Abstract
Endothelial migration is a crucial aspect of a variety of physiologic and pathologic conditions including atherosclerosis and vascular repair. Reactive oxygen species (ROS) function as second messengers during endothelial migration. Multiple intracellular sources of ROS are regulated by cellular context, external stimulus, and the microenvironment. However, the predominant source of ROS during endothelial cell (EC) migration and the mechanisms by which ROS regulate cell migration are incompletely understood. In this study, we tested the hypothesis that mitochondria-derived ROS (mtROS) regulate EC migration. In cultured human umbilical vein endothelial cells, VEGF increased mitochondrial metabolism, promoted mtROS production, and induced cell migration. Either the targeted mitochondrial delivery of the antioxidant, vitamin E (Mito-Vit-E), or the depletion of mitochondrial DNA abrogated VEGF-mediated mtROS production. Overexpression of mitochondrial catalase also inhibited VEGF-induced mitochondrial metabolism, Rac activation, and cell migration. Furthermore, these interventions suppressed VEGF-stimulated EC migration and blocked Rac1 activation in endothelial cells. Constitutively active Rac1 reversed Mito-Vit-E-induced inhibition of EC migration. Mito-Vit-E also attenuated carotid artery reendothelialization in vivo. These results provide strong evidence that mtROS regulate EC migration through Rac-1.
Keywords: antioxidants, mitochondria, oxidant signaling, reactive oxygen species, endothelial cells
vascular endothelial growth factor (VEGF) is a critical regulator of endothelial cell (EC) migration. VEGF increases EC permeability, stimulates proliferation, and promotes migration through phosphatidylinositol 3-kinase and the small GTPase Rac-1 (40). Strategies that inhibit VEGF signaling are effective in the treatment of a wide spectrum of pathological conditions that involve EC migration, including atherosclerosis and tumor angiogenesis (34). New evidence suggests that intracellular reactive oxygen species (ROS) regulate cytokine or growth factor signaling (11, 26, 27). However, the predominant source and regulation of intracellular ROS production during VEGF-induced endothelial migration are unclear.
ROS are produced in the cytoplasmic compartment by a variety of sources including the NADPH oxidase (Nox) (6, 41), xanthine oxidase (37), and nitric oxide (43). Among these, Nox-derived ROS are recognized as important regulators of endothelial VEGF signaling (21, 46, 48). We previously demonstrated that Nox-derived ROS regulate the effects of TNF on EC cell-cell adhesion (22). However, gene silencing studies suggest that Nox-derived oxidants may not account for all the effects of VEGF on endothelial cells (1).
Emerging evidence suggests that mitochondrial ROS (mtROS) and related oxidative stress pathways are also important in the regulation of endothelial function (45, 52). Mitochondrial oxidants regulate VEGF receptor transactivation and redox-dependent downstream signaling (10), promote endothelial sprouting (12), and stimulate NF-κB and TNF-α-induced apoptosis (23). A recent study showed that VEGF stimulates mitochondrial gene expression in endothelial cells, thereby implicating VEGF in the regulation of mitochondrial biogenesis (50). However, the role of endothelial mtROS during VEGF-induced cell signaling is still unclear. In one study, mitochondrial H2O2 promoted endothelial cell migration and sprouting (12), whereas another study showed that suppressing mitochondrial ROS maintained the angiogenic capacity of endothelial cells (35). Given the known effects of oxidants on cell migration, we hypothesized that mitochondrial oxidants contribute to endothelial migration. In this study, we examined the effect of VEGF on endothelial mitochondrial ROS generation and determined the role of VEGF-induced mtROS on EC signaling during migration.
A major impediment of mitochondrial research has been the lack of reagents that specifically target mitochondrial function. The development of mitochondrial targeting technology has facilitated our ability to specifically regulate mitochondrial function. Mitochondria-targeted antioxidants have been shown to alter mitochondrial oxidative status in vitro and in vivo (2, 24). In animal experiments, pretreatment with mitochondria-targeted vitamin E (Mito-Vit-E) or coenzyme Q (Mito-Q) inhibited mitochondrial oxidative damage (25) and prevented cardiac dysfunction induced by ischemia-reperfusion (2). In cell culture, the mitochondria-targeted superoxide dismutase mimetic (Mito-CP) and peroxidase mimetic (Mito-peroxidase) inhibited apoptosis (15, 19). This mitochondrial targeting strategy has the potential for development of novel therapeutic approaches in diseases where mitochondrial function is abnormal.
In this study, we demonstrate that VEGF promotes endothelial migration at least in part by mitochondria-generated ROS. We further determined that mitochondria-targeted vitamin E prevents VEGF-induced mtROS production and endothelial migration in vitro and in vivo. These studies further expand our understanding of mitochondrial function and its contribution to vascular signaling and suggest that targeting mtROS is an effective strategy to modulate endothelial function.
MATERIALS AND METHODS
Cell culture.
Primary human umbilical vein endothelial cells (HUVEC, Clonetics, San Diego, CA) were cultured in endothelial cell basal medium (EBM-2, Clonetics) supplemented with an endothelial cell Bullet Kit (Clonetics). The HUVEC cell line (CRL 2922) was cultured with DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. Cells were serum starved overnight before experiments unless otherwise specified. Mito-Vit-E quantification experiments to confirm its selective mitochondrial accumulation were done with the HUVEC cell line. In all other experiments, primary HUVEC cells at passage 5–7 were used.
Adenovirus infection.
Adenovirus that express mitochondria-targeted catalase (AdmCat) and the control empty adenovirus (AdNull) were obtained from Dr. Andre Melendez through the University of Iowa Gene Transfer Vector Core. The infection of HUVEC with adenovirus was carried out as previously reported at the multiplicity of infection of 100 (3, 4). Briefly, the cells were plated and allowed to attach to the dishes overnight before the desired amount of viral particles was added. After 24 h, the media were changed to remove virus and the cells were cultured for another 24 h before each experiment. The efficiency of the infection and the mitochondrial accumulation of mitocatalase have been confirmed (3, 4).
Mito-Vit-E synthesis and quantification.
Mito-Vit-E was synthesized according to a previously published method with modification (39).The purity of our synthesized Mito-Vit-E was at least 90%. To confirm the selective mitochondrial accumulation of Mito-Vit-E, HUVEC cells at 90% confluency were exposed to vehicle or Mito-Vit-E (1 μM) for 6 h before they were washed and harvested by trypsinization. The mitochondria and cytoplasm fractions were prepared by deferential centrifugations (51). The levels of Mito-Vit-E in cytoplasm and mitochondria were determined by mass spectrometry by the use of Applied Biosystems 3200 QTRAP coupled to a Shimadzu Prominence LC (LC/MS/MS). Standard curves were prepared using blank cell homogenate or mitochondria spiked with varying concentrations of Mito-Vit-E dissolved in DMSO. The data were calculated to the mitochondria/cytoplasm ratio of Mito-Vit-E concentration (mass/volume) which indicates relative selective accumulation of Mito-Vit-E in mitochondria.
Immunoblots.
