Abstract
The present study explores the hypothesis that arterial smooth muscle cells are organized into layers with similar phenotypic characteristics that vary with the relative position between the lumen and the adventitia due to transmural gradients in vasotrophic factors. A corollary hypothesis is that vascular endothelial growth factor (VEGF) is a factor that helps establish transmural variations in smooth muscle phenotype. Organ culture of endothelium-denuded ovine carotid arteries with 3 ng/ml VEGF-A165 for 24 h differentially and significantly influenced potassium-induced (55% increase) and stretch-induced (36% decrease) stress-strain relations in adult (n = 18) but not term fetal (n = 21) arteries, suggesting that smooth muscle reactivity to VEGF is acquired during postnatal maturation. Because inclusion of fetal bovine serum significantly inhibited all contractile effects of VEGF (adult: n = 11; fetus: n = 11), it was excluded in all cultures. When assessed in relation to the distance between the lumen and the adventitia in immunohistochemically stained coronal artery sections, expression of smooth muscle α-actin (SMαA), myosin light chain kinase (MLCK), and 20-kDa regulatory myosin light chain exhibited distinct protein-dependent and age-dependent gradients across the artery wall. VEGF depressed regional SMαA abundance up to 15% in adult (n = 6) but not in fetal (n = 6) arteries, increased regional MLCK abundance up to 140% in fetal (n = 8) but not in adult (n = 10) arteries, and increased regional MLC20 abundance up to 28% in fetal arteries (n = 7) but decreased it by 17% in adult arteries (n = 9). Measurements of mRNA levels verified that VEGF receptor transcripts for both Flt-1 and kinase insert domain receptor (KDR) were expressed in both fetal and adult arteries. Overall, the present data support the unique hypothesis that smooth muscle cells are organized into lamina of similar phenotype with characteristics that depend on the relative position between the lumen and the adventitia and involve the direct effects of growth factors such as VEGF, which acts independently of the vascular endothelium in an age-dependent manner.
Keywords: myosin light chain kinase, organ culture, regulatory myosin light chain, smooth muscle, vascular endothelial growth factor receptors
arteries are composed of multiple different phenotypes of several major cell types organized into structures that are both highly heterogeneous and dynamic, particularly during periods of growth and development (20, 21, 26). Although a broad variety of evidence has carefully detailed this dynamic heterogeneity, the factors driving phenotypic changes in vascular mural cells remain uncertain. One major source of trophic factors governing vascular growth and differentiation is clearly the nonvascular parenchymal tissue surrounding the arteries (33, 34). Another important source is the vascular endothelium, which can modulate smooth muscle structure and function in relation to changes in artery perfusion and shear stress (5). Together, these two main sources of vasotrophic factors set up opposite gradients between the vascular endothelium and the tissue parenchyma, which in turn have the potential to govern the regional characteristics of smooth muscle phenotype across the artery wall. In support of this “gradient hypothesis,” evidence from studies in the rabbit aorta (21) and bovine pulmonary artery (20) demonstrate that smooth muscle cells are organized into lamina of similar phenotype with characteristics that depend on the relative position between the lumen and the adventitia. The identities of the factors responsible for these gradients in phenotype, however, remain uncertain.
One growth factor with great potential to help establish gradients in smooth muscle phenotype is vascular endothelial growth factor (VEGF). Owing to its pronounced effects on vascular permeability, VEGF was discovered in the mid-1970s and was initially known as vascular permeability factor or vasculotropin (17, 53). Following independent lines of investigation, other studies identified VEGF on the basis of its ability to promote endothelial cell transformation and angiogenesis (12, 34). The common identity of vascular permeability factor, vasculotropin, and VEGF was recognized in the mid-1990s (16), and since that time more than 30,000 studies have explored the function and properties of this molecule, most of which have focused on its role in angiogenesis (25, 50). Together, these studies support the view that hypoxic stimulation of the synthesis and release of VEGF from parenchymal tissues leads to new capillary formation, increases tissue perfusion, and attenuates local hypoxia in a classical negative feedback pattern of regulation. Correspondingly, numerous studies have focused on the therapeutic potential of VEGF in diseases where angiogenesis plays a central role, including tumor angiogenesis (12, 54) and wet age-related macular degeneration (40). A common theme among the majority of these studies is that the effects of VEGF are mediated by specific tyrosine kinase receptors located on the microvascular endothelium.
In addition to its well-established effects on angiogenesis, work in the late 1990s also began to suggest endothelium-independent roles for VEGF. Most prominent among these was the ability of VEGF to increase the resistance of neuronal cultures to oxygen and glucose deprivation (30). Interestingly, these protective effects appeared to arise from the ability of VEGF to depress neuronal apoptosis (3), which is similar to the mechanism whereby VEGF promotes endothelial survival and proliferation (2). More recent studies have further demonstrated a protective effect of VEGF for other central nervous system cell types including astrocytes (19). VEGF also appears to have important trophic and protective effects on cellular elements of the peripheral nervous system, including both Schwann cells (55) and perivascular sympathetic neurons (39). Virtually all of these effects of VEGF appear to be mediated by the highly specific dimeric receptor tyrosine kinases Flt-1 and Flk-1/KDR (25).
Apart from trophic effects on endothelia and cell types of the central and peripheral nervous systems, VEGF has also been implicated as a regulator of skeletal muscle growth and differentiation (9, 44). In parallel, recent studies have further suggested that VEGF has direct effects on smooth muscle, through which it can stimulate migration (22) and proliferation (45) of smooth muscle myocytes. Given the ability of VEGF to influence skeletal muscle differentiation (9, 44), it is possible that VEGF could also influence smooth muscle differentiation, although available studies of this hypothesis have thus far been limited to identification of VEGF receptors in smooth muscle cells (10, 29). Such an effect for VEGF would give it the simultaneous abilities to regulate endothelial growth and proliferation, stimulate sympathetic perivascular growth with subsequent trophic effects on smooth muscle development (8, 58), and influence upstream smooth muscle differentiation in a coordinate manner.
The present study was carried out to explore the hypothesis that smooth muscle cells are organized into lamina of similar phenotype with characteristics that depend on the relative position between the lumen and the adventitia and involve the direct effects of VEGF on arterial smooth muscle independent of the vascular endothelium. Given that the perinatal period is a time of rapid vascular differentiation and functional maturation, it is likely that the potential effects of VEGF on smooth muscle development would be most evident during this period. Correspondingly, the current experiments examined and compared arteries harvested from term fetal lambs and adult sheep. To identify potential smooth muscle effects of VEGF far removed from the microvasculature, the paradigm utilized common carotid artery segments. Denudation of the vascular endothelium from all segments minimized any possible contributions from endothelium-dependent mechanisms. The use of whole artery organ culture methods facilitated quantitation of the direct effects of VEGF on smooth muscle through comparisons of treated and untreated segments. Determination of active stress-strain relations and contractile protein abundances further enabled quantitation of VEGF effects from both functional and structural perspectives, respectively. To explore the gradient hypothesis the experimental approach incorporated customized methods to quantitate marker abundance as a function of relative position between the lumen and the adventitia. Together, these approaches revealed unprecedented effects of VEGF on large artery growth and functional maturation.
MATERIALS AND METHODS
All procedures used in these studies received approval from the Animal Research Committee of Loma Linda University and adhered to the policies and practices outlined in the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.” All procedures related to animal surgery and procurement of tissues have previously been described in detail (61). All sheep used in these studies were bred, raised, and maintained at sea level before transportation to Loma Linda University.
Tissue harvest and preparation.
All protocols utilized ring segments of common carotid arteries from fetal (139–142 days gestation) lambs of either sex and young nulliparous adult female sheep (18–24 mo old), harvested using sterile techniques. Ring segments were used instead of arterial strips to better preserve artery structure and minimize damage to the medial layer. Correspondingly, all rings included the adventitial layer. Pregnant ewes were anesthetized with 30 mg/kg pentobarbital sodium, intubated, and then placed on 1.5–2.0% halothane. The anesthetized fetus was then exteriorized through a midline vertical laparotomy and killed by rapid removal of the heart and exsanguination. Adult animals were killed by intravenous administration of 100 mg/kg pentobarbital sodium. Harvested arteries were placed in sterile HEPES buffer solution (in mM) (122.1 NaCl, 25 HEPES, 5.16 KCl, 2.4 MgSO4, 0.05 EDTA, 11.1 dextrose, 1.6 CaCl2). After gentle removal of extracellular and loose connective tissue, the arteries were denuded of endothelium, as previously described (61). The denuded carotid segments were cut into 3-mm lengths and then distributed to the various protocols.
Organ culture.
