Abstract
Glutamate is the major excitatory neurotransmitter in the mammalian CNS and mediates fast synaptic transmission upon activation of glutamate-gated ion channels. In addition, glutamate modulates a variety of other synaptic responses and intracellular signaling by activating metabotropic glutamate receptors (mGluRs), which are G protein-coupled receptors. The mGluRs are also expressed in nonneuronal tissues and are implicated in a variety of normal biological functions as well as diseases. To study mGluR-activated calcium signaling in neurons, we generated mGluR5 transgenic animals using a Thy1 promoter to drive expression in the forebrain, and one founder unexpectedly developed melanoma. To directly investigate the role of mGluR5 in melanoma formation, we generated mGluR5 transgenic lines under a melanocyte-specific promoter, tyrosinase-related protein 1. A majority of the founders showed a severe phenotype with early onset. Hyperpigmentation of the pinnae and tail could be detected as early as 3–5 d after birth for most of the mGluR5 transgene-positive mice. There was 100% penetrance in the progeny from the tyrosinase-related protein 1-mGluR5 lines generated from founders that developed melanoma. Expression of mGluR5 was detected in melanoma samples by RT-PCR, immunoblotting, and immunohistochemistry. We evaluated the expression of several cancer-related proteins in tumor samples and observed a dramatic increase in the phosphorylation of ERK, implicating ERK as a downstream effector of mGluR5 signaling in tumors. Our findings show that mGluR5-mediated glutamatergic signaling can trigger melanoma in vivo. The aggressive growth and severe phenotype make these mouse lines unique and a potentially powerful tool for therapeutic studies.
Keywords: Gq, mGluR1, melanin
Metabotropic glutamate receptors (mGluRs) are G protein-coupled receptors that are widely expressed in the brain and modulate many diverse signaling pathways. In the CNS, mGluR activation regulates ion channels, mediates slow excitatory and inhibitory responses, modulates neurotransmitter release, and regulates neuronal development and growth (1–3). In addition, various neurological disorders have been attributed to functional impairment of mGluRs in the CNS. The mGluRs (mGluR1–8) are classified into three groups on the basis of sequence identity and pharmacological properties. Group I mGluRs (mGluR1 and mGluR5) are Gq-coupled receptors that activate PLCβ, resulting in intracellular Ca2+ release and protein kinase C (PKC) activation (4).
Although glutamate signaling is usually investigated in the context of CNS function, it clearly plays a role in nonneuronal cells as well. In particular, glutamate signaling has been described in astrocytes, cerebral endothelial cells, bone, and skin (5, 6). In bone cells, glutamate or NMDA application increases NMDA receptor currents (7). Similarly, mGluR5 expression has been observed in many types of cells other than neurons, including astrocytes, hepatocytes, melanocytes, osteoblast cells (8–11), fibroblast cells (12), and more recently, stem cells (13, 14). In astrocytes, mGluR5 triggers intracellular Ca2+ release that is potentiated by adenosine receptor activation (8). Furthermore, mGluR3 and mGluR5 regulate proliferation, differentiation, and self-renewal of stem cells of different origin (14).
Glutamatergic signaling has also been implicated in the biology of cancer (5, 12). For example, glutamate has been demonstrated to stimulate proliferation and migration of tumor cell lines (15, 16). Gliomas with high glutamate release show a distinct growth advantage, and the NMDA receptor antagonists MK801 and memantine slow the growth of glutamate-secreting tumors, suggesting that NMDA receptor activation facilitates tumor expansion (17). In addition, the NMDA receptor antagonist, dizocilpine, and/or the AMPA receptor antagonist, GYKI52466, exert antiproliferative effects in human tumor cell lines, including colon adenocarcinoma, astrocytoma, breast and lung carcinoma, and neuroblastoma cells (15). Moreover, NMDA receptor and AMPA receptor antagonists inhibit the ERK pathway, resulting in suppression of cancer growth (16, 18). The mGluRs have also been studied, with mGluR5 expression reported in human tumors and cancer cell lines (5, 12), such as breast cancer (12), colon cancer (12), sarcomas (10), squamous cell carcinomas (19) and various types of brain tumors (20). Interestingly, ectopic expression of mGluR1 induces melanoma (21). Altogether, these data suggest that mGluRs may be a relevant factor for development, proliferation, and progression of certain types of cancers.
Previously, we reported that calmodulin (CaM) binding to mGluR5 enhances receptor surface expression and Ca2+ signaling, whereas PKC phosphorylation of serine 901 (S901) inhibits CaM binding and decreases surface expression (22, 23). We also showed that mGluR5 S901A, which cannot be phosphorylated, binds to CaM independently of PKC activity and triggers prolonged Ca2+ oscillations. To investigate the in vivo effects of S901 phosphorylation of mGluR5, we generated transgenic mouse strains for mGluR5 wild type and S901A under the control of the Thy-1 promoter. Unexpectedly, we observed a profound tumor/melanoma phenotype in one of the mGluR5 S901A transgenic founders, which was propagated to offspring. To determine if mGluR5 expression in melanocytes was sufficient to induce melanoma, we next generated transgenic mice expressing mGluR5 wild type or S901A using a melanocyte-specific promoter, tyrosinase-related protein 1 (TRP1). Strikingly, like the original Thy1 line, both founders and progenies from TRP1-mGluR5 transgenic lines developed severe melanoma. These lines all developed aggressive tumors with early onset. In this report, we characterized these melanoma mice with molecular and histopathological methods and found that mGluR5 expression alone drives melanoma formation, demonstrating a role for glutamatergic signaling and specifically for mGluR5 in melanoma in vivo. These mouse lines potentially provide a model for studying the role of glutamatergic signaling in cancer.
