Abstract
Angiotensin II is a modulator of myometrial activity; both AT1 and AT2 receptors are expressed in myometrium. Since in other tissues angiotensin II has been reported to activate intracellular receptors, we assessed the effects of intracellular administration of angiotensin II via microinjection on myometrium, using calcium imaging. Intracellular injection of angiotensin II increased cytosolic Ca2+ concentration ([Ca2+]i) in myometrial cells in a dose-dependent manner. The effect was abolished by the AT1 receptor antagonist losartan but not by the AT2 receptor antagonist PD-123319. Disruption of the endo-lysosomal system, but not that of Golgi apparatus, prevented the angiotensin II-induced increase in [Ca2+]i. Blockade of AT1 receptor internalization had no effect, whereas blockade of microautophagy abolished the increase in [Ca2+]i produced by intracellular injection of angiotensin II; this indicates that microautophagy is a critical step in transporting the peptide into the endo-lysosomes lumenum. The response to angiotensin II was slightly reduced in Ca2+-free saline, indicating a major involvement of Ca2+ release from internal stores. Blockade of inositol 1,4,5-trisphosphate (IP3) receptors with heparin and xestospongin C or inhibition of phospholipase C (PLC) with U-73122 abolished the response to angiotensin II, supporting the involvement of PLC-IP3 pathway. Angiotensin II-induced increase in [Ca2+]i was slightly reduced by antagonism of ryanodine receptors. Taken together, our results indicate for the first time that in myometrial cells, intracellular angiotensin II activates AT1-like receptors on lysosomes and activates PLC-IP3-dependent Ca2+ release from endoplasmic reticulum; the response is further augmented by a Ca2+-induced Ca2+ release mechanism via ryanodine receptors activation.
Keywords: calcium imaging, cytosolic calcium concentration, endoplasmic reticulum, microinjection
angiotensin ii is an eight amino acid peptide that exerts its actions by activation of G protein-coupled receptors, namely AT1 and AT2 receptors. Increasing evidence suggests that angiotensin II acts not only as an endocrine/paracrine factor, but also as an autacoid or intracrine peptide, with the ability to signal from within the cell, without stimulating plasma membrane receptors (21, 38). We previously reported that intracellular administration of angiotensin II via liposomes increases contractility of rat aorta via activation of intracellular receptors (7). Similarly, microinjection of angiotensin II increases cytosolic Ca2+ concentration ([Ca2+]i) in vascular smooth muscle cells (13, 16) or kidney proximal tubule cells (49). The intracellular angiotensin II can result either via uptake from the interstitium or by intracellular synthesis. In the latter case, intracellular renin converts angiotensinogen to angiotensin I, which is further converted to the active angiotensin II by chymase or by the angiotensin-converting enzyme, both of which are present intracellularly (21). The intracellular renin-angiotensin system has been identified in the heart (11), kidney (18, 19, 34, 48), and uterus (37).1
Autoradiography and binding studies reveal that the uterus expressed high levels of angiotensin II receptors; both AT1 and AT2 receptors are present in the uterus. The prevalence of myometrial AT1 versus AT2 receptors varies in different species and physiological states (42, 44). Extracellular angiotensin II induces dose-dependent contractions of the myometrium (12, 32, 44). This effect is sensitive to the AT1 blocker losartan and depends on the estrous cycle (1, 47). Since an increase in [Ca2+]i is essential in uterine contractility, in this study we assessed the effects of intracellular injection of angiotensin II on [Ca2+]i using calcium imaging.
MATERIALS AND METHODS
Primary culture of myometrial cells.
Primary myometrial cells were prepared from the rat uterus according to the procedure described by Krizsan-Agbas et al. (20) with some modifications. Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee. Adult (nonpregnant) female Sprague-Dawley rats (Ace Animals, Boyertown, PA) were anesthetized with urethane (1.2 g/kg ip); uterine horns were immediately excised and immersed in cold Hanks balanced salt solution (HBSS). The myometrium was rapidly separated from endometrium and stroma and then minced into fragments (about 1 mm3). Tissues were digested for 1.5 h at 37°C by collagenase type I (600 U/ml, Sigma-Aldrich, St. Louis, MO) in DMEM culture medium, followed by trituration with a sterile Pasteur pipette and filtration through a cell strainer with 70-μm pores. After collection, the cells were washed twice by centrifugation (1,000 RPM, 10 min) and resuspended in fresh DMEM medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cultures were maintained at 37°C in a humidified atmosphere with 5% CO2; the medium was changed every 2–3 days.
