Abstract
The mechanical stress due to shear flow has profound effects on cell proliferation, transport, gene expression, and apoptosis. The mechanisms for flow sensing and transduction are unclear, but it is postulated that fluid flow pulls upon the apical surface, and the resulting stress is eventually transmitted through the cytoskeleton to adhesion plaques on the basal surface. Here we report a direct observation of this flow-induced stress in the cytoskeleton in living cells using a parallel plate microfluidic chip with a fluorescence resonance energy transfer (FRET)-based mechanical stress sensor in actinin. The sensing cassette was genetically inserted into the cytoskeletal host protein and transfected into Madin-Darby canine kidney cells. A shear stress of 10 dyn/cm2 resulted in a rapid increase in the FRET ratio indicating a decrease in stress across actinin with flow. The effect was reversible, and cells were able to respond to repeated stimulation and showed adaptive changes in the cytoskeleton. Flow-induced Ca2+ elevation did not affect the response, suggesting that flow-induced changes in actinin stress are insensitive to intracellular Ca2+ level. The reduction in FRET ratio suggests actin filaments are under normal compression in the presence of flow shear stress due to changes in cell shape, and/or actinin is not in series with actin. Treatment with cytochalasin-D that disrupts F-actin reduced prestress and the response to flow. The FRET/flow method is capable of resolving changes of stress in multiple proteins with optical spatial resolution and time resolution >1 Hz. This promises to provide insight into the force distribution and transduction in all cells.
Keywords: cross-linking protein, live cell imaging, shear stress, fluorescence resonance energy transfer
fluid shear stress from tubular flow regulates cell proliferation, migration, and gene expression in renal epithelial cells (17, 30, 38, 40). Tubular flow directly interfaces with the apical surface of cells and is postulated to be converted to cytoskeletal stress by the resistance of substrate to drag force at focal adhesions (2, 3, 19). The cytoskeletal stresses may induce conformational changes of adhesion proteins that serve as mechanical sensors and transducers, activating cascades of biochemical pathways (6, 8, 35). The actin-based cytoskeleton likely plays a key role in stress distribution and via force-driven biochemistry will affect other transducers (3). The effective elasticity of actin filaments has been shown to depend on the time scale (7), the stress level (16), prestresses (14, 23), the flexibility of individual filaments, and interactions between filaments (5, 26, 32). In living cells, the dynamics of the cytoskeleton is not only acutely modulated by stress but also undergoes slower chronic rearrangements of the cytoskeleton (7, 9, 12). The causal/dynamic effects of stress can best be resolved by examining the time-resolved distribution of stress in response to rapid changes of flow. This helps reveal the primary stress sensors without interference from long-term cytoskeleton rearrangements.
While the traction force distribution generated by living cells has been studied using various methods in static conditions (24, 34), flow-induced change in internal stresses has rarely been measured due to the absence of appropriate tools or methods. Such a measurement requires a suitable platform that can exert precisely controlled shear flow on intact cells and stress-sensitive probes capable of detecting change in stresses in real time. In the present study, this has been achieved by combining a microfluidic chip and fluorescence resonance energy transfer (FRET)-based mechanical stress sensors genetically expressed in specific proteins (27, 28).
Activation of target proteins by pulling on magnetic beads attached to the cell surface has been observed in live cells using FRET sensors (20, 37). The FRET sensors have also been used to observe shear stress-induced activation of Src in smooth muscle cells, which showed that the activation was dependent on cytoskeletal prestress (29). Recently, using FRET sensors it was found that vinculin, a protein that links actin cytoskeleton to integrins, bears mechanical force that regulates focal adhesions recruitment (18). A direct observation of the flow-induced stress distribution in live cells can provide insight into the primary sensing mechanisms and transduction pathways. Using a microfluidic platform, in this study we have measured the stress relocation in space and time in live cells using a FRET-based mechanical stress sensor in one protein actinin. The sensor cassette sstFRET (spectrin-repeat stress-sensitive FRET) (27) was inserted in α-actinin, a cross-linking protein of F-actin, and expressed in Madin-Darby canine kideny (MDCK) cells. A stepwise increase in shear stress from 0.06 to 10 dyn/cm2 resulted in an increase in the FRET ratio, indicating a remarkable decrease in cytoskeletal stress with flow. Cells responded reversibly to repeated pulses showing that the cytoskeleton requires time to adapt.
METHODS
sstFRET sensor and actinin-sstFRET construction.