Artery segments destined for organ culture were maintained in untreated 12-well plates with DMEM (no. M56469C, Sigma Aldrich, St. Louis, MO) supplemented with the following: Na2HCO3 (3.7 g/l), 0.5% amino acid solution (no. M5550, Sigma Aldrich), 1% nonessential amino acid solution (no. M7145, Sigma Aldrich), 4 mM glutamine (no. G7513, Sigma Aldrich), 2% antibiotic-antimycotic solution (no. 15240-096, GIBCO, Carlsbad, CA), and gentamycin at 70 μg/ml (no. 15750-060, GIBCO). Cultures were maintained in a humidified incubator with 5% CO2 in room air at 37°C.
To assess the effects of fetal bovine serum (FBS, no. SH30070.01, Hyclone) on organ culture, matched serial segments of adult ovine carotids were cultured in either 0%, 1%, 2%, or 4% FBS in DMEM for 24, 48, or 72 h. To determine the combined effects of VEGF-A165 (no. 293-VE-010, R&D Systems, Minneapolis, MN), arteries were cultured in both the absence and presence of a low physiological concentration (3 ng/ml) of VEGF-A165 added only during the final 24 h of culture, together with FBS. Use of this low physiological concentration decreased the likelihood that VEGF would interact with non-VEGF receptors, such as the PDGF receptor recently shown to bind VEGF-A (4), and also replicated circulating levels of VEGF measured in sheep (60). For all conditions, the culture medium was changed once every 24 h, and upon completion of culture the arteries were examined for functional changes using the contractility protocol (see Contractility studies).
In separate experiments, matched serial artery segments were cultured without FBS in DMEM alone (starvation) for 24 h and then were cultured an additional 24 h in new media containing DMEM either with or without 3 ng/ml VEGF-A165. As revealed in our preliminary studies, the 24-h period of serum starvation before VEGF treatment aligned the cell cycle (23, 64), allowed for the removal of endogenous growth factors by diffusion into the media, and significantly reduced the variability of responses to VEGF. Arteries cultured under these conditions were then submitted for Western blots, fluorescent immunohistochemistry, and contractility studies. For all culture treatments, matched fresh uncultured artery segments were studied in parallel.
Contractility studies.
All artery ring segments used for contractility measurements were wire mounted in vitro between an isometric force transducer and a micrometer slide used to measure artery diameters. Mounted segments were first equilibrated for 30 min in calcium-replete Na+-Krebs buffer containing (in mM) 122 NaCl, 25.6 NaHCO3, 5.17 KCl, 2.49 MgSO4, 1.60 CaCl2, 2.56 dextrose, 0.027 EGTA, and 0.114 ascorbic acid. Continuous bubbling with 95% O2-5% CO2 at 38°C (normal ovine core temperature) maintained buffer pH at ≈7.4. After initial equilibration, unstressed artery diameter in each segment was measured at a passive tension of 0.03 g. Given this unstressed diameter (D0), the working diameters (D) needed to attain artery strain values (D/D0) of 1.5, 1.8, 2.1, 2.3, 2.7, 3.0, and 3.3 were calculated. Contractile stresses (in mN/cm2) were measured at each of these strain values, in increasing order, under resting conditions to determine spontaneous myogenic tone, and following contraction with a high potassium buffer containing (in mM) 5.16 NaCl, 122.1 KCl, 2.15 NaHCO3, 2.5 MgSO4, 0.027 EDTA, 11.08 dextrose, 0.114 ascorbic acid, and 1.6 ml CaCl2. Once contractile responses to the high K+ buffer had stabilized, the arteries were returned to Na+-Krebs buffer and then equilibrated at the next highest stretch ratio. Once the responses at a strain (D/D0) of 3.3 had been recorded, the arteries were then frozen in liquid nitrogen to rupture the cells and then incubated in a calcium-free Na+ Krebs buffer containing 3 mM EGTA. The level of passive stress produced at each strain ratio used was then recorded.
The active stresses produced by high K+ were calculated as the differences between the magnitudes of the active stresses recorded immediately before and after the application of the high K+ buffer. The active stresses attributable to spontaneous myogenic tone were calculated as the differences between the spontaneous active tone measured before, and the passive stresses measured after, liquid N2 and EGTA treatment at each level of strain. To determine arterial stiffness, the relations between strain and passive stresses (after liquid N2 and EGTA treatment) were determined using curve fitting with a monotonic exponential model to determine the coefficient of stiffness.
Fluorescent immunohistochemistry.
Artery segments were fixed in 4% neutral buffered EM-grade formaldehyde (no. 15713S, Electron Microscopy Sciences) overnight. Segments were dehydrated and embedded in paraffin and sectioned at 5 μm. Slides were deparaffinized in Histo-Clear (no. HS-200, National Diagnostic, Atlanta, GA) and rehydrated in decreasing grades of alcohol. Antigenic sites were exposed by microwaving samples in a solution of citrate buffer (pH 6.03). Permeabilization and blocking were achieved using 1% bovine serum albumin (no. SC-2323, Santa Cruz Biotechnology) and 0.1% Triton X-100 (no. T-8787, Sigma Aldrich). The primary antibodies, monoclonal anti-α actin smooth muscle (A5228, Sigma Aldrich) at 1:200, myosin light chain kinase (MLCK, SC-25428, Santa Cruz Biotechnology) at 1:50, and monoclonal anti-regulatory myosin light chain (no. M4401, Sigma-Aldrich) at 1:300 were applied and incubated at 4°C overnight. The next day, the slides were washed for two 10-min cycles in PBS. The appropriate secondary antibody with DyLight 488 Conjugated (no. 35502, Pierce Chemical, Rockford, IL) was applied to the tissue for 2 h at room temperature. After this incubation, the slides were kept in the dark to minimize deterioration of the fluorescent dye. The slides were then covered and washed for two 10-min cycles in PBS. Tissue slides were mounted with coverslips and SlowFade Gold antifade reagent with DAPI (S36939, Invitrogen, Carlsbad, CA) and then stored until imaged. All images were captured using a Zeiss Imager. A1 AX10 Fluorescence microscope and Spot software (version 4.6.4.5, Diagnostic Instruments).
Transmural morphometry.
To explore the gradient hypothesis that smooth muscle cells are organized into lamina of similar phenotype with characteristics that depend on the relative position between the lumen and the adventitia (20, 21, 26), we developed a procedure to quantify regional protein abundance. Using Image Pro Plus (version 6.0, MediaCybernetics), six separate intensity scans were recorded along radial lines extending from the basal lamina to the adventitial-medial junction. The radial scan lines mapped fluorescent intensity to distance from the lumen and were distributed symmetrically at 60-degree increments around the lumen to provide even coverage of each coronal section. All distance measurements were normalized to medial thickness, with a value of “0” assigned to the region just inside the basal lamina and a value of “100” assigned to the region just inside the adventitial-medial junction. Because the relations between fluorescent intensity and fluorophore concentration are highly nonlinear, we prepared calibration curves with known concentrations and volumes of commercially prepared microspheres uniformly labeled with fluorophore (no. MP07219, Invitrogen/Molecular Probes, Eugene, OR). These calibration curves (see Fig. 3) enabled conversion of the recorded fluorescent intensities into relative concentrations, which in turn were averaged as a function of percent distance from the lumen across all line scans taken from the same section. The resulting transmural concentration profiles were then normalized so that the area beneath the concentration-distance curves were unity. The resulting individual transmural profiles were then averaged across all artery sections within the same experimental group. To calibrate these transmural estimates of regional marker concentration, the normalized local concentration profiles from each section were multiplied by the average content of each marker in each experimental group determined using calibrated Western blots. Finally, the calibrated values of local marker concentration were averaged to provide regional estimates of marker abundance. The “lumen,” “media,” and “adventitial” regions were averaged across values from 5% to 15%, from 45% to 55%, and from 85% to 95% of the normalized distance from the lumen, respectively.
Fig. 3.
Calibration of relations between fluorophore concentration and fluorescent intensity. The relations between fluorophore concentrations and fluorescence intensities were determined using commercially prepared labeled microbeads applied to microscope slides in known volumes at multiple known concentrations. To accommodate the range of conditions employed during imaging, multiple exposure times were used as indicated in the legend. The data were fitted with the logistic equation, and the inverse forms of these calibration equations were used to convert fluorescent intensities into relative mass values for all images examined. The values shown represent averages for 3 measurements at each concentration-time combination.
Western blot analyses.