Calcium imaging.
Measurements of intracellular Ca2+ concentrations were performed as previously described (8). Briefly, cells were incubated with 5 μM fura-2 AM (Invitrogen, Carlsbad, CA) in HBSS at room temperature for 45 min, in the dark, washed three times with dye-free HBSS, and then incubated for another 45 min to allow for complete deesterification of the dye. Coverslips (25 mm diameter) were subsequently mounted in an open bath chamber (RP-40LP, Warner Instruments, Hamden, CT) on the stage of an inverted microscope Nikon Eclipse TiE (Optical Apparatus, Ardmore, PA). The microscope was equipped with a Perfect Focus System and a Photometrics CoolSnap HQ2 CCD camera (Roper Scientific, Optical Apparatus). During the experiments the Perfect Focus System was activated. Fura-2 AM fluorescence (emission = 510 nm), following alternate excitation at 340 and 380 nm, was acquired at a frequency of 0.25 Hz. Images were acquired and analyzed using NIS-Elements AR 3.1 software (Nikon/Optical Apparatus). The ratio of the fluorescence signals (340/380 nm) was converted to Ca2+ concentrations (14). In Ca2+-free experiments, CaCl2 was omitted and 2.5 mM EGTA was added.
Intracellular microinjection.
Injections were performed using Femtotips II, InjectMan NI2, and FemtoJet systems (Eppendorf) as previously reported (8). Pipettes were back filled with an intracellular solution composed of 110 mM KCl, 10 mM NaCl, and 20 mM HEPES (pH 7.2) (14) or angiotensin II. The injection time was 0.4 s at 60 hPa with a compensation pressure of 20 hPa to have <0.1% of cell volume microinjected (14). Blockade of AT1 receptors internalization was done by cells pretreatment for 5 h with phenylarsine oxide (PAO, 30 μM), an agent that is known to inhibit the AT1 internalization in rat myometrium (24). Blockade of microautophagy was done by preincubating the cells for 1 h with 30 μM rapamycin (22).
Statistical analysis.
One-way ANOVA or two-way ANOVA were used; a P value of <0.05 was considered statistically significant.
RESULTS
Intracellular injection of angiotensin II increases [Ca2+]i in myometrial cells.
The mean basal [Ca2+]i of rat myometrial cells was 67 ± 1.5 nM (n = 154 cells). Intracellular microinjection of control buffer produced a small but not significant increase in [Ca2+]i by 19 ± 3 nM (n = 6 cells) (Fig. 1, A, C, D). Microinjection of angiotensin II (10−9 M, 10−8 M, and 10−7 M final intracellular concentrations) produced a robust and transitory increase in [Ca2+]i by 131 ± 12 nM, 382 ± 17 nM, and 865 ± 44 nM, respectively (n = 6 for each concentration tested) (Fig. 1, B–D).
Fig. 1.
Intracellular administration of ANG II produced a dose-dependent increase in intracellular Ca2+ concentration ([Ca2+]i) in rat myometrial cells. A and B: fluorescent images of fura-2 AM-loaded myometrial cells illustrating the levels of basal [Ca2+]i (left) during injection of control buffer or ANG II (10−7 M, middle) and 6 min after injection (right). The fluorescence intensity scale (0–4) is illustrated on each panel and magnified in A (left). C: intracellular administration of ANG II (10−9, 10−8, and 10−7 M) produced a fast, robust and transitory increase in [Ca2+]i; averaged traces from 6 experiments are shown. D: comparison of the increase in [Ca2+]i produced by control injection and ANG II; ANG II (10−9, 10−8, and 10−7 M, intracellular concentrations) produced a dose-dependent increase in [Ca2+]i. *P < 0.05 compared with control.
