Aldosterone alters the open probability of amiloride-blockable sodium channels in A6 epithelia. Am J Physiol Cell Physiol 263: C825–C837, 1992 [DOI] [PubMed] [Google Scholar]
- 36. Kishore BK, Chou CL, Knepper MA. Extracellular nucleotide receptor inhibits AVP-stimulated water permeability in inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 269: F863–F869, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Kishore BK, Ginns SM, Krane CM, Nielsen S, Knepper MA. Cellular localization of P2Y2 purinoceptor in rat renal inner medulla and lung. Am J Physiol Renal Physiol 278: F43–F51, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Kishore BK, Isaac J, Fausther M, Tripp SR, Shi H, Gill PS, Braun N, Zimmermann H, Sevigny J, Robson SC. Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am J Physiol Renal Physiol 288: F1032–F1043, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Kishore BK, Krane CM, Miller RL, Shi H, Zhang P, Hemmert A, Sun R, Nelson RD. P2Y2 receptor mRNA and protein expression is altered in inner medullas of hydrated and dehydrated rats: relevance to AVP-independent regulation of IMCD function. Am J Physiol Renal Physiol 288: F1164–F1172, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE. P2Y2 receptors and water transport in the kidney. Purinergic Signal 5: 491–499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kohan DE. The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr Opin Nephrol Hypertens 15: 34–40, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Koster HP, Hartog A, van Os CH, Bindels RJ. Inhibition of Na+ and Ca2+ reabsorption by P2u purinoceptors requires PKC but not Ca2+ signaling. Am J Physiol Renal Fluid Electrolyte Physiol 270: F53–F60, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J 19: 142–143, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Lazarowski ER, Harden TK. Quantitation of extracellular UTP using a sensitive enzymatic assay. Br J Pharmacol 127: 1272–1278, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lazarowski ER, Homolya L, Boucher RC, Harden TK. Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J Biol Chem 272: 24348–24354, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Lehrmann H, Thomas J, Kim SJ, Jacobi C, Leipziger J. Luminal P2Y2 receptor-mediated inhibition of Na+ absorption in isolated perfused mouse CCD. J Am Soc Nephrol 13: 10–18, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Ling BN, Kemendy AE, Kokko KE, Hinton CF, Marunaka Y, Eaton DC. Regulation of the amiloride-blockable sodium channel from epithelial tissue. Mol Cell Biochem 99: 141–150, 1990 [DOI] [PubMed] [Google Scholar]
- 49. Lu M, MacGregor GG, Wang W, Giebisch G. Extracellular ATP inhibits the small-conductance K channel on the apical membrane of the cortical collecting duct from mouse kidney. J Gen Physiol 116: 299–310, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ma HP, Eaton DC. Acute regulation of epithelial sodium channel by anionic phospholipids. J Am Soc Nephrol 16: 3182–3187, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Ma HP, Li L, Zhou ZH, Eaton DC, Warnock DG. ATP masks stretch activation of epithelial sodium channels in A6 distal nephron cells. Am J Physiol Renal Physiol 282: F501–F505, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC). J Biol Chem 277: 7641–7644, 2002 [DOI] [PubMed] [Google Scholar]
- 53. McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol 289: F1304–F1312, 2005 [DOI] [PubMed] [Google Scholar]
- 54. McDonough AA, Leong PK, Yang LE. Mechanisms of pressure natriuresis: how blood pressure regulates renal sodium transport. Ann NY Acad Sci 986: 669–677, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Mironova E, Peti-Peterdi J, Bugaj V, Stockand JD. Diminished paracrine regulation of the epithelial Na+ channel by purinergic signaling in mice lacking connexin 30. J Biol Chem 286: 1054–1060, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mo J, Fisher MJ. Uridine nucleotide-induced stimulation of gluconeogenesis in isolated rat proximal tubules. Naunyn Schmiedebergs Arch Pharmacol 366: 151–157, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Moeller HB, Olesen ET, Fenton RA. Regulation of the water channel aquaporin-2 by posttranslational modifications. Am J Physiol Renal Physiol 300: F1062–F1073, 2011 [DOI] [PubMed] [Google Scholar]