Protein samples were subjected to 4–12% gradient SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% nonfat milk-Tris-buffered saline-Tween at room temperature for 1 h and subsequently probed with primary antibodies against DNA polymerase gamma (POLG; Santa Cruz Biotechnology, Santa Cruz, CA), cytochrome-c oxidase (COX) II (Molecular Probes, Carlsbad, CA), phospho-Akt(Ser-473), phospho-p38MAP(Thr-180/Tyr-182), phospho- p21-activated kinase (PAK1 Thr-423, PAK2 Thr-402), phospho-ERK1/2(T202/Y204) (Cell Signaling Technology), and GAPDH (Millipore, Temecula, CA). The membranes were then rinsed and incubated with corresponding horseradish peroxidase (HRP)-conjugated secondary antibody (Bio-Rad, Hercules, CA). Antibody dilutions and incubation time were according to manufacturer's instructions. Signals were detected by using ChemiGlow West (Alpha Innotech, San Leandro, CA).
POLG gene knockdown.
The sequences of small interfering RNA (siRNA) are as follows: POLG: 5′-GGAUGGUAAUAGCUGUAAUTT-3′ and 5′-AUUACAGCUAUUACCAUCCTT-3′; scrambled: 5′-UUCUCCGAACGUGUCACGUTT-3′; 5′-ACGUGACACGUUCGGAGAATT-3′ (Shanghai GenePharma, Shanghai, China). Transfection was performed using DharmaFECT 1 transfection reagents (Thermo Fisher Scientific, Lafayette, CO) according to the manufacturer's instructions. Briefly, HUVEC were cultured in six-well plates to ∼80% confluence. In total, 200 pmol siRNA was diluted in 200 μl of Opti-MEM I Reduced Serum Medium, and 4 μl of DharmaFECT 1 was diluted in 200 μl of the same medium. After 5 min, the diluted siRNA and DharmaFECT 1 were mixed and left at room temperature for 20 min before the mixture was added to the well with cells and 1.6 ml cultural medium. After 12 h, the medium was replaced with fresh medium without the siRNA and the cells were used 3 or 7 days after the transfection. The cells were replated before the experiments or when they are confluent. POLG is essential for the faithful maintenance of mtDNA (42), and POLG knockdown with siRNA has been used in the study of mtDNA replication during differentiation of murine ES cells (18).
Cytochrome-c oxidase activity.
Mitochondrial fractions were prepared by differential centrifugation according to a previously described procedure (51). Cytochrome-c oxidase activity was measured using a commercial assay kit according to the manufacturer's instructions (Sigma-Aldrich, St. Louis, MO). Cytochrome-c oxidase activities were normalized by the amount of mitochondrial protein per reaction.
Detection of mitochondrial ROS.
Mitochondrial superoxide was detected by labeling the cells with MitoSox Red (Invitrogen, Carlsbad, CA). HUVEC were loaded with MitoSox Red (28) (5 μM, 20 min) and stimulated with VEGF (50 ng/ml, 5 min) or vehicle before flow cytometry.
To detect mitochondrial H2O2, HUVEC were transiently transfected with a plasmid, pHyPer-dMito, which encodes a derivative of hydrogen peroxide-specific sensor protein Hyper (9) tagged with mitochondrial signal peptide sequence (Evrogen, Moscow, Russia). Cells were grown in fibronectin-coated glass-bottom microwell dishes (Ashland, MA) and the transfection was done with FuGENE 6 (Roche Applied Science). Cells were serum starved overnight before mitochondrial H2O2 levels were determined by sequential acquisition of images using 405/40 and 492/18 nm band-pass excitation filters with a 530/35 nm band-pass emission filter. Images were acquired using the Nikon ECLIPSE TE2000-U (Melville, NY) with a ×63 1.2 objective before and after the cells were exposed to VEGF (50 ng/ml). Ratio images of 492/405 nm absorbance were analyzed with NIS-Elements AR 3.0 to determine mitochondrial H2O2 levels. To show the specificity of the effect of VEGF on ROS production, we preincubated VEGF with VEGF-Trap before VEGF was used to stimulate HUVEC. VEGF-Trap is a synthetic protein that specifically binds to VEGF, rendering it unable to bind to its receptor.
Detection of intracellular ROS.
Total intracellular ROS was detected by loading cells with the fluorophore 2′,7′-dichloro-dihydrofluorescein (H2DCF) (Molecular Probes, Eugene, OR). Cells were labeled with 20 μM DCF for 20 min and visualized using fluorescence microscopy before and after they were exposed to VEGF (50 ng/ml) for 15 min (Nikon Instruments, Melville, NY). The fluorescence intensity was quantified with the NIS-Elements software. In some experiments, cells were pretreated with Mito-Vit-E (1 μM) or vehicle for 6 h.
To determine intracellular H2O2 levels, HUVEC were transiently transfected with plasmid pHyper-cyto vector, which is a mammalian expression vector encoding a fluorescent H2O2 sensor Hyper. Intracellular H2O2 levels were determined at 37°C by sequential acquisition of images using 405/40 and 492/18 nm band-pass excitation filters with a 530/35 nm band-pass emission filter. Images were acquired using the Nikon ECLIPSE TE2000-U with a ×63 1.2 objective before and after the cells were exposed to VEGF (50 ng/ml). Ratio images of 492/405 nm absorbance were analyzed with NIS-Elements AR 3.0 to determine intracellular H2O2 levels. In some experiments, VEGF was preincubated with VEGF-Trap before VEGF was used to stimulate the cells.
Cell migration.
We first used the electric cell-substrate impedance sensing (ECIS) system (Applied Biophysics, Troy, NY) to conduct cell migration assay as reported previously (8, 30). HUVEC cells were cultured in 8W1E ECIS arrays (Applied Biophysics), in which each well for cell culture contains a small working electrode (5 × 10−4 cm2) and a larger (0.15 cm2) counter electrode. Because of the difference in surface area (300-fold), the total impedance of the system is determined mainly by the impedance of the small working electrode. Equivalent numbers of cells were seeded on fibronectin-coated gold electrode arrays and permitted to adhere overnight to form the cell monolayer. The cells were treated with VEGF (50 ng/ml) or vehicle for 10 min before a wounding was performed by electroporation using voltage pulses of 6 V and 60 kHz for 60 s, which causes death and detachment of cells on the working electrode. Cells surrounding the working electrode that had not been submitted to the wounding then migrate inward to replace the killed cells. After the wounding, cell migration was assessed by continuous measurement of transmonolayer electrical resistance for 24 h. The cell migration was expressed as change of resistance from immediately after to 6 h after the wounding.
As another way to study endothelial cell migration, HUVEC were grown to near confluence, pretreated with vehicle, Vit-E (1 μM), or Mito-Vit-E (1 μM) before a defined region of cells was removed with a razor blade as previously reported (7). Cells were then treated with vehicle or VEGF (50 ng/ml) for an additional 36 h before the number of cells that had migrated past the wound edge was counted in low-power fields. The data represent the numbers of all cells that migrated past 2-mm length of the wound edge. Each image represents 400 μm of the length of the wound edge, which is one fifth of the low-power field in which the cells were counted.
Cell proliferation assay.