Angiotensin II activates intracellular AT1 receptors in myometrial cells.
Since both AT1 and AT2 receptors are present in the myometrium, the next series of experiments was designed to identify which type of receptor mediates the Ca2+-mobilizing effects of angiotensin II. Pretreatment with and concomitant injection of losartan (1 μM, 15 min), an AT1 receptor antagonist, abolished the effect of angiotensin II on [Ca2+]i, (Fig. 2, B, E, F). Coinjection of saralasin (1 μM final concentration inside the cells) with angiotensin II largely reduced the angiotensin II-evoked response; in the presence of saralasin, angiotensin II increased [Ca2+]i only by 127 ± 6 nM (Fig. 2, D–F). Pretreatment with and coinjection of PD-123319 (1 μM, 15 min), an AT2 receptor antagonist, did not significantly affect the response to angiotensin II. In the presence of PD-123319, intracellular injection of angiotensin II increased [Ca2+]i by 836 ± 61 nM versus 865 ± 44 nM, in the absence of PD-123319 (Fig. 2, C–E).
Fig. 2.
ANG II activates intracellular AT1 receptors in myometrial cells. A–D: fluorescent images of fura-2 AM-loaded myometrial cells illustrating the levels of basal [Ca2+]i (left) during microinjection of ANG II (10−7 M, middle), alone (A), or in the presence of losartan (B), PD-123319 (C), or saralasin (D), and 6 min after injection (right). The fluorescence intensity scale (0–4) is illustrated on each panel and magnified in A (left). E: averaged traces from 6 experiments indicating the increase in [Ca2+]i produced by ANG II alone or in the presence of AT1 receptor antagonist, losartan, AT2 receptor antagonist (PD 123319), or of AT1 and AT2 receptors antagonist, saralasin; losartan and saralasin abolished the increase in [Ca2+]i produced by injection of ANG II. F: comparison of the increase in [Ca2+]i produced by ANG II in the absence and presence of losartan, PD-123319, and saralasin. *P < 0.05 compared with ANG II alone.
Intracellular AT1 receptors are located on endo-lysosomes.
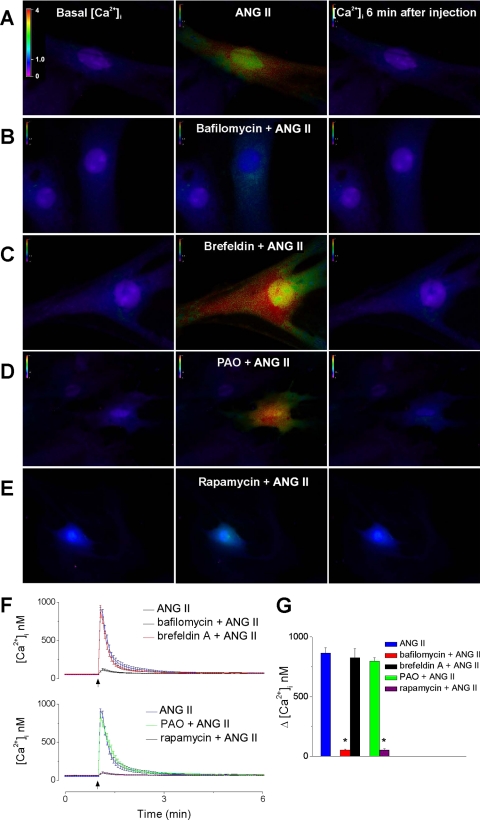
Pretreatment with bafilomycin A1 (1 μM, 1 h), a V-type ATPase inhibitor (6), abolished the Ca2+ response to angiotensin II (Fig. 3, B, F, G) (n = 6). In cells pretreated with brefeldin A (10 μM, 1 h), which disrupts Golgi apparatus, angiotensin II (10−7 M) increased [Ca2+]i by 827 ± 76 nM (n = 6) (versus 865 ± 44 nM produced angiotensin II alone) (Fig. 3, C, F, G). Blockade of AT1 receptor endocytosis with PAO (30 μM) had no significant effect on intracellular angiotensin II-evoked [Ca2+]i increase (798 ± 28 vs. 865 ± 44 nM; Fig. 3, D, F, G). In contrast, the blockade of microautophagy with rapamycin (30 μM) abolished the elevation in [Ca2+]i induced by intracellular injection of angiotensin II (Fig. 3, E–G).