We constructed the sstFRET sensor consisting of Venus, a yellow fluorescent protein (YFP), as the FRET acceptor and Cerulean, a cyan fluorescent protein (CFP), as the FRET donor, and as a linker, a spectrin repeat domain subcloned from nonerythrocytic spectrin isoform 1 (27). To generate force-reporting α-actinin, we first subcloned α-actinin into pEYFP-C1 vector (Clontech, Mountain View, CA) by replacing the original YFP gene. Primers and restriction enzymes used for subcloning are the following: sense, 5′-CAGATCCGCTAGCATGGACCATTATGATTCTCAGCAAACC-3′ with NheI; anti-sense, 5′-GATCCCGGGCCCGCGGTACCTTAGAGGTCACTCTCGCCGTAC-3′ with KpnI. We named the new construct PEG-actinin. To insert sstFRET into PEG-actinin, we introduced two restriction enzyme sites, AgeI and NotI, between first and second spectrin repeat domains, amino acid position 300, by a site-directed mutagenesis kit from Stratagene (La Jolla, CA). AgeI and NotI were added to the ends of sstFRET gene by primers 5′-GCTAGCGCTACCGGTGCCACCATGGTG-3′ and 5′-GATCCGGTGCGGCCGCTTGGATCCCGGGCCCTCTTCTTGTACAGCTCG-3′. We named the new construct PEG-actinin-sstFRET. Because of the conservative nucleic acid sequence of Venus and Cerulean, primers targeting to any sequence inside sstFRET would have two binding sites in the template and would yield little sstFRET gene fragments. Hence we designed all PCR primers to target the downstream and upstream flanking sequences of the sstFRET template. Figure 1, A–C, shows the schematic of sstFRET. The stress sensitivity of sstFRET has been characterized in solution using DNA springs and in cells previously (27), where we showed that the sensor probes pose no adverse effect on the host proteins, and the host proteins with the reporter will function and target in the same manner as terminal green fluorescent protein (GFP)-labeled proteins (27).
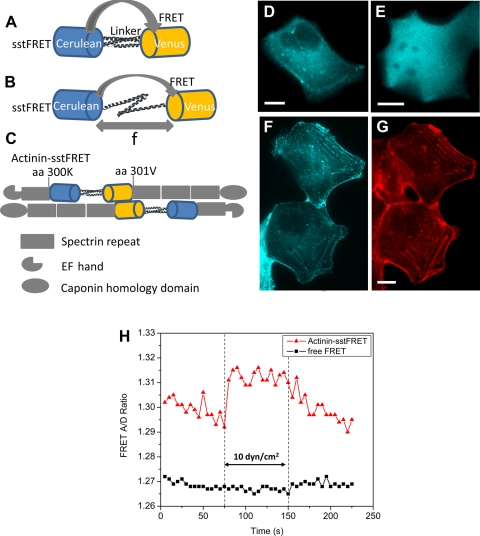
Fig. 1.
Spectrin-repeat stress-sensitive fluorescence resonance energy transfer (FRET) (sstFRET) sensor construction and calibration. A: sstFRET consists of Cerulean, donor, Venus, acceptor, and a linker. Resting sensor shows higher FRET. B: under axial force (f) the distance between donor and acceptor is extended leading to lower FRET. C: actinin-sstFRET, showing actinin hosting sstFRET at amino acid 300, close to the middle of actinin. D and E: fluorescence images [cyan fluorescent protein (CFP) channel] of Madin-Darby canine kidney (MDCK) cells expressing actinin-sstFRET and free sstFRET, respectively. F and G: fluorescence images of actinin-sstFRET in MDCK cells (F) and actin stained with phalloidin-Alexa Fluor568 (G), showing a colocalization of actinin-sstFRET with actin. H: change of FRET ratio in response to an increase of shear stress from 0.6 to 10 dyn/cm2, showing an increase of FRET in actinin-sstFRET expressing cells, not in free sstFRET expressing cells. The scale bar represents 10 μm.
Shear stress control in microfluidic chamber.
The microfluidic flow chamber is a parallel plate system made of a 25-mm diameter cover glass substrate and a 1-mm thick glass slide of ∼7.5 × 7.5 mm separated by polydimethylsiloxane (PDMS) walls. The chamber is 500 μm wide, 15 mm long, and 100 μm in height. The device was fabricated using soft lithography and characterized previously (36). Holes were punched through PDMS at the end of the chamber for inlet and outlet. The chip is mounted on a homemade assembly for fluorescence imaging (36). Solutions were perfused through the chamber using a syringe pump (Harvard Apparatus PHD2000). The wall shear stress (τ) was calculated using τ = 6μQ/wh2, where μ is the dynamic viscosity of perfusate, Q is the volume flow rate, w and h are the width and the height of chamber, respectively (13). Viscosity was assumed to be 1.0 × 10−3 Pa s.
Cell culture and transfection.