Artery segments were homogenized using glass on glass in 8 M urea, 500 mM NaCl, 20 mM Tris, 23 mM glycine, 10 mM EGTA, and 10% glycerol at pH 8.6 with addition of a protease inhibitor cocktail at 5 μl per ml of buffer (no. M1745, Sigma-Aldrich). Supernatants were collected after centrifugation at 5,000 g for 20 min. Protein homogenates were separated by SDS-PAGE along with a pooled reference prepared from adult ovine common carotid arteries used to calibrate sample abundances. The separated proteins were transferred to nitrocellulose at 350 mA for 90 min in Towbin's buffer (25 mM Tris, 192 mM glycine, 10 and 20% methanol). After transfer, the membranes were blocked with 5% milk in Tris-buffered saline at pH 7.5 (M-TBS) for 1 h at room temperature using continuous shaking. All subsequent washes and incubations were performed in M-TBS with 0.1% Tween-20. Primary antibodies were incubated for 3 h with the following dilutions for smooth muscle α-actin (SMαA) at 1:3,000, MLCK at 1:10,000, regulatory myosin light chain at 1:200, and VEGF-A165 at 1:1,000. Primary antibodies for SMαA, MLCK, and regulatory myosin light chain were obtained as described for immunohistochemistry. The anti-VEGF antibody was purchased from Abcam (no. AB119, Cambridge, MA). For visualization, membranes were incubated for 90 min with a secondary antibody conjugated to DyLight 800 (no. 46422, Pierce Chemical). Membranes were imaged on LI-COR Bioscience's Odyssey system.
Quantitation of mRNA for Flt-1, KDR, and endothelial nitric oxide synthase.
Freshly dissected carotid artery segments were denuded of endothelium and stored in RNAlater (AM7020, Invitrogen) at −20°C until homogenized. To extract RNA, uncultured segments weighing ∼25 mg were prepared using the RNeasy Fibrous Tissue Mini Kit (no. 74704, Qiagen, Valencia, CA). Total RNA extracts were prepared from uncultured fetal and adult carotid arteries, as well as cultured ovine endothelial cells used as a positive control. To minimize RNA degradation during sample preparation, all instruments were treated with RNAse Away (no. 7002, Molecular BioProducts). Total mRNA was quantified with the NanoDrop 2000 Spectrophotometer (Thermo Scientific); all samples exhibited RNA concentrations of at least 50 ng/μl and a 260/280 nm ratio between 1.7 and 2.1.
To select the optimum primer sets, multiple primer pairs were assayed, and for each 1.0 μg of total RNA was cloned with the AMV Reverse Transcriptase kit (no. 12328-019, Invitrogen) and then amplified via PCR in a 20-μl reaction volume using JumpStart REDTaq DNA Polymerase (no. D8187; Sigma-Aldrich). After amplification, the products were separated on a 3% agarose gel, visualized with GelStar-Nucleic Acid Gel Stain (no. 50535, Fischer), and imaged using AlphaEase by Alpha Innotech (Santa Clara, CA). Final primer selections were as follows: for Flt1, the forward primer was 5′-ACCTTGGTTGTGGCTGATGCT-3′ and the reverse was 5′-GCTCACCTGAAATCCATGGGGCA-3′; for kinase insert domain receptor (KDR) the forward primer was 5′-GGGACCTGGCAGCGCGAA-3′ and the reverse was 5′-TCGTTTCTGGGGCCATCCAC-3′ (P6); and for endothelial nitric oxide synthase (eNOS) the forward primer was 5′-TCTTCCACCAGGAGATGGTC-3′ and the reverse was 3′-GTTGGTAGGACATGCGGAGA-5′. The Flt1 and kinase insert domain receptor (KDR) primers were designed using the NCBI Primer-Blast software for GenBank AF488351.1 and AF233076.1, respectively.
Once optimal primer sets and conditions had been identified, mRNA samples were quantitated using a single step iQ SybrGreen Supermix (no. 170-8880, Bio-Rad, København, Denmark). Melting and efficiency curves were determined to optimize the Tm (59.4), cycle number, and reagent ratios. The final samples included 100 ng mRNA and 300 nM primers (for Flt-1, KDR, and eNOS) in a 25-μl reaction volume. Reactions were run using a Bio-Rad IQ5 at 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min for 40 cycles. The PCR products were separated on 3% agarose gels in 8TAE, stained with SybrGold (Molecular Probes), and imaged using AlphaEase.
Data analysis and statistics.
Contractile stresses were calculated as ratios of force per cross-sectional area. Forces were calculated as contractile tensions measured in grams times the acceleration due to gravity. Cross-sectional areas were calculated as the products of wall thicknesses and segment lengths, corrected for stretch as previously described in detail (27, 49). Values of contractile stresses were compared using a balanced two-way repeated measures ANOVA; each animal contributed equally to the fresh, starved, and VEGF treatment groups. Relations between fluorophore concentration and fluorescent signal intensity were determined using nonlinear regression with the logistic equation. For each relation, the inverse form of the calibration equation was used to directly calculate fluorophore concentrations from intensity values. The intensity values were normalized within each segment to yield an area beneath the intensity-distance curve of unity and calibrated against marker abundances measured with Western blots. All Western measures of protein abundance were calibrated against a standard curve prepared from common pooled references prepared from adult common carotids. Values of local abundance were compared using a three-way ANOVA with age, treatment, and region as factors. Post hoc comparisons between means within the ANOVA were performed using a Duncan's Multiple Range analysis. For mRNA abundances, fold differences were calculated as the slope of the efficiency curve raised to the power of the difference in Ct values between unknowns and corresponding positive control values. Fold difference values were divided into 100 to calculate percentages of positive control mRNA. Normal distributions were verified in all data sets using a D′Agostino-Pearson K2 test, and homogeneity of variance within all ANOVA data sets was verified using a Bartlett's-Cochran test (63). Statistical power was at least 0.8 unless stated otherwise.
RESULTS
A total of 75 sheep were used in this study of which 43 were term fetal lambs and 32 were adult sheep. From these animals we harvested a total of 494 carotid artery segments, including 257 fetal segments and 237 adult segments. Throughout the text, “n” represents the number of animals, and not the number of segments, used in each experiment. Unless stated otherwise, statistical significance implies P < 0.05. All values are given as means ± SE.
Effects of FBS on organ culture with VEGF-A165.
In both fetal and adult arteries, organ culture significantly decreased contractile responses to K+ after 48 and 72 h in all FBS-treated fetal and adult segments (Fig. 1, left panels). Under serum starvation conditions, responses to K+ were significantly decreased relative to day 0 only at 48 h in adult segments but at 24, 48, and 72 h in fetal segments. For both fetal and adult segments, serum-starved contractile responses to K+ were stable after 48 h of culture.
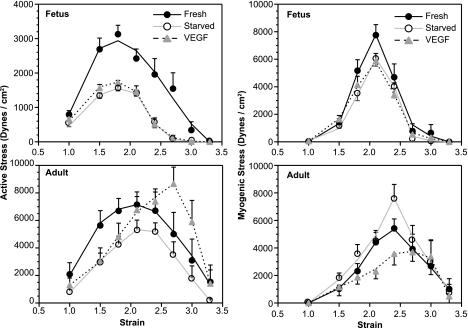
Fig. 1.
Organ culture with fetal bovine serum (FBS) alters common carotid contractility in an age- and concentration-dependent manner. Organ culture of endothelium-denuded fetal and adult common carotid segments with FBS significantly altered the maximum contractile response to 120 mM K+ (plotted as maximum active stresses), and this effect varied with duration of culture (P < 0.05 in both fetal and adult segments at 48 and 72 h for all FBS concentrations) and age (left panels). In serum-starved arteries, contractile responses to K+ were significantly depressed only at 48 h of culture in adult arteries and at 24, 48, and 72 h in fetal arteries. Contractile responses did not change significantly after 48 h of serum starvation in both fetal and adult arteries. When fetal and adult segments were organ cultured 24 h under serum starvation conditions, followed by 24 h of organ culture with a low physiological concentration (3 ng/ml) of vascular endothelial growth factor (VEGF)-A165, the presence of FBS significantly decreased contractile responses to VEGF in and age- and concentration-dependent manner. Culture with VEGF alone significantly enhanced contractile responses to 120 mM potassium (plotted as change in active stress), and increasing concentrations of FBS progressively reversed this effect (right panels). The effects of VEGF and FBS on contractility were significantly greater in adult than fetal arteries. Error bars indicate SE for 203 total fetal segments and 210 total adult segments, distributed among the various experimental groups, from a total of 11 fetuses and 11 adult sheep.
Serum starvation for 24 h followed by an additional 24 h of organ culture with 3 ng/ml VEGF-A165 also significantly influenced contractile responses to K+; in both fetal and adult arteries these responses were significantly increased in the absence of FBS and more so in adult than fetal arteries (Fig. 1, right panels). The presence of FBS significantly attenuated or reversed the effects of VEGF-A165 on contractile responses to K+.