Fig. 3.
Intracellular AT1 receptors are located in lysosomes in rat myometrial cells. A–E: fluorescent images of fura-2 AM-loaded myometrial cells illustrating the levels of basal [Ca2+]i (left) during microinjection of ANG II (10−7 M, middle) alone (A) or in cells pretreated with bafilomycin A1 (B), brefeldin A (C), phenylarsine oxide (PAO, D), or rapamycin (E) and 6 min after injection (right). The fluorescence intensity scale (0–4) is illustrated on each panel and magnified in A (left). F: pretreatment with bafilomycin A1 (1 μM, 1 h), or rapamycin (30 μM, 1 h), but not with brefeldin A (10 μM, 1 h), or PAO (30 μM, 5 h) prevented the ANG II-induced increase in [Ca2+]i; averaged traces from 6 experiments for each treatment are shown. G: comparison of the increase in [Ca2+]i produced by ANG II in the absence and presence of brefeldin A, bafilomycin A1, PAO, or rapamycin. *P < 0.05 compared with ANG II alone.
Angiotensin II mobilizes Ca2+ from endoplasmic reticulum pool.
In Ca2+-free EGTA (2.5 mM)-containing HBSS, intracellular injection of angiotensin II produced a fast and transitory increase in [Ca2+]i by 609 ± 27 nM (n = 6). The response was insensitive to the blockade of NAADP-signaling by Ned-19 (5 μM, 15 min) (35) (Fig. 4A). The response to angiotensin II was markedly reduced by blocking IP3 receptors with xestospongin C and heparin (48 ± 12 nM, n = 6, Fig. 4A) or by inhibiting PLC by pretreatment with U-73122; pretreatment with the inactive derivative U-73343 did not affect the response (Fig. 4A), supporting the PLC specificity of the response. Pretreatment with ryanodine (1 μM, 15 min) slightly reduced the response (438 ± 21 nM, n = 6, Fig. 4A). These results indicate the major involvement of IP3-dependent Ca2+ pathways and a participation of Ca2+-induced Ca2+ release mechanism.
Fig. 4.
ANG II-induced Ca2+ mobilization involves release of Ca2+ from endoplasmic reticulum (ER). A: averaged responses (n = 6) to intracellular injection of ANG II in the presence of the mentioned antagonists of lysosomal and ER Ca2+ release; experiments were carried out in Ca2+-free saline (0 Ca2+). The response to ANG II was not affected by blocking NAADP signaling with Ned-19 or the inactive derivative of PLC inhibitor U-73343; markedly reduced by inositol 1,4,5-trisphosphate (IP3) receptor (IP3R) antagonists (xestospongin C, XeC, and heparin) or phospholipase C (PLC) inhibitor U-73122, slightly reduced by pretreatment with the ryanodine receptor antagonist (ryanodine, Ry). B: comparison of the amplitude of the [Ca2+]i increases produced by intracellular injection of ANG II in the presence of the mentioned antagonists. *P < 0.05 compared with ANG II (10−7 M); **P < 0.05 compared with ANG II and Ned-19 and ANG II and xestospongin C and heparin or ANG II and Ned-19.
DISCUSSION
Previous studies indicate that angiotensin II induces depolarization and stimulates the contractility of nonpregnant rat myometrium (31). Increase of [Ca2+]i and stimulation of myosin light chain kinase via Ca2+-calmodulin is essential for myometrial contraction. Since in other tissues such as vasculature and kidney angiotensin II has been reported to activate intracellular receptors (7, 13, 16, 49), we assessed the effects of intracellular administration of angiotensin II via microinjection on myometrial cells, using calcium imaging. The first important observation of our study is that intracellular application of angiotensin II (10−9 to 10−7 M) resulted in dose-dependent increments in [Ca2+]i in rat myometrial cells. Intracellular AT2 receptor blockade by PD-123319 did not affect the angiotensin II-induced increase in [Ca2+]i, whereas losartan, an AT1 receptor antagonist, or saralasin (nonspecific AT1 and AT2 antagonist) abolished it. These results indicate that the observed response is dependent on AT1 receptors located inside the cell and support an intracrine role for angiotensin II in the myometrium. This finding is consistent with previous reports that describe [Ca2+]i elevation upon intracellular stimulation of angiotensin II receptors in renal proximal tubule cells (49) or vascular smooth muscle cells (16).