- 58. Namba K, Suzuki T, Nakata H. Immunogold electron microscopic evidence of in situ formation of homo- and heteromeric purinergic adenosine A1 and P2Y2 receptors in rat brain. BMC Res Notes 3: 323, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nishiyama A, Majid DS, Taher KA, Miyatake A, Navar LG. Relation between renal interstitial ATP concentrations and autoregulation-mediated changes in renal vascular resistance. Circ Res 86: 656–662, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Odgaard E, Praetorius HA, Leipziger J. AVP-stimulated nucleotide secretion in perfused mouse medullary thick ascending limb and cortical collecting duct. Am J Physiol Renal Physiol 297: F341–F349, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem 281: 22992–23002, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Paulais M, Baudouin-Legros M, Teulon J. Extracellular ATP and UTP trigger calcium entry in mouse cortical thick ascending limbs. Am J Physiol Renal Fluid Electrolyte Physiol 268: F496–F502, 1995 [DOI] [PubMed] [Google Scholar]
- 63. Pluznick JL, Sansom SC. BK channels in the kidney: role in K+ secretion and localization of molecular components. Am J Physiol Renal Physiol 291: F517–F529, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283: 36599–36607, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pochynyuk O, Rieg T, Bugaj V, Schroth J, Fridman A, Boss GR, Insel PA, Stockand JD, Vallon V. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. FASEB J 24: 2056–2065, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Praetorius HA, Frokiaer J, Nielsen S, Spring KR. Bending the primary cilium opens Ca2+-sensitive intermediate-conductance K+ channels in MDCK cells. J Membr Biol 191: 193–200, 2003 [DOI] [PubMed] [Google Scholar]
- 67. Praetorius HA, Leipziger J. Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol 72: 377–393, 2010 [DOI] [PubMed] [Google Scholar]
- 68. Rieg T, Bundey RA, Chen Y, Corriden R, Junger W, Insel PA, Vallon V. Absence of P2Y2 receptors facilitates free water excretion (Abstract). J Am Soc Nephrol 17, 31A 2006 [Google Scholar]
- 69. Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Rieg T, Gerasimova M, Boyer JL, Insel PA, Vallon V. P2Y2 receptor activation decreases blood pressure and increases renal Na+ excretion. Am J Physiol Regul Integr Comp Physiol (First published May 25, 2011). doi:10.1152/ajpregu.00148.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70a. Rieg T, Pothula K, Schroth J, Satriano J, Osswald H, Schnermann J, Insel PA, Bundey RA, Vallon V. Vasopressin regulation of inner medullary collecting ducts and compensatory changes in mice lacking adenosine A1 receptors. Am J Physiol Renal Physiol 294: F638–F644, 2008 [DOI] [PubMed] [Google Scholar]
- 71. Rieg T, Tang T, Murray F, Schroth J, Insel PA, Fenton RA, Hammond HK, Vallon V. Adenylate cyclase 6 determines cAMP formation and aquaporin-2 phosphorylation and trafficking in inner medulla. J Am Soc Nephrol 21: 2059–2068, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rieg T, Vallon V. ATP and adenosine in the local regulation of water transport and homeostasis by the kidney. Am J Physiol Regul Integr Comp Physiol 296: R419–R427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int 72: 566–573, 2007 [DOI] [PubMed] [Google Scholar]
- 74. Roman RM, Feranchak AP, Salter KD, Wang Y, Fitz JG. Endogenous ATP release regulates Cl- secretion in cultured human and rat biliary epithelial cells. Am J Physiol Gastrointest Liver Physiol 276: G1391–G1400, 1999 [DOI] [PubMed] [Google Scholar]
- 75. Rouse D, Leite M, Suki WN. ATP inhibits the hydrosmotic effect of AVP in rabbit CCT: evidence for a nucleotide P2u receptor. Am J Physiol Renal Fluid Electrolyte Physiol 267: F289–F295, 1994 [DOI] [PubMed] [Google Scholar]
- 76. Sabirov RZ, Okada Y. ATP-conducting maxi-anion channel: a new player in stress-sensory transduction. Jpn J Physiol 54: 7–14, 2004 [DOI] [PubMed] [Google Scholar]
- 77. Sage CL, Marcus DC. Immunolocalization of P2Y4 and P2Y2 purinergic receptors in strial marginal cells and vestibular dark cells. J Membr Biol 185: 103–115, 2002 [DOI] [PubMed] [Google Scholar]
- 78. Schwiebert EM. ATP release mechanisms, ATP receptors and purinergic signalling along the nephron. Clin Exp Pharmacol Physiol 28: 340–350, 2001 [DOI] [PubMed] [Google Scholar]
- 79. Shirley DG, Bailey MA, Unwin RJ. In vivo stimulation of apical P2 receptors in collecting ducts: evidence for inhibition of sodium reabsorption. Am J Physiol Renal Physiol 288: F1243–F1248, 2005 [DOI] [PubMed] [Google Scholar]
- 80. Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol 20: 1724–1732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stockand JD, Mironova E, Bugaj V, Rieg T, Insel PA, Vallon V, Peti-Peterdi J, Pochynyuk O. Purinergic inhibition of ENaC produces aldosterone escape. J Am Soc Nephrol 21: 1903–1911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sun R, Carlson NG, Hemmert AC, Kishore BK. P2Y2 receptor-mediated release of prostaglandin E2 by IMCD is altered in hydrated and dehydrated rats: relevance to AVP-independent regulation of IMCD function. Am J Physiol Renal Physiol 289: F585–F592, 2005 [DOI] [PubMed] [Google Scholar]