Effects of organ culture with VEGF-A165 on artery structure and stress-strain relations.
In fresh artery samples, unstressed diameters averaged 2.98 ± 0.15 and 3.80 ± 0.27 mm in fetal and adult arteries, respectively. Corresponding values of wall thickness averaged 341 ± 20 (fetal) and 685 ± 22 (adult) μm. For wall stiffness, the fresh values averaged 8.22 ± 0.52 (fetal) and 5.99 ± 0.46 (adult). The optimum stretch ratio values for myogenic tone averaged 2.13 ± 0.05 (fetal) and 2.42 ± 0.12 (adult). The optimum stretch ratio values for K+-induced tone averaged 1.87 ± 0.09 (fetal) and 2.08 ± 0.13 (adult). For all of these variables, the combined N values totaled 54 for fetal arteries and 29 for adult arteries. Corresponding fetal and adult values were significantly different for each of these variables. In addition, for both fetal and adult arteries, the magnitude of stretch required to attain optimum myogenic tone was significantly greater than required to reach optimum K+-induced tone. Serum starvation had no significant effect in either age group except in adult arteries where starvation significantly decreased unstressed diameter. Organ culture with 3 ng/ml VEGF-A165 had no significant effect on any of these structural variables in either the fetus or the adult.
Contractile responses to K+-induced depolarization exhibited typical bell-shaped relations between active stress and strain (Fig. 2, left panels) that agreed well with our previous findings (27, 49). Organ culture for 48 h under serum starvation conditions significantly depressed maximum active stress responses to K+-induced depolarization in both fetal and adult arteries. Organ culture for 24 h under serum starvation conditions followed by 24 h of culture with 3 ng/ml VEGF-A165 had no significant effect compared with serum starvation alone in fetal arteries but significantly enhanced maximum K+-induced active stresses and right shifted the stress-strain relation in adult arteries (Fig. 2, lower left panel). For corresponding treatments, maximum active stress responses to K+-induced depolarization were significantly greater in adult than in fetal arteries.
Fig. 2.
organ culture with VEGF differentially alters contractile responses to myogenic stretch and potassium depolarization in an age-dependent manner. Endothelium-denuded carotid arteries from 28 fetal lambs and 23 adult sheep were prepared as matched sets of 3 adjacent segments from each animal. One member of each set was studied the day of euthansia (Fresh), one was studied after 48 h of organ culture under serum starvation conditions (Starved), and one was studied after 24 h of serum starvation followed by 24 h of culture with 3 ng/ml VEGF-A165. Complete stress-strain relations were determined in all fetal (top panels) and adult (bottom panels) arteries. At each level of stress examined, the level of spontaneous, stretch-dependent tone was determined (right panels), as was the active response to depolarization with 120 mM potassium (left panels). As revealed by repeated measures ANOVA, organ culture with VEGF was without effect in fetal arteries but significantly increased maximum active stress and decreased maximum myogenic stress in adult arteries. Error bars indicate means ± SE.
Contractile responses to artery stretch alone (myogenic stress), defined as the difference in stresses measured before and after freezing in liquid N2 and incubation in 3 mM ETGA, also exhibited bell-shaped relations, although these were significantly right-shifted relative to those for the K+-induced active stress strain relations for both fetal and adult arteries (Fig. 2, right panels). Organ culture for 48 h under serum starvation conditions modestly but significantly depressed maximum myogenic stress in fetal arteries but significantly increased it in adult arteries. Organ culture for 24 h of serum starvation followed by 24 h of culture with 3 ng/ml VEGF-A165 again had no significant effect compared with serum starvation alone in fetal arteries but significantly depressed maximum myogenic stress in adult arteries. The magnitudes of maximum myogenic stress were similar in fetal and adult arteries and compared with the magnitudes of maximum K+-induced active stresses were similar in adult arteries but were more than twofold greater in fetal arteries. Overall, the stress-strain results revealed that organ culture with VEGF-A165 for 24 h had little effect in fetal arteries but significantly enhanced K+-induced active stresses and significantly depressed myogenic stresses in adult arteries.
Effects of organ culture with VEGF-A165 on regional expression of SMαA.
To enable a more quantitative analysis of the artery images, we determined the relations between fluorophore concentrations and fluorescent intensities for multiple exposure times (Fig. 3). When the calibration curves constructed from these measurements were used to convert the line scan data into apparent concentrations and then normalized to Western blot measurements of whole artery protein abundance, we obtained regional estimates of local marker abundance that were highly reproducible (see Figs. 4–6).
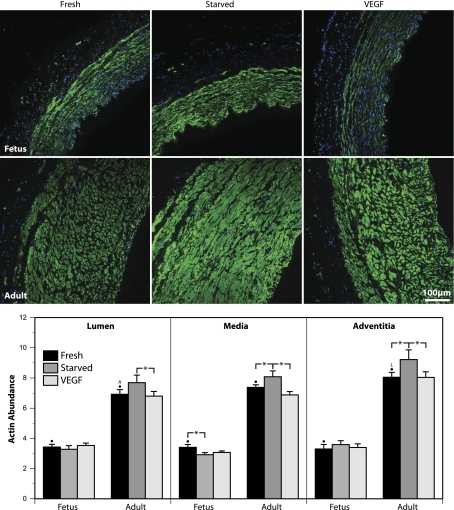
Fig. 4.
Transmural morphometry reveals an age-dependent influence of organ culture with VEGF on the regional expression of smooth muscle α-actin. Endothelium-denuded carotid arteries from fetal and adult sheep were organ cultured as described for the contractility studies and then were fixed in 4% paraformaldehyde, sectioned at 5 μm, and immunostained for smooth muscle α-actin (green signal). Cell nuclei were stained with DAPI (blue signal). For all images shown, the artery lumen faces rightward. Signal intensities were independently adjusted for each image to maximize dynamic range and do not indicate absolute marker abundance. Coronal sections were line scanned to determine the relations between fluorescent intensity and location in the artery wall. Three locations were examined in detail. The “lumen” region was defined as the area lying just inside the basal elastic lamina. The “media” region was defined as the area midway between the basal elastic lamina and the adventitial-medial border. The “adventitia” region was defined as the area of smooth muscle immediately adjacent to the adventitial-medial border. Values of fluorescent intensity recorded in each region were converted to apparent concentration using standardized calibration curves (see Fig. 3) and then averaged across multiple animals and normalized relative to Western blot measurements of smooth muscle α-actin abundance. For the Fresh segments, the letters “A” and “L” indicate significant differences compared with the adventitial and luminal regions, respectively. Asterisks indicate paired differences due to treatment (P < 0.05, ANOVA). Error bars indicate means ± SE for N = 6 for both fetal and adult arteries. Overall, smooth muscle α-actin abundance was significantly greater in adult than in fetal arteries for all corresponding treatments. Organ culture with 3 ng/ml VEGF-A165 significantly depressed smooth muscle α-actin abundance in adult but not fetal arteries. Note that organ culture altered the structure of both the medial and adventitial layers in an age-dependent manner.
Fig. 6.
Transmural morphometry reveals an age-dependent influence of organ culture with VEGF on the regional expression of regulatory myosin light chain. Coronal sections of ovine carotid arteries immunostained for 20 kDa regulatory myosin light chain were line scanned to determine the relations between fluorescent intensity and location in the artery wall. For all images shown, the artery lumen faces leftward. As in Fig. 4, signal intensities were independently adjusted for each image to maximize dynamic range and do not indicate absolute marker abundance. Regional abundances were quantified using calibrated standards, as described in Fig. 4. For the Fresh segments, the letters “A,” “M,” and “L” indicate significant differences compared with the adventitial, medial and luminal regions, respectively. Asterisks indicate paired differences due to treatment (P < 0.05, ANOVA). Error bars indicate means ± SE for N = 7 fetal arteries and N = 9 adult arteries. Overall, myosin light chain abundance was significantly greater in adult than in fetal arteries for all regions in Fresh arteries. Organ culture with 3 ng/ml VEGF-A165 significantly increased myosin light chain abundance in all regions of fetal arteries but decreased light chain abundance in the medial and adventitial regions of adult arteries. Note that the effects of organ culture were most evident in the luminal region of fetal arteries but in the adventitial region of adult arteries.