It is not unusual that a G protein-coupled receptor (GPCR) signals from within the cell. Upon internalization, some GPCRs have been shown to direct a β-arrestin-mediated activation of the mitogen-activated protein kinase cascade, which appears to happen selectively on the endosomes (26). Intracellular GPCRs are either located at the Golgi, where they undergo modifications that will further allow insertion and functionality at the plasma membrane, or targeted at the endo-lysosomal compartment upon internalization. The AT1A receptor is known to remain associated with β-arrestin upon endocytosis (2, 47), being further trafficked to cytosolic vesicular structures; however, it is neither dephosphorylated nor efficiently recycled back to the cell surface (2). In our experiments, angiotensin II-induced Ca2+ response was not affected by brefeldin A, a Golgi apparatus disruptor (4); however, pretreatment with bafilomycin A1, a V-type ATPase inhibitor that prevents endo-lysosomal acidification (6), abolished the response. These results indicate that functional intracellular angiotensin II receptors are located at endo-lysosomal level. Endosomes have previously been implicated as important sites for receptor-initiated signaling, as they contain a wide range of signaling molecules in their membranes and are able to recruit scaffolding proteins and signaling mediators (40, 43, 46). Apparently this is an intriguing observation because in endo-lysosomal system the NH2-terminal of receptors (binding site) is located in the lumenum and COOH-terminal is located in the cytoplasmic side. Moreover, saralasin, a nonspecific AT1 and AT2 blocker with peptidic structure largely reduced elevation of [Ca2+]i produced by intracellular injection of angiotensin II. These results suggest that the NH2-terminal of endo-lysosomal AT1 receptors are translocated, and both NH2- and COOH-terminal are on cytoplasmic side or both peptides (angiotensin II and saralasin) are transported into the endo-lysosomal lumenum. In our opinion, the translocation of NH2-terminal to the cytoplasmic side was very unlikely, and next step was to identify how those peptides are transported inside the endo-lysosomes.
Microautophagy is a process that transfers cytoplasmic components such as old or unfolded proteins into endo-lysosomes by direct invagination of endo-lysosomal membranes followed by scission of the vesicle (for review see Ref. 9). The invaginations phase occurs with a lag period of 20–30 min (41); meanwhile, the second phase is a very fast process (22). Using a luciferase assay, Kunz et al. (22) reported that after the invaginations are formed, 40% of the uptake occurred and luciferase was transported into the lysosomes even after an incubation of only 2 min. Viewed in this context, the intracellular injected angiotensin II or saralasin could be rapidly transported into endo-lysosomes via ongoing microautophagy. The fact that rapamycin, which blocks microautophagy (22), abolished the angiotensin II-induced increase in [Ca2+]i suggests that this process is a key step in transporting the peptides inside the endo-lysosomes.
Next, we characterized the calcium pathways and pools involved in the response to intracellular angiotensin II in the rat myometrium. In the absence of extracellular Ca2+, the response to angiotensin II is only slightly decreased indicating a major involvement of Ca2+ release from internal stores. Intracellular organelles reportedly involved in Ca2+ release include the endoplasmic reticulum (ER) and acidic Ca2+ stores such as lysosomes (3, 36). Nicotinic acid-adenine dinucleotide phosphate (NAADP) is a second messenger that releases Ca2+ from lysosomes (25, 36). Pretreatement with Ned-19, an inhibitor of NAADP signaling (35) did not affect angiotensin II-induced Ca2+ response. In contrast, xestospongin C and heparin that prevent IP3R-mediated Ca2+ release from the ER, significantly decreased, angiotensin II-induced response.
