Abstract
Transient receptor potential (TRP) ankyrin 1 (TRPA1) is a Ca2+-permeant, nonselective cationic channel. It is predominantly expressed in the C afferent sensory nerve fibers of trigeminal and dorsal root ganglion neurons and is highly coexpressed with the nociceptive ion channel transient receptor potential vanilloid 1 (TRPV1). Several physical and chemical stimuli have been shown to activate the channel. In this study, we have used electrophysiological techniques and behavioral models to characterize the properties of TRPA1. Whole cell TRPA1 currents induced by brief application of lower concentrations of N-methyl maleimide (NMM) or allyl isothiocyanate (AITC) can be reversed readily by washout, whereas continuous application of higher concentrations of NMM or AITC completely desensitized the currents. The deactivation and desensitization kinetics differed between NMM and AITC. TRPA1 current amplitude increased with repeated application of lower concentrations of AITC, whereas saturating concentrations of AITC induced tachyphylaxis, which was more pronounced in the presence of extracellular Ca2+. The outward rectification exhibited by native TRPA1-mediated whole cell and single-channel currents was minimal as compared with other TRP channels. TRPA1 currents were negatively modulated by protons and polyamines, both of which activate the heat-sensitive channel, TRPV1. Interestingly, neither protein kinase C nor protein kinase A activation sensitized AITC-induced currents, but each profoundly sensitized capsaicin-induced currents. Current-clamp experiments revealed that AITC produced a slow and sustained depolarization as compared with capsaicin. TRPA1 is also expressed at the central terminals of nociceptors at the caudal spinal trigeminal nucleus. Activation of TRPA1 in this area increases the frequency and amplitude of miniature excitatory or inhibitory postsynaptic currents. In behavioral studies, intraplantar and intrathecal administration of AITC induced more pronounced and prolonged changes in nociceptive behavior than those induced by capsaicin. In conclusion, the characteristics of TRPA1 we have delineated suggest that it might play a unique role in nociception.
Keywords: transient receptor potential vanilloid 1, synaptic transmission, patch-clamp
transient receptor potential (TRP) ankyrin 1 (TRPA1) is a nonspecific, Ca2+-permeable cationic channel, first identified in human fibroblasts (23). It is unique in its structure among TRP channels for having a large number of ankyrin repeat domains (16, 17, 19), which are known to impart a springlike action to proteins (59). It is activated by physical stimuli like noxious cold (<17°C) temperatures and mechanical force, and by extracellular Ca2+ (6, 8, 15, 16, 26, 31, 39, 42, 61, 65). TRPA1 is also activated by several classes of compounds by distinct mechanisms: 1) allyl isothiocyanate (AITC, mustard oil), allicin and diallyldisulfide (garlic derivatives), acrolein (present in tear gas and car exhaust fumes) and N-methyl maleimide (NMM) activate TRPA1 by covalent modification of cysteine residues; 2) cysteine-mutated channels are activated by δ-9-tetrahydrocannabinol and 2-aminophenyl borane; and 3) bradykinin (BK) activates TRPA1 by stimulating phospholipase C (PLC) and protein kinase A (PKA) (22, 38, 57, 64). TRPA1 is also activated by endogenous chemicals produced during oxidative stress including H2O2/hydroxyl radicals, aldehydes such as 4-hydroxynonenal, cyclopentenone prostaglandins such as 15d-PGJ2, and hypochlorite (3, 9). TRPA1 is predominantly expressed in trigeminal (TG) and dorsal root ganglion (DRG) neurons with a high degree of coexpression with transient receptor potential vanilloid 1 (TRPV1) and trk receptors (2, 5, 32, 56, 60, 61). The level of expression of TRPA1 increases in the presence of neurotrophic factors like nerve growth factor (5, 45, 46).
Behavioral studies in mice lacking TRPA1 (TRPA1−/−) confirmed its role in nociception to pungent substances (7, 34). Treatment with TRPA1 antisense oligodeoxynucleotide reduced behavioral hypersensitivity to cold after Complete Freund's adjuvant (CFA)-induced inflammation or sciatic nerve injury and decreased cold hyperalgesia following L5 spinal nerve ligation (27, 45). TRPA1 knockout mice exhibited impaired behavioral responses to a cold plate maintained at 0°C (34). However, experiments conducted by Bautista et al. (7) and Nagata et al. (42) failed to demonstrate this effect. Recent studies using TRPA1 knockout mice have confirmed that there is no difference in acute cold sensation, but mechanical sensitivity is significantly altered. It has been suggested that the mechanosensation of inner hair cells could be mediated by TRPA1 (15, 42), but TRPA1 knockout mice did not exhibit any deficiency in hearing (7, 34). TRPA1 knockout animals did not show any change in mechanical sensitivity as measured by paw withdrawal threshold using von Frey filaments. However, response to a forceful mechanical stimulus by a sharp blunt needle was significantly decreased (7, 34). It has been demonstrated that low threshold Aβ and D-hair mechanoreceptive fiber characteristics have also changed (28, 35). Recently, it has been shown that ablation of channels that are linked to TRPV1 lineage, which include TRPA1, resulted in a complete loss of thermal sensitivity but the mechanical sensitivity was unaffected (40). Interestingly, the Drosophila orthologue of TRPA1, in contrast to its mammalian counterpart, is activated by heat and regulates thermotaxis (44).
Inflammatory mediators stimulate protein kinase A (PKA) and protein kinase C (PKC) pathways, which have been shown to upregulate the activity of nociceptors like TRPV1 (13, 14, 29, 37, 44, 48). Stimulation of PLC and PKA has been shown to modulate TRPA1 channel activity (57). Translocation of TRPA1 from cytosol to plasma membrane has been shown to be promoted by increasing cAMP levels (64). Since TRPA1 is highly coexpressed with TRPV1, we wanted to examine the potential modulation of TRPA1 by second messenger pathways that have been shown to upregulate TRPV1 activity.
In addition to their role in the peripheral nerve terminals, several nociceptive ion channels have been shown to be expressed at the central terminals of sensory neurons, where they are able to modulate synaptic transmission at the first sensory synapse (24, 33, 43, 58). Sikand and Premkumar (58) have shown that the potentiation of glutamatergic transmission between DRG and dorsal horn neurons by capsaicin is regulated by PKC-mediated phosphorylation of TRPV1 and Ca2+-dependent desensitization of TRPV1 (58). Kosugi et al. (33) showed that TRPA1 channels in the lumbar spinal cord modulate synaptic transmission (33). We have studied TRPA1 function in the caudal spinal trigeminal nucleus (CSTN), the site of termination of nociceptors mediating craniofacial pain.
In this study, we have characterized the functions of TRPA1 in sensory neurons from wild-type and TRPV1 knockout mice (to rule out the possible involvement of TRPV1, because TRPV1 and TRPA1 are coexpressed), in Xenopus oocytes heterologously expressing the cloned receptor and in slices of CSTN from rats. We have also characterized the role of TRPA1 in nociception using animal models of pain.
MATERIALS AND METHODS
Electrophysiology.
All the animals used in these experiments were cared for according to the standards of the National Institutes of Health (NIH, Bethesda, MD). All animal use protocols were approved by Southern Illinois University School of Medicine Animal Care Committee.
