Abstract
Proteostasis is defined as the homeostatic mechanisms that maintain the function of all cytoplasmic proteins. We recently demonstrated that the capacity of the proteostasis network is a critical factor that defines the limits of cellular and organismal survival in hypertonic environments. The current studies were performed to determine the extent of protein damage induced by cellular water loss. Using worm strains expressing fluorescently tagged foreign and endogenous proteins and proteins with temperature-sensitive point mutations, we demonstrate that hypertonic stress causes aggregation and misfolding of diverse proteins in multiple cell types. Protein damage is rapid. Aggregation of a polyglutamine yellow fluorescent protein reporter is observable with <1 h of hypertonic stress, and aggregate volume doubles approximately every 10 min. Aggregate formation is irreversible and occurs after as little as 10 min of exposure to hypertonic conditions. To determine whether endogenous proteins are aggregated by hypertonic stress, we quantified the relative amount of total cellular protein present in detergent-insoluble extracts. Exposure for 4 h to 400 mM or 500 mM NaCl induced a 55–120% increase in endogenous protein aggregation. Inhibition of insulin signaling or acclimation to mild hypertonic stress increased survival under extreme hypertonic conditions and prevented aggregation of endogenous proteins. Our results demonstrate that hypertonic stress causes widespread and dramatic protein damage and that cells have a significant capacity to remodel the network of proteins that function to maintain proteostasis. These findings have important implications for understanding how cells cope with hypertonic stress and other protein-damaging stressors.
Keywords: protein aggregation, proteostasis, organic osmolytes, kidney, polyglutamine, macromolecular crowding, cell volume, Caenorhabditis elegans
osmotic homeostasis is a fundamental requirement for life. All cells are exposed to osmotic challenges from intracellular solute flux and/or extracellular osmolality shifts. Most mammalian cells are protected from extracellular osmotic perturbations by the kidney, which tightly regulates blood ionic and osmotic concentrations. However, there are important exceptions to this generalization. Renal medullary cells are subjected normally to extreme osmotic stress by the renal concentrating mechanism. Several disorders such as renal failure, diabetes, syndrome of inappropriate ADH secretion, and hypernatremia disrupt plasma osmolality. In addition, hypertonic solutions are widely used to treat diverse clinical problems such as intracranial hypertension, interstitial fluid accumulation after coronary artery bypass surgery, hemorrhagic shock, and cystic fibrosis.1
It has long been assumed from in vitro studies that hypertonic stress damages proteins (e.g., 47). Protein unfolding is thought to occur when cellular water loss causes a transient increase in cytoplasmic ionic strength. Exposure of normally buried hydrophobic surfaces on denatured proteins favors protein aggregation (23). Protein aggregation is likely further promoted by cell shrinkage, which increases macromolecular crowding and protein-protein interactions (18, 37, 55).
Numerous studies have demonstrated that the expression of various chaperone proteins is upregulated in response to cell shrinkage (4, 8). Expression of chaperones is triggered in part by accumulation of denatured cytoplasmic proteins (3, 40), suggesting indirectly that hypertonic stress damages cellular proteins in vivo. However, there is little direct evidence demonstrating the types and extent of in vivo hypertonic stress-induced protein damage. With the exception of our own recent work (10), we are aware of only one other study identifying protein damage in hypertonically stressed cells. Chun at al. (12) demonstrated that hypertonic stress enhances aggregation of a mutant huntingtin protein in cultured human neuroblastoma cells.
We recently carried out a genome-wide RNA interference (RNAi) screen in Caenorhabditis elegans with the goal of identifying genes required for survival in hypertonic environments (10). This screen identified 40 genes that when silenced reduce survival in hypertonic conditions, a phenotype we term hypertonic sensitive, or Hos. Twenty of the 40 Hos genes encode proteins that function to detect, transport, and degrade damaged proteins. We also demonstrated that hypertonic stress causes aggregation of an aggregation-prone fluorescent reporter protein, Q35::YFP. Knockdown of the expression of Hos genes involved in protein degradation increases Q35::YFP aggregation.
Protein homeostasis is the maintenance of the complement of properly functioning proteins within a cell. Like osmotic homeostasis, it is essential for life. Our previous studies (10) suggested strongly that protein damage in hypertonically stressed cells must be rapidly repaired or removed in order for cells to survive in hypertonic environments. The purpose of the current study was to carry out a more extensive and detailed characterization of the protein damage induced by hypertonic stress. Using multiple transgenic and mutant models, we demonstrate that protein damage in hypertonically stressed cells is widespread and occurs rapidly after exposure to hypertonic conditions. Importantly, we demonstrate that hypertonic stress causes striking aggregation of endogenous proteins and that this aggregation is prevented by inhibition of the insulin signaling pathway or acclimation to mild hypertonic stress. These results demonstrate that cells have a significant capacity to remodel the network of proteins that function to maintain protein homeostasis. Our studies establish C. elegans as an important model system in which to define the molecular mechanisms utilized by eukaryotic cells for protein homeostasis under osmotic stress conditions. In addition, our work provides new insights into the factors that limit survival of cells and organisms in hypertonic environments, and it has broad implications for understanding diseases that perturb plasma osmolality and for understanding possible complications of clinical therapies that employ hypertonic solutions.
MATERIALS AND METHODS
C. elegans strains.
The following strains were obtained from the Caenorhabditis Genetics Center: wild-type N2 Bristol, Q35::YFP - AM140 rmIs132[Punc-54::Q35::YFP], α-synuclein::YFP - NL5901 pkIs2386[Punc-54::α-synuclein::YFP], paramyosin(ts) - CB1402 [unc-15(e1402)], ras(ts) - SD551[let-60(ga89)], perlecan(ts) - HE250[unc-52(e669su250)], myosin(ts) - CB1301[unc-54(e1301)], DAF-2 loss-of-function - CB1370[daf-2(e1370)], DAF-2;DAF-16 double loss-of-function - DR1309[daf-2(e1370;daf-16(m26)]. The KIN-19::tagRFP strain, CF3166 muEx473[pC03C10.1::C03C10.1::TagRFP + ptph-1::GFP], was generously provided by Dr. C. Kenyon. Unless otherwise stated, worms were cultured at 20°C using standard methods (5).
Protein aggregate counts.
Synchronized L1-stage worms were grown to young adults on 51 mM NaCl nematode growth medium (NGM) and then transferred to hypertonic NGM agar plates containing 400 mM or 500 mM NaCl. Q35::YFP puncta were counted manually using a Zeiss Stemi SV11 microscope (Chester, VA) set for green fluorescent protein (GFP) excitation at ×8 magnification. Quantification of α-synuclein::YFP and KIN-19::tagRFP puncta was carried out using a Zeiss LSM510-Meta confocal microscope and Plan-Neofluar ×40/1.3 numerical aperture (NA) or Plan-Apochromat ×63/1.4 NA oil objective lenses. α-Synuclein::YFP puncta were quantified in a single body wall muscle cells located anterior to the nerve ring. KIN-19::tagRFP puncta were quantified in the anterior pharynx, which encompasses the procorpus and metacorpus.