Cells were plated in 96-well plates (5,000 cells/well). After overnight culture, the cells were pretreated with vehicle, Vit-E (1 μM), or Mito-Vit-E (1 μM) for 6 h. The cells were then treated with vehicle or VEGF (50 ng/ml) for an additional 48 h before the MTT assay was performed using Vybrant MTT Cell Proliferation Assay Kit (Invitrogen) according to the manufacturer's protocol. The data are expressed as absorbance at 570 nM.
Rac1 activity.
Rac1 activation was measured using G-LISA Rac Activation Assay kit (Cytoskeleton, Denver, CO) according to the manufacturer's protocol. Briefly, 70% to 80% confluent HUVEC cells were serum starved overnight before they were treated with vehicle or VEGF (50 ng/ml) for 5 min. Cell lysates were then prepared and incubated on Rac-GTP affinity plates for 30 min at 4°C. After antibody detection, Rac1 activation was measured by luminometry.
Carotid artery reendothelialization.
Carotid artery reendothelialization was studied in male C57BL/6 mice (12–16 wk of age, Charles River Laboratories, Wilmington, MA) using previously described methods (36). In brief, perivascular electric injury was induced using a bipolar microregulator to produce an electric current that denuded the carotid artery endothelium. Using Evans blue dye uptake, the area of initial denudation was evaluated on the day of injury, and the remaining area of denudation was determined 4 days postinjury to assess reendothelialization in a blinded manner by image analysis using Scion Image (free software from National Institutes of Health). Endothelial denudation and postinjury recovery in this model have been confirmed by immunohistochemistry for von Willebrand factor (vWF; 36). Study groups included Mito-Vit-E-treated and control vehicle-treated mice. Mito-Vit-E (or vehicle) was given via oral gavage at the dosage of 50 mg·kg−1·day−1 for 3 days before perivascular electric injury, and the treatment was continued until the animals were euthanized. The care and use of all study animals was approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
Immunohistochemistry of von Willebrand factor.
The carotid arteries were harvested on day 1 after sham operation or electric injury. The arteries were formalin fixed, paraffin embedded, and sectioned at 5-μm in thickness. Tissue sections on glass slides were deparaffinized, rehydrated, and placed in PBS with 0.05% Tween before immunostaining. The tissue sections were incubated with a rabbit anti-vWF antibody form Dako (Glostrup, Denmark), followed by an anti-rabbit IgG biotinylated secondary antibody from Vector Laboratories (Burlingame, CA). Tissue sections were then incubated in a dilution of streptavidin-HRP, followed by 3′3′-diaminobenzidine. The slides were counterstained with hematoxylin, dehydrated, and mounted with coverslips.
Statistical analysis.
All data are expressed as means ± SE of at least three independent experiments. Student's t-tests or analysis of variance (ANOVA) were used to assess the statistical significance of difference between groups of in vitro studies unless otherwise specified. Animal studies were analyzed by Mann-Whitney's rank sum test using 6–8 mice/group. P < 0.05 was considered statistically significant.
RESULTS
VEGF increases mitochondrial metabolism and mitochondrial ROS production.
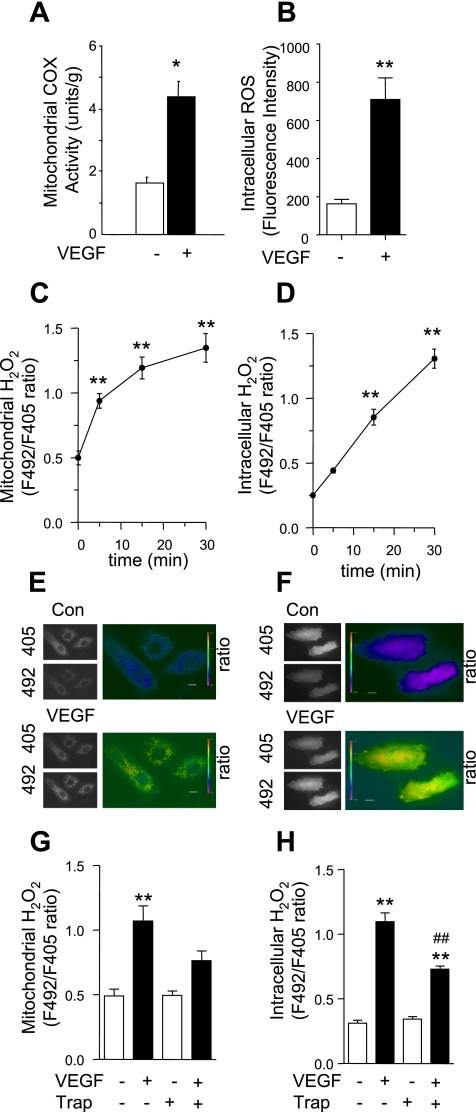
To examine the effect of VEGF on mitochondrial function in endothelial cells, we exposed HUVEC to VEGF (50 ng/ml, 5 min) or vehicle and determined mitochondrial metabolism by the measurement of mitochondrial cytochrome-c oxidase activity. As shown in Fig. 1A, COX activity in VEGF-stimulated cells was more than twofold the level in vehicle-treated cells.
Fig. 1.
VEGF increased mitochondria cytochrome-c activity, as well as mitochondrial and intracellular H2O2 production. A: human umbilical vein endothelial cells (HUVEC) were stimulated with vehicle or VEGF (50 ng/ml) for 5 min, and mitochondrial fractions were isolated and cytochrome-c oxidase (COX) activity was determined. B: HUVEC were loaded with dichlorofluorescein (DCF), and fluorescence intensity was determined after cells were stimulated with vehicle or VEGF (50 ng/ml) for 15 min. ROS, reactive oxygen species. C–H: HUVEC were transfected with pHyPer-dMito (C, E, and G) or pHyPer-cyto (D, F, and H), and mitochondrial (C, E, and G) and intracellular (D, F, and H) H2O2 levels were determined by fluorescence ratio (F492/F405 nm) imaging before and after VEGF (50 ng/ml) treatment. For the inhibition of VEGF effects, VEGF was preincubated with VEGF-Trap (300 ng/ml) for 30 min before VEGF was used to stimulate the cells (G and H). Data shown are means ± SE of at least 3 independent experiments (A–D, G, and H) and representative images at 15 (E) or 30 (F) min after VEGF treatment. Bars, 20 μm. Con, control. *P < 0.05, **P < 0.01 vs. vehicle control or before VEGF stimulation; ##P < 0.01 vs. without VEGF-Trap preincubation.
To characterize the mitochondrial oxidants produced in response to VEGF stimulation, we examined the levels of mitochondrial superoxide and H2O2 by ROS species-specific fluorescence labeling of the cells. To measure mitochondrial superoxide, HUVEC were prelabeled with MitoSox Red followed by VEGF exposure and subsequent flow cytometry. No significant difference in mitochondrial superoxide was detected in VEGF-exposed and unexposed cells (data not shown). Mitochondrial H2O2 production in HUVEC was measured by transient transfection with pHyPer-dMito plasmid and analyzed by ratio imaging before and after VEGF stimulation (Fig. 1, C and E). VEGF significantly increased mitochondrial H2O2 levels within 5 min of exposure, an effect that lasted up to 30 min postexposure (Fig. 1, C and E). To confirm specificity of this observation, we preincubated VEGF with VEGF-Trap. Preincubation of VEGF with VEGF-Trap reduced VEGF-induced mitochondrial H2O2 production (Fig. 1G).