Results
Generation of mGluR5 Transgenic Mice.
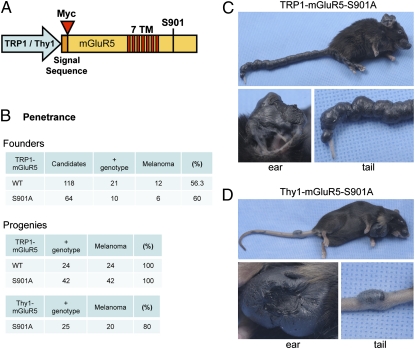
CaM binding to mGluR5 competes with PKC phosphorylation of S901 in the mGluR5 C-terminal domain (23). Mutating this residue to block phosphorylation, S901A affects CaM binding and the trafficking of mGluR5. To test the role of mGluR5 S901 in vivo, we produced transgenic mice expressing mGluR5 wild type or S901A under control of the Thy1 promoter, which is expressed in most regions of the forebrain (Fig. 1A) (24). Surprisingly, one Thy1-mGluR5 S901A founder developed pigmented tumors that were first identified as malignant melanoma in a pathology report. We have been able to propagate this melanoma-bearing transgenic line to date. To determine if mGluR5 expression in skin is a melanoma inducer, we constructed new transgenic lines in which the expression of mGluR5 is specifically targeted to melanocytes under control of the TRP1 promoter (25). By pronuclear injection of TRP1-mGluR5 (wild type or S901A) transgenes (Fig. 1A), we produced 21 genotypically positive founders for TRP1-mGluR5 wild type and 10 positive founders for TRP1-mGluR5 S901A (Fig. 1B). We found that 12 of the 21 mGluR5 wild-type founders displayed melanoma phenotypes (56.3% penetrance), and 6 of the 10 mGluR5 S901A founders developed melanoma (60% penetrance; Fig. 1B). We have maintained these TRP1-mGluR5 transgenic lines through several generations and find 100% penetrance of the melanoma phenotype in the progeny (Fig. 1B). In contrast, the original Thy1-mGluR5 S901A transgenic line showed 80% penetrance. The animals expressing TRP1-mGluR5 S901A developed severe melanoma on ears, nose, and tail. In particular, this strain displayed melanoma lesions encompassing the entire tail (Fig. 1C). The Thy1-mGluR5 S901A line also developed melanoma on ears, nose, and tail (Fig. 1D), which were typically focal and less severe.
Fig. 1.
mGluR5 expression induces melanoma formation. (A) Schematic of the mGluR5 transgene. A TRP1 or Thy1 promoter was used to drive expression of Myc-mGluR5. The Myc tag was inserted following the signal sequence. The seven transmembrane domains (7TM) are depicted in red. For each promoter, mGluR5 wild type and S901A were constructed. (B) Melanoma penetrance of founders and offspring. The mGluR5 wild-type or S901A strains, under the TRP1 or Thy1 promoter, were evaluated for melanoma formation among the genotypically positive mice. (C and D) Examples of severe melanoma observed in a transgenic TRP1-mGluR5 S901A mouse (C) and a Thy1-mGluR5 S901A mouse (D). The pinnae, nose, and tail showed numerous severe tumors in these mice. The mouse overexpressing mGluR5 under the TRP1 promoter has melanoma formation covering the entire tail (C, 11 mo old), whereas the mouse expressing mGluR5 under the Thy1 promoter displays focal tumors in the tail (D, 4 1/2 mo old). The magnified pictures of ear and tail are also shown below.
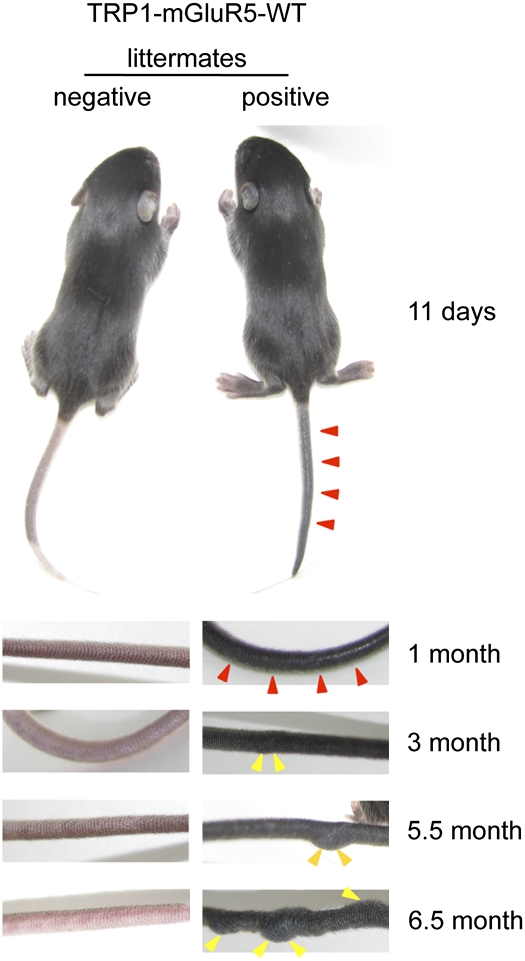
Transgenic Mice Display Hyperpigmentation Within Days and Later Develop Tumors.