Confocal microscopy.
Worms were anesthetized in NGM buffer containing 0.1% Tricaine and 0.01% tetramizole, mounted on 2% agarose pads on glass slides, and imaged using a Zeiss LSM510-Meta confocal microscope with Plan-Neofluar ×40/1.3 NA and Plan-Apochromat ×63/1.4 NA oil objective lenses (Carl Zeiss MicroImaging, Thornwood, NY). FRAP analysis was performed by photobleaching regions of body wall muscle cells or the anterior pharynx with 50–150 iterations of an argon laser set at 100% power and 514 nm or 543 nm for bleaching of YFP and tagRFP, respectively. Images were taken every 3 s for up to 60 s after photobleaching, and fluorescence intensity was measured with ImageJ software (National Institutes of Health, Bethesda, MD).
Aggregate volumes were estimated from optical z-stacks through body wall muscle cells by summing the fluorescence areas of the aggregate in each optical slice and multiplying that sum by slice thickness. Fluorescence area was measured using Metamorph software (Molecular Devices, Sunnyvale, CA).
Temperature-sensitive mutant phenotype assays.
Synchronized wild-type, paramyosin [temperature-sensitive (ts)] and ras (ts) worms were grown on 51 mM NaCl NGM at 16°C. Adult worms were picked to plates that were either 51 mM NaCl at 16°C (control), 51 mM NaCl at 25°C, or 300 mM NaCl at 16°C and were allowed to lay eggs for 24 h. The number of eggs was counted, and those that did not develop past early larval stages were scored as larval arrest or defective egg hatching phenotypes.
Motility assays.
Worm motility was measured as described previously (36). Briefly, adult worms grown on 51 mM NaCl NGM were transferred to 500 mM NaCl NGM for 6 h and then allowed to recover on 51 mM NaCl NGM for 24 h. Single worms were then placed on a fresh lawn of OP50 bacteria and removed after 30–60 s. Brightfield images of the lawns were obtained using a Zeiss Stemi 2,000-CS microscope (Thornwood, NY) equipped with a charge-coupled device camera (DAGE-MTI, Michigan City, IN). The length of the tracks in bacterial lawn made by individual worms was measured with ImageJ software.
Analysis of endogenous insoluble proteins.
Isolation of insoluble proteins was carried out using methods similar to those described by David et al. (14). Briefly, worms were grown on 51 mM NaCl NGM agar with either standard OP50 or RNAi feeding bacteria and transferred to hypertonic 400 mM or 500 mM NaCl plates for 4 h. Worms were then washed in NGM solution isotonic to control or high NaCl agar plates. Washed worms were transferred to a buffer containing 51, 400, 500, or 650 mM NaCl, 100 mM MES, 1 mM EGTA, 0.1 mM EDTA, 0.5 mM MgSO4, 20 mM NaF, drip frozen in liquid nitrogen, and ground to a powder with a mortar and pestle. Immediately upon thawing, 10 μl of the ground material was taken for analysis of total protein concentration using a bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL). Two additional 60-μl aliquots were placed in either a solubilization buffer (8 M urea, 2% SDS, 50 mM DTT, 50 mM Tris, Roche complete protease inhibitor, pH 7.4) for total protein determination or a RIPA buffer (50 mM Tris, 150 mM NaCl, 5 mM EDTA, 0.5% SDS, 0.5% SDO, 1% NP-40, Roche complete protease inhibitor, pH 8). Insoluble proteins were isolated from samples in RIPA buffer by centrifugation at 16,100 g for 10 min. After supernatant removal, the insoluble protein pellet was resuspended in 100 μl of RIPA buffer, centrifuged a second time, and then solubilized in solubilization buffer.
Protein samples were analyzed by SDS-PAGE. Gels were stained with Bio-Safe Coomassie (Bio-Rad, Hercules, CA), and the amount of protein present in each lane was quantified with ImageJ software. To assess changes in the amount of insoluble protein, total protein gels were loaded with a sample volume containing 20 μg of protein and the same sample volume was used to load the corresponding insoluble protein gel.
Survival studies.
Hypertonic stress was induced in various experimental settings by exposing worms for 4–6 h to growth media containing 400–650 mM NaCl. We monitored survival under these conditions by returning worms after the stress period to control growth medium for 24 h. In all cases, survival ranged between 98% and 100% (data not shown).
Statistical analyses.
Data are presented as means ± SE. Statistical significance was determined using Student's two-tailed t-test for paired or unpaired means. When comparing three or more groups, statistical significance was determined by one-way analysis of variance. P ≤ 0.05 was taken to indicate statistical significance. All graphs are plotted on common scales to facilitate comparison between experimental groups.
RESULTS
A polyglutamine tract containing fluorescent reporter undergoes rapid and irreversible aggregation in response to hypertonic stress.
Proteins that contain tracts of contiguous glutamine residues undergo spontaneous, age-dependent aggregation. These proteins give rise to neurodegenerative disorders such as Huntington's Disease and spinocerebellar ataxias (27). In our previous studies (10), we reported that yellow fluorescent protein (YFP) containing a tract of 35 glutamine residues (Q35::YFP) expressed in the body wall muscle cells of C. elegans aggregates in response to hypertonic stress. We carried out more detailed studies of this protein to determine the time course of aggregation and aggregate growth, and whether aggregate formation was reversible. As shown in Fig. 1A, numerous Q35::YFP aggregates are detected within 1 h after exposure of worms to 400 mM or 500 mM NaCl growth medium. We were able to detect small punctate structures that we believe were Q35::YFP aggregates within as little as 30 min of exposure to 500 mM NaCl (data not shown).
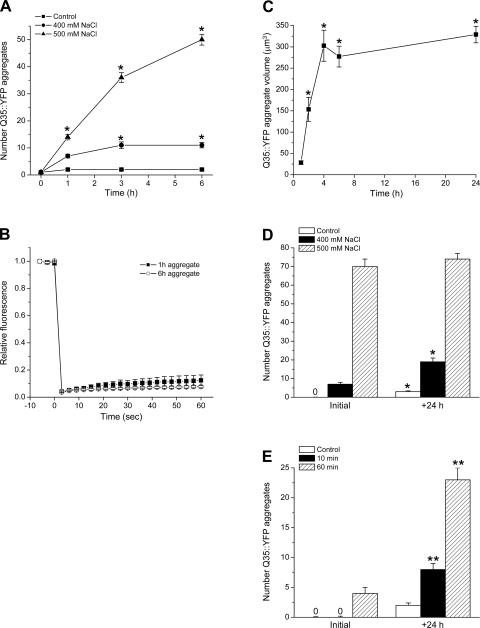
Fig. 1.