For SMαA, relative abundances (compared with our pooled standard reference) averaged 1.42 ± 0.08, 1.53 ± 0.10, and 1.38 ± 0.09 in fresh, starved, and VEGF-treated adult arteries (N = 7). Corresponding values in fetal arteries averaged 0.62 ± 0.07, 0.59 ± 0.05, and 0.64 ± 0.07 (N = 7). When these measurements were used to normalize the fluorescent line scan data, the results revealed a significantly greater expression of SMαA in the adventitial region than in the luminal region of fresh adult arteries (Fig. 4). For all three regions, the abundances of SMαA were significantly greater in adult than in fetal arteries. When treatments were compared relative to values in fresh arteries, 48 h of serum starvation increased SMαA expression in adult but not fetal arteries; starvation significantly depressed SMαA expression in fetal arteries but only in the medial region. Organ culture with VEGF had no effect on SMαA in fetal arteries but significantly decreased SMαA in all three regions of adult arteries.
Effects of organ culture with VEGF-A165 on regional expression of MLCK.
Western blot measurements of MLCK relative abundances averaged 1.34 ± 0.14, 0.09 ± 0.02, and 0.09 ± 0.04 in fresh, starved, and VEGF-treated arteries, respectively (N = 7). Corresponding values in fetal arteries averaged 0.60 ± 0.07, 0.06 ± 0.01, and 0.12 ± 0.03 (N = 7). When combined with the line scan data, these results revealed multiple significant age-related differences in regional abundances (Fig. 5). In fresh adult arteries, MLCK abundance was similar in the luminal and adventitial regions but was significantly depressed in the medial region. In contrast, in fresh fetal arteries MLCK abundance was greater in the luminal region and decreased progressively to its minimum value in the adventitial region. For all three regions, the abundances of MLCK were significantly greater in adult than in fetal arteries. When treatments were compared relative with values in fresh arteries, 48 h of serum starvation dramatically and significantly decreased MLCK abundance in all three regions of both age groups. Organ culture with VEGF modestly but significantly increased MLCK abundance in all three regions of fetal arteries but was without effect in any adult artery region.
Fig. 5.
Transmural morphometry reveals an age-dependent influence of organ culture with VEGF on the regional expression of myosin light chain kinase (MLCK). Coronal sections of ovine carotid arteries were prepared as described for Fig. 4, immunostained for MLCK, then line scanned to determine the relations between fluorescent intensity and location in the artery wall. For all images shown, the artery lumen faces rightward. As in Fig. 4, signal intensities were independently adjusted for each image to maximize dynamic range and do not indicate absolute marker abundance. Regional abundances were quantified using calibrated standards, as described in Fig. 4. Three locations were examined in detail, as described for smooth muscle α-actin in Fig. 4. For the Fresh segments, the letters “A,” “M,” and “L” indicate significant differences compared with the adventitial, medial, and luminal regions, respectively. Asterisks indicate paired differences due to treatment (P < 0.05, ANOVA). Error bars indicate means ± SE for N = 8 fetal arteries and N = 10 adult arteries. Overall, MLCK abundance was significantly greater in adult than in fetal arteries for all regions in Fresh arteries. Organ culture with 3 ng/ml VEGF-A165 significantly increased MLCK abundance in fetal but not adult arteries. Note that the effects of organ culture on the structure and organization of both the medial and adventitial layers were most evident in the fetal arteries.
Effects of organ culture with VEGF-A165 on regional expression of 20 kDa regulatory myosin light chain.
Relative abundances of 20 kDa regulatory myosin light chain (MLC20) averaged 0.65 ± 0.09, 0.44 ± 0.07, and 0.42 ± 0.08 in fresh, starved, and VEGF-treated adult arteries, respectively (N = 7). Corresponding values in fetal arteries averaged 0.41 ± 0.05, 0.23 ± 0.04, and 0.27 ± 0.05, respectively (N = 7). In combination with the line scan data, these results revealed multiple regional and age-dependent differences (Fig. 6). In fresh adult arteries, the relative abundance of MLC20 was similar in the luminal and medial regions but was significantly greater in the adventitial region. In fresh fetal arteries, the relative abundance of MLC20 was greatest in the luminal region and least in the medial region. In all three regions, MLC20 abundance was significantly greater in adult than in fetal arteries. Relative to values in fresh arteries, 48 h of serum starvation significantly decreased MLC20 abundance in all three regions of both age groups. Organ culture with VEGF significantly increased MLC20 abundance in all three regions of fetal arteries but decreased MLC20 abundance in both the medial and adventitial regions of adult arteries.
Expression of mRNA transcripts for Flt-1, KDR, and eNOS in fetal and adult carotids.
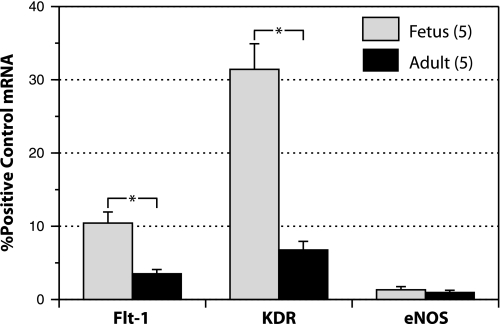
Real-time quantitative PCR measurements of the relative copy numbers of transcripts for the VEGF receptors Flt-1 and KDR and the endothelial marker eNOS strongly suggested the presence of VEGF receptors in both fetal and adult carotid artery smooth muscle (Fig. 7). Relative to expression levels in cultured ovine endothelial cells used as positive controls, the relative copy numbers for Flt-1 averaged 10.4 ± 1.5% and 3.5 ± 0.6% in fetal (n = 5) and adult (n = 5) arteries, respectively. For KDR the corresponding values averaged 31.2 ± 4.6% and 6.3 ± 1.7%, respectively. For both Flt-1 and KDR, transcript abundances were significantly greater in fetal than adult arteries. Relative copy numbers for the eNOS transcript were very small for both fetal (1.3 ± 0.4%) and adult (0.9 ± 0.4%) arteries, suggesting that the mechanical procedure used to remove the vascular endothelium was highly effective and that the measured abundances for the Flt-1 and KDR receptors cannot be explained by contamination from endothelial cells.
Fig. 7.
Transcripts for the VEGF receptors flt-1 and KDR are expressed in ovine carotid smooth muscle. Identical masses of total RNA extracted from freshly dissected, uncultured, endothelium-denuded ovine carotids were amplified using quantitative real-time PCR with primer sets specific for the VEGF receptors Flt-1 and KDR. To verify lack of contamination with endothelial cells, total RNA samples were also amplified with primer sets specific for endothelial nitric oxide synthase (eNOS). Relative copy numbers were calculated using efficiency curves prepared for each run, and the results are expressed relative to the abundances of each marker measured in cultured ovine endothelial cells (positive control). Asterisks indicate significant differences between corresponding fetal and adult values. Error bars indicate means ± SE for 5 fetal arteries and 5 adult arteries. Transcript abundances were significantly greater in fetal than adult arteries for both Flt-1 and KDR. In both fetal and adult arteries, the relative abundance of Flt-1 was less than for KDR. The relative absence of eNOS transcripts in the RNA extracts verifies that the sources of the measured transcripts did not originate from endothelial cells.
DISCUSSION
The present study offers six original observations. First, organ culture with FBS obscures or reverses the effects of VEGF on endothelium-denuded carotid arteries. Second, 24 h of organ culture in the absence of FBS followed by 24 h of culture with a physiologically relevant concentration of VEGF-A165 (3 ng/ml) increased K+-induced contractions and depressed stretch-induced contractions in adult arteries but was without effect in fetal arteries. Third, organ culture with VEGF decreased SMαA abundance throughout the artery wall in adult arteries but was without effect in fetal arteries. Fourth, organ culture with VEGF was without effect on MLCK abundance in adult arteries but uniformly increased MLCK abundance in fetal arteries. Fifth, organ culture with VEGF increased the abundance of regulatory light chain in fetal arteries but decreased MLC20 abundance in adult arteries. Sixth, transcripts for Flt-1 and KDR were expressed in arteries from both age groups and to a greater extent in fetal than adult arteries. Together, these observations support the hypothesis that smooth muscle cells are organized into lamina of similar phenotype with characteristics that depend on the relative position between the lumen and the adventitia and involve the direct effects of VEGF on arterial smooth muscle independent of the vascular endothelium in an age-dependent manner.
Effects of organ culture with FBS and VEGF on arterial contractility.