We have characterized the time course of tumor progression in transgenic mice expressing TRP1-mGluR5 wild type (Fig. 2). These mice show hyperpigmentation of the ear and tail on or before 11 d of age, compared with transgene negative littermates. These mice typically develop tumors on the tail around 3 mo of age, which later form sizable tumor lesions at several sites on the tail. At ∼6–7 mo of age, the mice possess nodular tumor masses throughout the entire tail. Upon incidence of ulceration, the affected mice are euthanized as described in our animal protocol (Materials and Methods). The other mGluR5 transgenic strains (Thy1-S901A and TRP1-S901A) also displayed hyperpigmentation at early stages and later developed bulky tumors on ear and/or tail. However, melanoma progression was variable among individual mice and between strains, depending on the parental lines or the origin of the initial tumor. Some mice showed faster melanomagenesis, with hyperpigmentation being detected as early as 4 d after birth and developing to a full-blown melanoma at only 2 mo of age.
Fig. 2.
Age-dependent melanoma development in transgenic mice expressing TRP1-mGluR5 wild type. TRP1-mGluR5 wild-type transgenic mice were evaluated through development (11 d, 1 mo, 3 mo, 5.5 mo, and 6.5 mo of age). Images were taken of the tail to document melanoma progression compared with negative littermates. (Lower) Left panels display genotyped transgene-negative animals, and right panels show transgene-positive mice. The positive mice have intense pigmentation and/or melanoma tumors on tails. Red arrowheads indicate the hyperpigmentation in the mice at 11 d or 1 mo of age. Yellow arrowheads indicate the overt volumetric tumor formations in the mice of 3, 5.5, or 6.5 mo of age.
Histopathology of Mouse Melanoma in the mGluR5 Transgenic Mice.
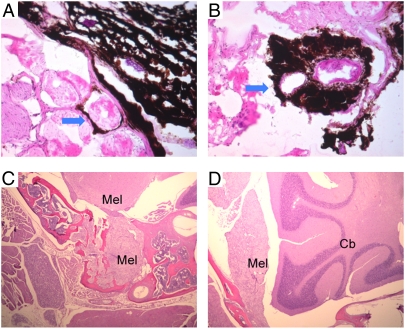
Typical melanoma lesions were first observed in glabrous skin such as pinnae of the ears, snout, perianal regions, eye, meninges, feet, and tail. Melanoma lesions were also detected in sentinel lymph nodes (cervical, axillary, aorta, and inguinal). In addition, lungs, spleen, and liver were found to have pigmented foci mixed with melanophages and melanoma cells. We observed two stages of mGluR5-induced melanoma (Fig. 3), including hyperpigmentation caused by horizontal growth of melanocytes at early stage (Fig. 3 C and D) and melanoma tumor formation initiated by vertical growth of melanoma cells in pinnae and tail at a later stage (Fig. 3 A, B, G, and H). The vertical growth of melanoma cells resulted in generalized thickening of the pinnae and tail with dramatically heavy deposits of dark melanin (Fig. 3 A, B, G, and H). The normal pinnae and tail showed minimal pigmentation (Fig. 3 E, F, I, and J). Melanin intensity or the pigmentation level of melanoma cells varied, as did the cell density. Spindle-shaped and rounded melanoma cells were dominant in the tumors, together with little mesenchymal structure. This was better demonstrated in the melanin-bleached samples with higher magnification.
Fig. 3.
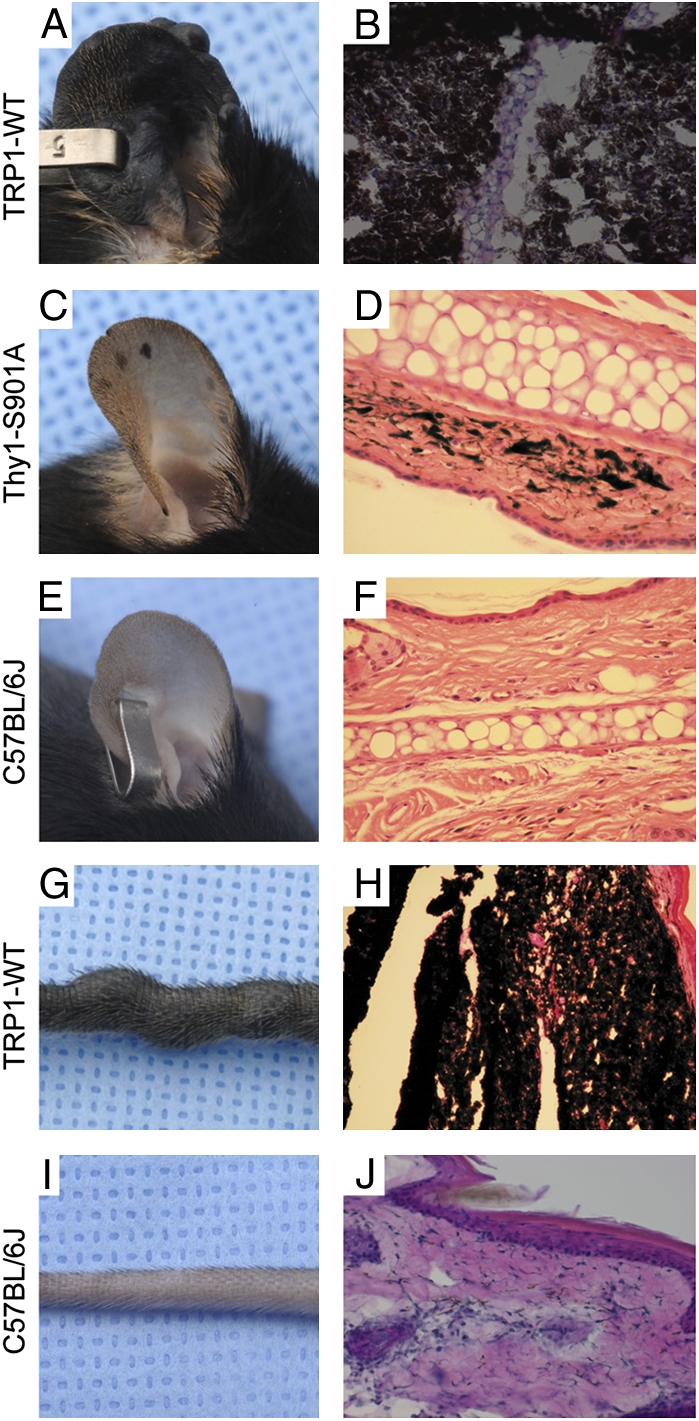
Gross and histologic examination of cutaneous melanomas in pinnae and tail of mGluR5 transgenic mice. The pinnae and tails from transgenic mice (TRP1-mGluR5 wild type or Thy1-mGluR5 S901A) or C57BL/6J were photographed and stained with H&E. Melanomas showed the nodular form of tumor burdens, generalized dermal thickness, and hyperpigmented tumor cells in the pinnae or tail of TRP1-mGluR5 wild-type transgenic mice (A, B, G, and H), whereas only a limited number of pigmented melanocytes were observed in normal pinnae or tail (E, F, I, and J). A few flat hyperpigmented lesions, representative of the early stage of melanomagenesis, were shown in pinnae of Thy1-mGluR5 S901A transgenic mice (C and D).