Characteristics of hypertonic stress-induced aggregation of Q35::yellow fluorescent protein (YFP). A: rate of Q35::YFP aggregate formation in worms exposed acutely to 400 mM or 500 mM NaCl. (n = 30–90 worms); *P < 0.01 vs. control. B: time course of bleaching and fluorescence recovery in Q35::YFP aggregates detected 1 and 6 h after exposure to 500 mM NaCl. (n = 3 worms). C: rate of Q35::YFP aggregate growth in worms exposed to 500 mM NaCl. (n = 9–12 aggregates from 5 worms); *P < 0.01 vs. volume at 1 h. D: effect of a 24 h hypertonic stress on the reversibility of Q35::YFP aggregate formation. Worms were exposed to 400 mM or 500 mM NaCl for 24 h (initial) and then returned to control conditions for 24 h (+24 h). (n = 12–18 worms); *P < 0.01 vs. initial number of aggregates in worms exposed to 400 mM NaCl. E: effect of brief hypertonic stress on the reversibility of Q35::YFP aggregate formation. Worms were exposed to 500 mM NaCl for either 10 or 60 min (initial) and then returned to control conditions for 24 h (+24 h). (n = 24 worms); **P < 0.001 vs. initial number of aggregates detected 10 min and 60 min after exposure to 500 mM NaCl.
To confirm that the early hypertonicity-induced changes in Q35::YFP distribution were due to bona fide aggregation, we carried out fluorescence recovery after photobleaching (FRAP) analysis as described previously (10). Worms were exposed to 500 mM NaCl for 1 or 6 h and then imaged by confocal microscopy. Small regions of presumed aggregates were bleached, and fluorescence recovery in the bleached region was quantified over time. As shown in Fig. 1B, FRAP was undetectable in punctate YFP structures detected 1 and 6 h after hypertonic stress was induced. These results demonstrate that under hypertonic conditions, Q35::YFP is localized to aggregates where individual proteins are immobile and that formation of insoluble Q35::YFP aggregates occurs with <1 h of hypertonic stress.
Hypertonicity-induced Q35::YFP aggregates increase rapidly not only in number, but also in size. To quantify aggregate growth, we exposed groups of worms to hypertonic growth medium for 1–24 h and estimated aggregate volume by confocal microscopy. As shown in Fig. 1C, aggregate volume increased ∼12-fold after a 4-h exposure to 500 mM NaCl growth medium. Aggregate volume remained stable between 4 and 24 h. Similar aggregate growth was observed when single worms were imaged continuously over a 3-h period (data not shown).
Numerous studies have demonstrated that polyglutamine protein aggregates can be degraded and cleared by autophagy and other cellular mechanisms (e.g., 15, 35, 52). To determine whether hypertonicity-induced Q35::YFP aggregates can be cleared, we exposed worms to either 400 mM or 500 mM NaCl growth media for 6 h and quantified the number of aggregates immediately and 24 h after returning the animals to control growth medium. No significant (P > 0.05) reduction in the number of aggregates was observed when animals exposed to either 400 mM or 500 mM NaCl were returned to control conditions (Fig. 1D).
We also examined the effects of short-term hypertonic stress on Q35::YFP aggregate clearance. Worms were exposed to 500 mM NaCl growth medium for either 10 min or 60 min and then returned to control medium. As shown in Fig. 1E, no Q35::YFP aggregates were detected after 10 min of hypertonic stress. However, even though animals were returned to control conditions, Q35::YFP aggregates formed. The mean number of aggregates detected per worm was 8, which was significantly (P < 0.001) greater than that observed in unstressed animals.
The effects of a 60-min exposure to 500 mM NaCl were even more striking. An average of four aggregates/worm were detected immediately after returning animals to control growth medium (Fig. 1E). Striking Q35::YFP aggregation continued under control conditions. By 24 h, the number of aggregates increased by nearly sixfold (P < 0.001; Fig. 1E). Taken together, data in Fig. 1, D and E, demonstrate that hypertonicity-induced Q35::YFP aggregates cannot be cleared after removal of the hypertonic stress. Indeed, as shown in Fig. 1E, short-term hypertonic stress triggers processes that drive continued Q35::YFP aggregation.
The gross morphology of C. elegans body wall muscle cells includes a contractile myofilament lattice and a noncontractile cell body that contains the cytoplasm and cell organelles. We optically sectioned muscle cells with confocal microscopy to define where Q35::YFP aggregates form in response to hypertonic stress. Two types of aggregates were observed: elongated, dense aggregates that aligned with myofilaments and were localized to the contractile lattice, and more diffuse aggregates in the cell body that frequently colocalized with nuclei (Fig. 2A). This morphology and localization is similar to that observed for GFP-tagged polyglutamine aggregates that form spontaneously in C. elegans muscle cells during aging (43).
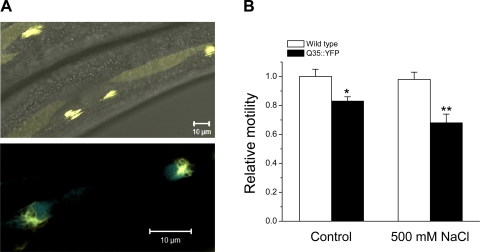
Fig. 2.
Morphology and toxicity of hypertonic stress-induced Q35::YFP aggregates. A: confocal micrographs of muscle cells expressing Q35::YFP. Worms were exposed to 500 mM NaCl for 6 h. Top: combined differential interference contrast/fluorescence micrograph showing Q35::YFP aggregates localized to myofilament lattice. Bottom: fluorescence micrograph showing Q35::YFP localized to muscle cell body. Q35::YFP aggregates (yellow) are associated with cell nuclei (blue) expressing cyan fluorescent protein with a nuclear localization signal. B: toxicity of hypertonic stress-induced Q35::YFP aggregates. Worm motility was used to assess toxicity of Q35::YFP aggregates to body wall muscle cells. Motility was quantified in wild-type and Q35::YFP worms exposed to 500 mM NaCl for 6 h and then returned to control conditions for 24 h, and in age-matched, unstressed wild-type and Q35::YFP animals. (n = 9 worms); *P < 0.05 vs. wild-type worms. **P < 0.05 vs. unstressed Q35::YFP worms.