The organ culture of arteries is a convenient method employed for more than 40 years (37) to identify how compounds and pathogens, both endogenous and exogenous, directly influence vascular smooth muscle structure and composition. Optimization of culture conditions can be challenging, however, in light of the broad variety of conditions used. Of particular importance is the concentration of FBS in the culture media, which can vary from 10% (43) to 1% (1) or even less (35). To optimize the concentration of FBS for the present studies, the experimental approach relied on the functional endpoint of contractility measured as stresses (force per cross-sectional area). Measurement of active stresses was essential owing to the many possible effects of organ culture on ion channel expression (38), calcium metabolism (56, 57), and overall contractility (62) that together precluded any method of normalization of contractile force. This approach thus incorporated any changes in artery wall thickness and contractility caused by organ culture. Not unexpectedly, the presence of FBS in the organ culture medium significantly altered contractile responses to K+-induced depolarization in a concentration- and time-dependent manner; contractility was stable after 48 h of culture in both fetal and adult arteries but only in serum-starved arteries (Fig. 1, left panels). When the arteries were cultured for 24 h to align the cell cycles (23, 64) and facilitate washout of endogenous growth factors, exposure to 3 ng/ml VEGF in the absence of FBS significantly enhanced contractile stresses in both fetal and adult arteries (Fig. 1, right panels). More importantly, inclusion of FBS in the culture medium reversed the contractile effects of VEGF, indicating that FBS can obscure the vascular effects of VEGF, even at low concentrations. These results further demonstrated that VEGF at a low physiologically relevant concentration (60) with only 24 h of treatment can significantly alter artery contractility through direct, endothelium-independent effects. By extrapolation, the use of higher VEGF concentrations and/or longer durations of treatment, as commonly reported in the literature (10, 22), might be expected to produce significantly greater, if less physiologically relevant, vascular smooth muscle effects. Together, the results with FBS and 3 ng/ml VEGF strongly suggest that variations in the concentrations of both of these factors could help explain much of the inconsistency reported in previous studies of the effects of VEGF on smooth muscle cultures (10, 35). From these considerations, the organ culture model selected for the present studies avoided the use of FBS, included an initial 24 h of serum starvation, and explored the effects of culture with 3 ng/ml VEGF for only 24 h.
Consistent with our previous findings (27, 49), fetal arteries exhibited significantly smaller values of unstressed diameter and wall thickness and greater values of stiffness, compared with adult arteries. None of these parameters were significantly affected by either serum starvation or treatment with VEGF, suggesting that all contractile changes produced by VEGF were independent of structural changes. In adult arteries, treatment with VEGF enhanced contractile responses to K+ depolarization but depressed responses to myogenic stretch (Fig. 2, lower panels). These opposite effects of VEGF strongly support previous suggestions that very different mechanisms govern K+-induced and stretch-induced contractions (14, 46, 52). These findings further suggest that VEGF differentially influences the expression, organization, and/or function of the vascular proteins that separately mediate contractile responses to K+-induced depolarization and myogenic stretch. In sharp contrast to the findings in adult arteries, treatment of fetal arteries with VEGF was without effect on contractile responses to either K+ depolarization or myogenic stretch (Fig. 2, top panels). This finding suggests that coupling between VEGF, its receptors, and contractile protein function for either K+-induced or stretch-induced contraction is acquired with postnatal vascular maturation.
Effects of organ culture with VEGF on contractile protein abundance and distribution.
To further explore intramural differences in the expression and distribution of contractile proteins and how these regional patterns might be influenced by organ culture with VEGF, our experimental approach included preparation of serial sections of fresh and organ-cultured arteries for fluorescent immunohistochemistry. Images from these sections revealed major differences between fetal and adult arteries in the organization of the medial and adventitial layers, as expected (Figs. 4–6). To enable identification of the effects of organ culture with VEGF on these structural differences, we developed a morphometric technique to quantify the transmural distribution of the protein markers studied. Conversion of fluorescent intensities measured via radial line scans into apparent concentrations using calibration curves prepared with the same microscope and fluorophores used for our images (Fig. 3) was an essential feature of this paradigm. This approach normalized the intensity results, eliminated complications due to nonlinear relations between fluorophore concentration and fluorescent signal intensity, and made possible the use of standard parametric statistical methods. With these methods, we calculated regional marker concentrations by normalizing the resulting transmural profiles relative to whole artery marker abundances measured via Western blot. This original morphometric paradigm provided several unique perspectives of how marker expression varies across the artery wall and how treatment with VEGF influences these patterns of contractile protein expression in ovine carotids.
SMαA was the first marker analyzed with our transmural morphometry paradigm. As thoroughly discussed by Owens et al. (47), SMαA is present in all smooth muscle cells, is their most abundant single protein, and is a critical determinant of cell shape and function is this cell type. In the present studies, the overall abundance of SMαA was more than twofold greater in adult than in fetal arteries (Fig. 4), which may be explained at least in part by evidence that average cell volume was smaller, and the fraction of extracellular volume was significantly greater in studies of fetal compared with adult ovine carotid arteries (18). In other studies, SMαA expression also correlated closely with the proportion of smooth muscle cells in a fully differentiated contractile phenotype, which increased with postnatal age (11, 48). In fresh, uncultured adult arteries, SMαA abundance was significantly less in the luminal region than the adventitial region, indicating that endogenous expression rose as a gradient relative to distance from the lumen. This finding is consistent with previous descriptions of transmural gradients for other vascular proteins (20, 21) and supports the general hypothesis that vascular smooth muscle characteristics are governed by opposing gradients of growth factors originating from the vascular endothelium and the extravascular parenchyma, respectively.
Consistent with the findings of Kato et al. (32), serum starvation elevated SMαA expression in adult arteries (Fig. 4). Kato et al. (32) further reported that treatment with platelet-derived growth factor reversed the effect of serum starvation on SMαA, which parallels our novel finding that culture with VEGF also reversed the effects of serum starvation in adult arteries. This latter finding suggests that expression of SMαA is tonically inhibited by multiple growth factors in adult arteries. From a functional perspective, the effects of VEGF on SMαA were consistent with its effect on myogenic contraction (Fig. 2) but not on depolarization-induced contraction. This divergence of effects suggests that similar mechanisms might mediate the effects of VEGF on both myogenic tone and SMαA expression, but different mechanisms must mediate the effects of VEGF on depolarization-induced tone in adult arteries. In contrast, VEGF had no effect on either SMαA expression or contractility in fetal arteries. The fetal arteries also exhibited little evidence of the expression gradients observed in adult arteries. These results thus further support the interpretation that coupling between VEGF and contractility is acquired largely during postnatal maturation.
The second contractile protein analyzed with the transmural morphometry paradigm was MLCK. Our previous work with this enzyme has demonstrated that MLCK is far more abundant and is regulated very differently in adult than in fetal ovine carotid arteries (28). Consistent with our earlier findings, MLCK abundance was greater in adult than in fetal arteries (Fig. 5). In the adult arteries, no transmural gradient was observed, although MLCK abundance was depressed in the media layer compared with the luminal and adventitial layers; endothelial and parenchymal trophic factors may enhance MLCK expression in the outer smooth muscle layers. The complete withdrawal of all trophic factors produced a major depression in MLCK in all artery regions within 48 h, suggesting that MLCK expression is highly dynamic, counterbalanced by high rates of protein turnover, and dependent on near-continuous support from one or more vasotrophic factors. VEGF does not appear to be among the factors that support maximal levels of MLCK expression, because treatment with VEGF had no effect on MLCK in any region of adult arteries. VEGF thus had very different effects on SMαA and MLCK expression in adult arteries. In marked contrast to the adult artery responses, MLCK expression in fetal arteries exhibited a significant gradient with the highest expression in the luminal region and the lowest in the adventitial region; these results again support the gradient hypothesis but further suggest that gradients in contractile protein expression can either increase or decrease with distance from the lumen and are highly dependent on both age and the specific protein examined. As observed in adult arteries, serum starvation also markedly decreased MLCK expression in fetal arteries, suggesting that the mechanisms regulating MLCK expression and turnover are probably operational early in vascular development. This finding also suggests that MLCK is typically present in great excess beyond the levels required to support contraction. In contrast to the adult arteries, however, MLCK expression in fetal arteries was significantly enhanced by VEGF treatment. This finding suggests not only that VEGF receptors may be present on fetal smooth muscle cells but also that VEGF exerts fundamentally different influences on immature and mature arteries. In turn, the ability of VEGF to enhance MLCK expression in fetal arteries may represent an early developmental stage of reactivity to VEGF. That VEGF- and starvation-induced changes in MLCK expression were not correlated with contractility in either fetal or adult arteries emphasizes that MLCK abundance alone is not a strong predictor of contractile behavior; other factors are clearly involved.