Adult rats and wild-type and TRPV1 knockout mice (Jackson Laboratories) were euthanized by isoflurane. Dorsal root ganglia (DRG) were dissected and the cells were dissociated by triturating with a fire-polished glass pipette. Cells were cultured in Neurobasal medium (Life Technologies, Buffalo, NY), supplemented with fetal bovine serum (10%), and grown on poly-d-lysine-coated glass coverslips. Cells were used within 5 to 15 h of plating. Small diameter (<30 μm) rounded neurons were selected for patch-clamp experiments. More than 70% of these neurons responded to capsaicin. Giga-seal patch-clamp technique (21) was used to record whole cell and single-channel currents. For whole cell patch-clamp recordings, the bath solution contained (in mM) 140 Na gluconate, 2.5 KCl, 10 HEPES, 1 MgCl2, and 1.5 EGTA, and pH was adjusted to 7.35 with NaOH; the pipette solution contained (in mM) 130 K-gluconate, 10 NaCl, 2.5 KCl, 10 HEPES, and 1 MgCl2, and pH was adjusted to 7.35 with KOH. For current-clamp experiments, the pipette solution contained (in mM) 130 K-gluconate, 10 NaCl, 1 MgCl2, 10 BAPTA, 1 K2ATP, and 10 HEPES, and pH was adjusted to 7.35 with KOH. For current-clamp experiments in the presence of Ca2+, EGTA was replaced by 1.8 mM CaCl2. Currents were recorded using a WPC 100 patch-clamp amplifier (E. S. F. Electronic, Gottingen, Germany, or Axopatch 200B, Axon Instruments, Union City, CA). Data were filtered at 10 kHz, digitized (VR-10B, Instrutech; Great Neck, NY), and stored on videotapes or directly stored in the computer using a LabView interface (National Instruments, Austin, TX). For analysis of whole cell currents, data were filtered at 1 kHz (−3 db frequency with an 8-pole low-pass Bessel filter, LPF-8, Warner Instruments, Hamden, CT) and digitized at 2 kHz (LabView-based hardware and software).
For single-channel recordings, the bathing solution contained (in mM) 140 Na- gluconate, 2.5 KCl, 5 HEPES, and 1.5 EGTA, and pH was adjusted to 7.35 with NaOH. For cell-attached patches, Na-gluconate was replaced with K-gluconate. The pipette solution contained (in mM), unless indicated otherwise, 140 K-gluconate, 10 NaCl, 10 BAPTA, 10 HEPES, 2 K2ATP, and 0.25 GTP, and pH was adjusted to 7.35 with KOH. For cell-attached patches, the pipette solution was Na-gluconate (140 mM) containing extracellular solution. Data were collected using Axopatch 200B amplifier with the filter set at 10 kHz, digitized (VR-1B), and stored in a computer. For analysis of amplitude and open probability (Po), the data were filtered at 2.5 kHz (LPF-8) and digitized at 5 kHz using LabView based hardware and software. Single channel analyses were performed using Channel 2, Australian National University, and QUB software (www.qub.buffalo.edu) (47, 53).
For slice patch-clamp experiments, Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were bred locally. Rats 2–3 wk old were used for the recording of synaptic currents. Horizontal slices of brain stem were prepared using methods similar to those previously published (19). Rats were deeply anesthetized with isoflurane (5%) and then decapitated. A block of brain stem from the obex to the upper cervical cord was removed, placed in cold (4°C), oxygenated physiological solution, and cut with a vibrating tissue slicer into 200-μm-thick horizontal sections. Two or three sections containing CSTN were obtained from the dorsal aspect of each brain. For recording, brain stem slices were placed on the stage of an upright near-infrared differential interference contrast microscope (Olympus BX-50wi). In horizontal slices, the spinal trigeminal nucleus caudalis was visible as a translucent band just medial to the spinal trigeminal tract. Recordings were made at 32°C.
Responses from brain stem slices were recorded in a superfusion chamber. Extracellular medium contained (in mM) 126 NaCl, 2.5 KCl, 1.2 MgCl2, 11 dextrose, 1.4 NaH2PO4, 2.4 CaCl2, and 25 NaHCO3, and pH was adjusted to 7.35 with NaOH at 32°C; the medium was continually gassed with 95% O2-5% CO2. Intracellular recording solution for recording miniature excitatory postsynaptic currents (mEPSCs) and inhibitory postsynaptic currents (IPSCs) had the following composition (in mM): 140 CsCl, 2 CaCl2, 10 EGTA, 5 HEPES, and 2 MgATP, and pH was adjusted to 7.35 with CsOH. Recording electrodes were pulled from capillary glass (1.5 mm outer diameter, 1.0 mm inner diameter). After filling with intracellular recording solution, an electrode impedance of 4 MΩ is usual. The electrode tip potential was canceled and the electrode's component of the series resistance was fully compensated before the electrode was placed against an individual neuron visualized using infrared differential interference contrast optics. Tight seals of 2 to 10 GΩ were routinely obtained by light suction, and whole cell mode was obtained by further light suction. After entry into whole cell mode, access resistance usually increased to 5–10 MΩ, but was not further compensated. Neurons were voltage clamped at −60 mV to record mEPSCs and at 0 mV to record mIPSCs. Current- and voltage-clamp recordings were conducted using an EPC10 amplifier (HEKA Elektronic). Amplification was done with a CyberAmp programmable signal conditioner and digitization was done with a Digidata 1200 computer interface. mEPSCs and IPSCs were digitized at 5 kHz and low-pass filtered at 2 kHz. The digitized signals were recorded with an Axon Instruments PClamp 7 program (Axon Instruments) running on an IBM PC compatible computer. Data were analyzed using Mini Analysis Program (Synaptosoft, Decatur, GA). The amplitude and frequency of the synaptic events were determined from 20-s data segments to avoid desensitization of responses over time. Only mEPSCs with amplitudes ≥5 pA were analyzed.
Oocytes were obtained by an abdominal incision after anesthetization of the frog by immersion in a 0.05% solution of 3-aminobenzoic acid ethyl ester (MS222). Frogs were euthanized by a subcutaneous injection of a 2% solution of MS222 according to NIH guidelines. One day after separation of the oocytes from the follicular layer, 50–70 nl of TRPA1 cRNA were injected using a Drummond Nanoject (Drummond Scientific, Broomall, PA). Three days after the injection, oocytes were used for recordings. Double-electrode voltage-clamp technique was performed using a Warner amplifier (model OC725C, Warner Instruments). Experiments were performed at 21–23°C. Oocytes were placed in a chamber perfused (5–10 ml/min) with Ca2+-free Ringer solution containing (in mM) 100 NaCl, 2.5 KCl, and 5 HEPES, and pH was adjusted to 7.35 with NaOH. Current-voltage relationships were measured using 1-s voltage ramps from −80 mV to +80 mV. Data were digitized and stored on videotape or directly stored on the computer using a LabView interface (National Instruments).
Behavioral studies.
Nocifensive behavior was examined in wild-type and TRPV1 knockout mice after intraplantar injection of TRPV1 and TRPA1 agonists, capsaicin (2 mM) and AITC (1 mM), respectively. Nocifensive behavior was characterized by the latency, duration, and number of licks and/or shakes of the injected hindlimb in a given period of time. For the measurement of thermal and mechanical sensitivities, the rats were administered intrathecal capsaicin (25 μg/20 μl) and AITC (25 μg/20 μl). Thermal and mechanical sensitivities were determined using a plantar test instrument (Ugo Basile, Camerio, Italy) and dynamic plantar anesthesiometer instrument using von Frey probe (Ugo Basile, Camerio, Italy), respectively. For thermal nociceptive response, a mobile radiant heat source was located under the table and focused onto the desired paw. Paw withdrawal latencies (PWLs) were recorded three times for each hind paw and the average was taken as the baseline value. For mechanical nociceptive response, a 0.5 mm diameter von Frey probe was applied to the plantar surface of the rat hind paw with pressure increasing by 0.05 g/s and the pressure at which a paw withdrawal occurred was recorded and this was taken as paw withdrawal threshold (PWT). For each hind paw, the procedure was repeated three times and the average pressure to produce withdrawal was calculated (11). PWLs/PWTs were determined 15 min, 30 min, and 1, 2, and 4 h after capsaicin/AITC administration.
Data analysis.
All data are shown as means ± SE. Significance is tested using unpaired Student's t-test, and the data were considered significant at P < 0.05. For analysis of synaptic currents, Kolmogorov-Smirnov (KS) test was used to compare the cumulative probability curves for inter-event intervals and amplitude between various treatment groups. Data are represented as means ± SE and expressed as percentage of control, which is scaled to 100%. For experiments that involved manipulation of one of the legs (capsaicin or AITC injection), the data were normalized for each animal and the data obtained were subjected to a one-way ANOVA followed by Tukey's test. All the chemicals used in this study were obtained from Sigma (St. Louis, MO).