MDCK cells (ATCC) were trypsinized and suspended in Dulbecco's modified Eagle medium (DMEM) containing fetal bovine serum and 1% penicillin and streptomycin to a concentration of ∼300,000 cells/ml. The suspension was perfused into flow chamber with a 200-μl pipette and incubated at 37°C for 2 h to allow cell attachment before adding new media. Cell culture medium was then changed every 24 h. Cells were transfected with sstFRET-labeled α-actinin (1.2 μg) at 48 h after seeding. Cells were incubated for another 24 h in transfection solution. The transfection efficiency was typically 20–40%.
FRET recording and image analysis.
Fluorescence images were acquired using a Zeiss inverted microscope with a ×63 oil immersion objective (Axiovert 200m, Zeiss) and a Hamamatsu EM-CCD Camera (ImagEM C9100-13, Hamamatsu, Japan). A Dual-View optical system (Optical Insights) acquired simultaneous images from both donor and receptor channels. The excitation filter set included a bandpass filter (436 ± 20 nm) and a dichroic mirror (455DCLP); the emission filter set included two emission filters (480 ± 30 nm and 535 ± 40 nm) and a dichroic mirror (505DCXR). Time-lapse fluorescence images were recorded using Zeiss software (AxioVision, Ziess). Images obtained from the CFP/YFP channels were aligned and processed using Image-J (NIH) following previously published methods (28, 39). The ratio of acceptor to donor intensity was obtained using Image-J and displayed in a 16-color map. The color represents the acceptor-to-donor ratio of a FRET image. Red color illustrates a higher ratio, indicating lower tension or compression; blue color shows a lower ratio, a higher tension. The bleed through from CFP to YFP was calibrated before experiments and subtracted during image processing (28, 39). The baseline shift due to fluorescence quenching was measured at the beginning of each recording under static conditions, and this was subsequently subtracted from the plots. The peak value of each challenge was used for FRET ratio comparison. A fresh cell culture in flow chamber was used for each measurement. Statistics are calculated as means ± SE of n measurements.
To evaluate cytosolic Ca2+, isotonic solution containing 6 μM Fluo-4 AM (Invitrogen, Carlsbad, CA) was perfused slowly into the chamber. The cells were incubated at 37°C for 40 min and then washed and kept in the dark at room temperature for 15 min before the experiments. Fluorescence micrographs were recorded using the same Zeiss fluorescence imaging system as above and a FITC filter set (Ex: 470 ± 40 nm; dichroic filter: 495 nm; Em: 525 ± 50 nm, Zeiss).
Solutions.
Isotonic solution consisted of (in mM) 75 NaCl, 5 KCl, 1 CaCl2, 2 MgCl2, and 10 HEPES; pH 7.4. The osmolarity was adjusted to 335 mosM with mannitol. For solutions of different osmolarity, mannitol concentration was adjusted while maintaining constant ionic strength. To remove extracellular Ca2+, CaCl2 was replaced with MgCl2. Cytochalasin-D stock was diluted to 10 μM in cell culture media before experiments.
Finite element analysis.
We built a simple two-dimensional model of a hemispherical resting cell and calculated the stress and strain under laminar flow conditions using finite element analysis (ANSYS and ANSYS/CFX, ANSYS). The model had a flow chamber with the same dimensions as the physical chamber and a deformable elastic hemispheric cell attached to the bottom of the chamber. We assumed steady flow with nonslip boundary and employed two-way coupling of fluid structure interaction method. The flow domain was calculated by ANSYS/CFX and the deformable cell domain by ANSYS. The flow domain and the cell domain are coupled through continuous boundary conditions for displacement, traction, and velocity at the fluid-cell interface.
RESULTS
FRET observation of flow-induced stress in actinin.
Using the flow chamber, we expressed sstFRET labeled α-actinin in MDCK cells (Fig. 1D) and measured the FRET signal with changes in flow rate. The stress sensitivity of sstFRET has been characterized in solution using DNA springs previously (27). As controls we transfected with the free sstFRET cassette (Fig. 1E) or a mixture of 1:1 Venus and Cerulean fluorophores. The fluorescence images in Fig. 1, D and E, show that actinin-sstFRET reporters exhibit a nonuniform distribution (Fig. 1D), unlike the free FRET sensors that distribute uniformly in the cytosol (Fig. 1E). In sstFRET-actinin-expressing cells, increasing the flow rate from 0.3 to 50 μl/min (0.06 to 10 dyn/cm2) caused a stable and reversible increase in FRET ratio (Fig. 1H) meaning that stress in the sensor had decreased. In contrast, cells expressing free Venus and Cerulean or the free sstFRET cassette did not show a noticeable change in FRET (Fig. 1H). To examine whether actinin-sstFRET is colocalized to actin fibers, we immunostained cells with phalloidin-Alexa Fluor568 and compared the probe distribution (Fig. 1F) to F-actin in fixed cells (Fig. 1G). The results show that actinin-sstFRET and F-actin colocalize in MDCK cells (Fig. 1, F and G).