The third contractile protein analyzed using our transmural morphometry paradigm was MLC20. This protein is a critical regulator of smooth muscle contraction expressed at similar levels in fetal and adult ovine arteries, although regulation of its levels of phosphorylation appears to be highly age dependent (28, 41, 51). Consistent with those findings, the present results revealed a slightly greater abundance of MLC20 in adult than in fetal arteries across all three regions (Fig. 6). Adult arteries exhibited a modest transmural gradient in MLC20 expression, with maximum levels in the adventitial region, suggesting that MLC20 expression also may be influenced by growth factor gradients across the artery wall. Compared with MLCK, starvation-induced decreases in MLC20 were much smaller in adult arteries, suggesting that MLC20 is dynamically regulated and dependent on continuous trophic support but to a smaller extent than observed for MLCK and to a much greater extent than observed for SMαA. Similar to the effect of VEGF on SMαA abundance in adult arteries, treatment with VEGF depressed MLC20 expression in adult arteries, and the magnitude of this effect was least in the luminal region and greatest in the adventitial region. In sharp contrast, VEGF increased MLC20 expression in fetal arteries even though the response to serum starvation was similar to that observed in adult arteries. Together, these findings reinforce the hypothesis that VEGF influences vascular smooth muscle composition and function through direct endothelium-independent mechanisms.
Age-dependent expression of VEGF receptor transcripts.
A central question raised by the observed effects of VEGF on contractility and contractile protein expression concerns the pathway through which VEGF exerts these effects. As suggested by our qPCR measurements of very low levels of eNOS transcripts in both age groups (Fig. 7), the methods used to remove the endothelium were effective and thus minimize the likelihood that our results could be explained by direct effects of VEGF on endothelial cells, with secondary effects on the underlying vascular smooth muscle. Although it is possible that VEGF could also act directly on adventitial fibroblasts, such effects are typically associated with increased fibroblast migration, but not proliferation, and thus would probably not exert secondary effects on adjacent smooth muscle (31). Instead, our findings that transcripts for Flt-1 and KDR were measured at significant levels in both fetal and adult arteries predict that the observed direct effects of VEGF were mediated by activation of specific VEGF receptors. In support of this interpretation, other studies have reported the presence of VEGF receptor protein and transcripts in cultured vascular smooth muscle from multiple sources (10, 22, 29, 45). Although the relative mRNA abundances for both Flt-1 and KDR were significantly greater in fetal than in adult arteries, the physiological significance of these differences is challenging to interpret owing the possible variations in mRNA half-life, inhibition of translation by microRNA, and other mechanisms that influence translation efficiency. Direct measurements of VEGF receptor protein expression remain necessary to determine the extent to which differences in VEGF receptors can explain the observed age-related differences in responses to VEGF. Aside from these limitations, the mRNA results strongly support the hypothesis that VEGF can act on VEGF receptors to influence arterial contractility and contractile protein expression in an age-dependent manner.
Overview.
Altogether, the present results strongly support the general hypothesis that smooth muscle cells are organized into lamina of similar phenotype with characteristics that depend on the relative position between the lumen and the adventitia and involve the direct effects of VEGF on arterial smooth muscle independent of the vascular endothelium in an age-dependent manner. Although the present results do not directly elucidate the important functional consequences of this highly laminar structure of the artery wall, the results do show that VEGF can directly alter artery structure and function through endothelium-independent effects on smooth muscle phenotype. Effects of VEGF on contractility were evident only in adult arteries, which advances the corollary hypothesis that smooth muscle reactivity to VEGF is acquired during postnatal maturation. VEGF also produced multiple heterogeneous patterns of change in contractile protein expression that supported the gradient hypothesis but also demonstrated that regional differences in protein expression were highly protein specific. VEGF depressed SMαA abundance only in adult arteries, increased MLCK abundance only in fetal arteries, and increased MLC20 abundance in fetal arteries but decreased it in adult arteries. Measurements of mRNA levels verified that VEGF receptor transcripts are expressed in large artery smooth muscle, which is consistent with the hypothesis that VEGF receptors mediate the observed effects, although the present data cannot exclude the participation of other receptors. Together, these findings extend previous studies of intramural differences in protein expression, which have been limited almost exclusively to cultured arterial smooth muscle, often in the presence of FBS, which can dramatically alter smooth muscle responses to trophic factors. The present use of organ cultures of fresh whole arteries also minimized the time-dependent changes in cell phenotype, which are typical of cultured smooth muscle. As a whole, the present results raise the possibility that physiological and pathophysiological changes in arterial smooth muscle structure and function associated with hypoxia, ischemia, and wound repair are also strongly associated with increased expression of VEGF and other growth factors (6, 24) and may involve the integrated influences of transmural gradients in these growth factors across the artery wall. Whereas the vascular endothelium is a potential source for many vasotrophic factors, the source of growth factors arriving first at the serosal surface of large arteries is less clear. For these arteries, the primary source of VEGF may be adventitial fibroblasts (25), although this idea awaits experimental confirmation.
Beyond the present results, many questions remain. What other vasotrophic factors contribute to transmural gradients in smooth muscle phenotype? How might the presence of an intact endothelium influence responses to VEGF either directly or indirectly through the release of secondary trophic factors such as endothelin (13) or nitric oxide (65)? Could possible autocrine effects of VEGF on endothelial cells (36, 42, 59) in some way amplify the vasotrophic effects of VEGF on vascular smooth muscle? In view of the potential effects of estrogens on VEGF function (7, 15), to what extent can the observed age-related differences in VEGF effects be explained by the fact that estrogen concentrations are typically far greater in female adults than in fetuses of either gender? In turn, might the effects of VEGF on adult arteries vary with gender or estrogen status postmenopause? Might higher concentrations or longer durations of VEGF treatment yield more pronounced effects on contractility and contractile protein expression? Can the differential effects of VEGF on depolarization-induced and stretch-induced contractions be exploited to elucidate the molecular differences between these two very different types of contraction? Do variations in transcript translation efficiency or receptor coupling efficiency contribute to the marked age-related differences in the effects of VEGF on smooth muscle? Regardless of the answers to these questions, the present data reinforce the unique hypothesis that smooth muscle cells are organized into lamina of similar phenotype with characteristics that depend on the relative position between the lumen and the adventitia and involve the direct effects of growth factors such as VEGF independent of the vascular endothelium in an age-dependent manner.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The work reported in this manuscript was supported by National Institutes of Health Grants HL-54120, HD-31266, and HL-64867 and by the Loma Linda University School of Medicine.
REFERENCES
- 1. Abraham NG, Scapagnini G, Kappas A. Human heme oxygenase: cell cycle-dependent expression and DNA microarray identification of multiple gene responses after transduction of endothelial cells. J Cell Biochem 90: 1098–1111, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1: 1024–1028, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Bagnard D, Vaillant C, Khuth ST, Dufay N, Lohrum M, Puschel AW, Belin MF, Bolz J, Thomasset N. Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J Neurosci 21: 3332–3341, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol 177: 489–500, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev 89: 481–534, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 16: 585–601, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Bausero P, Ben-Mahdi M, Mazucatelli J, Bloy C, Perrot-Applanat M. Vascular endothelial growth factor is modulated in vascular muscle cells by estradiol, tamoxifen, and hypoxia. Am J Physiol Heart Circ Physiol 279: H2033–H2042, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Bevan RD. Effect of sympathetic denervation on smooth muscle cell proliferation in the growing rabbit ear artery. Circ Res 37: 14–19, 1975 [DOI] [PubMed] [Google Scholar]
- 9. Bryan BA, Walshe TE, Mitchell DC, Havumaki JS, Saint-Geniez M, Maharaj AS, Maldonado AE, D'Amore PA. Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Mol Biol Cell 19: 994–1006, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chandra A, Angle N. Vascular endothelial growth factor stimulates a novel calcium-signaling pathway in vascular smooth muscle cells. Surgery 138: 780–787, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Charles SM, Zhang L, Cipolla MJ, Buchholz JN, Pearce WJ. The roles of cytosolic Ca2+ concentration and myofilament Ca2+ sensitization in age-dependent cerebrovascular myogenic tone. Am J Physiol Heart Circ Physiol 299: H1034–H1044, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest 84: 1470–1478, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dao HH, Bouvet C, Moreau S, Beaucage P, Lariviere R, Servant MJ, de Champlain J, Moreau P. Endothelin is a dose-dependent trophic factor and a mitogen in small arteries in vivo. Cardiovasc Res 71: 61–68, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999 [DOI] [PubMed] [Google Scholar]