RESULTS
TRPA1-mediated whole cell currents in DRG neurons.
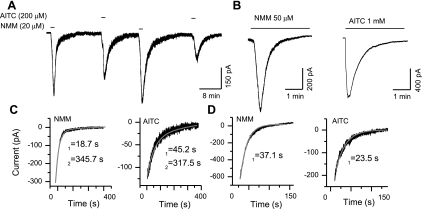
TRPA1-mediated whole cell currents in adult dissociated DRG neurons from rats or mice were recorded at −60 mV in response to application of agonists. TRPA1 agonists AITC and NMM have been shown to activate the channel by covalent modification of cysteine and lysine residues (22). Since covalent modification is an irreversible process within the time course of electrophysiological experiments (15–60 min), therefore, it is expected that NMM and AITC activate the current in an irreversible manner, as seen with a high-affinity TRPV1 agonist, resiniferatoxin (53). Surprisingly, brief application (20–50 s) of NMM (20 μM) or AITC (200 μM) induced responses that were readily reversible (Fig. 1A), but continuous application of higher concentrations of NMM (>50 μM) and AITC (>500 μM) induced a complete desensitization of the current response (Fig. 1B). We determined the deactivation (current decay during washout) and desensitization (current decay in the continuous presence of higher concentrations of agonists) rates by fitting exponential functions. NMM-induced current deactivation could be well-fitted with a double exponential function with time constants of 30.3 ± 11.2 s and 434.7 ± 131.8 s, n = 4; AITC-induced current deactivation could be fitted with a double exponential function with a time constant of 46.8 ± 11.2 s and 444.5 ± 103.7 s, n = 3 (Fig. 1C). Then, we determined the desensitization rate constants of NMM (>50 μM)- and AITC (>500 μM)-induced currents, which could be well-fitted with a single exponential function with a time constant of 55.7 ± 13.0 s, n = 5, and 33.8 ± 7.7 s, n = 5, respectively (Fig. 1D). These observations suggest that the TRPA1 channels have at least two closed states while the agonist is being removed and the channels enter the desensitized state monotonically with a constant rate.
Fig. 1.
Deactivation and desensitization characteristics of transient receptor potential ankyrin 1 (TRPA1). A: N-methyl maleimide (NMM)- and allyl isothiocyanate (AITC)-induced currents are readily reversible by agonist washout. B: at higher concentrations, continued presence of NMM and AITC caused complete desensitization of the responses. C: deactivation phases can be well-fitted with two exponential functions. D: the desensitization phases can be well-fitted with a single exponential function.
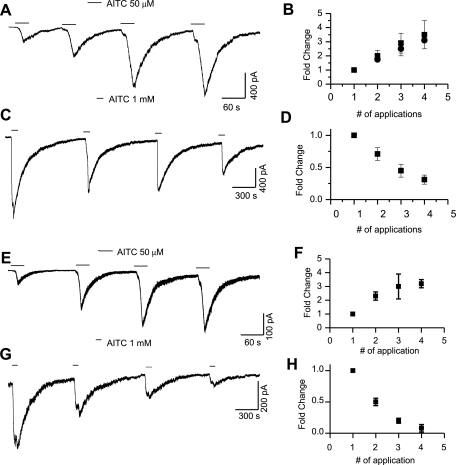
We also observed that repeated application of lower concentrations of the agonist AITC (20 or 50 μM) increased the amplitude of the currents with subsequent applications. The amplitude of the current after the second (2 ± 0.4-fold, n = 3), third (2.9 ± 0.7-fold, n = 3), and fourth (3.5 ± 1-fold, n = 2) applications of AITC (20 μM) was significantly larger (P < 0.01) than the first response. Similarly, at a concentration of AITC of 50 μM, the second (1.74 ± 0.1-fold, n = 9), third (2.5 ± 0.5-fold, n = 3), and fourth (3.1 ± 0.1-fold, n = 3) responses increased significantly (P < 0.01) as compared with the first response (Fig. 2, A and B). There was no significant difference in fold change between 20 and 50 μM AITC. Interestingly, at saturating concentrations (1 mM), AITC-induced currents exhibited tachyphylaxis with repeated application (Fig. 2, C and D). The current amplitude decreased significantly (P < 0.05) for the second (0.71 ± 0.1-fold, n = 6), third (0.45 ± 0.1-fold, n = 6), and fourth applications (0.31 ± 0.07-fold, n = 5).
Fig. 2.
Native TRPA1-mediated whole cell currents from wild-type and transient receptor potential vanilloid 1 (TRPV1) knockout mice dorsal root ganglion (DRG) neurons in the absence of extracellular Ca2+. A and B: AITC (50 μM)-induced current amplitude increased with repeated application. C and D: repeated application of a saturating concentration of AITC (1 mM) induced tachyphylaxis. E and F: in TRPV1 knockout mice, AITC (50 μM)-induced current amplitude increased with repeated application. G and H: saturating concentration of AITC (1 mM)-induced currents exhibited tachyphylaxis with repeated application.
TRPA1-mediated currents in TRPV1 knockout mice.
It is possible that TRPV1 subunits combine with TRPA1 subunits to form heterotetramers (2, 56, 60). We have found that in the wild-type animals, out of the total number of cells exposed to AITC and capsaicin during patch-clamp experiments, 9% responded to AITC alone, 38% to capsaicin alone, and 51% to both, AITC and capsaicin. Several studies have suggested an interaction between TRPV1 and TRPA1 that range from cross-desensitization to coassembly as heterotetramers (60). To evaluate the properties of native TRPA1-mediated currents in isolation, without the possibility of TRPV1 channel interference, we recorded whole cell currents in response to AITC from adult dissociated neurons harvested from TRPV1 knockout mice. AITC-induced currents exhibited an increase in current amplitude with repeated application of AITC (50 μM) in the absence of extracellular Ca2+. The increase in current amplitude with repeated application after the second (2.3 ± 0.3-fold, n = 15), third (3 ± 0.9-fold, n = 12), and fourth (3.2 ± 0.3-fold, n = 5) applications was similar to that seen in DRG neurons obtained from wild-type animals (Fig. 2, E and F). At a saturating concentration of AITC (1 mM), the current amplitudes of second (0.5 ± 0.06-fold, n = 4), third (0.2 ± 0.04-fold, n = 4), and fourth (0.08 ± 0.06-fold, n = 2) applications were smaller than that of the first application (Fig. 2, G and H).
Dose-response curve and current-voltage relationship.
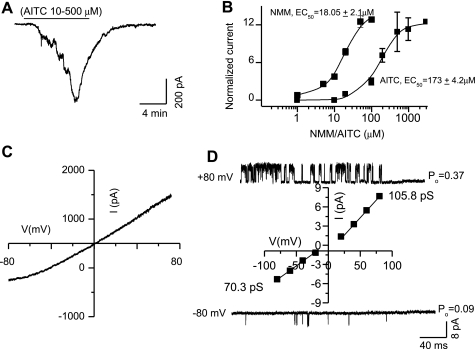
Dose-response curves were constructed by applying increasing concentrations of NMM (3–100 μM) and AITC (10–1,000 μM) sequentially in the same cell, which yielded EC50 values of 18 ± 2.1 μM and 173 ± 4.2 μM, and a saturating concentration of approximately 50 μM and 1 mM, respectively (Fig. 3, A and B).
Fig. 3.
Dose-response and current-voltage relationship curves of TRPA1- induced currents. A: cumulative dose response to increasing concentrations of AITC. B: dose-response curves of NMM and AITC. C: a ramp current-voltage relationship. D: single-channel current-voltage relationship. Note that the single-channel open probability (Po) is lower at −80 mV as compared with +80 mV.