Melanoma Observed in Muscle and Bone.
Local invasion of melanoma cells is an important indicator of aggressive growth and malignancy. In some severe cases of melanoma from the mGluR5 transgenic mice, melanoma cells from tail skin were present under the basement membranes and underlying muscles as observed in horizontal sections of the tail (Fig. 4 A and B). In addition, severe forms of melanoma often affect tissues of uvea and meninges. Meningeal melanoma was often present at the bottom of the brain and penetrated deep into the skull bone (“Mel” in Fig. 4 C and D). The melanin hyperpigmentation frequently obscured the tissue features, which could be more clearly identified after melanin bleaching (Fig. 4 C and D). The morphology of these invading melanoma cells was similar to that of the primary melanoma. The invasive growth of these melanoma cells demonstrates the aggressive nature of the tumors and is consistent with malignant and/or metastatic melanomas.
Fig. 4.
Local invasion of tail and meningeal melanomas. In the late stage of melanomagenesis, highly aggressive melanoma cells from tail or meninges penetrate the underlying muscular layer (A and B) or bone tissues (C and D). (A and B) The H&E staining of tails from TRP1-mGluR5 wild-type transgenic mice. Arrows indicate invasion of melanoma cells to skeletal muscle. (C and D) Melanin-bleached specimen followed by H&E staining of Thy1-mGluR5 S901A transgenic mice. Mel, melanoma; Cb, cerebellum.
mGluR5 Expression Detected in Melanoma, but Not in Normal Pinnae.
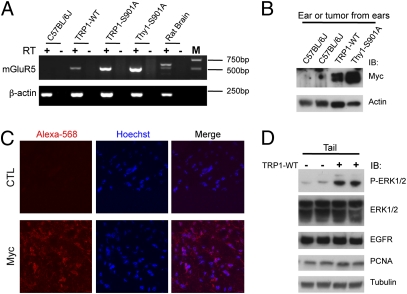
Melanoma tissues from the three different strains of mGluR5 transgenic mice, together with pinnae from normal C57BL/6J mouse and normal rat brain, were tested for rat mGluR5 mRNA expression using one-step RT-PCR with primers specific for rat mGluR5. Ectopic mRNA expression of mGluR5 was as high or higher than endogenous mGluR5 in rat brain (positive control) compared with β-actin expression, which was evaluated as a control (Fig. 5A). No positive bands were seen in reverse transcriptase-free PCR, indicating that there is no detectable genomic DNA contamination in the RT-PCR samples. To confirm the sequence of the RT-PCR products, several PCR products were subcloned into a TA cloning vector, and the resultant inserts were verified by DNA sequencing to be rat mGluR5 fragments encoding the first half of the C terminus of mGluR5. Normal skin of the C57BL/6J mice was used as a negative control for RT-PCR and found to be negative for ectopic rat mGluR5 expression. Furthermore, Myc-mGluR5 expression was evaluated by Western blotting of melanoma tumor samples from ears (Fig. 5B). The tumor samples from different transgenic lines (TRP1-mGluR5 wild type or Thy1-mGluR5 S901A) showed strong expression of Myc-mGluR5, in contrast to control ear samples from C57BL/6J mice. In addition, we conducted fluorescence-based immunohistochemistry to evaluate mGluR5 expression in tissues from the transgenic animals. By staining sections of tumors, we observed robust expression of Myc-mGluR5 compared with control (Fig. 5C). Taken together, the results from RT-PCR, Western blotting, and immunohistochemistry demonstrate that the mGluR5 transprotein is strongly expressed in tumor tissues.
Fig. 5.