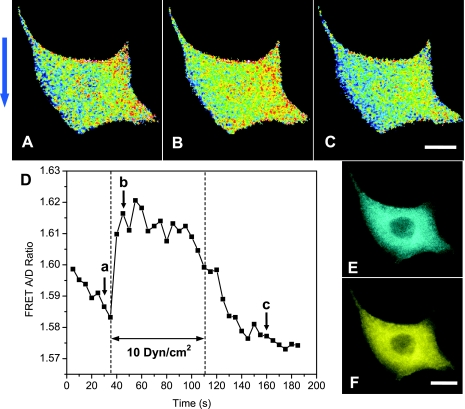
Figure 2, A–C, shows the change of FRET ratio in response to an increase of flow rate from 0.06 to 10 dyn/cm2 and at the various times indicated in Fig. 2D (also see online supplementary movie SI-1 at the AJP-Cell Physiol website). The signal was averaged over the whole cell and is shown as a function of time in Fig. 2D. An increase in flow resulted in a transient increase in FRET suggesting a release of tension in actinin. This change in intracellular stress was reversible with removal of flow. Shear stresses up to 20 dyn/cm2 resulted in only a slightly higher FRET change, suggesting 20 dyn/cm2 is close to saturation stress (data not shown). The reversible increase of FRET with shear stress appears nonintuitive, but Poisson ratio in solid mechanics causes transverse compression with axial extension so that if flow shear stress stretches the actin network in some region of the cell along flow direction, it could cause compression normal to flow. This effect has been previously observed in biopolymers including F-actin (21). Most importantly, flow shear stress may cause nonuniform distribution of internal stress and F-actin filaments are mostly located in a compression region. Since the FRET measurements are at optical resolution, the image will contain multiple molecules and the mean stresses of all of the molecules within the optical voxel are being measured. Thus the measured FRET signal represents the majority of attached molecules that sense the force.
Fig. 2.
Response of actinin-FRET expressing MDCK cells to an increase of shear stress from 0.06 to 10 dyn/cm2. A–C: FRET ratio taken at the times indicated in corresponding letters in D. The color in A–C represents the acceptor-to-donor ratio of a FRET image. Red color illustrates a higher ratio, indicating lower tension or compression; blue color shows a lower ratio, a higher tension. The arrow at the left indicates the flow direction. D: time course of the average FRET ratio in response to flow change showing a reversible increase in FRET. E and F: fluorescence images of actinin-sstFRET, Cerulean (CFP donor channel) (E) and Venus [yellow fluorescent protein (YFP) accepter channel] (F). The scale bar represents 10 μm.
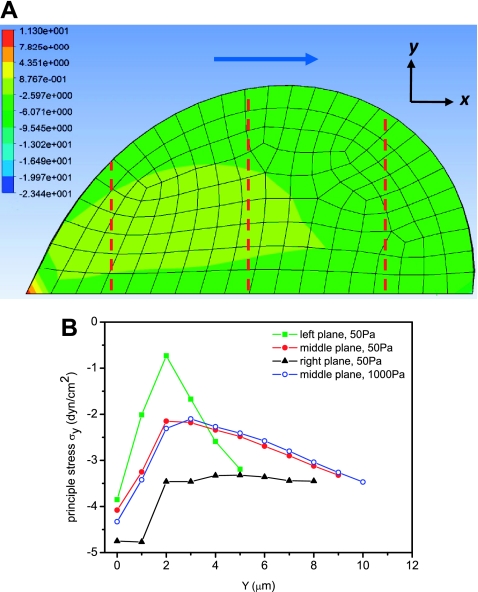
The finite element model of an elastic cell under flow predicts an overall negative stress in normal direction (σy) and a negative stress region close to the basal surface along flow direction (σx). The distribution of normal stress is shown in Fig. 3A, using Young's modulus of the cell to be 50 Pa and a shear stress of 12 dyn/cm2. The normal stress is plotted in Fig. 3B for three planes indicated by dashed lines in Fig. 3A for the cell with 50 Pa modulus and is compared with the cell with 1,500 Pa modulus. We assumed Young's modulus of 50 to 1,500 Pa based on microrheology measurements in epithelial cells (1, 10). The sign of the normal stress remained the same with different modulus values, although softer cells showed larger deformation along the flow direction and a broader compression region (Fig. 3A).
Fig. 3.
Two-dimensional finite element analysis of stress distribution in a cell under flow shear stress of 12 dyn/cm2. The cell was 40 μm in diameter and deformable with modulus of 50 Pa. B: distribution of normal stress (σy) along the y-direction (from basal to apical surface) at three planes indicated by red dashed lines in A for cell modulus of 50 Pa (solid symbols) and 1,000 Pa (open circles). The arrow in A indicates flow direction.