AITC-induced membrane currents recorded at −60 mV were smaller than at +60 mV. To determine the current-voltage relationship, a ramp protocol (−80 to +80 mV in 1 s) was used. Native TRPA1-mediated whole cell currents in response to AITC exhibited a slight outward rectification and reversed around 0 mV, which is consistent with nonspecific cation flux (Fig. 3C). The extent of outward rectification exhibited by TRPA1-mediated currents was less pronounced than that observed with TRPV1-mediated currents (47, 49). We recorded single-channel currents using cell-attached/outside-out patches to determine the mechanism of outward rectification. We found that in Ca2+-free conditions, TRPA1-mediated single-channel currents had a slope conductance of 105.8 pS and 70.3 pS at positive and negative potentials, respectively (Fig. 3D). We also determined the probability of channel opening at different voltages. The Po at +80 mV was 0.37 and at −80 mV was 0.09. These results show that both the single-channel conductance and the Po contribute to the slight outward rectification observed in our experiments. This observation suggests that activation of TRPA1 may induce a larger membrane conductance and a greater depolarization, while at resting membrane potentials as compared with outwardly rectifying channels such as TRPV1.
Role of extracellular Ca2+ in TRPA1-mediated whole cell currents.
It has been shown that cross-desensitization occurs between TRPA1 and TRPV1 with the use of TRPA1- and TRPV1-selective agonists (2, 25). TRPV1-mediated currents exhibit desensitization and tachyphylaxis in the presence of extracellular Ca2+. To avoid the potential effect of TRPV1 subunits and evaluate the effect of Ca2+ on TRPA1-mediated currents, we recorded AITC-evoked currents in DRG neurons from TRPV1 knockout mice in the presence of extracellular Ca2+. TRPA1-mediated currents induced by repeated application of 50 μM AITC in the presence of extracellular Ca2+ did exhibit a significant increase in the amplitude for the second (1.3 ± 0.2-fold, n = 8), but not the third (1.02 ± 0.1-fold, n = 6) and fourth (0.92 ± 0.2-fold, n = 4) applications (Fig. 4, A and B). Thus, in the presence of extracellular Ca2+, TRPA1-mediated currents exhibit tachyphylaxis. We have demonstrated earlier that a saturating concentration of AITC (1 mM) induced tachyphylaxis even in the absence of extracellular Ca2+. We wanted to determine whether the presence of extracellular Ca2+ would exacerbate the degree of tachyphylaxis. Indeed, in the presence of 1.8 mM extracellular Ca2+, 1 mM AITC-induced TRPA1 currents exhibited a dramatic reduction in amplitude after the second (0.07 ± 0.03-fold, n = 7), third (0.04 ± 0.01-fold, n = 5), and fourth (0.005 ± 0.005-fold, n = 4) applications (Fig. 4, C and D). Thus, in the presence of extracellular Ca2+, the increase in the current amplitude reversed with repeated application of lower concentrations of AITC, whereas it exacerbated the degree of tachyphylaxis at saturating concentrations of AITC. Although it has been shown that Ca2+ is able to activate TRPA1 or sensitize its response to agonists (16, 65), subsequent to activation, a profound desensitization follows (31).
Fig. 4.
Effect of extracellular Ca2+ on TRPA1-mediated whole cell currents in DRG neurons from TRPV1 knockout mice. A and B: AITC (50 μM)-induced whole cell currents exhibited an initial increase followed by a decrease. C and D: saturating concentration of AITC (1 mM)-induced currents exhibited profound tachyphylaxis.
Modulation of TRPA1 by PKC and PKA in DRG neurons.
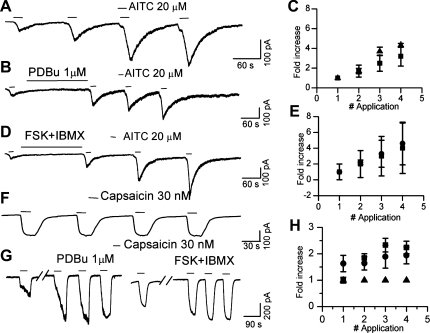
PKC-/PKA-mediated phosphorylation is a critical component of chronic inflammatory pain and has been shown to modulate the nociceptive ion channel TRPV1. In light of the robust modulation of TRPV1 by PKC activation, we wanted to examine the effect of PKC activation on TRPA1-mediated whole cell currents in DRG neurons from wild-type animals. Since TRPA1-mediated currents exhibited an increase in amplitude with repeated application of AITC and NMM (lower concentration) in the absence of extracellular Ca2+, we evaluated the effect of PKC activation by comparing the amplitude of the first response with that of the subsequent responses either in the presence or absence of phorbol 12,13-dibutyrate (PDBu). We did not observe a difference in the extent of increase in AITC-evoked whole cell current with repeated application in the presence of PDBu (1 μM) (Fig. 5, A–C). In similar experimental conditions, TRPV1-mediated currents had constant current amplitude with repeated application of agonists (Fig. 5F). As expected, TRPV1-mediated whole cell currents evoked by capsaicin (30 nM) were strongly potentiated (2.3 ± 0.24-fold, n = 4) following PDBu application (Fig. 5, F–H).
Fig. 5.
Lack of modulation of TRPA1- but not TRPV1-mediated currents by activation of PKC and PKA pathways. A: increase in amplitude of AITC (20 μM)-induced currents with repeated applications. B: AITC (20 μM)-induced currents in the presence of the PKC activator phorbol 12,13-dibutyrate (PDBu; 1 μM). C: plot comparing the normalized amplitude of AITC-induced currents in the presence (■) or absence (▴) of PDBu. D: AITC (20 μM)-induced currents in the presence of PKA-activating cocktail [forskolin (FSK), 50 μM + IBMX, 50 μM]. E: plot comparing the normalized amplitude of AITC-induced currents in the presence (●) or absence (■) of FSK (50 μM) + IBMX (50 μM). F: capsaicin (30 nM)-induced current amplitude remained constant with repeated application. G: capsaicin (30 nM)-induced currents were significantly potentiated following the application of PDBu (1 μM) or FSK (50 μM) + IBMX (50 μM). H: plot comparing the normalized amplitude of capsaicin-induced currents in the absence (▴) or presence of PDBu (■) or FSK + IBMX (●).
Activation of PKA is also involved in chronic inflammatory conditions by modulating several ion channels that are critical for nociception, including TRPV1. PKA-mediated phosphorylation reverses Ca2+-induced desensitization of TRPV1 and is thought to be involved in maintaining the basal activity of this channel (10, 37, 41). Incubation of the DRG neurons with 50 μM forskolin + 50 μM IBMX did not significantly alter the fold change in current amplitude with repeated application of 20 μM AITC (Fig. 5, D and E). However, TRPV1 currents were potentiated 1.9 ± 0.32-fold in Ca2+-free extracellular solution (n = 7) (Fig. 5, F–H). Recently, Schmidt et al. (57) have shown that activation of PKA causes translocation of TRPA1 from the cytosol to the plasma membrane in a Ca2+-dependent manner (57). We did not see a clear potentiation, although a trend was noted by the large SE at the third and fourth applications. One possible explanation for the lack of effect with PKA in our experiments may be related to the dialysis of the interior as a result of conventional whole cell recording and that the experiments were done in the absence of Ca2+ to avoid Ca2+-induced desensitization and tachyphylaxis.
Whole oocyte currents mediated by cloned TRPA1.
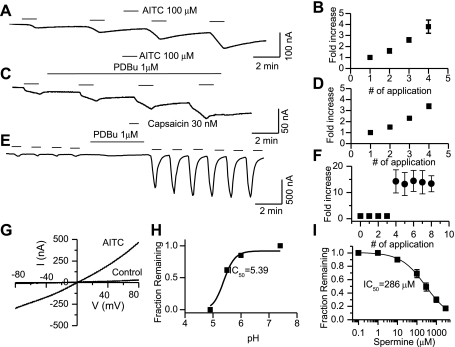
Cloned TRPA1-mediated whole-oocyte currents were recorded in response to AITC in Xenopus oocytes heterologously expressing the channel. Uninjected and oocytes injected with cRNA other than TRPA1 did not exhibit a current in response to application of AITC. Oocytes were voltage clamped at −60 mV, the current evoked in response to AITC (100 μM) exhibited an increase in the amplitude with repeated application of the same concentration similar to the findings from DRG neurons. The amplitudes of the second (1.6 ± 0.2) third (2.6 ± 0.3), and fourth (3.8 ± 0.6) responses were significantly greater than the first response (P < 0.05, n = 7) (Fig. 6, A and B). This is in contrast to TRPV1-mediated currents, which were constant with repeated application of agonists (Fig. 6E). A ramp protocol was used to determine the current-voltage relationship for TRPA1 (Fig. 6G).