Protein expression in melanoma tissue of transgenic mice. (A) Total RNA from normal pinnae, rat brain, and three different mGluR5 transgenic strains (TRP1-mGluR5 wild type, S901A, or Thy1-mGluR5 S901A) were DNase I-treated before they were used in PCR with or without reverse transcriptase (RT). Using RT-PCR, rat mGluR5 was expressed in the melanoma tissues from all three strains of transgenic mice, but not in the pinnae of normal nontransgenic mice (C57BL/6J pinnae) used as a negative control. mGluR5 is also detected in normal rat brain. (Upper) The strong band represents mGluR5b. (Lower) The weaker band is rat mGluR5a, which is the cDNA used as the transgene. β-Actin was used as a positive control that is present in all RNA in RT-PCR, and RT-free PCR were used as a negative control for assessing possible genomic DNA contamination, if any, in the RNA preparations. (B) The expression of the Myc-mGluR5 transgene. Tissue was analyzed by Western blotting using anti-Myc antibody. Ear samples from C57BL/6J mice and the tumors on ears of transgenic mice expressing TRP1-mGluR5 wild type or Thy1-mGluR5 S901A were tested for transgene expression. The melanoma tumors expressed Myc-mGluR5, whereas C57BL/6J mice did not. β-Actin was used for the internal control. (C) Ear sections of tumors from Thy1-Myc-mGluR5 transgenic mice (6 mo of age) were stained with Myc antibody to detect Myc-mGluR5, and receptor expression was visualized with Alexa 568-conjugated anti-rabbit IgG secondary antibody (red) and counterstained with Hoechst (blue). (Right panels) Merged images. Buffer alone (CTL) was used in place of primary antibody for the negative control. (D) Phosphorylation of ERK1/2 is increased in the TRP1-mGluR5 transgenic mice. The mouse tails from littermates, either positive or negative for TRP1-mGluR5 wild type (6 mo of age), were subjected to Western blotting with phospho-ERK1/2, ERK1/2, EGFR, and PCNA antibodies. Tubulin was used as a loading control.
Moreover, to investigate whether mGluR5 is present in human melanoma, a sensitive real time RT-PCR analysis was carried out using a pair of intron-spanning primers complementary to human mGluR5 exons. We used mGluR1, normal human melanocytes, and human brain as controls (Fig. S1). All of the mGluR1 and mGluR5 values were normalized with the level of β-actin for each sample. mGluR5 was detected in both human melanoma cell lines and in metastatic melanoma tissues (Fig. S1 A and B). Note that the amount of mGluR5 transcripts in human metastatic melanoma tissues (Fig. S1B) was generally higher than in human melanoma cell lines (Fig. S1A). Although mGluR5 mRNA was detected in normal human melanocytes (“Melanocytes” in Fig. S1A), each melanoma cell line also had higher amounts of mGluR5 than of mGluR1. In fresh human metastatic melanoma tissue, mGluR5 was highly expressed in all seven samples (Fig. S1B), whereas mGluR1 expression was detected in only one of seven samples examined (“HMM3” in Fig. S1B).
ERK1/2 Activation in mGluR5-Mediated Melanoma.
Many cellular proteins have been implicated in melanoma formation. For example, human melanoma cells frequently exhibit ERK activation, suggesting that constitutively activated ERK contributes to melanoma cell proliferation, invasion, and metastasis (26, 27). The tails from melanoma mice expressing TRP1-mGluR5 wild type were subjected to Western blotting to evaluate the expression of several proteins implicated in a variety of cancers. In particular, we monitored the phosphorylation state of ERK. The samples from transgene-positive mice displayed increased ERK1/2 phosphorylation, compared with those of negative littermates, whereas the total ERK1/2 expression level was not changed (Fig. 5D). Other oncogenic proteins, EGFR and PCNA, were also tested but did not show any striking changes in their expression levels. The analyses of the expression of other proteins, including N-cadherin, E-cadherin, NF-κB, tyrosinase, TRP1, and PTEN, revealed inconsistent changes in protein levels. We thus found variations between individuals and between strains that were likely due to tumor heterogeneity and different stages of tumor progression.
Discussion
In this study, we demonstrate that mGluR5 induces melanoma formation in vivo, providing evidence for the role of glutamatergic signaling in cancer. We generated transgenic mice expressing mGluR5 under the Thy1 or TRP1 promoter. At early stages, these mice displayed hyperpigmentation on ears and tails; and, at later stages, they developed dramatic tumor phenotypes, which were heritable with a high penetrance (over 80%). Moreover, melanoma cells were observed in the muscle, bone, and regional lymph nodes of the affected animals.
Signaling of group I mGluRs is regulated by CaM binding and PKC phosphorylation (22, 23). Specifically, phosphorylation of mGluR5 on S901 regulates CaM binding and receptor surface expression, and the mGluR5 S901A mutant, which cannot be phosphorylated, displays altered Ca2+ signaling (23). To investigate the S901A mutation in vivo, we generated mGluR5 transgenic mice (wild type and S901A). We unexpectedly observed melanoma in an mGluR5 S901A transgenic founder and suspected that this residue might be involved in the pathology. To address this hypothesis, we generated mGluR5 transgenic mice under the melanocyte-specific TRP1 promoter. We found that transgenic mice expressing either wild-type or S901A mGluR5 developed severe melanoma. The time course and severity of melanoma varied a great deal depending on the site of the initial tumor. However, the percentage of progeny displaying melanoma was the same with TRP1-mGluR5 wild type or S901A, suggesting that overexpression of mGluR5 in melanocytes induces the melanoma, irrespective of the phosphorylation of S901. The transgenic mGluR5 wild-type lines under the Thy1 promoter never produced a founder with melanoma, so we could not compare Thy1-mGluR5 wild type to mutant.