Age-induced Q35::YFP aggregates are toxic and cause muscle cell damage that reduces worm motility (6, 36). To assess the toxicity of hypertonic stress-induced aggregates, we quantified motility in wild-type and Q35::YFP worms exposed to 500 mM NaCl for 6 h and then returned to control medium for 24 h. As shown in Fig. 2B, no motility defects were detected in wild-type worms. Motility in control Q35::YFP worms was reduced ∼17% (P < 0.05) compared with control wild-type animals. Exposure of Q35::YFP worms to hypertonic stress significantly (P < 0.05) reduced motility by an additional ∼15% (Fig. 2B). These results suggest that hypertonicity-induced Q35::YFP aggregates and/or their intermediates are toxic to muscle cells.
Diverse proteins are damaged rapidly by hypertonic stress.
To determine whether hypertonicity-induced protein damage is unique to Q35::YFP, we examined the effects of hypertonic stress on other fluorescent reporters. α-Synuclein is an inherently disordered protein expressed abundantly in the brain and is found in protein inclusions associated with various neurodegenerative disorders. Familial forms of Parkinson's Disease are caused by mutations and duplication of the α-synuclein gene (49).
Human α-synuclein fused to YFP accumulates in so-called inclusion bodies in an age-dependent manner when expressed in C. elegans body wall muscle cells (51). As shown in Fig. 3A, the number of α-synuclein::YFP-containing inclusions increases four- to fivefold when C. elegans is exposed to 500 mM NaCl for 6 h.
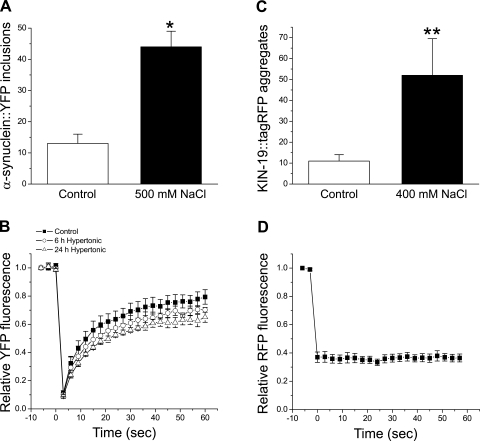
Fig. 3.
Effects of hypertonic stress on α-synuclein::YFP and KIN-19::tagRFP aggregation. A and C: α-synuclein::YFP inclusions in body wall muscle cells and KIN-19::tagRFP aggregates in the anterior pharynx under control and hypertonic stress conditions. (n = 5–7 worms); *P < 0.001 vs. control. RFP, red fluorescent protein. B and D: time course of bleaching and fluorescence recovery in α-synuclein::YFP inclusions and KIN-19::tagRFP aggregates. (n = 3–4 aggregates); **P < 0.05 vs. control.
We used FRAP to determine whether the α-synuclein-YFP within inclusion bodies was aggregated. As shown in Fig. 3B, YFP fluorescence intensity recovered 70–80% within 60 s after bleaching. This recovery is similar to that described previously by van Ham et al. (51) and indicates that α-synuclein::YFP remains mobile within inclusion bodies. No obvious differences were observed in either the rate or the extent of fluorescence recovery in inclusions of control and hypertonically stressed worms.
kin-19 encodes a predicted C. elegans casein kinase homolog. Monomeric red fluorescent protein (tagRFP)-tagged KIN-19 (KIN-19::tagRFP) is expressed abundantly in the worm pharynx. KIN-19::tagRFP expressed in the anterior pharynx undergoes spontaneous age-dependent aggregation (14). As shown in Fig. 3C, KIN-19 aggregates increased approximately fivefold when worms were exposed to 400 mM NaCl for 24 h. The mean ± SE total fluorescence in the anterior pharynx relative to control worms was 1.04 ± 0.24, indicating that aggregation was due to hypertonic stress rather than changes in KIN-19::tagRFP expression. FRAP was undetectable in KIN-19::tagRFP (Fig. 3D) aggregates, indicating that individual proteins are immobile.
Hypertonic stress causes protein misfolding in vivo.
It is widely assumed from in vitro studies that cell shrinkage and the attendant increases in cytoplasmic ionic strength cause proteins to misfold and misfunction in vivo. To the best of our knowledge, this idea has not previously been examined in living cells. We used worm strains harboring temperature-sensitive (ts) mutations to begin assessing whether hypertonic stress causes in vivo protein misfolding. Temperature-sensitive mutations are mutations that have little or no effect on protein function and phenotype at low or “permissive” temperatures. However, at elevated temperatures, ts mutations give rise to a mutant phenotype. It is widely accepted that ts mutant proteins fold and function correctly at low temperatures (e.g., 7, 22, 50). At elevated temperatures, ts mutant proteins misfold and therefore misfunction, giving rise to mutant phenotypes.
Figure 4 shows the effect of elevated temperature and hypertonic stress on the fraction of let-60(ga89) or unc-15(el402) ts mutant worms expressing the mutant phenotype. let-60 encodes a ras GTPase and unc-15 encodes paramyosin. These mutations give rise to egg hatching defects and larval arrest.
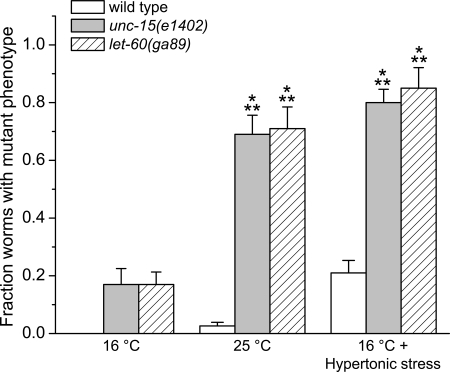
Fig. 4.
Effects of elevated temperature and hypertonic stress on expression of mutant phenotypes in unc-15 and let-60 temperature-sensitive (ts) mutant worms. unc-15 and let-60 encode paramyosin and a ras GTPase, respectively. Temperature-sensitive mutant phenotypes are induced by elevated temperature (25°C) and by hypertonic stress (300 mM NaCl) at low or permissive temperature (16°C), demonstrating that cell water loss also causes ts protein misfolding and misfunction. (n = 4–6 experiments performed on groups of 20–40 worms); *P < 0.001 vs. wild-type and mutant worms at 16°C, **P < 0.001 vs. wild-type worms at 25°C and at 16°C with hypertonic stress.
Worms were grown on 51 mM NaCl agar plates at the permissive temperature of 16°C and at 25°C for 4 days, or they were grown on 300 mM NaCl plates at 16°C for 4 days. As shown in Fig. 4, only 10–20% of the let-60(ga89) and unc-15(el402) worms exhibited the mutant phenotype at 16°C, whereas at 25°C, over 70% of the animals show larval arrest and lethality. Worms grown at 16°C on 300 mM NaCl resembled 25°C animals with >70% exhibiting the mutant phenotype. These results demonstrate that hypertonic stress causes misfolding and misfunction of LET-60 and UNC-15 in vivo.