AT1 receptors are known to couple with Gq/11 proteins that can further activate various signal transduction mediators, including phospholipase C (PLC) (45). PLC has been identified in lysosomal membranes (30, 39), thus we hypothesized it is activated following angiotensin II stimulation of AT1 receptors at this site. Accordingly, we tested the effect PLC inhibitor U-73122 (5) or its negative control U-73343 on angiotensin II response. Pretreatment with U-73122, but not with U-73343, abolished angiotensin II-induced increase in [Ca2+]i, thereby confirming that PLC activation is a required step for ER release of Ca2+ following intracellular AT1 receptor activation.
Antagonism of ryanodine receptors, another type of Ca2+ release channels located on the ER, slightly reduced the response to angiotensin II; this indicates that Ca2+ release via Ca2+-induced Ca2+ release (CICR) mechanism may amplify the IP3-dependent Ca2+ release. In summary, our findings indicate that intracellular angiotensin II transferred to lysosomes via microautophagy, activates AT1-like receptors located on lysosomes, which in turn stimulates lysosomal PLC to hydrolyze phosphatidylinositol-(4,5)-bisphosphate (PIP2) and form IP3. IP3 activates IP3R to release Ca2+ from the ER; CICR may further increase [Ca2+]i levels via ryanodine receptor activation (Fig. 5). A similar mechanism has been demonstrated in renal proximal tubule cells (12, 23, 32, 44, 49) and is consistent with the accepted contractile effect of angiotensin II on the myometrium, which involves AT1 receptor activation and elevation of [Ca2+]i (23, 28, 32).
Fig. 5.
Proposed mode of action of ANG II via activation of intracellular AT1 receptors. Intracellular ANG II transferred into the endo-lysosomes (Lys) via microautophagy acts on AT1 receptors situated on acid-filled Ca2+ stores and activates lysosomal PLC followed by production of IP3 from phosphatidylinositol 4,5-bisphosphate (PIP2). IP3 activates IP3R on ER, increasing [Ca2+]i. The increase in [Ca2+]i may activate ryanodine receptors (RyR) and further potentiates Ca2+ release from the ER via a Ca2+-induced Ca2+ release (CICR) mechanism.
Although the presence of a local renin-angiotensin system (RAS) in the uterus has been suggested, the significance of an uterine RAS is not clear. It appears that in pregnant women (29) and ewes (33) plasma angiotensin II levels present a fivefold increase compared with nonpregnant controls, suggesting a possibility that angiotensin II receptors may suffer internalization. Important differences in levels of expression of AT1 and AT2 receptors and reactivity to angiotensin II were reported in the myometrium of pregnant versus nonpregnant women (10). Whether or not the location of functional angiotensin II receptors plays a role in these differences it is not known. While most of evidence for functional intracellular angiotensin II receptors is provided by in vitro, or ex vivo studies, very recently, an elegant study using an innovative approach established the physiological in vivo effects of cytoplasmic angiotensin II-AT1 interaction on proximal tubule epithelial function (27). The authors used adenoviral transfer of intracellular cyan fluorescent fusion of angiotensin II selectively in proximal tubules and provided evidence that trapping angiotensin II intracellulary increased the blood pressure in rats and mice (46). This novel approach can distinguish the intracrine actions of angiotensin II in the physiological responses in an intact animal model (17).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1HL-90804 (to E. Brailoiu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address of A. A. Tica: Dept. of Pharmacology, School of Medicine, University of Medicine and Pharmacy, Craiova, Romania.
Footnotes
This article is the topic of an Editorial Focus by R. N. Re (38a).