Although both mGluR1 and mGluR5 are Gq-coupled receptors that stimulate intracellular Ca2+ release, they have distinct signaling properties. For example, activation of mGluR5 evokes Ca2+ oscillations, whereas mGluR1 activation results in a single peak Ca2+ response (28). In addition, mGluR5 strongly binds to CaM, but mGluR1 does not (22). Furthermore, expression of mGluR1 and mGluR5 varies throughout the CNS, consistent with unique roles for these two receptors. Previous studies have implicated mGluR1 in melanoma (21, 29), but, until now, there was no evidence of a similar role for mGluR5. The studies on mGluR1 and melanoma began with the characterization of an insertional mouse mutant line, TG3, that was predisposed to develop multiple melanomas affecting the pinnae of the ear, perianal reion, eyelid, snout, trunk, and legs (30–32). Physical mapping of this TG3 line revealed that intron 3 of the Grm1 (mGluR1) gene was affected and resulted in aberrant expression of mGluR1 in skin tissues (21). Driving mGluR1 expression under the dopachrome taumerase (Dct) promoter in melanocytes also resulted in animals that developed melanoma (21). In addition, another melanoma mouse model was generated using a tetracycline regulatory system to induce mGluR1 expression (29). However, our findings that mGluR5 causes melanoma were not predicted. In fact, one study specifically tested the potential role of mGluR5 in the TG3 mGluR1-expressing melanoma mice by breeding the mGluR1 transgenic mice with mGluR5 knockout mice (33). Although the absence of mGluR5 did not eliminate mGluR1-induced melanoma, we now clearly show that mGluR5 overexpression alone induces severe melanoma, consistent with parallel mGluR pathways being capable of driving aggressive tumor formation.
The melanoma induced by our transgenic TRP1-mGluR5 mouse lines appears to be more severe than that reported for the Dct-mGluR1 lines. The TRP1-mGluR5 wild-type mice showed hyperpigmentation on the ear and tail at 4 d of age, developed tumor lesions by 3–4 mo of age, and formed large pigmented masses by 6–7 mo of age (as shown in Fig. 2). In contrast, Dct-mGluR1 E lines reportedly displayed pigmented lesions at 5–6 mo old, which progressed into tumors by 6–7 mo (21). Although the time course and severity of the lesions vary between the mGluR1 and mGluR5 mice, the affected tissues and histopathological evaluation are remarkably similar. Both mGluR1 and mGluR5 melanoma mice (TG3, Dct-mGluR1, TRE-mGluR1, TRP1-mGluR5, and Thy1-mGluR5) exhibited tumor formation on the hairless skin, including pinnae and tails, rather than on trunk areas (21, 29, 32). Taken together, these results implicate both group I mGluRs in melanocytic neoplasia. Several studies of the mGluR1 melanoma mice demonstrate that pharmaceutical inhibition can diminish the tumor progression. For example, treatment with mGluR1 antagonists (LY367385 or BAY36-7620) or a glutamate release inhibitor (riluzole) suppressed the proliferation of mGluR1-expressing human melanoma cells and inhibited the tumor growth in mGluR1 transgenic mice (29, 34). We predict that the mGluR5 transgenic mice will enable similar studies to investigate mGluR signaling in tumor formation.
Although mGluR5 has been largely investigated in the central nervous system, a variety of studies report the expression and/or function of mGluR5 in nonneuronal cells and cancer cells (8, 10, 13, 14, 19, 20). In particular, patients with oral squamous cell carcinoma tissues that are strongly positive for mGluR5 immunoreactivity had a decreased 5-y survival rate compared with mGluR5-negative patients (19). In addition, the mGluR5 agonist DHPG increased tumor cell migration, invasion, and adhesion in HSC3 oral tongue cancer cells, which was reversed by the mGluR5-specific antagonist MPEP (19). Taken together, these findings suggest that mGluR5 may be a relevant factor for the development, proliferation, and progression of multiple types of cancers and that mGluR5 might have potential as a biomarker for cancer cell proliferation.
mGluR1 has been studied extensively as a transforming factor for mouse melanomas using mGluR1 transgenic models (21, 29). Our current study reveals that expression of a related group I metabotropic glutamate receptor, mGluR5, leads to melanomagenesis in mice. Although mGluR5 is expressed in primary cultured human melanocytes (9) and mGluR1 is expressed in human melanoma samples (21), neither mGluR1 nor mGluR5 have been detected in human melanoma tissue via various transcriptomic studies (35). Using a sensitive real-time RT-PCR method and intron-spanning primers, we detected mGluR5 mRNA transcripts in both human melanoma cell lines and fresh human melanoma tissues at a level comparable to or higher than human brain tissue (Fig. S1). The amount of mGluR5 transcripts in human metastatic melanoma tissues (Fig. S1B) was generally higher than in human melanoma cell lines (Fig. S1A), suggesting that long-term culture of melanoma cells might alter the level of mGluR5 gene expression. Perhaps the normal expression of mGluR5 in human melanocytes is the reason that no difference in mGluR5 expression has been reported in microarrays of melanoma.
It is well established that group I mGluRs (mGluR1 and mGluR5) activate ERK in neuronal systems. In the spinal cord, intrathecal injection of DHPG, a group I mGluR agonist, activates ERK1/2, resulting in enhanced pain sensitivity (36). In addition, ERK activation is required for mGluR-LTD in area CA1 of the hippocampus (37, 38). Here, we show that tumor samples from the mGluR5 melanoma mice exhibit a high level of ERK1/2 phosphorylation compared with wild-type littermates. Our findings are consistent with studies on mGluR1-mediated melanoma, in which persistent mGluR1 expression correlated with high ERK1/2 phosphorylation in immunohistochemical analysis (29). Previous reports have shown that mGluR1 activates ERK in melanoma cells and, vice versa, that the ERK pathway plays a key role in melanoma growth (34, 39, 40). Stimulation of mGluR1 with l-quisqualate, a group I mGluR agonist, leads to IP3 accumulation, and activates ERK1/2 via PKCε in melanoma cells derived from the tumors expressing mGluR1 (40). In addition, in human melanoma cell lines expressing mGluR1, l-quisqualate–induced mGluR1 stimulation led to the ERK activation (34). Taken together, these results suggest that group I mGluRs activate the ERK pathway and that ERK activation is involved in mGluR-mediated melanoma growth.