Fig. 6.
Whole cell currents recorded from oocytes heterologously expressing cloned TRPA1. A and B: AITC (100 μM)-induced current amplitude increased with repeated application. C: AITC (100 μM)-induced currents in the presence of PKC activator PDBu (1 μM). D: plot comparing the normalized amplitude of AITC- induced currents in the presence of PDBu. E: application of PDBu strongly potentiated capsaicin (30 nM)-induced currents. Note that capsaicin (30 nM)-induced current amplitude remained constant with repeated application. F: TRPV1-induced currents before (■) and after PDBu (●). G: a ramp current-voltage (I-V) curve for AITC currents demonstrating minimal outward rectification. H: proton-induced inhibition of TRPA1-mediated currents in oocytes (IC50 = pH 5.39). I: spermine-induced inhibition of TRPA1-mediated whole-oocyte currents (IC50 = 286 ± 12 μM).
Oocyte expression system is an excellent system to study the effects of PKC-mediated phosphorylation. The PKC-mediated pathway can be robustly activated in the oocytes (48), hence we used this system to further validate our findings in DRG neurons that TRPA1 is not sensitized by PKC. PKC activator PDBu (1 μM) was applied following the first AITC-mediated response. A comparison of the ratio of the amplitude of the first response to that of the second, third, and fourth responses in the presence and absence of PKC activator PDBu did not exhibit a significant difference (Fig. 6, C and D). However, TRPV1 responses did not increase with repeated application, but the responses were strongly potentiated (>10-fold, n = 4) following application of PDBu (Fig. 6, E and F). A ramp protocol was used to determine the current-voltage relationship for TRPA1-induced currents. In the presence of AITC (100 μM), the current amplitude increased significantly (Fig. 6G). As in DRG neurons (Fig. 3C), the outward rectification in cloned TRPA1 was not pronounced as compared with other TRP channels such as TRPV1 or the melastatin transient receptor potential channel TRPM8 (49). Oocytes do not have a strong PKA system; therefore we could not test the role of PKA in the modulation of cloned TRPA1.
Modulation by protons and polyamines.
TRPV1 is activated by protons, and this has been shown to be a critical mechanism for ischemia-induced pain and proton-mediated CGRP release in the heart (62). Since TRPA1 is highly coexpressed with TRPV1, we wanted to examine the effect of protons on this channel. Expression of channels in oocytes is an excellent system to test the modulation of a channel by protons, since DRG neurons have several channels that are activated by protons. Surprisingly, an increase in the proton concentration (lowering pH) inhibited AITC-induced TRPA1-mediated currents in a concentration-dependent manner with an IC50 of 5.39 ± 0.03. A complete block was observed below pH 5 (Fig. 6H). This is an interesting finding since protons are an important component of any inflammatory process and raises an intriguing possibility of opposing modulation of nociceptive channels expressed in the same sensory neurons. Since polyamines such as spermine has been shown to potentiate TRPV1 current (1), we determined the effect of spermine on cloned TRPA1 in oocytes. Spermine blocked TRPA1-mediated whole-oocyte currents in a concentration-dependent manner with an IC50 of 286 ± 12 μM (Fig. 6I). Blockade of TRPA1 by protons and positively charged molecules like spermine is intriguing since TRPV1 expressed in the same neurons is activated by these agonists.
TRPA1-mediated membrane depolarization and action potential generation.
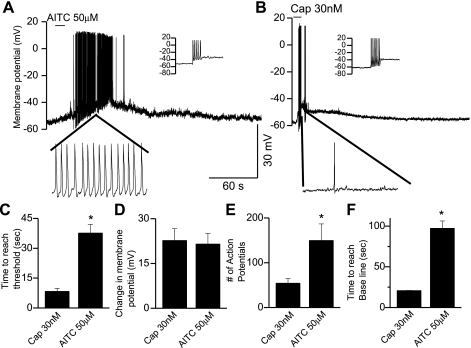
To attribute a physiological significance and to study the effect of TRPA1 activation on DRG (from wild-type animals) neuronal membrane potential, we employed current-clamp techniques. The concentrations of AITC and capsaicin used to compare the characteristics of activation of TRPA1 and TRPV1 respectively were based on the observation that 50 μM AITC and 30 nM capsaicin induced membrane depolarization to the same extent (Fig. 7D). The average membrane potential was −61 ± 4 mV (n = 9). Neuronal membrane excitability was tested by injecting current (10–100 pA) to induce action potentials before application of AITC or capsaicin (Fig. 7, A and B). In the absence of Ca2+, application of AITC (50 μM) or capsaicin (30 nM) induced a membrane depolarization, which generated multiple action potentials upon reaching threshold (Fig. 7, A and B). The average time needed to reach threshold after application of AITC (37.6 ± 4 s, n = 6) was significantly longer as compared with capsaicin (8.3 ± 1.6 s, n = 3) (P < 0.01, Fig. 7C). The extent of membrane depolarization induced by AITC (50 μM) (21.5 ± 2.6) mV was not significantly different from that induced by capsaicin (30 nM) (22.6 ± 3.9 mV, Fig. 7D). Quantification and comparison of the number of action potentials induced by AITC and capsaicin revealed a significantly (P < 0.01) greater number of action potentials induced by AITC (149 ± 4.7, n = 6) as compared with capsaicin (39 ± 4, n = 3) (Fig. 7E). The average time needed for the membrane potential to reach baseline value after depolarization was significantly longer (P < 0.05) following AITC (97.2 ± 9.2 s, n = 6) as compared with capsaicin (20.6 ± 0.5 s, n = 3) (Fig. 7F). Hence, major differences exist between the activation characteristics of these two channels with respect to their rate of membrane depolarization and deactivation; this could lead to enhanced nociceptive transmission by activation of TRPA1 as compared with TRPV1.
Fig. 7.
TRPA1-mediated membrane depolarization in DRG neurons. A: application of AITC (50 μM) caused membrane depolarization and generated action potentials. Inset: generation of action potentials following current injection to confirm neuronal excitability. B: application of capsaicin (30 nM) caused membrane depolarization and generated action potentials. Inset: generation of action potentials following current injection to confirm neuronal excitability. C: bar graph showing the time to reach threshold membrane potential for 50 μM AITC and 30 nM capsaicin. D: extent of the change in membrane potential following application of 50 μM AITC or 30 nM capsaicin. E: mean number of action potentials in response to 50 μM AITC or 30 nM capsaicin. F: time to reach baseline following application of 50 μM AITC or 30 nM capsaicin.
Modulation of synaptic transmission by activation of TRPA1.