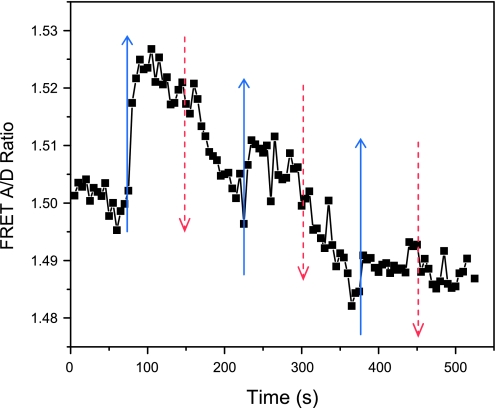
Repeated stimulation caused gradual adaptation as shown in Fig. 4 where we applied three 75 s duration flow pulses separated by 75 s with a peak stress of 10 dyn/cm2 (see online supplementary movie SI-2). The responding changes in stress decreased over time. This reduction in FRET peaks may represent a conformational change of force bearing proteins or a partial disruption of actin filaments by large forces.
Fig. 4.
FRET ratio in response to repeated increase in flow rate. The flow rate was increased from 0.3 to 50 μl/min (0.06 to 10 dyn/cm2) for a period of 75 s and repeated three times as indicated by arrows (blue solid: increase flow rate; red dished: decrease flow rate). The cells responded to repeated stimuli but with reduced peak amplitude.
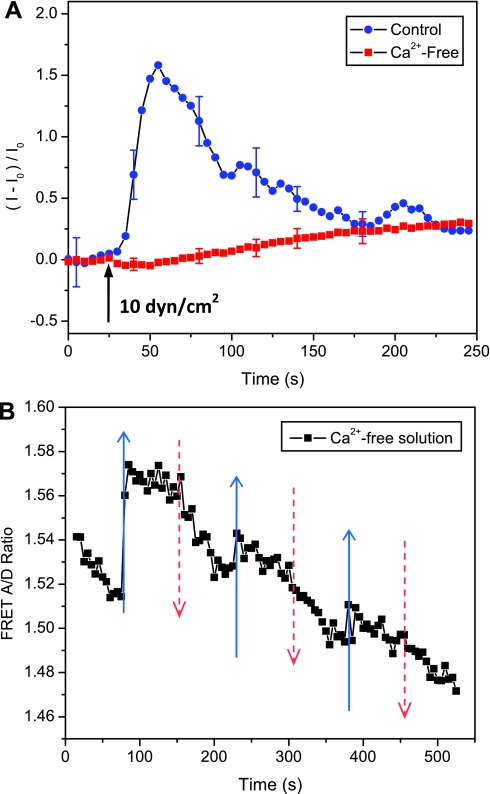
To determine whether flow-induced change in cytoskeletal stresses is associated with Ca2+ increase under flow, we removed Ca2+ from extracellular solution. Using Ca2+-sensitive dye (Fluo-4), we observed that a stepwise increase in flow shear stress from 0.06 to 10 dyn/cm2 caused a rapid Ca2+ rise followed by slow oscillations with a trend to lower Ca2+ (Fig. 5A). This Ca2+ response was diminished in Ca2+-free solution (Fig. 5A). We measured the response of actinin stress to flow simulation in Ca2+-free solution and observed a similar reversible response (Fig. 5B) compared with Fig. 4. This result shows that flow-induced changes in cytoskeletal stress are insensitive to intracellular Ca2+ levels.
Fig. 5.
Effect of intracellular Ca2+ on change of actinin-sstFRET stresses with flow. A: time course of Ca2+ increase in response to an acute change in flow stress from 0.06 to 10 dyn/cm2 in control (blue dots) and Ca2+-free solutions (red cubes) showing extracellular is necessary for flow-induced Ca2+ elevation. B: FRET ratio in response to repeated increase in flow rate in Ca2+-free solution. The flow chamber was perfused with Ca2+-free solution at 0.06 dyn/cm2 for 20 min to washout extracellular Ca2+, followed by three flow stimuli of 0.06 to 10 dyn/cm2 using the same protocol as Fig. 4 for FRET measurements. Flow-induced Ca2+ elevation did not affect the changes in actinin stress. The error bars show the means SE of 6 individual cells.
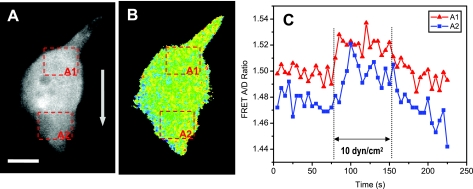
A more detailed analysis showed that local stresses near the upstream face (A1 in Fig. 6, A and B) had different kinetics than the downstream face (A2 in Fig. 6, A and B) so we see that the cell interior is governed not simply by elastic components but also by viscous components.