VEGF increases intracellular ROS.
To determine the effect of VEGF on total intracellular ROS production, HUVEC were prelabeled with DCF-DA and exposed to VEGF for 15 min. Intracellular ROS levels were determined by analyzing the fluorescence intensity before and after VEGF stimulation. VEGF significantly increased overall intracellular ROS production (Fig. 1B).
To measure intracellular H2O2, cells were transiently transfected with pHyPer-cyto plasmid, and cytoplasmic H2O2 levels were monitored by ratio imaging before and after VEGF stimulation. VEGF significantly increased cytoplasmic H2O2 levels 15 min after stimulation. Intracellular H2O2 levels remained high for 30 min after VEGF exposure (Fig. 1, D and F). Preincubation of VEGF with VEGF-Trap diminished the VEGF-induced increase in intracellular H2O2 (Fig. 1H).
Relative contribution of mitochondrial ROS to intracellular ROS.
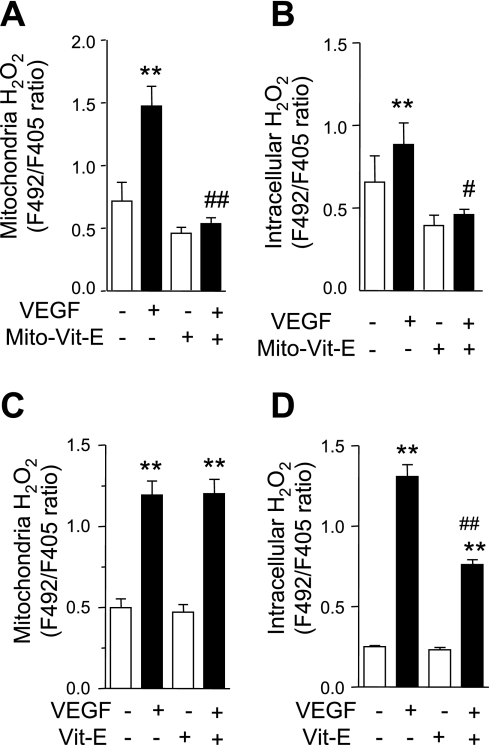
Given the temporal relationship of mitochondrial and intracellular H2O2, we chose to examine the relative contribution of VEGF-induced mitochondrial H2O2 to overall intracellular H2O2 production. We suppressed mitochondrial H2O2 production using two strategies as follows: 1) by delivery of Mito-Vit-E and 2) by mitochondrial DNA depletion. Specifically, we used Mito-Vit-E to inhibit H2O2. Secondly, we inhibited POLG using siRNA against POLG to deplete mitochondrial DNA. POLG is selectively encoded in mitochondria, and POLG disruption leads to mitochondrial DNA depletion and improper mitochondrial respiratory function (13). Selective accumulation of Mito-Vit-E within mitochondria was confirmed by mass spectrometry after exposing HUVEC to Mito-Vit-E (1 μM) for 6 h. Mitochondrial to cytoplasmic Mito-Vit-E ratio was calculated as 58.24 ± 6.58.
As expected, Mito-Vit-E completely inhibited VEGF-induced mitochondrial H2O2 production (Fig. 2A), and intracellular H2O2 production (Fig. 2B). Mito-Vit-E also caused a modest decrease of baseline mitochondrial H2O2; however, the difference was not statistically significant. In contrast, nontargeted Vit-E (1 μM, 6 h) only partially inhibited VEGF-induced increase of intracellular H2O2 (Fig. 2D), and it had no effect on VEGF-induce mitochondrial H2O2 production (Fig. 2C).
Fig. 2.
Mitochondria-targeted vitamin E (Mito-Vit-E) inhibits VEGF-induced mitochondrial and intracellular H2O2. HUVEC were transfected with pHyPer-dMito (A and C) or pHyPer-cyto (B and D). Cells were pretreated with Mito-Vit-E (1 μM) (A and B), Vit-E (1 μM) (C and D), or vehicle for 6 h before mitochondrial and intracellular H2O2 was determined by fluorescence ratio imaging (F492/F405 nm) before and after VEGF (50 ng/ml) stimulation for 15 (A and C) or 30 (B and D) min. Data are expressed as means ± SE of at least 3 independent experiments. **P < 0.01 vs. before VEGF stimulation. #P < 0.05, ##P < 0.01 vs. VEGF-stimulated cells without Mito-Vit-E or Vit-E pretreatment.
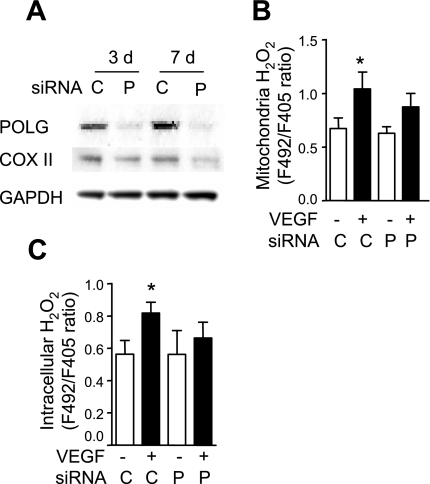
Similarly, POLG knockdown partially suppressed VEGF induced mitochondrial H2O2 production (Fig. 3B), and it decreased VEGF-induced intracellular H2O2 (Fig. 3C). Knockdown efficiency was confirmed by immunoblotting for POLG and COX II (Fig. 3A).
Fig. 3.
DNA polymerase gamma (POLG) knockdown decreases VEGF-induced mitochondrial and intracellular H2O2 production. A: at 3 and 7 days (d) after HUVEC cells were transfected with scrambled control small interfering (si)RNA (C) or POLG siRNA (P), the expression of POLG and COX II was analyzed using Western blot. B and C: HUVEC cells were transfected with scrambled control or POLG siRNA on day 0, and they were transfected with pHyPer-dMito (B) or pHyPer-cyto (C) on day 5. On day 7, mitochondrial and intracellular H2O2 levels were determined by fluorescence ratio imaging before and after VEGF stimulation (50 ng/ml) for 15 (B) or 30 (C) min. Data are expressed as means ± SE of at least 3 independent experiments. *P < 0.05 vs. before VEGF stimulation.
Mitochondrial oxidants regulate endothelial migration.