- 83. Sun R, Miller RL, Hemmert AC, Zhang P, Shi H, Nelson RD, Kishore BK. Chronic dDAVP infusion in rats decreases the expression of P2Y2 receptor in inner medulla and P2Y2 receptor-mediated PGE2 release by IMCD. Am J Physiol Renal Physiol 289: F768–F776, 2005 [DOI] [PubMed] [Google Scholar]
- 84. Taylor AL, Kudlow BA, Marrs KL, Gruenert DC, Guggino WB, Schwiebert EM. Bioluminescence detection of ATP release mechanisms in epithelia. Am J Physiol Cell Physiol 275: C1391–C1406, 1998 [DOI] [PubMed] [Google Scholar]
- 85. Thomas J, Deetjen P, Ko WH, Jacobi C, Leipziger J. P2Y2 receptor-mediated inhibition of amiloride-sensitive short circuit current in M-1 mouse cortical collecting duct cells. J Membr Biol 183: 115–124, 2001 [DOI] [PubMed] [Google Scholar]
- 86. Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs 175: 105–117, 2003 [DOI] [PubMed] [Google Scholar]
- 87. Unwin RJ, Bailey MA, Burnstock G. Purinergic signaling along the renal tubule: the current state of play. News Physiol Sci 18: 237–241, 2003 [DOI] [PubMed] [Google Scholar]
- 88. Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294: F10–F27, 2008 [DOI] [PubMed] [Google Scholar]
- 89. Vallon V, Hummler E, Rieg T, Pochynyuk O, Bugaj V, Schroth J, Dechenes G, Rossier B, Cunard R, Stockand J. Thiazolidinedione-induced fluid retention is independent of collecting duct alphaENaC activity. J Am Soc Nephrol 20: 721–729, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 86: 901–940, 2006 [DOI] [PubMed] [Google Scholar]
- 91. Vekaria RM, Shirley DG, Sevigny J, Unwin RJ. Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Renal Physiol 290: F550–F560, 2006 [DOI] [PubMed] [Google Scholar]
- 92. Vekaria RM, Unwin RJ, Shirley DG. Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol 17: 1841–1847, 2006 [DOI] [PubMed] [Google Scholar]
- 93. Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci USA 93: 12020–12025, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang Z, Nakayama T, Sato N, Izumi Y, Kasamaki Y, Ohta M, Soma M, Aoi N, Ozawa Y, Ma Y. The purinergic receptor P2Y, G-protein coupled, 2 (P2RY2) gene associated with essential hypertension in Japanese men. J Hum Hypertens 24: 327–335, 2010 [DOI] [PubMed] [Google Scholar]
- 95. Welch BD, Carlson NG, Shi H, Myatt L, Kishore BK. P2Y2 receptor-stimulated release of prostaglandin E2 by rat inner medullary collecting duct preparations. Am J Physiol Renal Physiol 285: F711–F721, 2003 [DOI] [PubMed] [Google Scholar]
- 96. Wildman SS, Boone M, Peppiatt-Wildman CM, Contreras-Sanz A, King BF, Shirley DG, Deen PM, Unwin RJ. Nucleotides downregulate aquaporin 2 via activation of apical P2 receptors. J Am Soc Nephrol 20: 1480–1490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wildman SS, Marks J, Turner CM, Yew-Booth L, Peppiatt-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol 19: 731–742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001 [DOI] [PubMed] [Google Scholar]
- 99. Woda CB, Leite M, Jr, Rohatgi R, Satlin LM. Effects of luminal flow and nucleotides on [Ca2+]i in rabbit cortical collecting duct. Am J Physiol Renal Physiol 283: F437–F446, 2002 [DOI] [PubMed] [Google Scholar]
- 100. Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem 277: 11965–11969, 2002 [DOI] [PubMed] [Google Scholar]
- 101. Zhang Y, Kohan DE, Nelson RD, Carlson NG, Kishore BK. Potential involvement of P2Y2 receptor in diuresis of postobstructive uropathy in rats. Am J Physiol Renal Physiol 298: F634–F642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang Y, Listhrop R, Ecelbarger CM, Kishore BK. Renal sodium transporter/channel expression and sodium excretion in P2Y2 receptor knockout mice fed a high-NaCl diet with/without aldosterone infusion. Am J Physiol Renal Physiol 300: F657–F668, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang Y, Nelson RD, Carlson NG, Kamerath CD, Kohan DE, Kishore BK. Potential role of purinergic signaling in lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 296: F1194–F1201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhang Y, Sands JM, Kohan DE, Nelson RD, Martin CF, Carlson NG, Kamerath CD, Ge Y, Klein JD, Kishore BK. Potential role of purinergic signaling in urinary concentration in inner medulla: insights from P2Y2 receptor gene knockout mice. Am J Physiol Renal Physiol 295: F1715–F1724, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zimmermann H. Ectonucleotidases in the nervous system. Novartis Found Symp 276: 113–128, 2006 [PubMed] [Google Scholar]