In conclusion, we show a profound effect of mGluR5 expression in melanocytes, resulting in severe tumors. These mouse lines are striking because of the severe phenotype, early onset, and complete penetrance. Our findings show an important role for glutamatergic signaling in melanoma development in vivo. Together with the previous studies on mGluR1 melanoma mice, our mGluR5-mediated melanoma lines show that Gq-coupled signaling has profound effects on tumor progression in melanocytes. These mGluR5 transgenic mice provide a potentially valuable tool for drug screening as well as for preclinical testing in the future.
Materials and Methods
Detailed information on histopathological analysis, RT-PCR, Western blotting, and immunohistochemistry are available in SI Materials and Methods.
A Myc epitope was inserted into the N terminus of mGluR5 between amino acids 22 and 23 (23). To prepare TRP1 promoter-Myc-mGluR5, TRP1 promoter (1,201 bp), the ORF of Myc-tagged rat mGluR5 (3,558 bp), and a SV-40 poly(A) signal sequence (293 bp) were ligated into the pBluescript SK vector (Stratagene). The point mutation, S901A, was introduced using the QuikChange site-directed mutagenesis system (Stratagene). The construct was verified by DNA sequencing, and the expression of Myc-mGluR5 was evaluated in the heterologous cells by Western blotting. Similarly, Thy1-Myc-mGluR5 was constructed by combining the Thy1 promoter, Myc-mGluR5 ORF, and poly(A) sequence into the pUC18 vector. The diagram of transgenic constructs is shown in Fig. 1A. Each Myc-mGluR5 transgenic construct was gel-purified with the QIAGEN gel extraction kit. The DNA was filtered with a 0.22-μm Millipore filter and diluted with 1 mM Tris⋅HCl (pH 7.5), 0.05 mM EDTA buffer to 10 μg/mL. The DNA was microinjected into the pronuclei of fertilized embryos from the mating of C57BL/6J mice. The microinjected embryos were implanted into pseudopregnant recipients. The founder mice were identified by PCR genotyping with rat mGluR5-specific primers (5′-AGTGCCACAGTGGCCCTGGGTTGC-3′ and 5′-TGCCTCCGCCACATCATAAAGCGC-3′). The use and care of animals in this study followed the guidelines of the National Institutes of Health Animal Research Advisory Committee (animal protocol no. 1313–10).
Supplementary Material
Acknowledgments
We thank Michael Spencer and Bill Branson (National Institutes of Health Medical Art) for assistance with high-resolution photography; James Nagle and Debbie Kauffman (National Institute of Neurological Disorders and Stroke Sequencing Facility) for DNA sequence analysis; and the animal care staff of the Porter Neuroscience Center. The National Institute of Neurological Disorders and Stroke Intramural Research Program supported this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107304108/-/DCSupplemental.
References
- 1.Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 2.Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: Prototypic family C G-protein-coupled receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim CH, Lee J, Lee JY, Roche KW. Metabotropic glutamate receptors: Phosphorylation and receptor signaling. J Neurosci Res. 2008;86:1–10. doi: 10.1002/jnr.21437. [DOI] [PubMed] [Google Scholar]
- 4.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 5.Kalariti N, Pissimissis N, Koutsilieris M. The glutamatergic system outside the CNS and in cancer biology. Expert Opin Investig Drugs. 2005;14:1487–1496. doi: 10.1517/13543784.14.12.1487. [DOI] [PubMed] [Google Scholar]
- 6.Skerry TM, Genever PG. Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci. 2001;22:174–181. doi: 10.1016/s0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- 7.Laketić-Ljubojević I, Suva LJ, Maathuis FJ, Sanders D, Skerry TM. Functional characterization of N-methyl-D-aspartic acid-gated channels in bone cells. Bone. 1999;25:631–637. doi: 10.1016/s8756-3282(99)00224-0. [DOI] [PubMed] [Google Scholar]
- 8.Cormier RJ, Mennerick S, Melbostad H, Zorumski CF. Basal levels of adenosine modulate mGluR5 on rat hippocampal astrocytes. Glia. 2001;33:24–35. doi: 10.1002/1098-1136(20010101)33:1<24::aid-glia1003>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Frati C, et al. Expression of functional mGlu5 metabotropic glutamate receptors in human melanocytes. J Cell Physiol. 2000;183:364–372. doi: 10.1002/(SICI)1097-4652(200006)183:3<364::AID-JCP9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Kalariti N, Lembessis P, Papageorgiou E, Pissimissis N, Koutsilieris M. Regulation of the mGluR5, EAAT1 and GS expression by glucocorticoids in MG-63 osteoblast-like osteosarcoma cells. J Musculoskelet Neuronal Interact. 2007;7:113–118. [PubMed] [Google Scholar]
- 11.Storto M, et al. Selective blockade of mGlu5 metabotropic glutamate receptors protects rat hepatocytes against hypoxic damage. Hepatology. 2000;31:649–655. doi: 10.1002/hep.510310315. [DOI] [PubMed] [Google Scholar]
- 12.Stepulak A, et al. Expression of glutamate receptor subunits in human cancers. Histochem Cell Biol. 2009;132:435–445. doi: 10.1007/s00418-009-0613-1. [DOI] [PubMed] [Google Scholar]