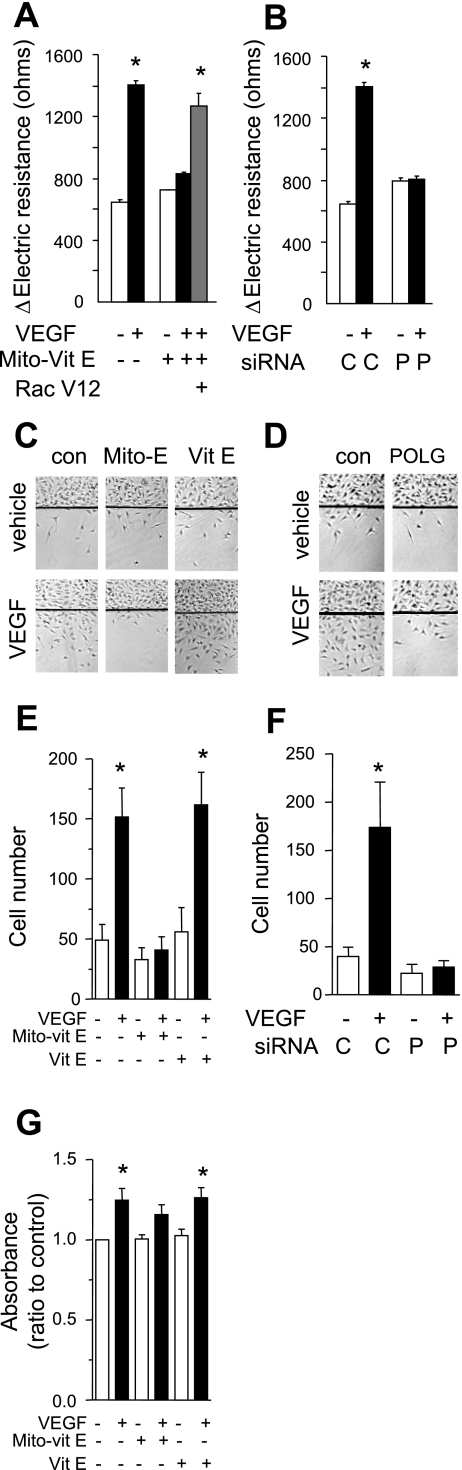
VEGF is recognized as a potent inducer of EC migration. Therefore we chose to examine the effect of mtROS on EC migration. We examined the effects of Mito-Vit-E and POLG knockdown on in vitro cell migration using migration assays. Cells were pretreated with Mito-Vit-E or transfected with POLG siRNA. After exposure to VEGF or vehicle, cell migration was measured by the ECIS migration assay as detailed in materials and methods. VEGF significantly increased HUVEC migration, consistent with prior studies (38). Mito-Vit-E pretreatment and mtDNA depletion with POLG knockdown prevented VEGF-induced cell migration (Fig. 4, A and B).
Fig. 4.
Mitochondrial ROS are involved in VEGF-induced endothelial migration. A: HUVEC infected or uninfected with adenovirus expressing mutationally activated Rac1 [Rac(V12)] were pretreated with vehicle or Mito-Vit-E (1 μM) for 6 h before they were exposed to vehicle or VEGF (50 ng/ml, 10 min). Cell migration was measured for 6 h by determining the change of transmonolayer electrical resistance after a wounding. B: HUVEC with (P) or without (C) POLG gene knockdown with siRNA were stimulated with vehicle or VEGF (50 ng/ml, 10 min) before cell migration was measured for 6 h by transmonolayer electrical resistance after a wounding. C and E: HUVEC were pretreated with vehicle, Vit-E (1 μM), or Mito-Vit-E (1 μM) for 6 h before a defined area of the cells was removed with a razor blade. Cells were then treated with vehicle or VEGF (50 ng/ml) for 36 h, and the number of cells that migrated past the wound edge was counted. D and F: HUVEC with or without POLG knockdown were treated with vehicle or VEGF, and migration assay was done in a similar way as in C and E. G: HUVEC were pretreated with vehicle, Vit-E (1 μM), or Mito-Vit-E (1 μM) for 6 h before they were stimulated with vehicle or VEGF (50 ng/ml) for 48 h and the MTT assay was performed. Data are expressed as means ± SE of at least 3 independent experiments (A, B, E, F, and G) or representative images (C and D). *P < 0.05 vs. without VEGF stimulation.
In separate experiments, HUVEC were grown to near confluence, pretreated with vehicle, Vit-E (1 μM), or Mito-Vit-E (1 μM) before a defined region of the EC monolayer was removed with a razor blade as previously reported (7). Cells were then exposed to vehicle or VEGF (50 ng/ml). After 36 h the cells that migrated past the wounded edge were counted manually. Again, VEGF increased cell migration and Mito-Vit-E significantly inhibited VEGF-induced cell migration. In contrast, nontargeted Vit-E had no effect on VEGF-induced cell migration (Fig. 4, C and E). POLG knockdown also completely prevented VEGF-induced cell migration (Fig. 4, D and F).
To determine whether Mito-Vit-E affects cell proliferation, HUVEC were pretreated with vehicle, Vit-E (1 μM), or Mito-Vit-E (1 μM) for 6 h, then they were stimulated with VEGF for 48 h before MTT assay was performed. As expected, VEGF stimulation significantly enhanced cell proliferation. Mito-Vit-E partially reduced VEGF-induced cell proliferation; however, this effect was not statistically significant. Nontargeted Vit-E did not cause any change in VEGF-stimulated proliferation (Fig. 4G).
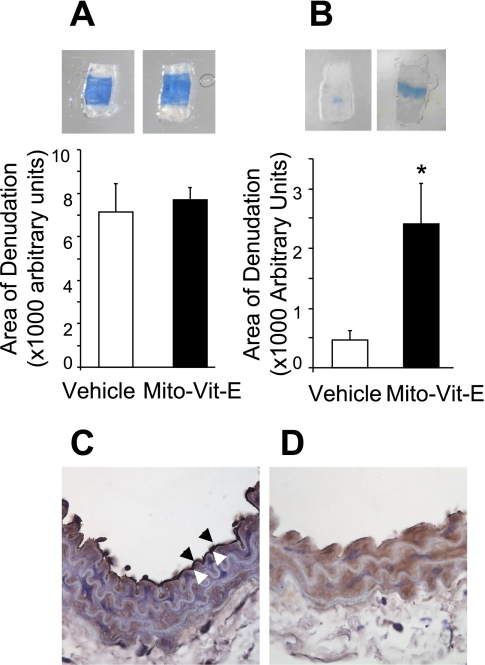
The role of mtROS in endothelial cell migration was further evaluated in vivo using a mouse carotid artery reendothelialization model. Mice were pretreated with Mito-Vit-E or vehicle for 3 days before subjected to perivascular electric injury to the common carotid artery, as previously reported by our group (36). The initial area of denudation indicated by the area of Evans blue dye incorporation was similar in the two groups, indicating that Mito-Vit-E treatment did not affect the area of injury at baseline (Fig. 5A). However, examination of the excised arteries on postinjury day 4 revealed that the area of remaining injury was fivefold greater in the Mit-Vit-E-treated mice, compared with the vehicle-treated group (Fig. 5B), confirming that Mito-Vit-E caused a marked attenuation in reendothelialization in vivo. Denudation of the endothelium by electric injury was confirmed by vWF immunostaining of carotid arteries harvested on day 1 after sham operation or electric injury (Fig. 5, C and D). The intact endothelium with positive vWF staining was shown in sham normal carotid arteries (arrow, Fig. 5C) but not in the electrically injured carotid artery (Fig. 5D).
Fig. 5.