We also conducted similar experiments with two other ts mutants: unc-52(e669su250) and unc-54(e1157). unc-52 and unc-54 encode perlecan and myosin, respectively. Mutant worms grown at 16°C and 300 mM or 400 mM NaCl exhibited no mutant phenotypes (data not shown). These results suggest that cell shrinkage does not cause misfolding significant enough to alter function of all cellular proteins.
Hypertonic stress causes aggregation of endogenous proteins.
Data in Figs. 1–4 provide compelling evidence that hypertonic stress causes rapid and dramatic protein damage. However, a potential concern is that the proteins examined are either transgenic or contain mutations that make them prone to misfolding and aggregation. To determine whether endogenous proteins are damaged in vivo by hypertonic stress, we quantified the impact of a 4-h exposure to either 400 mM or 500 mM NaCl on the relative amount of total cellular protein that is present in detergent-insoluble extracts. Aggregated proteins are solubilized in 8 M urea, but they remain insoluble in strong detergents (14, 31).
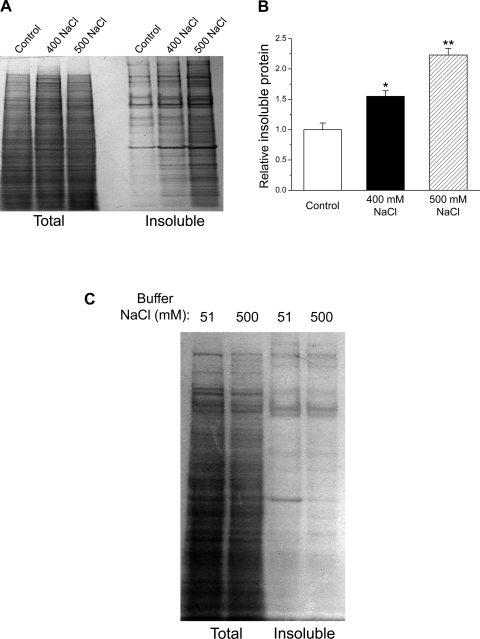
Figure 5A shows SDS-PAGE gels of total and detergent-insoluble proteins isolated from control and hypertonically stressed worms. As is clearly evident from the gels, the detergent-insoluble fraction increases strikingly in worms exposed to high NaCl. Quantification of results from three independent experiments is shown in Fig. 5B. Under control conditions, ∼12% of the total protein was present in the insoluble fraction. When worms were exposed for 4 h to either 400 mM or 500 mM NaCl, the insoluble protein fraction increased ∼55% (P < 0.05) and ∼120% (P < 0.01), respectively, relative to that observed in control animals (Fig. 5B).
Fig. 5.
Effects of hypertonic stress on aggregation of endogenous proteins. A: examples of SDS-PAGE gels of total and detergent-insoluble proteins isolated from control worms and worms exposed for 4 h to either 400 mM or 500 mM NaCl. B: quantification of insoluble protein. Insoluble protein was quantified as a percentage of total protein and is plotted relative to control animals. (n = 3); *P < 0.05 and **P < 0.01 vs. control worms. C: example of an SDS-PAGE gel of total and detergent-insoluble proteins isolated from control worms grown in 51 mM NaCl agar that were washed and frozen in a buffer containing either 51 or 500 mM NaCl. Results are representative of two independent experiments.
As described in materials and methods, total protein was extracted from worms by washing and freezing them in a buffer with a NaCl concentration equivalent to that of the agar to which they were exposed. It is thus conceivable that washing and freezing worms in high-NaCl buffers causes proteins to aggregate. To test for this, we washed and froze control worms grown on 51 mM NaCl agar with a 500 mM NaCl buffer. As shown in Fig. 5C, extracting protein from control worms in a high-salt buffer had no effect on the amount of protein in the insoluble fraction.
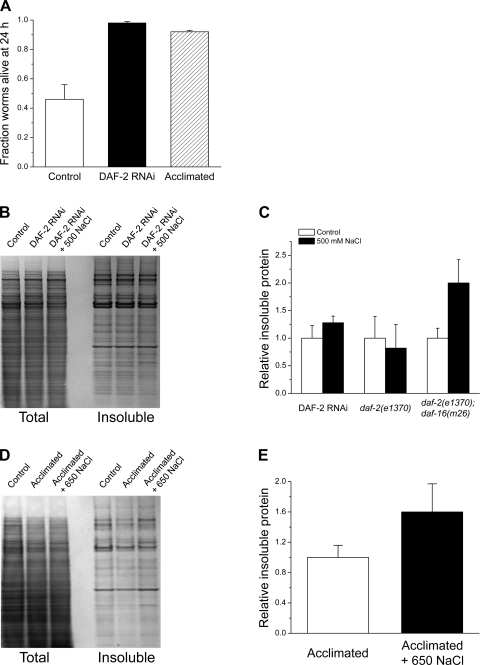
Inhibition of the C. elegans DAF-2 insulin signaling pathway dramatically increases survival under hypertonic conditions (Fig. 6A) (34). Increased survival occurs without significant changes in whole animal glycerol levels (34). We tested the role of DAF-2 signaling on aggregation of endogenous proteins. Expression of the DAF-2 insulin receptor was silenced by feeding worms bacteria expressing daf-2 double-stranded RNA (dsRNA) for 2 days. As shown in Fig. 6, B and C, no significant (P > 0.3) increase in protein aggregation was observed in DAF-2 RNAi worms exposed to 500 mM NaCl for 4 h.
Fig. 6.
Effects of inhibition of insulin signaling and acclimation to mild hypertonicity on survival and aggregation of endogenous proteins. A: fraction of worms alive 24 h after exposure to 500 mM NaCl. Worms were fed daf-2 double-stranded RNA or acclimated to 200 mM NaCl for 2 days before exposure to 500 mM NaCl; (n = 3 experiments performed on groups of 20–40 worms). B: example of SDS-PAGE gels of total and detergent-insoluble proteins in DAF-2 RNA interference (RNAi) worms. C: quantification of relative insoluble protein in DAF-2 RNAi worms and daf-2(e1370) and daf-2(e1370);daf-16(m26) mutant worms (n = 3). D and E: example of SDS-PAGE gels of total and detergent-insoluble proteins (D) and quantification of relative insoluble protein (E) in worms acclimated to 200 mM NaCl for 2 days (n = 3).