REFERENCES
- 1.Accorsi-Mendonca D, Correa FM, Anselmo-Franci JA, Paiva TB, de Oliveira AM. Angiotensin actions on the isolated rat uterus during the estrous cycle: influence of resting membrane potential and uterine morphology. Pharmacology 65: 162–169, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Anborgh PH, Seachrist JL, Dale LB, Ferguson SS. Receptor/beta-arrestin complex formation and the differential trafficking and resensitization of beta2-adrenergic and angiotensin II type 1A receptors. Mol Endocrinol 14: 2040–2053, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32: 235–249, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Betina V. Biological effects of the antibiotic brefeldin A (decumbin, cyanein, ascotoxin, synergisidin): a retrospective. Folia Microbiol (Praha) 37: 3–11, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Bleasdale JE, Bundy GL, Bunting S, Fitzpatrick FA, Huff RM, Sun FF, Pike JE. Inhibition of phospholipase C dependent processes by U-73,122. Adv Prostaglandin Thromboxane Leukot Res 19: 590–593, 1989 [PubMed] [Google Scholar]
- 6.Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA 85: 7972–7976, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brailoiu E, Filipeanu CM, Tica A, Toma CP, de Zeeuw D, Nelemans SA. Contractile effects by intracellular angiotensin II via receptors with a distinct pharmacological profile in rat aorta. Br J Pharmacol 126: 1133–1138, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brailoiu E, Hooper R, Cai X, Brailoiu GC, Keebler MV, Dun NJ, Marchant JS, Patel S. An ancestral deuterostome family of two-pore channels mediates nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J Biol Chem 285: 2897–2901, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cierchanover A. Proteolysis: from the lysosomes to ubiquitin and the proteasome. Nat Rev Mol Cell Biol 6: 79–87, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Cox BE, Word RA, Rosenfeld CR. Angiotensin II receptor characteristics and subtype expression in uterine arteries and myometrium during pregnancy. J Clin Endocrinol Metab 81: 49–58, 1996 [DOI] [PubMed] [Google Scholar]
- 11.De Mello WC, Danser AH. Angiotensin II and the heart: on the intracrine renin-angiotensin system. Hypertension 35: 1183–1188, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Dudley DT, Panek RL, Major TC, Lu GH, Bruns RF, Klinkefus BA, Hodges JC, Weishaar RE. Subclasses of angiotensin II binding sites and their functional significance. Mol Pharmacol 38: 370–377, 1990 [PubMed] [Google Scholar]
- 13.Filipeanu CM, Brailoiu E, Kok JW, Henning RH, De Zeeuw D, Nelemans SA. Intracellular angiotensin II elicits Ca2+ increases in A7r5 vascular smooth muscle cells. Eur J Pharmacol 420: 9–18, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 15.Guse AH, Berg I, da Silva CP, Potter BV, Mayr GW. Ca2+ entry induced by cyclic ADP-ribose in intact T-lymphocytes. J Biol Chem 272: 8546–8550, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Haller H, Lindschau C, Erdmann B, Quass P, Luft FC. Effects of intracellular angiotensin II in vascular smooth muscle cells. Circ Res 79: 765–772, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Harrison-Bernard LM. Trapping intracellular ANG II to the proximal tubule: powerful in vivo effects on sodium handling and blood pressure. Am J Physiol Renal Physiol 300: F1074–F1075, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Imig JD, Navar GL, Zou LX, O'Reilly KC, Allen PL, Kaysen JH, Hammond TG, Navar LG. Renal endosomes contain angiotensin peptides, converting enzyme, and AT(1A) receptors. Am J Physiol Renal Physiol 277: F303–F311, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Ingelfinger JR, Jung F, Diamant D, Haveran L, Lee E, Brem A, Tang SS. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol Renal Physiol 276: F218–F227, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Krizsan-Agbas D, Pedchenko T, Smith PG. Neurotrimin is an estrogen-regulated determinant of peripheral sympathetic innervation. J Neurosci Res 86: 3086–3095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: a new paradigm. Trends Endocrinol Metab 18: 208–214, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kunz JB, Schwarz H, Mayer A. Determination of four stages during microautophagy in vitro. J Biol Chem 279: 9987–9996, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Lalanne C, Mironneau C, Mironneau J, Savineau JP. Contractions of rat uterine smooth muscle induced by acetylcholine and angiotensin II in Ca2+-free medium. Br J Pharmacol 81: 317–326, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazari MF, Porto