Mito-Vit-E inhibited endothelial cell migration in vivo. C57BL/6 mice received Mito-Vit-E by oral gavage for 3 days before perivascular carotid electric injury (day 0), and the Mito-Vit-E treatment was continued until the end point of the experiment. Animals were euthanized at day 1 (A) or day 4 (B), and the area of denudation was quantified. C and D: immunostaining of von Willebrand Factor (vWF) was carried out on carotid arteries on day 1 after sham operation (C) or electric injury (D). Arrowheads indicate endothelium positively stained for vWF. Results from 6–8 mice/group were analyzed by Mann-Whitney's rank sum test. *P < 0.05.
VEGF-induced Rac1 activation is regulated by mitochondrial ROS.
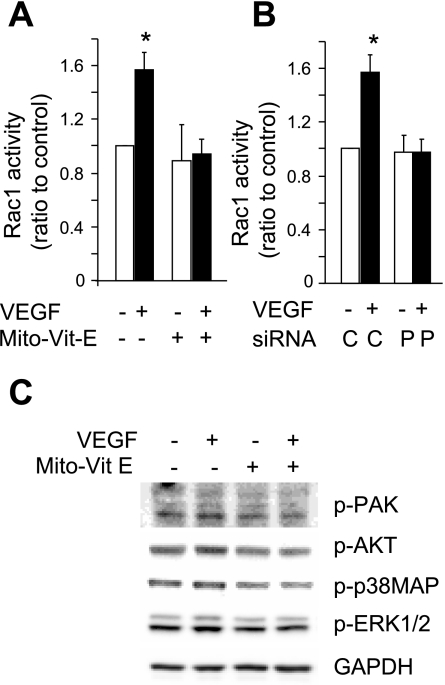
The Rho-related small GTPase Rac1 plays a central role in endothelial cell migration (44). We therefore examined the effect of mtROS on VEGF-induced Rac1 activation. VEGF significantly increased Rac1 activity compared with vehicle controls. However, this response was abolished by pretreatment of the cells with Mito-Vit-E (Fig. 6A). Similarly, mtDNA depletion by POLG knockdown also prevented VEGF-induced Rac1 activation (Fig. 6B). Infection of the cells with adenovirus expressing mutationally activated Rac1 [Rac(V12)] abolished Mito-Vit-E caused inhibition of VEGF-induced increase in migration (Fig. 4A). These data suggest that mtROS signal upstream of Rac1 during VEGF-induced EC migration.
Fig. 6.
Inhibition of mitochondrial ROS production prevents VEGF-induced Rac1 activation. A: HUVEC pretreated with vehicle or Mito-Vit-E (1 μM) for 6 h were stimulated with vehicle or VEGF (50 ng/ml) for 5 min, and Rac1 activity was determined. B: at 7 days after transfection with control (C) or POLG siRNA (P), HUVEC cells were stimulated with vehicle or VEGF (50 ng/ml) for 5 min and Rac1 activity was determined. C: HUVEC were pretreated with vehicle or Mito-Vit-E (1 μM) for 6 h and then stimulated with VEGF for 60 min, and whole cell lysates were collected for Western blot analysis of phosphorylated PAK, Akt, p38MAP, and ERK1/2. *P < 0.05 vs. vehicle.
Active Rac is known to bind to and phosphorylate PAK, which in turn phosphorylates and activate downstream signaling partners including Akt and p38MAP, both of which regulate cell survival and migration. Therefore we examined the effect of Mito-Vit-E on phosphorylation of PAK, Akt, and p38MAP after VEGF stimulation. VEGF increased phosphorylation of PAK, Akt, and p38MAP. Mito-Vit-E pretreatment inhibited VEGF-induced phosphorylation of PAK, Akt, and p38MAP (Fig. 6C). Mito-Vit-E also inhibited VEGF-induced phosphorylation of ERK1/2 (Fig. 6C).
Effect of mitochondria-targeted catalase on endothelial migration.
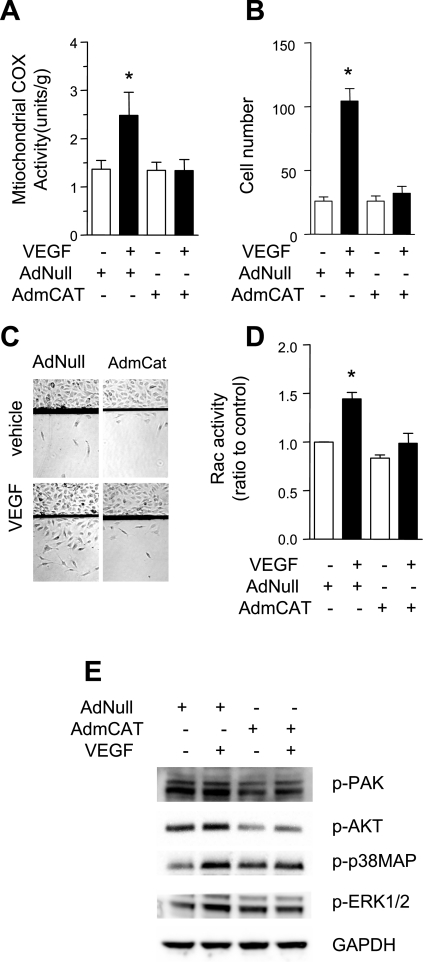
To further confirm the role of mitochondrial H2O2 on cell migration, we overexpressed mitochondria-targeted catalase (AdmCat) in HUVEC using adenoviral vectors. Cells infected with empty virus (AdNull) served as control. As expected, AdmCat significantly inhibited VEGF-induced activation of mitochondrial cytochrome-c oxidase activity (Fig. 7A), and VEGF-stimulated endothelial cell migration (Fig. 7, B and C). Overexpression of catalase in mitochondria also inhibited VEGF-induced activation of Rac activity (Fig. 7D) and phosphorylation of PAK, AKT, p38MAP, and ERK (Fig. 7E). These effects of mitochondria-targeted catalase are similar to those of Mito-Vit E treatment.
Fig. 7.
Mitochondrially targeted catalase inhibited endothelial migration. HUVEC were infected with AdNull or AdmCat 48 h before experiments. A: HUVEC were stimulated with vehicle or VEGF (50 ng/ml) for 5 min, mitochondrial fractions were isolated, and cytochrome-c oxidase activity was determined. B and C: a defined area of the cells was removed. Cells were then treated with vehicle of VEGF (50 ng/ml) for 36 h and the number of cells that migrated past the wound edge was counted. D: HUVEC were stimulated with VEGF (50 ng/ml) for 5 min and Rac1 activity was determined. E: cells were stimulated with VEGF (50 ng/ml) for 60 min before whole cell lysates were collected for Western blot analysis of phosphorylated PAK, Akt, ERK1/2, and p38MAP. *P < 0.05 vs. vehicle.
DISCUSSION
In this study, we report that VEGF increases mitochondrial oxidant production in endothelial cells. Inhibiting mitochondrial ROS by targeted delivery of mitochondria-targeted vitamin E to mitochondria, overexpression of mitochondria-targeted catalase, or depletion of mtDNA prevented VEGF-induced EC migration. In addition to that, Mito-Vit-E and mtDNA depletion inhibited VEGF-induced mitochondrial and intracellular ROS production. The effect on migration appears to involve the small GTPase Rac1, because inhibition of mitochondrial oxidants resulted in Rac1 inactivation and Mito-Vit-E could not inhibit VEGF-induced migration in cells expressing constitutively active Rac1. These observations provide new evidence that mitochondria-derived oxidants are important regulators of VEGF-induced endothelial migration.