We also assessed the effect of DAF-2 signaling on protein aggregation using a worm strain carrying a daf-2 loss-of-function allele (e1370). As shown in Fig. 6C, no significant (P > 0.8) protein aggregation was detected in daf-2(e1370) mutant worms exposed to 500 mM NaCl. Thus, loss of DAF-2 activity by RNAi knockdown or loss-of-function mutation prevents endogenous protein aggregation during hypertonic stress.
Increased resistance to hypertonic stress in daf-2 loss-of-function worms is mediated by activation of the forkhead box transcription factor DAF-16 and the resultant increased expression of DAF-16-regulated genes (34). To determine whether DAF-16 plays a role in reducing protein aggregation, we exposed daf-2(e1370);daf-16(m26) double loss-of-function mutant worms to 500 mM NaCl for 4 h. In three separate experiments, we observed hypertonic stress-induced increases in the relative amount of insoluble protein of 1.65- to 2.4-fold (Fig. 6C). The mean ± SE fold increase observed was 1.98 ± 0.22. This value was significantly (P < 0.01) elevated compared with daf-2(e1370) mutant worms. These results demonstrate that DAF-16 activity is required for inhibition of hypertonic stress-induced protein aggregation in DAF-2 RNAi or loss-of-function animals.
Acclimation to moderate hypertonicity increases survival (Fig. 6A) (33) and suppresses aggregation of Q35::YFP in worms subjected to extreme hypertonic stress (10). The effect of acclimation on endogenous protein aggregation is shown in Fig. 6, D and E. Worms were acclimated to 200 mM NaCl for 2 days and then exposed to 650 mM NaCl for 4 h. Exposure to 650 mM NaCl was used to mimic the change in NaCl concentration control animals would experience when placed on growth medium containing 500 mM NaCl. While the mean relative level of insoluble protein increased somewhat in acclimated worms exposed to 650 mM NaCl, this change was not statistically significant (P > 0.2; Fig. 6C).
DISCUSSION
Protein homeostasis or “proteostasis” is defined as the homeostatic mechanisms that maintain the conformation, concentration, interactions, localization, and hence function, of cytoplasmic proteins. The proteostasis network is highly conserved across evolutionarily divergent species and comprises the tightly integrated activities of gene transcription, RNA metabolism and protein synthesis, folding, assembly, trafficking, disassembly, and degradation (1, 13).
Errors in protein translation occur at a rate of one in every 1,000–10,000 translated codons, indicating that ∼15% of all average size proteins will contain at least one incorrect amino acid (16). If they are misfolded and dysfunctional, mistranslated proteins must be detected and repaired or destroyed. Mistranslation alone places the proteostasis network under a constant burden. The proteostasis network is further taxed by gene mutations that disrupt protein structure and function, by numerous protein-damaging stressors, and by bacterial and viral infection that hijack protein synthesis and folding machinery. Thus, as noted by Ghosh and Dill (21), “cells live on the edge of a proteostasis catastrophe.”
We recently carried out a genome-wide RNAi screen in C. elegans to identify genes required for survival in hypertonic environments (10). To our surprise, this screen failed to identify the usual suspects associated with hypertonic stress resistance such as ion transporters required for cell volume recovery and genes that mediate organic osmolyte accumulation. Instead, we found that half or 20 of the identified genes encode proteins that function to detect, transport, and degrade damaged proteins. These results indicate that the capacity of the proteostasis network is an essential factor that determines the limits to survival in hypertonic environments.
The current studies were carried out with the goal of determining the extent of hypertonic stress-induced protein damage. We found that three fluorescent reporter proteins, Q35::YFP, α-synuclein::YFP, and KIN-19::tagRFP, undergo rapid aggregation during hypertonic stress (Figs. 1–3). Q35::YFP and α-synuclein::YFP are aggregation-prone proteins while KIN-19 is a native C. elegans casein kinase. Hypertonic stress causes misfolding of ts mutant LET-60 and UNC-15 proteins (Fig. 4). Q35::YFP and α-synuclein::YFP are expressed in body wall muscle cells and KIN-19::tagRFP is expressed in the pharynx. LET-60 is present in numerous diverse cell types, but larval lethality appears to be due to failure of the excretory system to form properly in mutant animals (54). UNC-15 is expressed in essentially all C. elegans muscle cell types. The origin of the larval and embryonic lethality in unc-15 mutants has not been defined precisely, but it is likely due to failure of contractile apparatuses to develop properly (19). Taken together, results shown in Figs. 1–4 demonstrate that hypertonic stress damages diverse proteins and that this damage occurs in multiple diverse cell types.
Our studies with Q35::YFP reveal several important features of hypertonic stress-induced protein damage. First, protein aggregation is very rapid. Q35::YFP aggregates are detectable within a few tens of minutes after exposure of C. elegans to hypertonic conditions (Fig. 1A), and aggregate volume doubles approximately every 10 min until aggregates reach a stable size or all free protein has been aggregated (Fig. 1C).
Aggregate formation is not reversible (Fig. 1D) and exposure to as little as 10 min of hypertonic stress triggers Q35::YFP aggregation even when animals are returned to normotonic conditions (Fig. 1E). There are two possible explanations for these observations. Protein damage induced by hypertonic stress may rapidly saturate the proteostasis network thus limiting its ability to prevent aggregation. It is also likely that protein aggregates represent a mechanism utilized by cells to isolate toxic intermediates including misfolded monomers and oligomeric structures (e.g., 9, 28, 45).
Hypertonicity-induced Q35::YFP aggregates exhibit mild toxicity to muscle cells as measured by a whole animal motility assay (Fig. 2B). Interestingly, aggregates that form as worms age appear to be much more toxic. Morley et al. (36) observed that 10-day-old worms have ∼50 Q35::YFP aggregates per animal. Individual worms exposed to 500 mM NaCl for 6 h and returned to control medium for 24 h have ∼70 aggregates. Motility in the hypertonic stress Q35::YFP worms is reduced ∼30% compared with wild-type animals. In contrast, motility in 10-day-old Q35::YFP worms is reduced 65–70% (36).
There are several possible explanations for this apparent difference. Aging-induced aggregates form over a period of days whereas formation of hypertonic stress-induced aggregates is complete in a matter of hours (Fig. 1, A and C). The more rapid formation of Q35::YFP aggregates under hypertonic conditions may minimize the time that cells are exposed to toxic intermediate structures. It is also possible that proteostasis pathways activated in response to hypertonic stress may limit toxicity and/or that younger worms are better able to repair damage induced by toxic protein structures.
Importantly, we demonstrated in these studies that a 4-h exposure to hypertonic stress causes aggregation of numerous unidentified endogenous proteins (Fig. 5). Thus, the damage we observe is not associated simply with transgenic or mutant protein properties. To our knowledge, this is the first demonstration that native proteins are damaged by cellular water loss in vivo.