- 13.Castiglione M, et al. Group I metabotropic glutamate receptors control proliferation, survival and differentiation of cultured neural progenitor cells isolated from the subventricular zone of adult mice. Neuropharmacology. 2008;55:560–567. doi: 10.1016/j.neuropharm.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Melchiorri D, et al. Metabotropic glutamate receptors in stem/progenitor cells. Neuropharmacology. 2007;53:473–480. doi: 10.1016/j.neuropharm.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Rzeski W, Turski L, Ikonomidou C. Glutamate antagonists limit tumor growth. Proc Natl Acad Sci USA. 2001;98:6372–6377. doi: 10.1073/pnas.091113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stepulak A, et al. NMDA antagonist inhibits the extracellular signal-regulated kinase pathway and suppresses cancer growth. Proc Natl Acad Sci USA. 2005;102:15605–15610. doi: 10.1073/pnas.0507679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takano T, et al. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7:1010–1015. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- 18.Stepulak A, et al. AMPA antagonists inhibit the extracellular signal regulated kinase pathway and suppress lung cancer growth. Cancer Biol Ther. 2007;6:1908–1915. doi: 10.4161/cbt.6.12.4965. [DOI] [PubMed] [Google Scholar]
- 19.Park SY, et al. Clinical significance of metabotropic glutamate receptor 5 expression in oral squamous cell carcinoma. Oncol Rep. 2007;17:81–87. [PubMed] [Google Scholar]
- 20.Brocke KS, et al. Glutamate receptors in pediatric tumors of the central nervous system. Cancer Biol Ther. 2010;9:455–468. doi: 10.4161/cbt.9.6.10898. [DOI] [PubMed] [Google Scholar]
- 21.Pollock PM, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- 22.Choi KY, Chung S, Roche KW. Differential binding of calmodulin to group I metabotropic glutamate receptors regulates receptor trafficking and signaling. J Neurosci. 2011;31:5921–5930. doi: 10.1523/JNEUROSCI.6253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, et al. Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5. Proc Natl Acad Sci USA. 2008;105:12575–12580. doi: 10.1073/pnas.0712033105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han W, et al. C-terminal ECFP fusion impairs synaptotagmin 1 function: Crowding out synaptotagmin 1. J Biol Chem. 2005;280:5089–5100. doi: 10.1074/jbc.M408757200. [DOI] [PubMed] [Google Scholar]
- 25.Lowings P, Yavuzer U, Goding CR. Positive and negative elements regulate a melanocyte-specific promoter. Mol Cell Biol. 1992;12:3653–3662. doi: 10.1128/mcb.12.8.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huntington JT, et al. Overexpression of collagenase 1 (MMP-1) is mediated by the ERK pathway in invasive melanoma cells: Role of BRAF mutation and fibroblast growth factor signaling. J Biol Chem. 2004;279:33168–33176. doi: 10.1074/jbc.M405102200. [DOI] [PubMed] [Google Scholar]
- 27.Satyamoorthy K, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–759. [PubMed] [Google Scholar]
- 28.Kawabata S, et al. Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature. 1996;383:89–92. doi: 10.1038/383089a0. [DOI] [PubMed] [Google Scholar]
- 29.Ohtani Y, et al. Metabotropic glutamate receptor subtype-1 is essential for in vivo growth of melanoma. Oncogene. 2008;27:7162–7170. doi: 10.1038/onc.2008.329. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Zhu H, Wetzel WJ, Philbert MA. Spontaneous melanocytosis in transgenic mice. J Invest Dermatol. 1996;106:1145–1151. doi: 10.1111/1523-1747.ep12340194. [DOI] [PubMed] [Google Scholar]
- 31.Zhu H, et al. Development of early melanocytic lesions in transgenic mice predisposed to melanoma. Pigment Cell Res. 2000;13:158–164. doi: 10.1034/j.1600-0749.2000.130307.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhu H, et al. Development of heritable melanoma in transgenic mice. J Invest Dermatol. 1998;110:247–252. doi: 10.1046/j.1523-1747.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- 33.Marín YE, et al. Grm5 expression is not required for the oncogenic role of Grm1 in melanocytes. Neuropharmacology. 2005;49(Suppl 1):70–79. doi: 10.1016/j.neuropharm.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Namkoong J, et al. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res. 2007;67:2298–2305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- 35.Hoek KS. DNA microarray analyses of melanoma gene expression: A decade in the mines. Pigment Cell Res. 2007;20:466–484. doi: 10.1111/j.1600-0749.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 36.Karim F, Wang CC, Gereau RW., IV Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci. 2004;24:4859–4864. doi: 10.1523/JNEUROSCI.5407-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiels E, Kanterewicz BI, Norman ED, Trzaskos JM, Klann E. Long-term depression in the adult hippocampus in vivo involves activation of extracellular signal-regulated kinase and phosphorylation of Elk-1. J Neurosci. 2002;22:2054–2062. doi: 10.1523/JNEUROSCI.22-06-02054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdel-Daim M, et al. Pharmacogenomics of metabotropic glutamate receptor subtype 1 and in vivo malignant melanoma formation. J Dermatol. 2010;37:635–646. doi: 10.1111/j.1346-8138.2010.00833.x. [DOI] [PubMed] [Google Scholar]
- 40.Marín YE, et al. Stimulation of oncogenic metabotropic glutamate receptor 1 in melanoma cells activates ERK1/2 via PKCepsilon. Cell Signal. 2006;18:1279–1286. doi: 10.1016/j.cellsig.2005.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.