Endothelial migration is critical in a variety of physiologic and pathologic processes including wound healing, tumor angiogenesis, atherosclerosis, and vascular repair. Therefore, an understanding of the mechanisms of endothelial migration has important implications for these diseases. Oxidants are recognized as important regulators of endothelial cell migration and adhesion (21). Intracellular ROS are generated by a variety of intracellular pathways including nitric oxide, NADPH oxidase, xanthine oxidase, as well as from mitochondrial respiration (20). However, the complexity of this pathway has become apparent because Nox-derived ROS do not account for all the effects of cytokines and growth factors on endothelial signaling (1). Mitochondria were previously considered to represent organelles with limited function. However, recent studies suggest that mitochondria may be important for the localized delivery of metabolic energy in subcellular locations during periods of cellular stress or high cellular metabolic demand. Although mtROS were considered to be inactive byproducts of cellular respiration, recent studies suggest that mtROS may function as intracellular signaling mediators depending on external stimuli, cell type, or cellular context (52). This paradigm seems logical because mitochondria, by their intracellular mobility, can deliver low levels of oxidants to specific subcellular locations during periods of increased demand such as migration or adhesion. Despite this, our understanding of the precise role of mtROS in endothelial cell signaling is scant. The studies summarized in this report suggest that mtROS are crucial during endothelial migration in response to VEGF.
Little is known about the direct activators of mtROS production, their specific sites of activation, mechanisms of regulation, and targets of oxidation (27). Interestingly, new studies using gene expression analysis show that VEGF stimulation increases mitochondrial biogenesis to meet higher cellular energy demands (50). Consistent with this observation, we detected VEGF-induced increases in mitochondrial metabolism and mtROS production. It is possible that this represents an adaptive mechanism by which cellular energy production can be increased to meet metabolic demands during periods of cellular stress. We have demonstrated in this study that delivery of the antioxidant vitamin E specifically to mitochondria diminished VEGF-induced total intracellular ROS, suggesting that mitochondria contribute a measurable and significant component of total intracellular ROS during VEGF stimulation. This does not preclude the possibility that the NADPH oxidase is an important ROS contributor in this context, but suggests that mitochondrial and Nox pathways may be complementary. Some studies suggest that VEGF may facilitate cross talk between mitochondria and Nox during periods of increased ROS production (14, 49). However, this phenomenon is poorly defined and requires further study. Nox may function downstream of mitochondria because depletion of mtDNA or inhibition by the mitochondrial respiratory complex inhibitor rotenone or antimycin significantly reduced Nox expression in tumor cells (14). On the other hand, observations from an animal model of nitroglycerin-triggered vascular dysfunction implied that mtROS and Nox-derived ROS may function differentially, because mtROS was important for the initial development of nitrite tolerance whereas Nox-derived ROS were involved in the subsequent endothelial dysfunction (49). Continued investigation is needed of the relationship between mitochondria and Nox in endothelial cells.
Rac1 is an important regulator of cytoskeletal dynamics (5), and its activation is required for VEGF-induced endothelial permeability (29, 31). Rac1 is not only activated by Nox-derived ROS (16, 33), but also contributes to Nox-related ROS production (47, 53). Gene knockout of mitochondrial membrane protein prohibitin 1 (PHB1) or hypoxia increased mtROS production and Rac1 activation, suggesting an association between mtROS and Rac1 signal (17, 35). However, it is not known whether Rac1 responds directly to signals through mtROS. In this report, we show that Mito-Vit-E suppressed VEGF-induced Rac1 activity, suggesting that the Rac1 pathway is regulated, at least in part, by VEGF-induced mtROS production. To our knowledge this is the first report to provide direct evidence showing that mitochondrial oxidants regulate Rac1 activity during VEGF stimulation of endothelial cells. It would be interesting to further determine whether Rac1 activation is regulated through mitochondrial specific oxidation and whether other mitochondrial signaling molecules are involved in the regulation of Rac1 by mtROS.
The relationship between mitochondrial activation and endothelial cell migration has been suggested by several previous studies (12, 32). Histochemical analysis of corneal endothelial cells in a wound healing model showed a significant enhancement of mitochondrial activity within migrating cells (32), whereas overexpression of mitochondria-specific antioxidant enzymes promoted angiogenesis (12). However, the mechanism that links mitochondrial activation and cellular migration is unknown. In the current study, we provide direct evidence to support the functional significance of mtROS in the regulation of endothelial cell migration and further implicate small GTPase Rac1 as an important mediator in this process. In this report, we describe different approaches to specifically target mitochondria, including the use of a mitochondria-targeted antioxidant and mtDNA depletion, both of which were shown to inhibit mtROS production and downstream signaling.
Our results reveal a direct functional role of mitochondria in the regulation of overall intracellular ROS production, Rac1 activation, and endothelial cell migration. We believe that these findings will facilitate future studies using specific mitochondrial targeting interventions to better understand the functional significance of mitochondrial signaling in vascular diseases. In addition, the validation of mitochondria-targeted technology will potentially allow the future design of therapeutic interventions for diseases of endothelial dysfunction.
GRANTS
This study was supported by research funding from National Institutes of Health/National Institute of General Medical Sciences Grant R01-GM067674-01 (to F. E. Nwariaku).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Saiqa Khan for help with manuscript preparation and proofreading, and John M. Shelton and Kevin Hancock for assistance in immunostaining of vWF. We also thank Dr. Andre Melendez and University of Iowa Gene Transfer Vector Core for kindly providing us with AdNull and AdmCat adenovirus.
Present address of D. E. Frantz: Department of Chemistry, The University of Texas at San Antonio, San Antonio, TX 78249-0698.