- 15. de Araujo LF, Grozovsky R, dos Santos Pereira MJ, de Carvalho JJ, Vaisman M, Carvalho DP. Expressions of vascular endothelial growth factor and nitric oxide synthase III in the thyroid gland of ovariectomized rats are upregulated by estrogen and selective estrogen receptor modulators. Thyroid 20: 85–92, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146: 1029–1039, 1995 [PMC free article] [PubMed] [Google Scholar]
- 17. Dvorak HF, Dvorak AM, Manseau EJ, Wiberg L, Churchill WH. Fibrin gel investment associated with line 1 and line 10 solid tumor growth, angiogenesis, and fibroplasia in guinea pigs. Role of cellular immunity, myofibroblasts, microvascular damage, and infarction in line 1 tumor regression. J Natl Cancer Inst 62: 1459–1472, 1979 [PubMed] [Google Scholar]
- 18. Elliott CF, Pearce WJ. Effects of maturation on cell water, protein, and DNA content in ovine cerebral arteries. J Appl Physiol 79: 831–837, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Freitas-Andrade M, Carmeliet P, Stanimirovic DB, Moreno M. VEGFR-2-mediated increased proliferation and survival in response to oxygen and glucose deprivation in PlGF knockout astrocytes. J Neurochem 107: 756–767, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Frid MG, Moiseeva EP, Stenmark KR. Multiple phenotypically distinct smooth muscle cell populations exist in the adult and developing bovine pulmonary arterial media in vivo. Circ Res 75: 669–681, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Giuriato L, Scatena M, Chiavegato A, Tonello M, Scannapieco G, Pauletto P, Sartore S. Nonmuscle myosin isoforms and cell heterogeneity in developing rabbit vascular smooth muscle. J Cell Sci 101: 233–246, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Grosskreutz CL, Anand-Apte B, Duplaa C, Quinn TP, Terman BI, Zetter B, D'Amore PA. Vascular endothelial growth factor-induced migration of vascular smooth muscle cells in vitro. Microvasc Res 58: 128–136, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Gustincich S, Schneider C. Serum deprivation response gene is induced by serum starvation but not by contact inhibition. Cell Growth Differ 4: 753–760, 1993 [PubMed] [Google Scholar]
- 24. Hermann DM, Zechariah A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J Cereb Blood Flow Metab 29: 1620–1643, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56: 549–580, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Holifield B, Helgason T, Jemelka S, Taylor A, Navran S, Allen J, Seidel C. Differentiated vascular myocytes: are they involved in neointimal formation? J Clin Invest 97: 814–825, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hull A, Long D, Longo L, Pearce W. Pregnancy-induced changes in ovine cerebral arteries. Am J Physiol Regul Integr Comp Physiol 262: R137–R143, 1992 [DOI] [PubMed] [Google Scholar]
- 28. Injeti ER, Sandoval RJ, Williams JM, Smolensky AV, Ford LE, Pearce WJ. Maximal stimulation-induced in situ myosin light chain kinase activity is upregulated in fetal compared with adult ovine carotid arteries. Am J Physiol Heart Circ Physiol 295: H2289–H2298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishida A, Murray J, Saito Y, Kanthou C, Benzakour O, Shibuya M, Wijelath ES. Expression of vascular endothelial growth factor receptors in smooth muscle cells. J Cell Physiol 188: 359–368, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA 97: 10242–10247, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jin X, Ge X, Zhu DL, Yan C, Chu YF, Chen WD, Liu J, Gao PJ. Expression and function of vascular endothelial growth factor receptors (Flt-1 and Flk-1) in vascular adventitial fibroblasts. J Mol Cell Cardiol 43: 292–300, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Kato M, Kyogoku M. Competence growth factors evoke the phenotypic transition of arterial smooth muscle cells. Ann NY Acad Sci 598: 232–237, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc Med 17: 140–143, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306–1309, 1989 [DOI] [PubMed] [Google Scholar]
- 35. Li D, Zhang C, Song F, Lubenec I, Tian Y, Song QH. VEGF regulates FGF-2 and TGF-beta1 expression in injury endothelial cells and mediates smooth muscle cells proliferation and migration. Microvasc Res 77: 134–142, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ Res 77: 638–643, 1995 [DOI] [PubMed] [Google Scholar]
- 37. MacDougall JD, Biswas S, Cook RP. The effects of certain C27 steroids on organ cultures of rabbit aorta. Br J Exp Pathol 46: 549–553, 1965 [PMC free article] [PubMed] [Google Scholar]
- 38. Manoury B, Etheridge SL, Reid J, Gurney AM. Organ culture mimics the effects of hypoxia on membrane potential, K(+) channels and vessel tone in pulmonary artery. Br J Pharmacol 158: 848–861, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marko SB, Damon DH. VEGF promotes vascular sympathetic innervation. Am J Physiol Heart Circ Physiol 294: H2646–H2652, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Miller JW. Vascular endothelial growth factor and ocular neovascularization. Am J Pathol 151: 13–23, 1997 [PMC free article] [PubMed] [Google Scholar]
- 41. Nauli SM, Williams JM, Gerthoffer WT, Pearce WJ. Chronic hypoxia modulates relations among calcium, myosin light chain phosphorylation, and force differently in fetal and adult ovine basilar arteries. J Appl Physiol 99: 120–127, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Nilsson I, Shibuya M, Wennstrom S. Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp Cell Res 299: 476–485, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Oishi K, Kobayashi A, Fujii K, Kanehira D, Ito Y, Uchida MK. Angiogenesis in vitro: vascular tube formation from the differentiation of neural stem cells. J Pharm Sci 96: 208–218, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Olfert IM, Breen EC, Mathieu-Costello O, Wagner PD. Chronic hypoxia attenuates resting and exercise-induced VEGF, flt-1, and flk-1 mRNA levels in skeletal muscle. J Appl Physiol 90: 1532–1538, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Osada-Oka M, Ikeda T, Imaoka S, Akiba S, Sato T. VEGF-enhanced proliferation under hypoxia by an autocrine mechanism in human vascular smooth muscle cells. J Atheroscler Thromb 15: 26–33, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Osol G, Laher I, Kelley M. Myogenic tone is coupled to phospholipase C and G protein activation in small cerebral arteries. Am J Physiol Heart Circ Physiol 265: H415–H420, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Owens GK, Thompson MM. Developmental changes in isoactin expression in rat aortic smooth muscle cells in vivo. Relationship between growth and cytodifferentiation. J Biol Chem 261: 13373–13380, 1986 [PubMed] [Google Scholar]
- 49. Pearce WJ, Hull AD, Long DM, Longo LD. Developmental changes in ovine cerebral artery composition and reactivity. Am J Physiol Regul Integr Comp Physiol 261: R458–R465, 1991 [DOI] [PubMed] [Google Scholar]
- 50. Peters KG, DeVries C, Williams LT. Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proc Natl Acad Sci USA 90: 8915–8919, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sandoval RJ, Injeti ER, Williams JM, Georthoffer WT, Pearce WJ. Myogenic contractility is more dependent on myofilament calcium sensitization in term fetal than adult ovine cerebral arteries. Am J Physiol Heart Circ Physiol 293: H548–H556, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Schubert R, Lidington D, Bolz SS. The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res 77: 8–18, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219: 983–985, 1983 [DOI] [PubMed] [Google Scholar]
- 54. Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, Dvorak HF. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev 12: 303–324, 1993 [DOI] [PubMed] [Google Scholar]
- 55. Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci 19: 5731–5740, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tai K, Vandenberg G, Hamaide MC, Wibo M, Morel N. Effect of organ culture on noradrenaline- evoked contraction, calcium signalling and TRPC expression in rat mesenteric artery. J Vasc Res 46: 353–364, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Thorne GD, Paul RJ. Effects of organ culture on arterial gene expression and hypoxic relaxation: role of the ryanodine receptor. Am J Physiol Cell Physiol 284: C999–C1005, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Todd ME, Gowen B. Arterial wall and smooth muscle cell development in young Wistar rats and the effects of surgical denervation. Circ Res 69: 438–446, 1991 [DOI] [PubMed] [Google Scholar]
- 59. Tsoi SC, Wen Y, Chung JY, Chen D, Magness RR, Zheng J. Co-expression of vascular endothelial growth factor and neuropilin-1 in ovine feto-placental artery endothelial cells. Mol Cell Endocrinol 196: 95–106, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Vonnahme KA, Wilson ME, Li Y, Rupnow HL, Phernetton TM, Ford SP, Magness RR. Circulating levels of nitric oxide and vascular endothelial growth factor throughout ovine pregnancy. J Physiol 565: 101–109, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Williams JM, White CR, Chang MM, Injeti ER, Zhang L, Pearce WJ. Chronic hypoxic decreases in soluble guanylate cyclase protein and enzyme activity are age dependent in fetal and adult ovine carotid arteries. J Appl Physiol 100: 1857–1866, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Yamawaki H, Sato K, Hori M, Ozaki H, Nakamura S, Nakayama H, Doi K, Karaki H. Morphological and functional changes of rabbit mesenteric artery cultured with fetal bovine serum. Life Sci 67: 807–820, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Zar JH. Biostatistical Analysis. Upper Saddle River, NJ: Prentice Hall, 1999 [Google Scholar]
- 64. Zetterberg A, Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci USA 82: 5365–5369, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 99: 2625–2634, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]