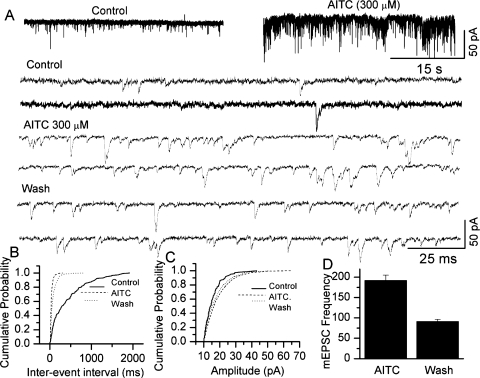
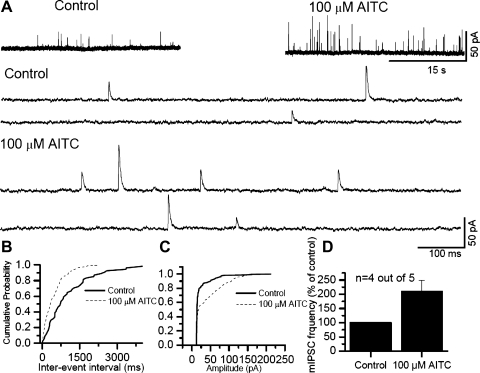
It is becoming increasingly apparent that nociceptive ion channels that are expressed in the peripheral terminals of sensory neurons that perceive noxious stimuli are also present in the central terminals of these neurons. In this study, we have used brain stem slices and recorded currents from the CSTN neurons. Giga-seal patch-clamp technique was used to record fast mEPSCs mediated by AMPA receptors after blocking the action potentials with TTX (1 μM) and GABA, glycine and NMDA currents with bicuculline (20 μM), strychnine (2 μM), and APV (20 μM), respectively. Application of AITC (100–300 μM) induced an increase in synaptic activity (Fig. 8A). The increase in the frequency of miniature events is revealed as a decrease in inter-event interval in 7 out of 11 cells (KS test P < 0.05) (Fig. 8B). AITC treatment also increased the amplitude of mEPSCs in 4 out of 11 cells (KS test P < 0.05) (Fig. 8C). The mean increase in mEPSC frequency is shown in the summary graph (Fig. 8D). The increase in mEPSC frequency suggests that TRPA1 activation increases glutamate release at central sensory afferent terminals. Similarly, we recorded mIPSCs in the absence of bicuculline and strychnine. mIPSCs were recorded at a holding potential of 0 mV. Following application of AITC (100–300 μM), the synaptic activity increased (Fig. 9A). Both the frequency and the amplitude of IPSCs were significantly increased in four out of five cells (KS test P < 0.05) (Fig. 9, B–D). The increase in the frequency of mEPSCs is similar to that observed with TRPV1 activation, but activation of TRPV1 did not alter the amplitude of mEPSCs (24). Furthermore, TRPV1 activation altered neither the frequency nor the amplitude of mIPSCs (24, 43, 58). A study by Kosugi et al. (33) shows an increase in mEPSC and mIPSC frequency and amplitude from slices taken from the lumbar region of the spinal cord (33). The extent of increase in frequency is lower in our experiments as compared with the increase shown by Kosugi et al. (33). This may be due to the age of the animals used (40 days) in these studies as well as the region from which slices were prepared (lumbar spinal cord dorsal horn). Several studies have been performed using TG neurons, and there seems to be a significant expression of TRPA1-expressing neurons in TG (30, 54). This result may have significance in the transduction of craniofacial pain, where CGRP-induced vasodilation impacts the nerve terminals exerting a mechanical force, which is manifested as pulsatile and throbbing headache with the change in the blood pressure (4, 18, 36).
Fig. 8.
Modulation of miniature excitatory postsynaptic currents (mEPSCs) by activation of TRPA1 at the brain stem. A: application of AITC (300 μM) to a slice, where the caudal spinal trigeminal nucleus (CSTN) neuron is patch clamped at −60 mV, increased mEPSC frequency and amplitude. The mEPSCs are shown in an expanded time scale below. B: cumulative probability histogram for inter-event intervals. AITC significantly decreased the inter-event interval (KS test P < 0.05). C: cumulative probability histogram for amplitude. AITC significantly increased the amplitude (KS test, P < 0.05). D: summary graph showing the percent increase in mEPSC frequency.
Fig. 9.
Modulation of miniature inhibitory postsynaptic currents (mIPSCs) by activation of TRPA1 at the brain stem. A: application of AITC (100 μM) to a slice where the CSTN neuron is patch clamped at 0 mV, increased mIPSC frequency and amplitude. The recordings are shown in an expanded time scale below. B: cumulative probability histogram for inter-event interval. AITC significantly decreased the inter-event interval (KS test, P < 0.05). C: cumulative probability histogram for amplitude. AITC significantly increased amplitude (KS test, P < 0.05). D: summary graph showing the percent increase in mIPSC frequency.
TRPA1-mediated behavioral effects.
To evaluate the behavioral effects of TRPA1 activation in vivo, intraplantar AITC- and capsaicin-induced nocifensive behavior was characterized and quantified. To induce nocifensive behavior, a higher concentration of AITC (1 mM) is needed. At higher concentrations, other effects, which include release of CGRP, BK, SP, and other proinflammatory agents, will sensitize or activate other ion channels, thereby increasing the complexity of explaining the data. On the other hand, it has been shown that there is an interaction between TRPV1 and TRPA1 activity, which includes the suggestion of coassembly as heterotetramers (2, 56, 60). Furthermore, recently it has been shown that higher concentrations of AITC activate TRPV1 (17). To avoid these complexities, we used TRPV1 knockout animals. AITC-induced nocifensive behavior in TRPV1 knockout mice exhibited a mean latency of 6.1 ± 0.7 s (n = 8, Fig. 10A) with a mean total duration of 366 ± 47 s (Fig. 10B). The animals exhibited a mean of 18.1 ± 3.7 licks and 20.6 ± 8 shakes (Fig. 10, C and D). On the other hand, intraplantar capsaicin in wild-type mice evoked nocifensive behavior with a mean latency of 6.3 ± 1 s (n = 19, Fig. 10A) with a mean total duration of 42 ± 5 s (Fig. 10B). The animals exhibited a mean of 4.8 ± 0.5 licks and 7.2 ± 1.4 shakes (Fig. 10, C and D, respectively). Thus, the total duration of nocifensive behavior in response to TRPA1 activation was significantly longer than that of TRPV1 activation (P < 0.05). The number of licks and shakes was also significantly greater (P < 0.05) than that seen following capsaicin injection (Fig. 10, C and D).
Fig. 10.
Comparison of TRPA1- and TRPV1-mediated nociceptive behavior. A: latency of onset of nocifensive behavior was not significantly different for TRPV1 knockout mice injected with intraplantar AITC (1 mM) or wild-type mice injected with capsaicin (2 mM). B: total duration of nocifensive behavior after injection of AITC was significantly longer that of induced by capsaicin injection. C: mean number of hind paw licks after AITC injection was significantly higher than that induced by capsaicin injection. D: mean number of hind paw shakes after AITC injection was significantly higher than that observed with capsaicin injection. E: intrathecal administration of 25 μg/20 μl AITC induced a long-lasting thermal hypersensitivity as compared with intrathecal administration of capsaicin. F: intrathecal administration of 25 μg/20 μl AITC caused a long-lasting change in mechanical sensitivity, whereas intrathecal administration of capsaicin had no effect.
Furthermore, we compared the thermal sensitivity after intrathecal injection of AITC (25 μg/μl) and capsaicin (25 μg/μl) in rats. AITC induced thermal hypersensitivity within 30 min (pretreatment, 11.32 ± 0.17 s vs. 30 min, 8.98 ± 1.42 s, n = 6, P < 0.05; Fig. 10E) following intrathecal administration and the animals exhibited thermal hypersensitivity even after 4 h (pretreatment, 11.32 ± 0.17 s vs. 4 h, 8.47 ± 0.31 s, n = 6, P < 0.05; Fig. 10E). Capsaicin also induced thermal hypersensitivity within 30 min (pretreatment, 10.19 ± 1.32 s vs. 30 min, 7.36 ± 0.62 s, n = 6, P < 0.05; Fig. 10E) following intrathecal administration, but at 4 h, thermal sensitivity reversed to pretreatment levels (pretreatment, 10.19 ± 1.32 s vs. 4 h, 9.46 ± 1.42 s, n = 6, Fig. 10E). Intrathecal administration of AITC induced mechanical hypersensitivity (pretreatment, 34.52 ± 2.88 g; 30 min, 24.66 ± 3.10 g; 4 h, 26.75 ± 1.28 g, n = 6, P < 0.05; Fig. 10F). However, intrathecal administration of capsaicin did not induce any change in mechanical sensitivity over the course of 4 h (pretreatment, 37.92 ± 1.63 g; 30 min, 36.03 ± 1.42 g; 4 h, 33.61 ± 2.75 g, n = 6, Fig. 10F).