We have begun mass spectrometry studies in an effort to identify proteins that are prone to aggregation when cells are subjected to hypertonic stress. Interestingly, a protein that was identified in one of the gel bands that showed particularly striking hypertonic stress-induced increases in the insoluble fraction (see Fig. 5A) was paramyosin or UNC-15 (K. Burkewitz and K. Strange, unpublished observations). This result is consistent with the UNC-15 ts mutant experiments shown in Fig. 4 and indicates that wild-type paramyosin is also damaged by hypertonic conditions.
Inhibition of DAF-2 insulin signaling in C. elegans activates the DAF-16 transcription factor and dramatically increases longevity and resistance to numerous stressors including hypertonic stress (20, 34) (Fig. 6A). Hypertonicity-induced aggregation of endogenous proteins is completely blocked in DAF-2 RNAi worms and worms carrying a daf-2 loss-of-function allele (Fig. 6, B and C). Loss of DAF-16 activity reverses the protective effect of DAF-2 inhibition (Fig. 6C). Consistent with these findings, we have shown previously that enhanced survival of DAF-2 mutants in hypertonic environments is due in part to DAF-16-regulated increases in the expression of small heat shock proteins (sHSPs) as well as enzymes involved in trehalose synthesis (34). sHSPs bind to unfolded proteins and thereby minimize their interaction and subsequent aggregation (24). Several studies have shown that trehalose inhibits protein aggregation in vivo (e.g., 2, 44, 46, 48). These results further support our contention that maintenance of proteostasis is essential for survival during hypertonic stress. Additional studies of the role of DAF-2-regulated proteostasis mechanisms in mediating increased survival during hypertonic stress are warranted.
Acclimation to mild hypertonic stress increases the ability of cells and organisms to survive more extreme hypertonicity that would normally be lethal (Fig. 6A). We showed previously that acclimation to 200 mM NaCl completely suppresses Q35::YFP aggregate formation when worms are exposed to 500 mM NaCl, a salt concentration where maximal aggregation is normally observed (10). Similarly, 200 mM NaCl acclimation prevents aggregation of endogenous proteins (Fig. 6, D and E). One explanation for these results is that accumulated glycerol prevents aggregation. However, ongoing studies (K. Choe, K. Burkewitz, A. Deonarine, and K. Strange, unpublished observations) suggest that glycerol plays little or no role in slowing or preventing the aggregation of Q35::YFP, a finding that is consistent with in vitro studies of several proteins (42, 46, 53). We suggest that while organic osmolytes like glycerol play important roles as chemical chaperones in vivo, remodeling of the proteostasis network to cope with protein damage induced by hypertonic stress is of at least equal importance.
Our findings have important physiological and pathophysiological implications. The extent and speed of the protein damage induced by hypertonic stress in C. elegans are striking. In osmotically unstable natural environments, organisms are frequently exposed to multiple protein-damaging stressors. For example, intertidal organisms and organisms like C. elegans that live in vicinal water around soil and organic matter often experience hypertonic stress, elevated temperatures, and hypoxia concurrently. The ability of an organism's proteostasis network to cope with rapid, extensive, and diverse forms of protein damage thus certainly plays a central role in defining the boundaries of its ecological niche. The evolution of populations of organisms and species in unstable environments is also directly impacted by cellular mechanisms that manage protein damage (26, 41).
The renal medulla is a hostile environment where the concentrating mechanism subjects medullary cells to large fluctuations in extracellular osmolality. Medullary osmolality in humans can vary from ∼600 mosM during diuresis to over 1,200 mosM under conditions of maximal water reabsorption. In addition to osmotic stress, renal medullary cells are exposed to hypoxia due to low blood flow, oxidative stress, and high concentrations of urea (8, 11, 38). All of these stressors can cause macromolecular damage. Understanding the types of protein damage induced by hypertonic stress and how that damage is prevented and repaired is critical for understanding renal medullary physiology and pathophysiology, particularly during senescence where proteostasis mechanisms decline (29, 30).
Finally, as noted earlier, hypertonic solutions are used widely to treat diverse disorders including hemorrhagic shock (39), airway edema, and mucous plugging associated with diseases such as bronchiolitis and cystic fibrosis (17, 32) and intracranial hypertension (25). Detailed understanding of hypertonicity-induced protein damage and how that damage could be exacerbated by other underlying conditions such as infection and senescence is thus essential for avoiding injurious side effects of hypertonic solution administration.
GRANTS
This work was supported by National Institutes of Health Grant R01 DK61168 (to K. Strange).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Experiments described in this study were proposed and designed by K. Burkewitz, K. Choe, and K. Strange. K. Burkewitz performed the experimental studies. All of the authors participated in the analysis and interpretation of data, writing of the manuscript, and approval of the final version of the manuscript for publication.
Footnotes
This article is the topic of an Editorial Focus by T. Hoppe (24a).