Fig. 6.
Spatiotemporal distribution of flow-induced stress in a single cell. A and B: fluorescence image (A, YFP channel) and FRET ratio (B) of actinin-sstFRET expression cell at time 0. FRET ratio was measured from two selected areas, upstream edge (A1) and downstream edge (A2), the time course of FRET signals are shown in C. The arrow in A indicates the flow direction. The scale bar represents 10 μm.
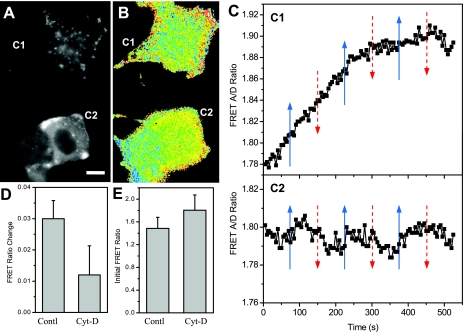
To evaluate the role of the actin cytoskeleton on stress distribution, we disrupted F-actin with 10 μM cytochalasin-D. Figure 7, A and B, shows the fluorescence image (YFP) and the FRET ratio of two treated cells present in one frame (see online supplementary movie SI-3). The top cell (C1 in Fig. 7, A and B) showed a punctuate distribution of actinin and a more evenly distributed background than the bottom cell (C2, Fig. 7, A and B). The mean stress of this cell (C1) showed a continuous and saturating increase with time (Fig. 7C, top). The bottom cell (C2) showed blurry and more uniform fluorescence, but there is no obvious fiber-like structure (Fig. 7A). With increasing shear stress, this cell showed very small change in he FRET ratio (Fig. 7C, bottom). Note that the bottom cell (C2) was joined with a weakly transfected cell on the left (weak fluorescence) that had minimum contribution to FRET. The blue region at the bottom left of the weakly transfected cell arises from a partially attached cell that was pulled off and later flowed away in the recording (see online supplemental movie 3). Under shear stress of 10 dyn/cm2, cytochalasin-D-treated cells had a FRET ratio change of 0.012 (n = 9), much smaller than the control cells that had a change of 0.03 (n = 7) (Fig. 7D). A typical time course of FRET in cytochalasin-D-treated cells is shown in the online supplementary movie SI-4. The net body force on the two groups of cells was similar since they were of similar size and remained attached. The stress in actinin and the sensitivity to cytochalasin-D were different, most likely reflecting differences in the structure of F-actin. The fluorescence image of the top cell indicated more disruption of actin, the increase of FRET may indicate the detachment of the cell from substrate due to the application of the drug. In separate experiments we observed that cell detachment from the substrate resulted in a progressive increase of FRET as tension was released (data not shown).
Fig. 7.
Cytoskeletal stress measured in cytochalasin-D-treated MDCK cells. A and B: yellow fluorescent protein image (A) and FRET ratio (B) of cells treated with 10 μM cytochalasin-D for 30 min. Two cells (C1 and C2) are imaged in the same field. C: responses of top cell (C1) and bottom cell (C2) to repeated increases in flow shear stress, 0.06 to 10 dyn/cm2 showing cytochalasin-D diminished the response to flow. The shear stress changes are indicated by arrows in C (blue: increase flow rate; red: decrease flow rate). D: statistics of change in FRET ratio (peak value) in response to shear stress for control (n = 7) and cytochalasin-D-treated (n = 9) cells, showing a reduction of FRET response due to the disruption of actin filaments. E: FRET ratio of control and cytochalasin-D-treated cells at rest showing how drug treatment reduced the prestress. The scale bar represents 10 μm.
The adherent cells are prestressed, and we postulated that most energy is stored in actin via interactions with the extracellular matrix. We estimated the prestress of actinin in MDCK cells by measuring the initial FRET ratio in actinin and compared them with cytochalasin-D-treated cells. The summary statistics of the FRET ratio in cells with and without treatment of cytochalasin-D is shown in Fig. 7E. Cytochalasin-treated cells showed a higher FRET ratio than control cell (1.8 ± 0.27, n = 10 vs. 1.5 ± 0.19, n = 7) indicating the control cells are relatively prestressed and cytochalasin-D reduced the stress by disruption of the cytoskeleton.