REFERENCES
- 1. Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem 282: 35373–35385, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J 19: 1088–1095, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Ahmad IM, Aykin-Burns N, Sim JE, Walsh SA, Higashikubo R, Buettner GR, Venkataraman S, Mackey MA, Flanagan SW, Oberley LW, Spitz DR. Mitochondrial O2*− and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J Biol Chem 280: 4254–4263, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bai J, Rodriguez AM, Melendez JA, Cederbaum AI. Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J Biol Chem 274: 26217–26224, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Burridge K, Wennerberg K. Rho and Rac take center stage. Cell 116: 167–179, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 8: 691–728, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Nonnuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest 120: 2319–2330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan CM, Fang JY, Lin HH, Yang CY, Hung CF. Lycopene inhibits PDGF-BB-induced retinal pigment epithelial cell migration by suppression of PI3K/Akt and MAPK pathways. Biochem Biophys Res Commun 388: 172–176, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Chen H, Xu G, Zhao Y, Tian B, Lu H, Yu X, Xu Z, Ying N, Hu S, Hua Y. A novel OxyR sensor and regulator of hydrogen peroxide stress with one cysteine residue in Deinococcus radiodurans. PLoS One 3: e1602, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen K, Thomas SR, Albano A, Murphy MP, Keaney JF., Jr Mitochondrial function is required for hydrogen peroxide-induced growth factor receptor transactivation and downstream signaling. J Biol Chem 279: 35079–35086, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem 277: 3101–3108, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, Aplin AE, Tai YT, Aguirre-Ghiso J, Flores SC, Melendez JA. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem 280: 16916–16924, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Copeland WC, Longley MJ. DNA polymerase gamma in mitochondrial DNA replication and repair. ScientificWorldJournal 3: 34–44, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Desouki MM, Kulawiec M, Bansal S, Das GM, Singh KK. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol Ther 4: 1367–1373, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Dhanasekaran A, Kotamraju S, Karunakaran C, Kalivendi SV, Thomas S, Joseph J, Kalyanaraman B. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Biol Med 39: 567–583, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Diebold I, Djordjevic T, Petry A, Hatzelmann A, Tenor H, Hess J, Gorlach A. Phosphodiesterase 2 mediates redox-sensitive endothelial cell proliferation and angiogenesis by thrombin via Rac1 and NADPH oxidase 2. Circ Res 104: 1169–1177, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Dougherty CJ, Kubasiak LA, Frazier DP, Li H, Xiong WC, Bishopric NH, Webster KA. Mitochondrial signals initiate the activation of c-Jun N-terminal kinase (JNK) by hypoxia-reoxygenation. FASEB J 18: 1060–1070, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Facucho-Oliveira JM, Alderson J, Spikings EC, Egginton S, St John JC. Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J Cell Sci 120: 4025–4034, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Filipovska A, Kelso GF, Brown SE, Beer SM, Smith RA, Murphy MP. Synthesis and characterization of a triphenylphosphonium-conjugated peroxidase mimetic. Insights into the interaction of ebselen with mitochondria. J Biol Chem 280: 24113–24126, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Forstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med 5: 338–349, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal 11: 791–810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu Y, Xu YC, Wu RF, Souza RF, Nwariaku FE, Terada LS. TNFalpha activates c-Jun amino terminal kinase through p47(phox). Exp Cell Res 272: 62–74, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Hughes G, Murphy MP, Ledgerwood EC. Mitochondrial reactive oxygen species regulate the temporal activation of nuclear factor kappaB to modulate tumour necrosis factor-induced apoptosis: evidence from mitochondria-targeted antioxidants. Biochem J 389: 83–89, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 276: 4588–4596, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Kelso GF, Porteous CM, Hughes G, Ledgerwood EC, Gane AM, Smith RA, Murphy MP. Prevention of mitochondrial oxidative damage using targeted antioxidants. Ann NY Acad Sci 959: 263–274, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol 287: R1014–R1030, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol 280: C719–C741, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc 2: 2295–2301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nahari D, Satchi-Fainaro R, Chen M, Mitchell I, Task LB, Liu Z, Kihneman J, Carroll AB, Terada LS, Nwariaku FE. Tumor cytotoxicity and endothelial Rac inhibition induced by TNP-470 in anaplastic thyroid cancer. Mol Cancer Ther 6: 1329–1337, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Nwariaku FE, Liu Z, Zhu X, Nahari D, Ingle C, Wu RF, Gu Y, Sarosi G, Terada LS. NADPH oxidase mediates vascular endothelial cadherin phosphorylation and endothelial dysfunction. Blood 104: 3214–3220, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Nwariaku FE, Rothenbach P, Liu Z, Zhu X, Turnage RH, Terada LS. Rho inhibition decreases TNF-induced endothelial MAPK activation and monolayer permeability. J Appl Physiol 95: 1889–1895, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Ogita Y, Nakamura T, Higuchi S, Mori T, Shimo-Oku M. Histochemical studies of mitochondrial activities of cultured corneal endothelial cells of cat during wound-healing. Jpn J Ophthalmol 34: 200–215, 1990 [PubMed] [Google Scholar]
- 33. Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal 11: 841–860, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pourgholami MH, Morris DL. Inhibitors of vascular endothelial growth factor in cancer. Cardiovasc Hematol Agents Med Chem 6: 343–347, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Schleicher M, Shepherd BR, Suarez Y, Fernandez-Hernando C, Yu J, Pan Y, Acevedo LM, Shadel GS, Sessa WC. Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. J Cell Biol 180: 101–112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, Hahner LD, Cummings ML, Kitchens RL, Marcel YL, Rader DJ, Shaul PW. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res 98: 63–72, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Shin MH, Moon YJ, Seo JE, Lee Y, Kim KH, Chung JH. Reactive oxygen species produced by NADPH oxidase, xanthine oxidase, and mitochondrial electron transport system mediate heat shock-induced MMP-1 and MMP-9 expression. Free Radic Biol Med 44: 635–645, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Shizukuda Y, Tang S, Yokota R, Ware JA. Vascular endothelial growth factor-induced endothelial cell migration and proliferation depend on a nitric oxide-mediated decrease in protein kinase Cdelta activity. Circ Res 85: 247–256, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci USA 100: 5407–5412, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soga N, Connolly JO, Chellaiah M, Kawamura J, Hruska KA. Rac regulates vascular endothelial growth factor stimulated motility. Cell Commun Adhes 8: 1–13, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail 8: 132–140, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Spelbrink JN, Toivonen JM, Hakkaart GA, Kurkela JM, Cooper HM, Lehtinen SK, Lecrenier N, Back JW, Speijer D, Foury F, Jacobs HT. In vivo functional analysis of the human mitochondrial DNA polymerase POLG expressed in cultured human cells. J Biol Chem 275: 24818–24828, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest 115: 1221–1231, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for Rac1 in endothelial cell function and vascular development. FASEB J 22: 1829–1838, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 10: 1713–1765, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, Ushio-Fukai M. Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103: 212–220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Usatyuk PV, Gorshkova IA, He D, Zhao Y, Kalari SK, Garcia JG, Natarajan V. Phospholipase D-mediated activation of IQGAP1 through Rac1 regulates hyperoxia-induced p47phox translocation and reactive oxygen species generation in lung endothelial cells. J Biol Chem 284: 15339–15352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 9: 731–739, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JM, Muller J, Schuhmacher S, Hortmann M, Baldus S, Gori T, Brandes RP, Munzel T, Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal 10: 1435–1447, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Wright GL, Maroulakou IG, Eldridge J, Liby TL, Sridharan V, Tsichlis PN, Muise-Helmericks RC. VEGF stimulation of mitochondrial biogenesis: requirement of AKT3 kinase. FASEB J 22: 3264–3275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zang Q, Maass DL, White J, Horton JW. Cardiac mitochondrial damage and loss of ROS defense after burn injury: the beneficial effects of antioxidant therapy. J Appl Physiol 102: 103–112, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292: H2023–H2031, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Zhang Q, Chatterjee S, Wei Z, Liu WD, Fisher AB. Rac and PI3 kinase mediate endothelial cell-reactive oxygen species generation during normoxic lung ischemia. Antioxid Redox Signal 10: 679–689, 2008 [DOI] [PubMed] [Google Scholar]