REFERENCES
- 1.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science 319: 916–919, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Beranger F, Crozet C, Goldsborough A, Lehmann S. Trehalose impairs aggregation of PrPSc molecules and protects prion-infected cells against oxidative damage. Biochem Biophys Res Commun 374: 44–48, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bjork JK, Sistonen L. Regulation of the members of the mammalian heat shock factor family. FEBS J 277: 4126–4139, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Borkan SC, Gullans SR. Molecular chaperones in the kidney. Annu Rev Physiol 64: 503–527, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Brenner S. The genetics of Caenorhabditis elegans. Genetics 77: 71–94, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brignull h, Morley JF, Morimoto RI. The stress of misfolded proteins: C. elegans models for neurodegenerative disease and aging. Adv Exp Med Biol 594: 167–189, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Brown CR, Hong-Brown LQ, Welch WJ. Correcting temperature-sensitive protein folding defects. J Clin Invest 99: 1432–1444, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Campioni S, Mannini B, Zampagni M, Pensalfini A, Parrini C, Evangelisti E, Relini A, Stefani M, Dobson CM, Cecchi C, Chiti F. A causative link between the structure of aberrant protein oligomers and their toxicity. Nat Chem Biol 6: 140–147, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Choe KP, Strange K. Genome-wide RNAi screen and in vivo protein aggregation reporters identify degradation of damaged proteins as an essential hypertonic stress response. Am J Physiol Cell Physiol 295: C1488–C1498, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christoph K, Beck FX, Neuhofer W. Osmoadaptation of mammalian cells - an orchestrated network of protective genes. Curr Genomics 8: 209–218, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun W, Lesort M, Lee M, Johnson GV. Transient osmotic stress facilitates mutant huntingtin aggregation. Neuroreport 13: 2543–2546, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci 9: 759–767, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol 8: e1000450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz-Hernandez M, Moreno-Herrero F, Gomez-Ramos P, Moran MA, Ferrer I, Baro AM, Avila J, Hernandez F, Lucas JJ. Biochemical, ultrastructural, and reversibility studies on huntingtin filaments isolated from mouse and human brain. J Neurosci 24: 9361–9371, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drummond DA, Wilke CO. The evolutionary consequences of erroneous protein synthesis. Nat Rev Genet 10: 715–724, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubus JC, Ravilly S. Inhaled therapies in cystic fibrosis. Rev Mal Respir 25: 989–998, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Ellis RJ, Minton AP. Protein aggregation in crowded environments. Biol Chem 387: 485–497, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Epstein HF, Casey DL, Ortiz I. Myosin and paramyosin of Caenorhabditis elegans embryos assemble into nascent structures distinct from thick filaments and multi-filament assemblages. J Cell Biol 122: 845–858, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science 328: 321–326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh K, Dill K. Cellular proteomes have broad distributions of protein stability. Biophys J 99: 3996–4002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gidalevitz T, Ben Zvi A, Ho KH, Brignull H, Morimoto RI. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science 311: 1471–1474, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Goldschmidt L, Teng PK, Riek R, Eisenberg D. Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proc Natl Acad Sci USA 107: 3487–3492, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol 12: 842–846, 2005 [DOI] [PubMed] [Google Scholar]
- 24a.Hoppe T. Too salty for worms: hypertonic stress challenges proteostasis networks. Focus on “Hypertonic stress induces rapid and widespread protein damage in C. elegans.” Am J Physiol Cell Physiol (June 29, 2011). doi:10.1152/ajpcell.00206.2011. [DOI] [PubMed] [Google Scholar]
- 25.Jantzen JP. Prevention and treatment of intracranial hypertension. Best Pract Res Clin Anaesthesiol 21: 517–538, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Jarosz DF, Taipale M, Lindquist S. Protein homeostasis and the phenotypic manifestation of genetic diversity: principles and mechanisms. Annu Rev Genet 44: 189–216, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Katsuno M, Banno H, Suzuki K, Takeuchi Y, Kawashima M, Tanaka F, Adachi H, Sobue G. Molecular genetics and biomarkers of polyglutamine diseases. Curr Mol Med 8: 221–234, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300: 486–489, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Kikis EA, Gidalevitz T, Morimoto RI. Protein homeostasis in models of aging and age-related conformational disease. Adv Exp Med Biol 694: 138–159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res Rev 10: 205–215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraemer BC, Zhang B, Leverenz JB, Thomas JH, Trojanowski JQ, Schellenberg GD. Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proc Natl Acad Sci USA 100: 9980–9985, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuzik BA, Al Qadhi SA, Kent S, Flavin MP, Hopman W, Hotte S, Gander S. Nebulized hypertonic saline in the treatment of viral bronchiolitis in infants. J Pediatr 151: 266–270, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Lamitina ST, Morrison R, Moeckel GW, Strange K. Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am J Physiol Cell Physiol 286: C785–C791, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Lamitina ST, Strange K. Transcriptional targets of the DAF-16 insulin signaling pathway protect C. elegans from extreme hypertonic stress. Am J Physiol Cell Physiol 288: C467–C474, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Menzies FM, Huebener J, Renna M, Bonin M, Riess O, Rubinsztein DC. Autophagy induction reduces mutant ataxin-3 levels and toxicity in a mouse model of spinocerebellar ataxia type 3. Brain 133: 93–104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morley JF, Brignull h, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci USA 99: 10417–10422, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munishkina LA, Ahmad A, Fink AL, Uversky VN. Guiding protein aggregation with macromolecular crowding. Biochemistry 47: 8993–9006, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neuhofer W, Beck FX. Survival in hostile environments: strategies of renal medullary cells. Physiology 21: 171–180, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Papia G, Burrows LL, Sinnadurai S, Marshall JC, Tawadros PS, Kapus A, Rotstein OD. Hypertonic saline resuscitation from hemorrhagic shock does not impair the neutrophil response to intraabdominal infection. Surgery 144: 814–821, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell 40: 253–266, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Rutherford SL. Between genotype and phenotype: protein chaperones and evolvability. Nat Rev Genet 4: 263–274, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Ryu J, Kanapathipillai M, Lentzen G, Park CB. Inhibition of beta-amyloid peptide aggregation and neurotoxicity by alpha-d-mannosylglycerate, a natural extremolyte. Peptides 29: 578–584, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Satyal SH, Schmidt E, Kitagawa K, Sondheimer N, Lindquist S, Kramer JM, Morimoto RI. Polyglutamine aggregates alter protein folding homeostasis in Caenorhabditis elegans. Proc Natl Acad Sci USA 97: 5750–5755, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seki T, Abe-Seki N, Kikawada T, Takahashi H, Yamamoto K, Adachi N, Tanaka S, Hide I, Saito N, Sakai N. Effect of trehalose on the properties of mutant gammaPKC, which causes spinocerebellar ataxia type 14, in neuronal cell lines and cultured Purkinje cells. J Biol Chem 285: 33252–33264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B. The most infectious prion protein particles. Nature 257–261, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singer MA, Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell 1: 639–648, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Somero GN. Protons, osmolytes, and fitness of internal milieu for protein function. Am J Physiol Regul Integr Comp Physiol 251: R197–R213, 1986 [DOI] [PubMed] [Google Scholar]
- 48.Tanaka M, Machida Y, Niu S, Ikeda T, Jana NR, Doi H, Kurosawa M, Nekooki M, Nukina N. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med 10: 148–154, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Uversky VN. Neuropathology, biochemistry, and biophysics of α-synuclein aggregation. J Neurochem 103: 17–37, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Van Dyk TK, Gatenby AA, LaRossa RA. Demonstration by genetic suppression of interaction of GroE products with many proteins. Nature 342: 451–453, 1989 [DOI] [PubMed] [Google Scholar]
- 51.van Ham TJ, Thijssen KL, Breitling R, Hofstra RM, Plasterk RH, Nollen EA. C. elegans model identifies genetic modifiers of α-synuclein inclusion formation during aging. PLoS Genet 4: e1000027, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto A, Cremona ML, Rothman JE. Autophagy-mediated clearance of huntingtin aggregates triggered by the insulin-signaling pathway. J Cell Biol 172: 719–731, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang DS, Yip CM, Huang TH, Chakrabartty A, Fraser PE. Manipulating the amyloid-beta aggregation pathway with chemical chaperones. J Biol Chem 274: 32970–32974, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Yochem J, Sundaram M, Han M. Ras is required for a limited number of cell fates and not for general proliferation in Caenorhabditis elegans. Mol Cell Biol 17: 2716–2722, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou HX, Rivas G, Minton AP. Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu Rev Biophys 37: 375–397, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]