DISCUSSION
Using FRET-based stress sensors, we measured in living cells the stress in actinin, a cross-linker of F-actin. In vitro experiments have shown that cross-linking proteins are highly compliant to mechanical forces (15), and the stresses in linking proteins reasonably indicate the local stresses in the cytoskeleton networks. The results show the involvement of the cytoskeleton for transmitting flow shear stress from the cell surface to the substrate. Remarkably, shear stress increased the average FRET ratio in actinin, suggesting a reduction in mean tension or a net compression of actinin (Fig. 2). This observation is supported by two-dimensionable simulation results using a deformable cell (Fig. 3).
Actinin, an actin-binding protein, is abundant in many regions of the cell including the basal membrane and adherens junctions that link actin to the substrate through the membrane. Unlike endothelial cells where an actin layer is found above the nucleus, in epithelial cells actin fibers are mostly located close to the basal plane. Our two-dimensional simulation predicts compression in the normal stress (σy in Fig. 3, A and B) and in the flow direction (σx) in the bottom region of the cell. This suggests that actin bundles are under compression in transverse direction in the presence of flow shear stress, consistent with our experimental observations, that shear stress caused an average negative stress in actinin (Figs. 2 and 4). Shear-induced negative normal stress has been reported in semiflexible polymer gels including cross-linked F-actin (21). The decrease in normal stress under flow could be the result of changes cell shape. In addition, stretching actin fibers in some region of the cell may also cause compression in actinin since actinin is not in series with actin. The Poisson constant of basic mechanics describes how stretched objects get thinner, i.e., are compressed. Theoretical modeling of cytoskeleton reorganization under stretching has shown that the cytoskeleton resists loading mainly by changing the orientation and spacing of actin filaments (33). Longitudinal stretching along fibers causes reduction in the spacing between them (31). If actinin has a component normal to the tension axis, it should be compressed with stretch. Our results show an average compression in the cell suggesting the normal negative force dominates under shear stress.
Unbinding of actinin from actin fibers under load could also release the force in actinin causing an increase in FRET. However, the rupture force between actinin and actin is ∼40–80 pN (11), which is much higher than the force needed to stretch the linker (27). At optical resolution we are measuring the net stress in a population of fibers so that our results may include stretching and some dissociation.
The flow-induced stress is reversible on a time scale of ∼75 s; repeated stimulation caused adaptive changes in the cytoskeleton (Fig. 4). The reversible changes in cytoskeletal stress can be associated with elastic stretching of load-bearing proteins (22). Adaptation may involve many cellular processes including conformational changes of proteins and cytoskeleton reorganization that are Ca2+ and/or ATP dependent'(7). Elevated Ca2+ is predicted to decrease macroscopic actinin binding affinity to actin (25) that might contribute to the adaptation. We have observed the increase in intracellular Ca2+ level activated by shear stress; however, the elimination of flow-induced Ca2+ elevation did not affect actinin stress response to flow (Fig. 5B). This result shows that the decrease in actinin stresses and the following adaptation is not directly associated with Ca2+-dependent processes. Actin filament polymerization could also occur on the time scale of ∼40 s (12), which may alter cytoskeletal stress. However, early cytoskeleton rearrangement in shear stress was observed after 15 min of shear flow in endothelial cells (4).
Cytochalasin-D-treated cells showed much weaker FRET response to flow (Fig. 7C), indicating actin integrity is required to distribute stress. Our observations showed that adherent cells are highly stressed in normal culture condition, probably via actin. Cytochalasin-D that inhibits extension of F-actin caused an increase in FRET (a decrease in actinin stress) from an average of 1.5 to 1.8 demonstrating prestress in the MDCK cells (Fig. 7E), suggesting a release of tension in the actin cytoskeleton by the drug.
In conclusion, we observed time-dependent flow-induced changes in the internal stress in actinin-sstFRET. Remarkably, increases in shear stress decreased actinin stress showing that the actin cytoskeleton are under normal compression in the presence of flow shear stress, and actinins are not oriented parallel to the stretching axis. Shear stress produced an average compression of actinin. The response is reversible and reduced by cytochalasin-D. The spatiotemporal analysis of stress in live cells can provide new information about how mechanical forces modulate cell behavior.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) Grant DK-77302 (to S. Z. Hua). F. Meng was supported by NIH HL-054887.