DISCUSSION
It has been proposed that TRPA1 is activated by reactive molecules that covalently modify the cysteine and lysine residues. This interaction changes the conformation of the channel protein rendering the channel to its open state. Covalent modification is considered to be an irreversible process within the time frame of the electrophysiological experiments. However, it is puzzling that under our experimental conditions, responses induced by lower concentrations of AITC and NMM were readily reversible by washout. The deactivation phase of the currents could be well-fitted with a double exponential function. At higher concentrations of agonists, the current responses completely desensitized and the desensitization phase could be well-fitted with a single exponential function. It is not clear whether desensitization observed with reactive covalent modifying agonists can be equated with pharmacological desensitization with agonist such as glutamate, GABA, and ACh, where the channels enter an open state and with continued exposure of agonists, the channels enter a desensitized state. Upon agonist removal, the channels reenter the closed state with time, from which it can be reactivated again.
In the absence of extracellular Ca2+, native and cloned TRPA1-mediated whole cell currents exhibited an increase in amplitude with repeated application of low concentrations of the agonists, but at higher concentrations of agonists exhibited tachyphylaxis. However, in the presence of extracellular Ca2+, lower concentrations of agonists induced an initial increase in current amplitude followed by tachyphylaxis. In the presence of higher concentrations of agonists, the degree of tachyphylaxis was significantly greater in the presence of extracellular Ca2+. The effect of Ca2+ in modulating TRPA1 is complicated, given the fact that extracellular Ca2+ potentiates the current, but subsequently induces profound desensitization. It is difficult to understand the physiological relevance. In our experiments, we have observed that agonist concentration-dependent desensitization as well as Ca2+-dependent desensitization. The effect of extracellular Ca2+ on TRPA1-induced whole cell currents suggests that in physiological conditions, the strength of a stimulus that activates the channel might determine the effect of subsequent stimuli by influencing the intracellular levels of Ca2+. A stimulus of submaximal strength might lead to a phenomenon similar to wind-up and enhance nociceptive transmission, whereas a stimulus of higher strength might lead to a run-down of the response.
The current-voltage relationship of TRPA1-mediated whole cell currents exhibited minimal outward rectification as compared with other TRP channels such as TRPV1 and TRPM8 (49). The outward rectification in whole cell currents of TRPV1 is due to voltage-dependent reduction in single-channel conductance as well as Po (47). The minimal rectification exhibited by TRPA1 is due to a lesser extent of voltage-dependent decrease in amplitude and Po. The rectification is also shown to be altered by TRPV1 and TRPA1 association (56). Lack of rectification may induce larger currents at negative membrane potentials. It is possible that in the presence of an agonist, TRPA1 is more active near the resting membrane potential and plays a role in transduction of subthreshold nociceptive stimuli.
TRPV1 is modulated profoundly by the activation of PKC- and PKA-mediated phosphorylation. Stimulation of PKC strongly sensitizes TRPV1 to its agonists and potentiates its response. On the other hand, stimulation of PKA reverses Ca2+-induced desensitization (10, 37, 41). In light of the fact that TRPA1 and TRPV1 are coexpressed in a subpopulation of nociceptive neurons involved in inflammatory pain, we investigated the potential role of PKC and PKA activators on TRPA1-mediated currents. In our experiments, TRPA1-mediated currents were not modulated in a conventional manner by either PKA or PKC stimulation. In DRG neurons coexpressing TRPA1 and TRPV1, a profound potentiation of TRPV1-mediated currents was observed, consistent with the earlier work. The absence of PKC and PKA-mediated potentiation of TRPA1 may be due to the nature of agonists that are used to activate the receptor. AITC and NMM activate TRPA1 by covalent modification of cysteine residues. It is possible that this activation mechanism is not dictated by the receptor sensitization state, since these compounds oxidize SH groups, so their affinity for ligand binding site may not be altered by phosphorylation. It is speculated that TRPA1 activity is susceptible to modulation in a conventional manner by PKA and PKC stimulation, when activated by noncovalent modifying agonists and physical stimuli such as cold and mechanical force.
The mechanism of activation of TRPA1 by BK has been studied and suggested that stimulation of BK receptors may transactivate TRPA1, especially if they are in close association with each other (6, 60). It has been shown that BK does not activate TRPA1 directly, but both PLC and PKA are involved in the BK-induced modulation of TRPA1 (58). Recently, it has been shown that stimulation of PKA promotes translocation of the TRPA1 receptor from cytosol to plasma membrane (57). Therefore, it is not clear whether the sensitization is due to enhanced channel activity as a result of receptor phosphorylation or due to increased channel density. Unlike the potentiation of TRPV1-mediated responses, we were unable to observe a clear potentiation of TRPA1-mediated responses. This discrepancy may be because the experiments were conducted using conventional whole cell recordings and in the absence of extracellular Ca2+, where intracellular dialysis may have prevented the translocation of the receptors.
TRPA1-mediated whole cell currents were inhibited in a concentration-dependent manner by protons, but TRPV1 currents have been shown to be potentiated. This opposing effect of protons on TRPA1 and TRPV1 in the same neurons coexpressing the two channels raises interesting possibilities of their roles in inflammation, ischemia, and pathological conditions associated with a low pH. TRPA1, like TRPV1, is expressed in neurons innervating blood vessels, and selective activation of TRPA1 can induce vasodilation via the release of CGRP (50, 62). Proton-induced CGRP release has been shown to be exclusively mediated via TRPV1 activation (20, 50, 62). TRPV1 can release CGRP in response to ischemia-induced decrease in pH, however, the block of TRPA1 by protons could negatively modulate CGRP release.
In view of the differential modulation and dissimilar biophysical properties of TRPA1 and TRPV1 and their high level of coexpression, we wanted to evaluate the physiological relevance of activation of these channels using wild-type and TRPV1 knockout animals. Furthermore, it has been recently suggested that TRPV1 may be activated by AITC, although at higher concentrations (17). We performed current-clamp experiments on DRG neurons to study action potential generation, whole cell patch-clamp on slices of the CSTN to study the modulation of synaptic transmission at the first sensory synapse, and in vivo nociceptive behavioral studies. The concentration of AITC, which induced the same extent of depolarization as capsaicin, reached threshold at a significantly slower rate as compared with capsaicin. However, the number of action potentials induced by equipotent concentrations of AITC was significantly greater than that of capsaicin. This finding is consistent with the observation that TRPA1-mediated whole cell currents exhibit a slower activation and deactivation rate, which could be responsible for prolonged firing of the nociceptive neurons during painful conditions.
In brain stem slices of the CSTN, application of AITC increased the frequency of mEPSCs and IPSCs and also increased the amplitude in some cells. This is in contrast with TRPV1 activation that affects only the mEPSCs, but not the mIPSCs. These data suggest that TRPA1 is functional at central terminals of sensory neurons. Several endogenous ligands have been identified (3, 9). It is also possible that increases in intracellular Ca2+ could activate or sensitize TRPA1 followed by desensitization depending on the concentration of intracellular Ca2+ achieved (16, 65). Based on these observations, we suggest that during enhanced neuronal activity, TRPA1 could be modulated dynamically. This is in contrast to TRPV1, which shows only a Ca2+-dependent desensitization, but not activation. Bradykinin, produced at peripheral and central terminals, may also serve as an endogenous modulator of TRPA1 during inflammation (6, 57, 63, 64).