CS, Freymuller E, Abreu LC, Picarelii ZP. Receptor-mediated endocytosis of angiotensin II in rat myometrial cells. Biochem Pharmacol 54: 399–408, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Lee HC, Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J Biol Chem 270: 2152–2157, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem 273: 18677–18680, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Li XC, Cook JL, Rubera I, Tauc M, Zhang F, Zhuo JL. Intrarenal transfer of an intracellular cyan fluorescent fusion of angiotensin II selectively in proximal tubules increases blood pressure in rats and mice. Am J Physiol Renal Physiol 300: F1076–F1088, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackenzie LW, Word RA, Casey ML, Stull JT. Myosin light chain phosphorylation in human myometrial smooth muscle cells. Am J Physiol Cell Physiol 258: C92–C98, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Magness RR, Cox K, Rosenfeld CR, Gant NF. Angiotensin II metabolic clearance rate and pressor responses in nonpregnant and pregnant women. Am J Obstet Gynecol 171: 668–679, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Matsuzawa Y, Hostetler KY. Properties of phospholipase C isolated from rat liver lysosomes. J Biol Chem 255: 646–652, 1980 [PubMed] [Google Scholar]
- 31.Mironneau J, Mironneau C, Grosset A, Hamon G, Savineau JP. Action of angiotensin II on the electrical and mechanical activity of rat uterine smooth muscle. Eur J Pharmacol 68: 275–285, 1980 [DOI] [PubMed] [Google Scholar]
- 32.Moore AF, HA MM, Khairallah PA. A comparison of the effects of angiotensin II and heptapeptide on smooth muscle (vascular and uterine). Eur J Pharmacol 39: 101–107, 1976 [DOI] [PubMed] [Google Scholar]
- 33.Naden RP, Coultrup S, Arant BS, Rosenfeld CR. Metabolic clearance of angiotensin II in pregnant and nonpregnant sheep. Am J Physiol Endocrinol Metab 249: E49–E55, 1985 [DOI] [PubMed] [Google Scholar]
- 34.Navar LG, Harrison-Bernard LM, Imig JD, Wang CT, Cervenka L, Mitchell KD. Intrarenal angiotensin II generation and renal effects of AT1 receptor blockade. J Am Soc Nephrol 10, Suppl 12: S266–S272, 1999 [PubMed] [Google Scholar]
- 35.Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, Galione A, Churchill GC. Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol 5: 220–226, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel S, Marchant JS, Brailoiu E. Two-pore channels: regulation by NAADP and customized roles in triggering calcium signals. Cell Calcium 47: 480–490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poisner AM. The human placental renin-angiotensin system. Front Neuroendocrinol 19: 232–252, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Re RN, Cook JL. Mechanisms of disease: intracrine physiology in the cardiovascular system. Nat Clin Pract Cardiovasc Med 4: 549–557, 2007 [DOI] [PubMed] [Google Scholar]
- 38a.Rel RN. The lysosomal action of intacrine angiotensin II. Focus on “Intracellular angiotension II activates rat myometrium.” Am J Physiol Cell Physiol (July 6, 2011). doi:10.1152/ajpcell.00232.2011 [DOI] [PubMed] [Google Scholar]
- 39.Richards DE, Irvine RF, Dawson RM. Hydrolysis of membrane phospholipids by phospholipases of rat liver lysosomes. Biochem J 182: 599–606, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadowski L, Pilecka I, Miaczynska M. Signaling from endosomes: location makes a difference. Exp Cell Res 315: 1601–1609, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Sattler T, Mayer A. Cell-free reconstitution of microautophagic vacuole invagination and vesicle formation. J Cell Biol 151: 529–538, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schauser KH, Nielsen AH, Winther H, Dantzer V, Poulsen K. Dominance of type 1 angiotensin II receptor in the nonpregnant and pregnant bovine uterus. J Reprod Fertil 116: 403–413, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol 3: 600–614, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev 45: 205–251, 1993 [PubMed] [Google Scholar]
- 45.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 52: 639–672, 2000 [PubMed] [Google Scholar]
- 46.von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol 19: 436–445, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Barak LS, Anborgh PH, Laporte SA, Caron MG, Ferguson SS. Cellular trafficking of G protein-coupled receptor/beta-arrestin endocytic complexes. J Biol Chem 274: 10999–11006, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT(1) receptor. Hypertension 39: 116–121, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol 290: F1382–F1390, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]