REFERENCES
- 1.Alcaraz J, Buscemi L, Grabulosa M, Trepat X, Fabry B, Farre R, Navajas D. Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys J 84: 2071–2079, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alenghat FJ, Nauli SM, Kolb R, Zhou J, Ingber DE. Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp Cell Res 301: 23–30, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Asparuhova MB, Gelman L, Chiquet M. Role of the actin cytoskeleton in tuning cellular responses to external mechanical stress. Scand J Med Sci Sports 19: 490–499, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JGN. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol 26: 453–464, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Broedersz CP, Storm C, MacKintosh FC. Nonlinear elasticity of composite networks of stiff biopolymers with flexible linkers. Physical Rev Lett 101: 118103, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Brown AE, Discher DE. Conformational changes and signaling in cell and matrix physics. Curr Biol 19: R781–R789, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bursac P, Lenormand G, Fabry B, Oliver M, Weitz DA, Viasnoff V, Butler JP, Fredberg JJ. Cytoskeletal remodelling and slow dynamics in the living cell. Nature Materials 4: 557–561, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA 103: 15463–15468, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhuri O, Parekh SH, Fletcher DA. Reversible stress softening of actin networks. Nature 445: 295–298, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dailey H, Yalcin H, Ghadiali S. Fluid-structure modeling of flow-induced alveolar epithelial cell deformation. Comp Struct 85: 1066–1071, 2007 [Google Scholar]
- 11.Ferrer JM, Lee H, Chen J, Pelz B, Nakamura F, Kamm RD, Lang MJ. Measuring molecular rupture forces between single actin filaments and actin-binding proteins. Proc Natl Acad Sci USA 105: 9221–9226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Footer MJ, Kerssemakers JW, Theriot JA, Dogterom M. Direct measurement of force generation by actin filament polymerization using an optical trap. Proc Natl Acad Sci USA 104: 2181–2186, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox RW, McDonald AT. Introduction to Fluid Mechanics. New York: Wiley, 1992 [Google Scholar]
- 14.Gardel ML, Nakamura F, Hartwig J, Crocker JC, Stossel TP, Weitz DA. Stress-dependent elasticity of composite actin networks as a model for cell behavior. Physical Rev Lett 96: 088102, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Gardel ML, Nakamura F, Hartwig JH, Crocker JC, Stossel TP, Weitz DA. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc Natl Acad Sci USA 103: 1762–1767, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira P, Weitz DA. Elastic behavior of cross-linked and bundled actin networks. Science 304: 1301–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Graf R, Apenberg S, Freyberg M, Friedl P. A common mechanism for the mechanosensitive regulation of apoptosis in different cell types and for different mechanical stimuli. Apoptosis 8: 531–538, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466: 263–266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J 20: 811–827, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Itoh RE, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Molec Cell Biol 22: 6582–6591, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janmey PA, McCormick ME, Rammensee S, Leight JL, Georges PC, MacKintosh FC. Negative normal stress in semiflexible biopolymer gels. Nature Mat 6: 48–51, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Johnson CP, Tanbg HY, Carag C, Speicher D, Discher DE. Forced unfolding of proteins within cells. Science 317: 3914–3921, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim T, Hwang W, Lee H, Kamm RD. Computational analysis of viscoelastic properties of crosslinked actin networks. PLoS Comput Biol 5: e1000439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc Natl Acad Sci USA 102: 4300–4305, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Interactions of calmodulin and α-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J Neurosci 19: 1165–1178, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieleg O, Bausch AR. Cross-linker unbinding and self-similarity in bundled cytoskeletal networks. Physical Rev Let 99: 158105, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Meng F, Sachs F. Visualizing dynamic cytoplasmic forces with a compliance-matched FRET sensor. J Cell Sci 124: 261–269, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng F, Suchyna TM, Sachs F. A fluorescence energy transfer-based mechanical stress sensor for specific proteins in situ. FEBS J 275: 3072–3087, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci USA 105: 6626–6631, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinlan MR, Docherty NG, Watson RW, Fitzpatrick JM. Exploring mechanisms involved in renal tubular sensing of mechanical stretch following ureteric obstruction. Am J Physiol Renal Physiol 295: F1–F11, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Ritter MC, Jesudason R, Majumdar A, Stamenovic D, Buczek-Thomas JA, Stone PJ, Nugent MA, Suki B. A zipper network model of the failure mechanics of extracellular matrices. Proc Natl Acad Sci USA 106: 1081–1086, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin JH, Gardel ML, Mahadevan L, Matsudaira P, Weitz DA. Relating microstructure to rheology of a bundled and cross-linked F-actin network in vitro. Proc Natl Acad Sci USA 101: 9636–9641, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamenovic D, Lazopoulos KA, Pirentis A, Suki B. Mechanical Stability Determines Stress Fiber and Focal Adhesion Orientation. Cell Mol Bioeng 2: 475–485, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci USA 100: 1484–1489, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J 20: 4639–4647, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Heo J, Hua SZ. Spatially resolved shear distribution in microfluidic chip for studying force transduction mechanisms in cells. Lab Chip 10: 235–239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature 434: 1040–1045, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM. Mechanotransduction in the renal tubule. Am J Physiol Renal Physiol 299: F1220–F1236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia Z, Liu Y. Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophys J 81: 2395–2402, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, Wandinger-Ness A, Bacallao RL, Alper SL. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol 296: F1464–F1476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.