Finally, animal behavioral experiments showed that intraplantar injection of AITC induced nocifensive behavior for significantly longer than that seen following capsaicin injection. It is interesting to note that AITC-induced nocifensive behavior was more severe (greater number of licks and shakes) than that observed with capsaicin. This effect may be due to the tendency for TRPV1 to undergo robust desensitization in the presence of higher concentrations of the agonist due to larger Ca2+ influx. On the other hand, TRPA1 is not as Ca2+ permeant and a longer activation time may be necessary to induce desensitization. The rate of deactivation of the whole cell current mediated by TRPA1 is also significantly slower than that of capsaicin and this may lead to prolonged depolarization of the membrane leading to greater number of action potentials. Intrathecal AITC-induced thermal hypersensitivity lasted significantly longer than intrathecal administration of capsaicin. In these experiments, the concentration of AITC or capsaicin that reached the nerve terminal is uncertain. Neither AITC nor capsaicin had any effect on mechanical sensitivity. Hence it is not possible to determine whether the nerve terminals in injected animals are stimulated by submaximal or saturating concentrations of the agonist. As discussed earlier, in the case of AITC, the concentration of the agonist determines the extent of tachyphylaxis. AITC and capsaicin are highly lipid soluble and it is possible that membrane-partitioning properties of the two agonists also contributes to the difference in the extent of nocifensive behavior observed in the study.
In summary, all of the characteristics of TRPA1 we have uncovered suggest that TRPA1 is an integral ion channel that is involved in nociceptive transmission. So far, TRPV1 has had all of the attention and is considered to be a potential target for next-generation analgesic by evaluation of its antagonists/agonists (12, 51, 52). Some of the functional characteristics of TRPA1 are comparable to TRPV1, whereas others are in stark contrast, such as the minimal outward rectification, slower activation, and deactivation kinetics and the block by protons and spermine. Taken these findings together with the differences in the nociceptive behavioral studies, there is an intriguing possibility that blockade of TRPA1 alone or in conjunction with TRPV1 could be a potential strategy for treating certain modalities of pain.
GRANTS
This work was supported by National Institutes of Health Grants DK065742, NSO42296, and DA028017 (to L. S. Premkumar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Ardem Patapoutian and David Julius for providing us with the cDNA clones of TRPA1.
REFERENCES
- 1.Ahern GP, Wang X, Miyares RL. Polyamines are potent ligands for the capsaicin receptor TRPV1. J Biol Chem 281: 8991–8995, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependant and regulated by TRPV1-directed internalization. J Physiol 583: 175–193, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 28: 2485–2494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashina M, Bendtsen L, Jensen R, Schifter S, Jansen-Olesen I, Olesen J. Plasma levels of calcitonin gene-related peptide in chronic tension-type headache. Neurology 55: 1335–1340, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci 20: 2276–2282, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–857, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bautista DM, Jordt SE, Nikai T, suruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124: 1269–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Högestätt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 102: 12248–12252, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 118: 1899–910, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. CAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35: 721–731, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Bishnoi M, Bosgraaf C, Premkumar LS. Preservation of acute pain and efferent functions following intrathecal resiniferatoxin induced analgesia in rats. J Pain. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bishnoi M, Premkumar LS. Possible consequences of blocking transient receptor potential vanilloid. Curr Pharm Biotechnol 12: 102–114, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Cesare P, Dekker LV, Sardini A, Parker P, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron 23: 617–624, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Cesare P, Moriondo A, Vellani V, McNaughton PA. Ion channels gated by heat. Proc Natl Acad Sci USA 96: 7658–7663, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, Géléoc GS, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang DS. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432: 723–730, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient potential channel A1 is directly gated by calcium ions. J Biol Chem 151: 153–160, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Everaerts W, Gees M, Alpizar YA, Farre R, Leten C, Apetrei A, Dewachter I, van Leuven F, Vennekens R, De Ridder D, Nilius B, Voets T, Talavera K. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol 21: 316–321, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 33: 48–56, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Grudt TJ, Williams JT. mu-Opioid agonists inhibit spinal trigeminal substantia gelatinosa neurons in guinea pig and rat. J Neurosci 14: 1646–1654, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S, Lozano-Cuenca J, Villalón CM, de Vries R, Garrelds IM, Avezaat CJ, van Kats JP, Saxena PR, MaassenVanDenBrink A. Pharmacological characterisation of capsaicin-induced relaxations in human and porcine isolated arteries. Naunyn Schmiedebergs Arch Pharmacol 375: 29–38, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 22.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA 103: 19564–19568, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem 274: 7325–7333, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Jeffry JA, Yu SQ, Sikand P, Parihar A, Evans MS, Premkumar LS. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS One 4: e7021, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid Win 55, 212–2 regulates TRPV1 phosphorylation in sensory neurons. J Biol Chem 281: 32879–32890, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Sakagami M, Noguchi K. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol 200: 112–123, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kerstein PC, del CD, Moran MM, Stucky CL. Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol Pain 5: 19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron 24: 253–260, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YS, Son JY, Kim TH, Paik SK, Dai Y, Noguchi K, Ahn DK, Bae YC. Expression of transient receptor potential ankyrin 1 (TRPA1) in the rat trigeminal sensory afferents and spinal dorsal horn. J Comp Neurol 518: 687–698, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Klionsky L, Tamir R, Gao B, Wang W, Immke DC, Nishimura N, Gavva NR. Species-specific pharmacology of Trichloro(sulfanyl)ethyl benzamides as transient receptor potential ankyrin 1 (TRPA1) antagonists. Mol Pain 3: 39, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol 493: 596–606, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Kosugi M, Nakatsuka T, Fujita T, Kuroda Y, Kumamoto E. Activation of TRPA1 channel facilitates excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. J Neurosci 27: 4443–4451, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair cell transduction. Neuron 50: 277–289, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci 29: 4808–4819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia 22: 54–61, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci 18: 6081–6092, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445: 541–545, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol 15: 929–934, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J 30: 582–593, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohapatra DP, Nau CJ. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. Biol Chem 278: 50080–50090, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25: 4052–4061, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakatsuka T, Furue H, Yoshimura M, Gu JG. Activation of central terminal vanilloid receptor-1 receptors and alpha beta-methylene-ATP-sensitive P2X receptors reveals a converged synaptic activity onto the deep dorsal horn neurons of the spinal cord. J Neurosci 22: 1228–1237, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase C epsilon and identification of two target serine residues. J Biol Chem 277: 13375–13378, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest 115: 2393–2401, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Premkumar LS, Agarwal S, Steffen D. Single-channel properties of native and cloned rat vanilloid receptors. J Physiol 545: 107–117, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature 408: 985–990, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Premkumar LS, Raisinghani M, Pingle SC, Long C, Pimentel F. Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J Neurosci 25: 11322–11329, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Premkumar LS, Raisinghani M. Nociceptors in cardiovascular functions: complex interplay as a result of cyclooxygenase inhibition. Mol Pain 2: 26, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Premkumar LS, Sikand P. TRPV1: a target for next generation analgesics. Curr Neuropharmacol 6: 151–163, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Premkumar LS. Targeting TRPV1 as an alternative approach to narcotic analgesics to treat chronic pain conditions. AAPS J 12: 361–370, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raisinghani M, Pabbidi RM, Premkumar LS. Activation of transient receptor potential vanilloid 1 (TRPV1) by resiniferatoxin. J Physiol 567: 771–786, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain 144: 270–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev 19: 419–424, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci 29: 1568–1578, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron 64: 498–509, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sikand P, Premkumar LS. Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. J Physiol 581: 631–647, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sotomayor M, Corey DP, Schulten K. In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure 13: 669–682, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Staruschenko A, Jeske NA, Akopian AN. Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J Biol Chem 285: 15167–15177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Strecker T, Messlinger K, Weyand M, Reeh PW. Role of different proton-sensitive channels in releasing calcitonin gene-related peptide from isolated hearts of mutant mice. Cardiovasc Res 65: 405–410, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Wang H, Kohno T, Amaya F, Brenner GJ, Ito N, Allchorne A, Ji RR, Woolf CJ. Bradykinin produces pain hypersensitivity by potentiating spinal cord glutamatergic synaptic transmission. J Neurosci 25: 7986–7992, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, Cui X, Tominaga M, Noguchi K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain 131: 1241–1251, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 10: 277–279, 2007 [DOI] [PubMed] [Google Scholar]